Guthrie test
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
| ICD-11 |
|---|
Introduction
The Guthrie test (Newborn Blood Spot Screening, "Heel Prick" test, dried blood spots, dried-blood spots, DBS) is a neonatal blood screening test originally developed by Dr Robert Guthrie (1916-95) at the University of Buffalo. By 1963 the test had become a routine neonatal test for phenylketonuria.[1]
The Guthrie test or "Heel Prick" test is routinely carried out on neonatal (newborn 48-72 hours of age) blood for a variety of known genetic disorders. The clinical term "phlebotomy" describes the act of drawing or removing blood from the circulatory system through an incision or puncture to obtain a sample for analysis and diagnosis. In Australia screening currently includes 25 rare conditions.
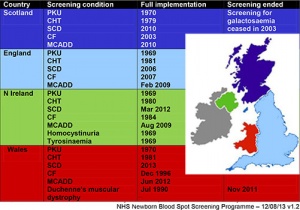
Note that different countries have different policies on:
- disorders included in the test
- archiving of this material
- deidentified availability for genetic research purposes.
An ultrasound study[2] has identified the shortest depth of perichondrium was in the centre of the heel and ranged from 3 to 8 mm. In 78 of the 80 infants in the study (GA24 to 42 weeks), the distance was 4 mm or more. Showing that the standard automated lancets for preterm use (puncture to a depth of 2.4 mm) may be safely used anywhere over the plantar surface of the heel avoiding the posterior aspect of the heel. A more recent study[3] identified the whole heel plantar surface is safe for obtaining blood in term and preterm infants of more than GA 33 weeks. A small amount of sucrose (0.012–0.12 g) can be given as an analgesic for newborns undergoing venepuncture or capillary heel-pricks.[4]
Blood is collected using a heelprick and spotted onto a test sheet to dry for later testing. Different countries and medical services have different policies on not only what will be tested for but also how long the test card will be kept following analysis. Check your local service for specific information.
| Historic Embryology |
|
Dr Robert Guthrie (1916-1995) was an American microbiologist at University of Buffalo who developed the collection of whole blood on filter paper "Guthrie cards" for transportation, storage, and testing for metabolic and genetic disorders of the newborn. The test today also has a number of names: "heel prick", "neonatal blood" test, and dried-blood spots. Guthrie is best known for developing the diagnostic test for phenylketonuria. |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: neonatal blood test | Guthrie test | heel prick test | congenital metaboloc disorder | phenylketonuria | hypothyroidism | cystic fibrosis | homocystinuria | | maple syrup urine disease | glutaric aciduria type 1 | spinal muscular atrophy |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Routine Screened Disorders
This list may differ between countries.
- phenylketonuria (PKU) (OMIM)
- Biotinidase Deficiency (OMIM)
- Congenital Adrenal Hyperplasia (CAH) (OMIM)
- Congenital Hypothyroidism (CH)
- Congenital Toxoplasmosis
- Cystic Fibrosis (CF) (OMIM)
- Galactosemia (GAL) (OMIM)
- Homocystinuria (OMIM)
- Maple Syrup Urine Disease (MSUD) (OMIM)
- Medium-Chain Acyl-CoA Dehydrogenase Deficiency (MCAD) (OMIM)
- Toxoplasma gondii IgM antibodies[15]
Phenylketonuria
| ICD-11 |
|---|
5C50.0 Phenylketonuria
5C50.00 Classical phenylketonuria | 5C50.01 Nonclassical phenylketonuria | 5C50.02 Embryofetopathy due to maternal phenylketonuria |
Incidence is about 1 in 10,000 live births (about 10 babies per year). PKU causes high blood levels of phenylalanine and severe intellectual disability. A diet low in phenylalanine, started in the first two to three weeks results in normal development.
5C50.00 Classical phenylketonuria - Classical phenylketonuria is a severe form of phenylketonuria (PKU, ) an inborn error of amino acid metabolism characterized in untreated patients by severe intellectual deficit and neuropsychiatric complications.
5C50.01 Nonclassical phenylketonuria - Mild phenylketonuria is a rare form of phenylketouria (PKU, ), an inborn error of amino acid metabolism, characterized by symptoms of PKU of mild to moderate severity.
5C50.02 Embryofetopathy due to maternal phenylketonuria - Maternal phenylalaninemia refers to developmental anomalies that may occur in offspring of women affected by phenylketonuria (PKU), and include fetal development disorders, including microcephaly, intrauterine growth retardation, and subsequent intellectual deficit, and embryo development disorders such as heart defects (usually conotruncal), corpus callosus agenesis, neuronal migration disorders, facial dysmorphism and more rarely cleft palate, tracheo-esophageal abnormalities.
- PubMed Search - Phenylketonuria
Galactosaemia
| ICD-11 |
|---|
5C51.4 Disorders of galactose metabolism
5C51.40 Galactose-1-phosphate uridyltransferase deficiency | 5C51.41 Galactokinase deficiency | 5C51.42 Glucose or galactose intolerance of newborn |
Galactosaemia incidence is about 1 in 40,000 births, about 1-3 cases per year. See a recent disease review[16] and a Cochrane Database review.[17] Babies cannot process galactose, a component of lactose. Life-threatening liver failure and infections can occur. A galactose-free diet instituted in the first week can be life saving.
5C51.41 Galactokinase deficiency - is a rare mild form of galactosemia characterized by early onset of cataract and an absence of the usual signs of classic galactosemia, i.e. feeding difficulties, poor weight gain and growth, lethargy, and jaundice.
- PubMed Search - Galactosaemia
Krabbe Disease
ICD-11 8A44.4 Krabbe disease
|
Krabbe Disease (globoid cell leukodystrophy, galactosylcerebrosidase deficiency, galactosylceramidase deficiency) Rare abnormality lysosomal disorderdue to mutation in the GALC gene producing less galactosylceramidase, an enzyme required for glial cells to make myelin that insulates nerve cells. This is also classified as a lysosomal disorder. There is a neonatal test for the disease that has an autosomal recessive inheritance
- Early-onset form - appears first months of life, lethal before age 2). See this recent review.[18]
- Late-onset form - appears in late childhood or early adolescence). pattern.
Maple Syrup Urine Disease
ICD-11 5C50.D0 Maple-syrup-urine disease
|
- Links: OMIM | Search PubMed
Biotinidase Deficiency
| ICD-11 5C50.E Organic aciduria - 5C50.E0 Classical organic aciduria This a term used to classify a group of metabolic disorders which disrupt normal amino acid metabolism, particularly branched-chain amino acids, causing a buildup of acids which are usually not present. |
Multiple carboxylase deficiency (MCD) is an autosomal recessive metabolic disorder characterized primarily by cutaneous and neurologic abnormalities. Symptoms result from the patient's inability to reutilize biotin, a necessary nutrient. (text from OMIM)
- Links: OMIM
Spinal Muscular Atrophy
ICD-11 8B61 Spinal muscular atrophy
8B61.0 Infantile spinal muscular atrophy, Type I | 8B61.1 Late infantile spinal muscular atrophy, Type II |
There are various forms of spinal muscular atrophy designated by the time of onset:
- Infantile - SMA type 1, onset of weakness may be prenatal (decreased fetal movements toward the end of pregnancy) or within the first six months of life.
- Late infantile - SMA type 2, muscle weakness is seen between the ages of 6 to 18 months.
- Juvenile - SMA type 3, weakness of muscles is seen after 18 months of age. The child is able to sit and stand independently.
- Adult - SMA type 4 weakness, most commonly develops after 35 years of age (less commonly between 18 to 35 years old).
Australia now (2018) includes heel prick screening for spinal muscular atrophy[8] (More? see ABC News)
Australia
<html5media width="600" height="400">https://www.youtube.com/embed/KVmLpVcnI1w</html5media>
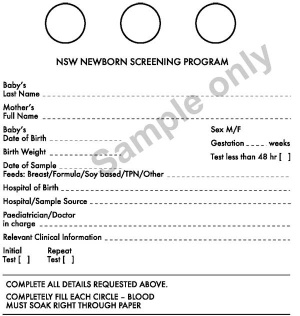
NSW Newborn Screening Programme
Each year test more than 90,000 babies and detects about 90 who need urgent assessment and treatment. In NSW and Victoria, the bloodspot cards are currently stored indefinitely.
- Phenylketonuria (PKU) - 1 in 10,000 live births (about 10 babies per year). PKU causes high blood levels of phenylalanine and severe intellectual disability. A diet low in phenylalanine, started in the first two to three weeks results in normal development.
- Primary congenital hypothyroidism - 1 in 3,500 live births (about 26 babies per year). It is caused by the absence or abnormal formation or function of the thyroid gland. This causes growth and intellectual disability if not treated. Medication with thyroid hormone started early, results in normal growth and development.
- Cystic Fibrosis (CF) - 1 in 2,500 live births (about 34 babies per year). Without treatment babies develop chest infections and often have very serious failure to thrive. Early institution of treatment greatly improves the health of babies with CF. Newborn bloodspot screening detects about 95% of babies with CF but also detects a few babies who may only be healthy carriers. For these babies a sweat test at about six weeks of age determines whether the baby has CF or is a healthy carrier.
- Galactosaemia - 1 in 40,000 births (about 1-3 cases per year). Babies cannot process galactose, a component of lactose. Life-threatening liver failure and infections can occur. A galactose-free diet instituted in the first week is life saving.
- Rarer metabolic disorders - Some fatty acid, organic acid and other amino acid defects can now be detected using Tandem Mass Spectrometry. These much rarer metabolic disorders affect about 15 – 18 babies per year. Early detection is important as diet and medications can treat most of these disorders. Without appropriate management they can cause severe disability or death.
Potential uses and access of stored bloodspots
- Identified cards may be used for family benefit or research and only with separate consent obtained before testing.
- Non-identifiable cards (identifiers permanently removed) may be used for research approved by a Health Research Ethics Committee – consent is not required.
- Parents have a right to access their child’s information. Other access requires parental consent except where there is a court order, to date this has not occurred.
Genetics services in NSW - coordinated by the NSW Genetics Service Advisory Committee, which is supported by the Statewide Services Development Branch of the Strategic Development Division, NSW Department of Health. (Information from NSW Health - Newborn Bloodspot Screening Policy 13-Nov-2006)
- Links: NSW Genetics Health
New Zealand
<html5media width="600" height="400">https://www.youtube.com/embed/xRbu8gV-JvI</html5media>
- Links: NZ newborn screening
USA
State laws mandate that blood be drawn from all newborn infants to screen for health-threatening conditions.
References
- ↑ GUTHRIE R & SUSI A. (1963). A SIMPLE PHENYLALANINE METHOD FOR DETECTING PHENYLKETONURIA IN LARGE POPULATIONS OF NEWBORN INFANTS. Pediatrics , 32, 338-43. PMID: 14063511
- ↑ Jain A & Rutter N. (1999). Ultrasound study of heel to calcaneum depth in neonates. Arch. Dis. Child. Fetal Neonatal Ed. , 80, F243-5. PMID: 10212093
- ↑ Arena J, Emparanza JI, Nogués A & Burls A. (2005). Skin to calcaneus distance in the neonate. Arch. Dis. Child. Fetal Neonatal Ed. , 90, F328-f331. PMID: 15871987 DOI.
- ↑ Stevens B, Yamada J & Ohlsson A. (2004). Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev , , CD001069. PMID: 15266438 DOI.
- ↑ van Vliet K, van Ginkel WG, van Dam E, de Blaauw P, Koehorst M, Kingma HA, van Spronsen FJ & Heiner-Fokkema MR. (2020). Dried blood spot versus venous blood sampling for phenylalanine and tyrosine. Orphanet J Rare Dis , 15, 82. PMID: 32245393 DOI.
- ↑ Pellegrinelli L, Alberti L, Pariani E, Barbi M & Binda S. (2020). Diagnosing congenital Cytomegalovirus infection: don't get rid of dried blood spots. BMC Infect. Dis. , 20, 217. PMID: 32164599 DOI.
- ↑ Bessey A, Chilcott JB, Leaviss J & Sutton A. (2018). Economic impact of screening for X-linked Adrenoleukodystrophy within a newborn blood spot screening programme. Orphanet J Rare Dis , 13, 179. PMID: 30309370 DOI.
- ↑ 8.0 8.1 Sampaio H, Wilcken B & Farrar M. (2018). Screening for spinal muscular atrophy. Med. J. Aust. , 209, 147-148. PMID: 30107765
- ↑ Wasim M, Awan FR, Khan HN, Tawab A, Iqbal M & Ayesha H. (2018). Aminoacidopathies: Prevalence, Etiology, Screening, and Treatment Options. Biochem. Genet. , 56, 7-21. PMID: 29094226 DOI.
- ↑ van Rijt WJ, Koolhaas GD, Bekhof J, Heiner Fokkema MR, de Koning TJ, Visser G, Schielen PC, van Spronsen FJ & Derks TG. (2016). Inborn Errors of Metabolism That Cause Sudden Infant Death: A Systematic Review with Implications for Population Neonatal Screening Programmes. Neonatology , 109, 297-302. PMID: 26907928 DOI.
- ↑ Hawkes N. (2014). Newborn babies will be tested for four more disorders, committee decides. BMJ , 348, g3267. PMID: 25134132
- ↑ Adam BW, Hall EM, Sternberg M, Lim TH, Flores SR, O'Brien S, Simms D, Li LX, De Jesus VR & Hannon WH. (2011). The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. , 44, 1445-50. PMID: 21963384 DOI.
- ↑ Nivoloni Kde A, da Silva-Costa SM, Pomílio MC, Pereira T, Lopes Kde C, de Moraes VC, Alexandrino F, de Oliveira CA & Sartorato EL. (2010). Newborn hearing screening and genetic testing in 8974 Brazilian neonates. Int. J. Pediatr. Otorhinolaryngol. , 74, 926-9. PMID: 20538352 DOI.
- ↑ Hardin J, Finnell RH, Wong D, Hogan ME, Horovitz J, Shu J & Shaw GM. (2009). Whole genome microarray analysis, from neonatal blood cards. BMC Genet. , 10, 38. PMID: 19624846 DOI.
- ↑ Schmidt DR, Hogh B, Andersen O, Fuchs J, Fledelius H & Petersen E. (2006). The national neonatal screening programme for congenital toxoplasmosis in Denmark: results from the initial four years, 1999-2002. Arch. Dis. Child. , 91, 661-5. PMID: 16861484 DOI.
- ↑ Demirbas D, Coelho AI, Rubio-Gozalbo ME & Berry GT. (2018). Hereditary Galactosemia. Metab. Clin. Exp. , , . PMID: 29409891 DOI.
- ↑ Lak R, Yazdizadeh B, Davari M, Nouhi M & Kelishadi R. (2017). Newborn screening for galactosaemia. Cochrane Database Syst Rev , 12, CD012272. PMID: 29274129 DOI.
- ↑ Beltran-Quintero ML, Bascou NA, Poe MD, Wenger DA, Saavedra-Matiz CA, Nichols MJ & Escolar ML. (2019). Early progression of Krabbe disease in patients with symptom onset between 0 and 5 months. Orphanet J Rare Dis , 14, 46. PMID: 30777126 DOI.
Reviews
van der Spek J, Groenwold RH, van der Burg M & van Montfrans JM. (2015). TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review. J. Clin. Immunol. , 35, 416-30. PMID: 25893636 DOI.
Articles
Abdelhakim M, McMurray E, Syed AR, Kafkas S, Kamau AA, Schofield PN & Hoehndorf R. (2020). DDIEM: drug database for inborn errors of metabolism. Orphanet J Rare Dis , 15, 146. PMID: 32527280 DOI.
Adam BW, Hall EM, Sternberg M, Lim TH, Flores SR, O'Brien S, Simms D, Li LX, De Jesus VR & Hannon WH. (2011). The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin. Biochem. , 44, 1445-50. PMID: 21963384 DOI.
Streetly A, Latinovic R, Hall K & Henthorn J. (2009). Implementation of universal newborn bloodspot screening for sickle cell disease and other clinically significant haemoglobinopathies in England: screening results for 2005-7. J. Clin. Pathol. , 62, 26-30. PMID: 19103854 DOI.
Whiteman PD, Clayton BE, Ersser RS, Lilly P & Seakins JW. (1979). Changing incidence of neonatal hypermethioninaemia: implications for the detection of homocystinuria. Arch. Dis. Child. , 54, 593-8. PMID: 507913
Search PubMed
Search Pubmed: Guthrie test | neonatal blood spot test
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Geneva: World Health Organization; 2010. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy.
- Drug Database for inborn errors of metabolism DDIEM a manually curated, ontologically formalized knowledgebase of drugs, therapeutic procedures, and mitigated phenotypes.
Australia
- PDF Screening Guidelines (2015)
- PDF Newborn Bloodspot Screening - NSW Health (2016)
- Australian Institute of Health and Welfare (AIHW)
- NSW Genetics Health
New Zealand
United Kingdom
- Screening Programmes
- UK National Screening Committee
- UK National Screening Committee - Meetings
- newborn blood spot
- newborn blood spot
USA
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Guthrie test. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Guthrie_test
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G