Book - Contributions to Embryology Carnegie Institution No.61
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Spaulding MH. The development of the external genitalia in the human embryo. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ. 81, 13: 69 – 88.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the External Genitalia in the Human Embryo
By Milo Herrick Spaulding,
Of the University of Montana, State College of Agriculture, Bozeman (1921). With four plates and two text-figures.
Introduction
The present investigation is concerned with the development of the external genitalia in the human embryo, the determination of the stage at which sex differences make their appearance, and how early sex can be recognized from these external structures. In addition to the morphological interest in the structural development of the phallus region, its embryology presents several other phases of almost equal importance. Thus the ability to recognize sex at an earlier period than has hitherto been possible is of great practical value to the clinician as well as to the embryologist. At the same time, this point is largely dependent upon the verification of the existence of a definite "indifferent" ("undifferentiated," Pohlman 1904) period through which all embryos have been generally believed to pass before assuming their definite male or female characteristics.
As a result of this study I have found that there is apparently no real indifferent period. On the contrary, the younger embryos show constant differences in the phallus and almost from the earliest differentiation of the genital tubercle (i. e., embryos 14 mm. GL) they can be divided into two groups. This division is based upon the marked difference in the length of the urethral groove upon the caudal slope of the phallus. This seems to be quite constant and without intergradations and can be traced backward from the older embryos, in which sex can be definitely recognized, to the younger stages which heretofore have been included in the socalled "indifferent" period. In one group the urethral groove extends from the base of the phallus practically to its apex, i. e., onto the region of the future glans; these I consider to be males. In the second group the groove is much shorter and terminates some distance below the apex of the phallus i. e., it does not extend onto the region of the future glans; these I consider to be females.
I wish to extend my sincerest thanks to Dr. G. L. Streeter for his kindness in according me the privilege of examining the extensive collection of human embryos in the Carnegie Laboratory of Embryology, as well as for the great interest he has taken and the many helpful suggestions he has made throughout the course of the investigation.
Materials and Methods
The material studied in the preparation of the present paper has been limited to the collection of human embryos in the Carnegie Laboratory of Embryology. This includes a large number of specimens which are graded 2 and 3; that is, they are unsuitable for sectioning because of faulty preservation, mutilation, or some other defect; also a considerable number of sectioned embryos up to 50 mm. in length. While perhaps more attention has been paid to the external features of these unsectioned embryos, as far as possible this evidence has been verified by a study of sectioned specimens of corresponding sizes, a number of these having been reconstructed in order to correlate the development of the internal urogenital organs with that of the external genitalia.
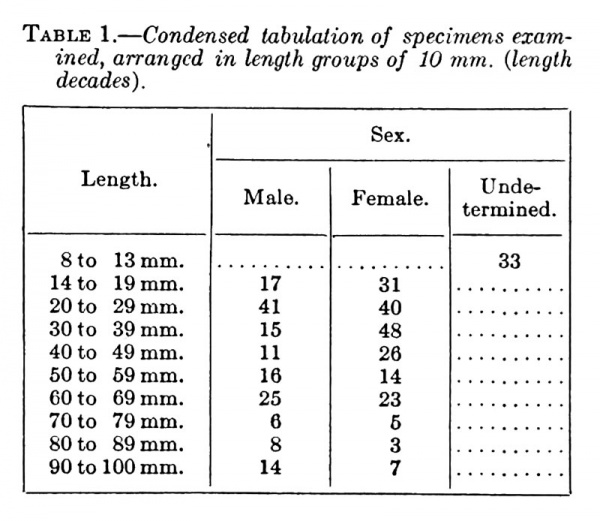
Table 1. Condensed tabulation of specimens examined, arranged in length groups of 10 mm. (length decades).
Only a condensed tabulation of the embryos examined, with the sex classified, is included in the present report, the complete list, including the serial number of each embryo with remarks upon its condition, having been placed on file among the records of the Carnegie Laboratory of Embryology. The sex grouping adopted in this table is based upon the diagnostic features brought out by the investigation.
Table 1 shows that there is considerable variation in the relative frequency of the sexes in each of the length decades and also that there is a greater number of females than males (145 to 84) in the period between 14 and 50 mm. length. This variation in the different groups would indicate that as yet a sufficient number of embryos has not been examined to permit definite deductions concerning the sex-ratio at this period of embryonic life. Conclusions, therefore, will have to be deferred until much more extensive data have been accumulated.
Critical examination has been made of practically all of the embryos in the collection between the lengths of 14 and 50 mm. About 80 of these, however, have been discarded from present consideration because, owing to mutilation or poor state of preservation, a clear picture of the external genitalia could not be obtained; therefore, no interpretation of sex was possible. Of the older stages (51 to 100 mm. CR) only selected specimens have been studied. The accompanying list, therefore, includes all the well-preserved embryos between the length of 14 and 50 mm., both sectioned and unsectioned, and a small proportion of the older specimens.
No attempt was made to identify the sex of embryos smaller than 14 mm., although it is believed that a careful study of a sufficient number of such younger stages will show that the sex differences here pointed out can be traced back to the very beginnings of the genital tubercle.
Historical
When the early scientists began the study of human embryology with the few specimens that came into their possession, the questions of sex recognition and the relations between the sexes attracted their attention to the study of the external genitalia. When we of the present day consider the paucity of their material, as well as the comparative crudity of their instruments and methods, we can not but be surprised at the accuracy of their observations and conclusions. That there were misinterpretations was due largely to insufficient material and defective apparatus, rather than to faulty observation.
As the result of this early work we find, in reviewing the literature on the subject, that various views were held by these early scientists concerning the sex of the younger embryos during the so-called indifferent period. Many of them considered that the younger embryos represented a stage intermediate between males and females. Others, for example Tiedemann (1813), who is conspicuous for the accuracy of his observations, believed that, because so many more females than males were observed, all human embryos were at first female and that the males resulted from an advance in development over this more primitive condition. The subsequent investigations of Joh. Muller (1830), Rathke (1832), and Bischoff (1842) resulted in but minor additions to the observations of Tiedmann. In fact, Muller considered that the latter were so remarkably complete that additional investigations could not be expected to produce anything new. Ecker (1851-59) published some excellent figures of the external genitalia of human embryos, which have served as a basis for the somewhat inaccurate Ecker-Ziegler wax models of this region. No other notable papers were published until about 1889, when the development of modern instruments, the introduction of modern methods of technique, and the consequent improvement in the quality of the preparations, resulted in a renewed interest in all branches of biological study. In the embryology of the human urogenital system this era was heralded by the work of Tourneux (1889) upon a series of 35 specimens ranging in length from 24 mm. to 35 cm. Only 10 of these were less than 40 mm. in length, while the majority were fetuses over 50 mm. in length. Although Tourneux described the external genitalia of the majority of these specimens, several of which are shown by fairly accurate figures, the number of young embryos was relatively so few that his attention was not directed toward possible sex differences in these early stages; hence, more consideration was given to the histological study of the urethral canal and the formation of the prostate. In the same year Nagel published the results of his study of a small series of human embryos. This was also devoted mainly to the internal urogenital system, although Nagel made some observations upon the external genitalia which, in part, predicted some of the results of the present study. He believed, for example, that the urethral groove should furnish a clue to the sex of an embryo at a much earlier period than it had previously been possible to recognize sex, although his observations indicated that the males possess the shorter groove, while my findings tend to show quite the opposite. Nagel furthermore found that the outer genital (labio-scrotal) swellings do not arise as a more or less complete ridge surrounding the phallus, as had previously been figured, but as separate, lateral swellings which only later become connected in front of the phallus in the female, and in the male are shifted to a caudal position to form the scrotum. The classical investigation of Keibel (1896), giving an exhaustive account of the development of the internal urogenital organs, based upon a series of 13 embryos (3 to 25 mm. in length) , was probably the first study of this system by the modern method of plate reconstruction whereby it has been possible to correlate the development of the external with the internal organs. While Keibel figures the external genitalia in several of his reconstructions, especially those of embryos 11.5 and 25 mm. long, which show the shorter urethral groove that I have found to be characteristic of female embryos, no special description is given of these structures. In fact, he realized that for the investigation of the development of the external genitalia a large series of embryos was necessary, and this portion of his paper is for the most part devoted to a plea to his colleagues for more specimens.
While I realize that I have been more fortunate than any of my predecessors in having at my disposal a larger and more complete series of embryos, I still would reiterate Keibel's plea for more material; because it seems that the more specimens one has at his disposal, the more one realizes the need of additional material in order to study some of the points concerning which there is still a conflict of opinion.
In 1904 Herzog pointed out that the direction of the phallus made it possible to recognize the sex of an embryo at about the beginning of the third month, instead of towards its close. This difference in direction he showed to consist in a greater caudal decurvation of the female phallus, while that of the male remained more nearly at right angles to the axis of the body. His series consisted of 16 embryos, varying in length from 20 to 190 mm. Of these, he figures the external genitalia of only 9, none of which bring out very clearly his point of directional difference of the phallus. Some excellent figures of the external genitalia are given by Otis (1906), although the discussion of the genitalia does not form a logical part of his paper.
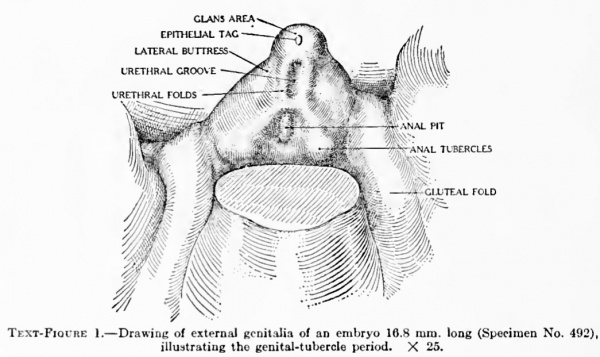
Text-Figure 1. Drawing of external genitalia of an embryo 16.8 mm long (Specimen No. 492), illustrating the genital-tubercle period. X 25.
From 1890 to 1907 a number of valuable contributions upon the development of the urogenital system in mammalia, especially the domesticated forms, made their appearance. While a number of these (notably the series of investigations carried out by Flcischmann and his pupils) pertained more or less to the development of the external genitalia, they have proved of but little use in the present investigation, since these papers failed to bring out any early sex differences in the forms studied. Felix (1912) gave an exhaustive summary of the accumulated observations upon the development of the human urogenital system, although his discussion of the external genitalia is rather meager as compared to that of the internal organs. Certain of my observations have led me to conclusions different from those reached by Felix ; the discussion of these moot points will be taken up in the section describing the development of the external genitalia.
Stages of Development of the External Genitalia
- Online Editor - Please note that the "stages" referred to in this paper are not Carnegie stages, use the embryo CRL to approximately convert to Carnegie Stages.
While the greater part of the investigation has been devoted to a study of the changes occurring after the embryos have attained a length of 14 mm., a few observations have been made upon younger specimens which are here included as forming a starting-point for the present description.
For the sake of convenience, the sequence of development of the external genitalia has been divided into three periods, each of which is represented by one or more stages:
- genital-tubercle period, characterized by the more or less conical form of the genital eminence prior to the formation of the labio-scrotal swellings.
- phallus period, beginning with the definite appearance of the labio-scrotal swellings which separate the conico-cjdindrical outgrowth of the genital tubercle from the surrounding body areas.
- definitive period, characterized (particularly in male embryos) by the transition of the primary external genitalia into what is essentially their final form.
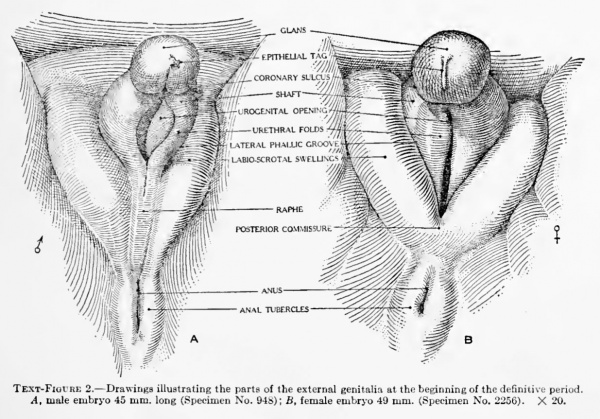
Text-Figure 2. Drawings illustrating the parts of the external genitalia at the beginning of the definitive period. A, male embryo 45 mm. long (Specimen No. 948); B, female embryo 49 mm. (Specimen No. 2256). X 20.
Genital-Tubercle Period
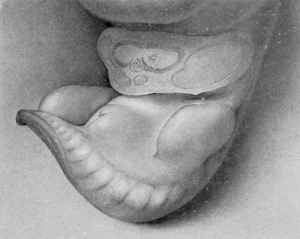
Stage 1
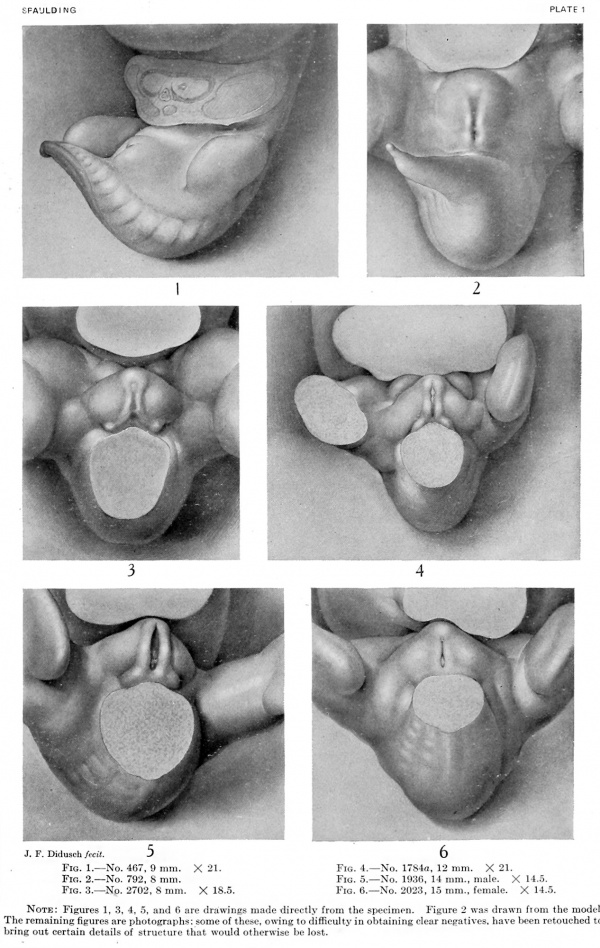
9 mm (fig. 1, plate 1). The earliest stage that I have studied is shown in an embryo 9 mm. long. In this specimen the genital tubercle is a very low conical eminence between the umbilical cord and the base of the tail. Its apex is separated from the rest of the tubercle by a pair of converging depressions which unite in the midventral line to form a longitudinal groove extending to the caudal border, giving to the entire groove a Y-shape. Caudal to the arms of the Y and lateral to its stem is a pair of broad, swollen areas. This condition is slightly different from the stage described by Keibel (1896) of the beginning development of the genitalia in a 3-mm. embryo. In his specimen he described the cloacal tubercle as consisting of a pair of eminences separated by the cloacal membrane. The difference between the stage described by Keibel and my first stage may be accounted for entirely by the difference in the size of the two specimens.
The term genital tubercle, which I use to describe the entire genital eminence, has a slightly different meaning from the one given to it by Felix (1912, p. 948). That author speaks of the genital eminence at first as the cloacal tubercle, which is later divided into a basal genital tubercle and a terminal phallus. According to his usage, the former develops into the genital (labio-scrotal) swellings, while the latter forms the phallus proper. My observations indicate that the entire primordial genital eminence should be considered as the precursor of the phallus and as such should be termed the genital tubercle. As will be shown later, my observations also indicate that the labio-scrotal swellings apparently originate from the outlying tissue and not from the basal portion of the primary tubercle. But even without this modified interpretation of their origin, the term genital tubercle seems most inapplicable to these swellings (which are decidedly secondary, both in genital structure and in function) and is therefore much more a propos of the genital eminence as the precursor of the phallus.
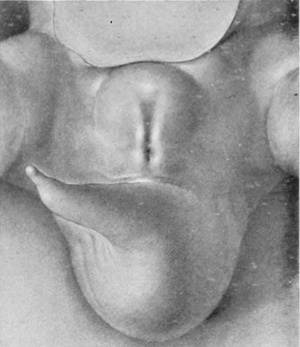
Stage 2
8 mm (fig. 2, plate 1). The second stage, represented by the reconstruction model of an 8-mm. embryo, shows considerable advance in the development of the genital tubercle. This now forms a somewhat rounded mass which occupies almost the entire area between the umbilical cord and the base of the tail. Its cranial margin is but barely indicated by a slight groove between its apex and the umbilical cord. The apex is broadly rounded and from it the slightly convex caudal surface slopes toward the base of the tail. The caudal slope is almost bisected by the urethral groove, a shallow depression extending from the base to the tip of the tubercle. The basal end of the groove is separated from the anal pit by a narrow transverse bar. It is significant that, while there is this external separation of the primitive cloacal groove into the urethral groove and anal pit, the internal division of the cloacal cavity into urogenital sinus and rectum is not yet complete, at least in this embryo. The margins of the urethral groove are but slightly elevated into the urethral folds, although the margins of the anal pit are more pronounced. Laterally the caudal surface of the tubercle is rounded out into pronounced swellings. Almost in front of the tubercle the ventral body-wall bears a pair of swellings Iying in the umbilico-phallic angles, the significance of which has not yet been definitely ascertained. These masses show a gradual increase in size until the embryo reaches a length of 16 mm. They then apparently disappear in some embryos, while in others they undergo a caudal shifting; this would seem to indicate that they are the primordia of the labio-scrotal swellings, which definitely make their appearance in embryos 17 to 19 mm. long. As yet, however, a sufficient number of these younger embryos has not been examined to permit definite conclusions on this point.
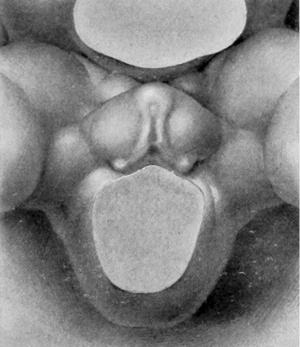
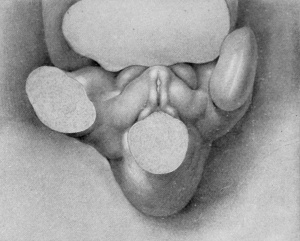
Stage 3
8 to 12 mm. (fig. 3 and fig. 4). As growth proceeds, the genital tubercle is transformed into a compressed, conical protuberance. This is brought about by the deepening of the umbihco-phallic groove, so that the cranial slope of the tubercle is nearly straight (i. e., approximately at right angles with the body axis), while the caudal slope remains decidedly convex. At the same time the caudal outline has become markedly triangular by the broadening of the base until it occupies practically the entire area between the bases of the legs. The conspicuous lateral slopes form the "lateral buttresses" which, arising from the cranial border of the tubercle just proximal to its apical area, have a decidedly caudal trend, so that they finally disappear basally opposite the caudal border of the tubercle. The apex of the tubercle is now clearly marked off from the more proximal portion by a shallow circular depression, indicating it as the future glans and separating it from the basal shaft. The urethral groove is a long, lancet-shaped depression, broadest and deepest basally, narrowing distally into a shallow slit limited by a very small "epithelial tag" just proximal to the primitive glans area. The urethral folds (margins of the groove) are elevated as shght rolls of tissue which distally merge into the glans region, while basally they become more tumid and broaden out to surround the anal pit as anal tubercles. Lateral to these urethral folds, the caudal surface of the tubercle is somewhat swollen in the younger specimen illustrating this stage (fig. 3), in marked contrast to the decided concavity of these regions in the older embryo (fig. 4) . In the former (fig. 3) the urethral and anal membranes have not ruptured. The older embryo (fig. 4) agrees with the finding that (in the majority of cases) these membranes rupture at about the stage of 12 to 13 mm., although several specimens were found, among both the sectioned and unsectioned material in which the membranes were still imperforate at 17 mm. The perforation of this membrane transforms the shallow urethral groove into a gutter-like, primitive urogenital opening as a direct communication between the phallic portion of the urogenital sinus and the exterior. This is accompanied by an increase in the definiteness of its outlines, as the result of which its sex difference in length is correspondingly emphasized. Because of the variation in the time of rupturing of this membrane, as well as on account of normal differences in the breadth of the opening thus formed, it seems advisable to continue to use the term urethral groove, except when referring to embryos that clearly show this feature as an opening.
While there is an apparent sex difference in the lateral outline of the tubercle in embryos 10 to 16 mm. in length, in that in some its tip is placed slightly more cranially than in others, thus producing a wider separation between the tubercle and the tail in the first group than in the second, a sufficient number of embryos has not been examined to permit of definite conclusions on this point, because of the possibility that some distortion of the embryos may have been brought about by manipulations during fixation.
Stage 4
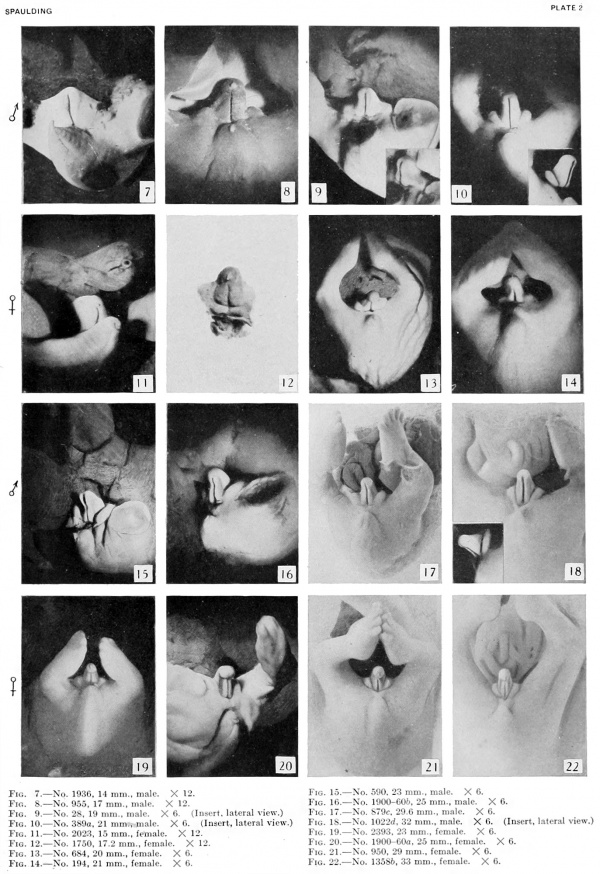
11 to 15 mm. (fig. 5, fig. 7, male; fig. 6, fig. 11, female). In this stage the sex difference in the length of the urethral groove becomes clearly evident. But slight changes have taken place in the general shape of the genital tubercle. In the male (figs. 5, 7) the glans area is more clearly indicated than in the female (figs. 6, 11) . In both embryos the urethral groove is sharply outlined, making it possible to contrast its difference in length in the tvwo specimens. In the female the epithelial tag is more pronounced than in the male and the entire tubercle is slightly more swollen.
Phallus Period
Stage 5
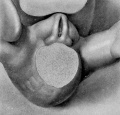
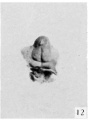
16 to 17 mm. (fig. 8, male; fig. 12, female). By the time the embryo has attained a length of 16 to 17 mm. the genital tubercle has elongated into a narrow, conical organ which, because of its modified shape and its separation from . the surrounding body areas by the newly formed labio-scrotal swellings, will now be called the phallus. The male embryo representing this stage (fig. 8) shows the phallus as more nearly cylindrical than in any of the younger embryos, the lateral buttresses having, to a marked extent, merged into its body. The groove limiting the glans is present, although not clearly shown in the photograph. The urogenital opening is a narrow orifice extending almost the full length of the phallus, limited distally by a pronounced epithelial tag. The urethral folds are considerably broader than in any of the younger stages, their outer margins being sharply separated from the remains of the lateral buttresses by a concave depression. The most pronounced change, however, is the presence of the labio-scrotal swellings as a pair of distinct, rounded ridges, one on each side of the base of the phallus and separated from it by a broad lateral phallic groove. As regards this feature, it is quite likely that this embryo represents a slightly older stage, as the male specimen of the next stage shows more clearly the probable beginnings of these swellings, and the female selected as the counterpart for this stage does not show them to any marked degree, if they are present at all. It is, of course, very possible that there is normally more or less variation in the time of development of these structures, and that this embryo (fig. 8) is merely slightly precocious in this respect.
The female of this stage is represented by the dissected-out phallus region of an embryo of the same length. The phallus has the same general configuration as that of the male just described, with the exception of a shorter urogenital opening terminated by a larger epithelial tag, a greater fullness in the genital folds, the presence of a straight postanal bar which is grooved by a slight median longitudinal depression flanked on each side by anal tubercles, and the apparent absence of the labioscrotal swellings. The presence or absence of these swellings in this specimen must, however, remain an open question, although the indications point strongly to their absence. In this respect the embryo seems to be more nearly normal (so far as sequence in the appearance of the modified parts of the external genitalia is concerned) than its coordinate male.
Stage 6
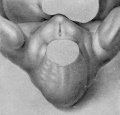
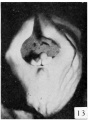
19 mm. CR (fig. 9, male; fig. 13, female). In the male of this stage the phallus is still of narrow, conical form, with the glans area rather more sharply indicated by a broad, band-like depression, not as clearly shown in the photograph as in the embryo itself. The remains of the lateral buttresses arise just proximal to this depression, spreading laterally for a short distance, then continuing basally almost parallel to the axis of the phallus until they finally merge into the tissue at its base. Unlike most of the younger embryos examined, there is almost no caudal trend to these buttresses. The urogenital opening has deepened and become more pronounced. It extends from the base of the phallus practically to its tip. In this specimen it is not limited distally by an epithelial tag. The lack of this appendage, useless as it at present seems, is probably due to some loss prior to or during fixation. The distal portion of the groove (the glans portion) forms a diamond-shaped dilatation extending to the proximal limits of the glans, the remainder of the groove being constricted into a deep, narrow slit. The urethral folds are rather pronounced elevations which diverge distally to merge into the glans, while proximally they are considerably thickened into a pair of caudally projecting, diverging masses (bulbo-urethral swellings?) which merge laterally into the outhing labio-scrotal swellings. They are separated from the cavernous portion of the phallus by broad concavities. Perhaps the most interesting feature of this embryo is that it shows the apparent beginnings of the labio-scrotal swellings. These are a pair of flat, elevated areas on either side of the base of the phallus with which they seem to be continuous. The cranial margins of these areas are nearly straight, extending at diverging angles into the inguinal regions, where their cranio-lateral angles continue for a short distance as ridges of tissue. The caudal margins appear as continuations of the diverging wings of the basal portions of the urethral folds, forming sinuous curves to the caudo-lateral angles, from which points they sweep cranially in rounding curves to the cranio-lateral angles, where they unite with the cranial margins. This is the only specimen which shows these swellings definitely connected with the base of the phallus. In all others in which they are developed there is a groove (lateral phallic groove) on each side which separates them from the base of the phallus.
As regards the formation of the labio-scrotal swellings, my observations diverge from the description given by Felix (1912, pp. 948-949) :
"The cloacal tubercle is thus divided into the almost circular base of the phallus and the semilunar genital tubercle (fig. 639). This latter represents an originally unpaired structure Iying cranial to the phallus and surrounding it on two sides; from it there are formed later the two genital swellings. The genital tubercle is separated from the phallus in female embryos by a deep groove which is the anlage of the later sulcus n}^npho-labialis, the groove between the labia majora and minora. In the male embryos this groove is absent from the beginning."
My own findings indicate that the labio-scrotal swellings arise as paired outgrowths from the outlying tissue and not from a basal segment of the primary tubercle. Furthermore, all but one of the specimens I have examined, both male and female, show these swellings as separated from the phallus by the lateral phallic grooves, a condition which is in striking contrast to the concluding statement made by Felix.
The coordinate female (fig. 13) shows, more clearly than any of the younger specimens, the distinction between the glans and shaft, although the division is indicated more by changes in the surface modeling than by the formation of a definite coronary sulcus. There is also a very slight indication of the caudal decurvation which was first pointed out by Herzog (1904) as an early sex difference. The urogenital opening is clearly limited to the shaft, being broader basally and narrowing distally into a mere slit. The urethral folds are still quite broad and are separated from the cavernous portion by shallow depressions. The labio-scrotal swellings resemble those already described for the male of the preceding stage.
Stage 7
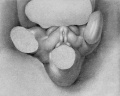
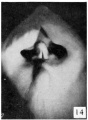
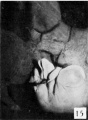
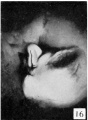
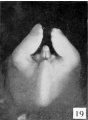
21 to 23 mm. CR (fig. 10, fig. 15, males; fig. 14, fig. 19, females). While there is a slight increase in the length of the phallus at this stage, the most conspicuous changes are the increase in the relative sex differences in the length of the urogenital opening and the greater development of the genital folds and the labio-scrotal swellings. In the males figured, the greater length of the urogenital opening is clearly seen to be in sharp contrast to the much shorter opening of the females. The urethral folds at this stage are more pronounced than in any of the embryos previously described. As a result of the deepening of the lateral depression between these folds and the cavernous portion of the shaft, their median portions now project from the shaft as compressed ridges. Distally they merge into the terminal glans area, while proximally they broaden out into the overhanging pre-anal enlargements (bulbo-urethral swellings?). As a result of this modification, the shaft of the phallus now shows clearly two distinct regions: the heavily thickened, cavernous portion, formed by the medial migration of the lateral buttresses, and the caudal urethral portion, represented by the projecting urethral folds outlining the urogenital opening, a condition which persists throughout the remainder of the phallus period. The labio-scrotal swellings have now grown into y-shaped enlargements, separated from the phallus by grooves more pronounced in the males than in the females. Cranially, these swellings are separated from each other by a triangular prolongation of the base of the phallus, so that there is no indication of the horseshoe shape which is so characteristic of most of the older illustrations. The caudal extremities of these swellings are joined together by a low, curving postanal ridge (slightly larger in the female), which is almost bisected by a median sagittal groove.
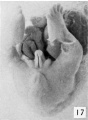
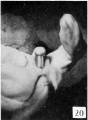
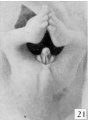
Stage 8
24 to 28 mm. CR (fig. 16, fig. 17, males; fig. 20, fig. 21, females). This stage is represented by an exceedingly interesting pair of twins. In the male (fig. 16) the nearly cylindrical phallus projects at approximately right angles to the body axis, in marked contrast to a pronounced decurvature of that of its mate (fig. 20). The glans is not so sharply demarked as in the female, although there are slight indications of its extent. The urogenital opening extends almost to the tip of the phallus as a shallow, open groove deepened basally by the caudally extended, platelike urethral folds. As a result of this increase in the folds, the base of the phallus is considerably longer than its apex. The labio-scrotal swellings are separated from the base of the phallus by broad, shallow grooves; their tips curve cranio-laterally away from the phallus, leaving a rather broad, unswollen area between the latter and the umbilical cord.
In the female (fig. 20) the glans has become more clearly defined, in a negative way, by changes which have taken place in the character of the urethral folds. As a result of this modification the entire shaft is terminally decurved upon the sharply demarked urethral folds, which now apparently form plate-like supports for the thicker cavernous shaft, the terminal portion of which (the future glans) is slightly more knob-like than that of the male. The inclosed urogenital opening is narrow and lanceolate, clearly restricted to the region of the genital folds and not extending onto the decurved glans. In general, the labio-scrotal swellings are similar to those of the male; their caudal prolongation into a curving, connecting postanal ridge is, however, more pronounced. Here it is without tubercular enlargements, but is almost bisected by a median groove.
Some of the older embryos in this stage show further changes of a minor nature in the phallic region. Thus the glans may be more strongly indicated by a further increase in the density of its tissue (corpus cavernosum glandis), so that it appears as an opaque white tip in sharp contrast to the more translucent shaft. The postanal ridge, joining the tips of the labio-scrotal swelUngs, becomes more V-shaped, while the inclosed anal opening shifts from a transverse to a longitudinal slit.
Reconstruction models of three embryos of this stage show quite clearly the correlation between the sex differences of the external and internal organs. In one specimen (No. 584a, 25 mm. CR) showing externally the long male type of urogenital opening, the tip of which extends onto the glans, the internal organs are male also. The gonad is rather short, barely reaching to the rim of the pelvis, while the tubar portion of the urogenital folds, containing the Wolffian and Mullerian ducts, unite with the dorsal wall of the bladder without the formation of a genital cord (primordium of the uterus).
In the females modeled (No. 840, 24.8 mm. CR, and No. 782, 28 mm.) the opposite condition is found. Externally the phallus of each shows a short urogenital opening. Internally the organs are decidedly more of the female type. The gonads are slightly longer, while the tubar portions of the urogenital folds (containing the Mullerian ducts) unite to form a genital cord before fusing with the dorsal wall of the bladder, thus producing a sinuous ridge which projects from the dorsal wall of the bladder and partially subdivides the cavity of the pelvis. In the older specimen the gonads are relatively larger and more compact.
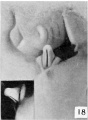
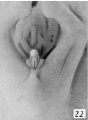
Stage 9
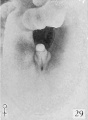
30 to 38, mm CR (figs. 18 to 23, males; figs. 22 and 29, females) .The changes in form between this and the previous stage are slight and consist mainly of a further decurvation of the female phallus and a great condensation of the glans area, although as yet the definite coronary sulcus has not been formed. In the male, the urogenital opening still extends the entire length of the phallus and there is no indication of the raphe.
Definitive Period
Stage 10
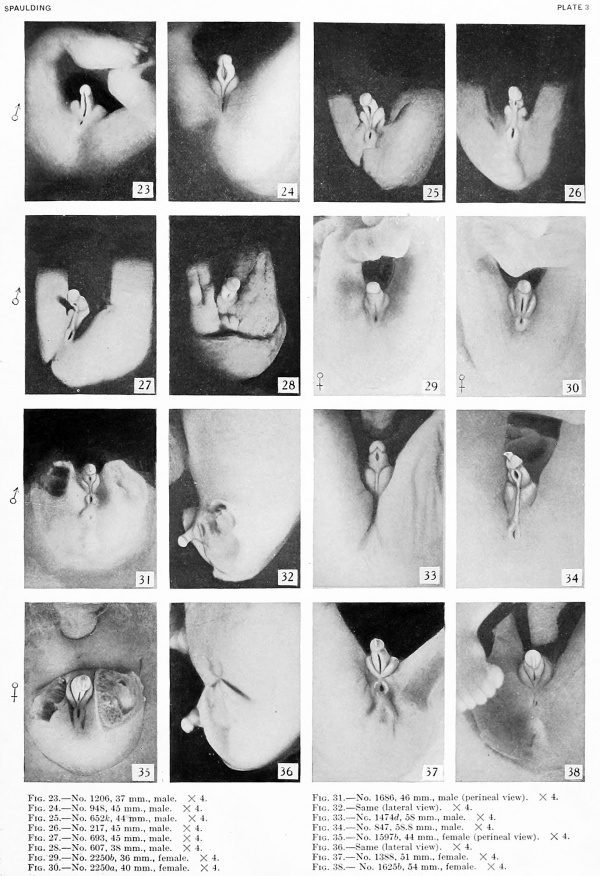
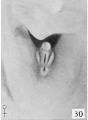
38 to 45 mm. CR (figs. 24 to 28, 31, 32, males; figs. 30, 35, 36, females) . The series of important changes occurring between the lengths of 38 and 45 mm. culminates in the final differentiation of the external genitalia. The formation of an open coronary sulcus demarks the terminal, knob-like glans from the remainder of the phallus. The most significant changes, however, are those involved in the separation ol the urogenital opening and anus. These modifications, more extensive in the male than in the female, arc brought about by the transformation of the open, gutter-like urogenital sinus into the tubular urethra, due to the almost complete fusion of the urethral folds to form the raphe. At the same time the labio-scrotal swellings migrate from their primitive lateral position to a new one caudal to the penis, forming the scrotum. An early stage in this series of modifications is shown in figure 24. The ridge-like raphe now separates the anal opening from the proximal end of the lenticular urogenital opening, the distal end of which continues to the tip of the glans as a narrowing slit. Coincident with this fusion of the urethral folds to form the raphe, the labio-scrotal swellings shift slightly, so that their greatest tumidity now lies caudal to the penis instead of lateral to it, as heretofore.
Regarding the formation of the scrotum, Felix (1912) makes such conflicting statements that it is not at all clear just what his conclusions are. In his preliminary account (p. 953) he states:
- "The basal growth of the pars pelvina must produce a new area, interposed between the base of the penis and the anal opening, and the best name for this is the unpaired scrotal area (fig. 642)."
The figure referred to by him, according to my interpretation, is that of a female specimen, and the area he labels as the unpaired scrotal area I should designate as the posterior commissure. He continues:
- "In embryos of 60 mm. head-foot length this (unpaired scrotal area) becomes raised up in toto and forms the unpaired scrotal swelling, into which the two genital swellings, which we may now term the scrotal swellings, extend from above .... As soon as the descensus is complete this unpaired scrotal area alone forms the scrotal sack, the paired scrotal swellings to the right and left of the penis fading out into the surrounding areas."
Later, in referring to the development of the labia majora in the female, he says (p. 955) :
- "The two genital swellings on this account are not prolonged anally to form an unpaired scrotal tubercle, and consequently they do not fade out as in the male but persist as the labia majora."
Finally, in his resume of the development of the external genitalia he concludes (p. 958) :
- "In the male the tubcrculum genitale fades out in all its parts and in its place there is formed at the anal periphery of the phallus, from the unpaired scrotal area, a new swelling, the unpaired scrotal sack."
An analysis of the above shows that he has made two statements implying that the labio-scrotal swellings are transformed into the scrotum (in toto or in part), and two other equally emphatic statements implying that they play no part in its formation. Furthermore, if we accept his first statement, it is still doubtful whether he considers that there is a caudal migration of the labio-scrotal swellings into the scrotum, or whether the final formation of the latter is accomplished by an anal prolongation of these swellings with an accompanying cranial atrophy.
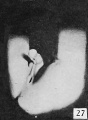
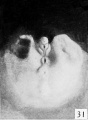
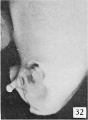
Subsequent growth results in the gradual reduction of the broad urogenital opening into a small aperture of varying size and shape, located just proximal to the coronary sulcus. The reduction in the size of this opening is usually accompanied by the development of a more or less pronounced epithelial rim and an increase in the size of the epithelial tag into a comb-like ridge of varying size and shape. This sequence of events is so clearly shown in figures 24 to 28, 31, and 32 that no detailed description of the individual steps is necessary. Attention should be called, however, to the ridge-like development of the penial raphe in the embryo illustrated in figure 27, where it forms a penial-scrotal frenulum, binding the penis to the scrotum with a consequent decurvation. The production of this frenulum is quite characteristic for most of the older embryos, although as a rule it does not become noticeably developed until after the embryos have attained a length of 55 mm.
The changes taking place in the female at this time are not so extensive. As in the male, a definite coronary sulcus is formed. The other modifications may be considered as a faint paralleling of the more profound metamorphosis which the male genitalia have been undergoing and consist chiefly of changes in the labioscrotal swellings. The caudal ends of these draw toward each other (fig. 35) and finally fuse to form the posterior commissure (figs. 37, 38), definitely separating the urogenital opening from the anus, although this is shght in comparison to the corresponding separation in the male. In this manner, the labio-scrotal swellings are transformed from separate, lateral swellings into a cranially open, horseshoeshaped rim partially encircling the phallus. Unlike the corresponding change in the male, this fusion is not accompanied by either a caudal migration of these swellings or by the formation of a definite raphe.
Regarding the formation of the posterior commissure, my observations are decidedly at variance with the conclusions drawn by Wood-Jones (1913) that in the adult (human) female this is not definitely present as a commissural bar separating the vaginal orifice and anus, but that it merely shows as such when the genitalia are placed in an unnatural position.
The posterior commissure may be considered as marking the advent of the definitive period in the female, and from now on we may refer to the component parts of the external genitalia by their adult terms. The labio-scrotal swellings form the labia majora, connected by the posterior commissure. The cavernous portion of the phallus becomes the clitoris, divided into glans and shaft. The urethral folds constitute the labia minora, margining the persisting primary urogenital opening (the urethro- vaginal orifice). It must be pointed out, however, that the use of these latter terms at this time is an arbitrary one, because the division of the female phallus into clitoris and labia minora is never entirely complete and is consummated only with the formation of the frenulum clitoridis at some time after the close of the period covered by the present investigation (100 mm. CR length). For this reason, the strict usage of these terms would require the continued use of the inchisivc term phallus until the separation is culminated.
The phallus period is thus terminated by the formation of the raphia and the scrotum in the male, and the posterior commissure of the labia majora in the female. Thus the definitive characteristics of the external genitalia are organized and the recognition of the sex of the embryos is correspondingly simplified. These observations, in the strict sense, diverge from the statement made by Felix (1912, p. 949) :
- " The beginning of the sexual differentiation can hardly be assigned to a definite period, since it takes place quite gradually and even in advanced stages presents difficulties to diagnosis. We base our diagnosis on the position of the ostium urogenitale relative to the coronarj' sulcus on the one hand and the anal opening on the other. Male embryos are distinguished by the distal {distal here has reference to the base of the phallus) circumference of the ostium urogenitale always lying in the coronary sulcus, while the proximal circumference becomes more and more distant from the anal opening. Female embryos may be recognized by the urogenital opening retreating away from the coronary sulcus by the gradual closure of its distal part, while its proximal part alwaj's lies close to the anal opening; the differentiating moment is, accordingly, both positive and negative in each sex."
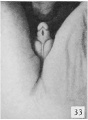
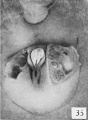
While this statement, if it be interpreted broadly, may to a certain extent be considered as containing the germ of the diagnostic point which I have found to be of value in the early recognition of sex in the human embryo, it plainly does not agree with my own observations, in that Felix limits it to the final period when I find definitive changes taking place in the external genitalia, and at which time the modifications are so characteristic that examination of the external genitalia almost at once enables one to definitely say that one embryo is male and another is female. It must be emphasized, however, that it is still necessary to make careful examination of the genitalia to obtain a correct diagnosis, because from this time throughout the rest of the fetal period individual variations are more pronounced than in the preceding embryonic period. In many of the females, for example, the clitoris attains considerable size, and if this alone is considered, or if only a cursory examination is made, such an embryo might easily be mistaken for a male. (A comparison of figs. 33 and 34, males, with figs. 37 and 46, females, will illustrate this point.) In fact, in the majority of males between the lengths of 50 and 100 mm. CR, the exposed penis seems to be comparatively shorter than the clitoris in females of the same size, an effect which is largely produced by the varying development of the penial-scrotal frenulum. So few pronounced morphological changes take place in the developing genitalia during the rest of the period covered in this study that it will not be necessary to divide them into successive stages.
Although I have not yet been able to make a complete study of the development of the prepuce, my observations upon its formation from the external examination of older embryos may be of value and are therefore included in the present account as suggesting the apparent formation of this structure, although the final corroboration of this will have to be left for future investigation. For this reason, it seems inadvisable at this time to enter into any comparison of the various explanations that have been made to account for its origin.
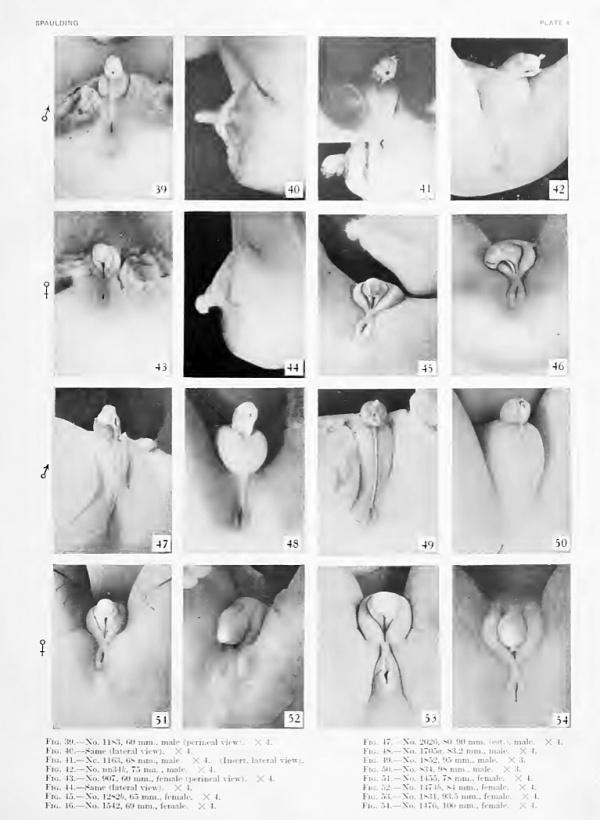
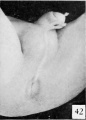
After the separation of the phallus into shaft and glans by the formation of a coronary sulcus, the glans remains as a naked, knob-like termination of the shaft (in both sexes) until the embryo reaches a length of about 65 mm. CR. In males of this size the first traces of the prepuce may be noted. The skin of the shaft becomes elevated into a pair of conspicuous rolls on either side of the urethral opening (fig. 41) . Gradually these rolls join on the dorsal side of the shaft (fig. 42) to form a flat ridge whose distal margin has grown out to cover the proximal edge (corona) of the glans (embryos 75 mm. CR). The continued outgrowth of this fold results in the gradual inclosure of the glans (figs. 47 to 50) until (embryos 98 to 100 mm. CR) the glans is completely covered by the prepuce.
Accompanying the development of the prepuce, there is a gradual shifting of the urethral opening until (in embryos 85 to 90 mm. CR) it occupies a subterminal position m the frenular notch of the glans. With the completion of the prepuce this primary opening is entirely closed. Some time later a new (the secondary or definitive) terminal urethral opening is formed near the tip of the glans.
But few important changes take place in the female genitalia in this later period (50 to 100 mm. CR length), these being mainly concerned with the continued growth of the labia majora and the beginnings of prepuce formation. The labia majora increase somewhat in height, so that the inclosed structures become more submerged in the rim which they form, although this submergence is by no means completed at this period.
The formation of the prepuce is considerably more involved than in the male and all of the folds included in its complete development do not make their appearance until some time after the close of the period under consideration. The only observations made are concerned with the glandular portion. This fold apparently develops in much the same way as it does in the male, although it has not been studied as closely and is not as well shown in the figures. Its growth is by no means as rapid as in the male, with the result that the glans of the clitoris is not completely covered by it at the close of this period (100 mm. CR) . As has already been noted, the complete separation between the clitoris proper and the labia minora does not take place until considerably later.
Summary
A more comprehensive idea of the sequence of events in the development of the external genitalia may be gained if we briefly review their development in each sex.
Development of the Male Genitalia
The genital tubercle, from which is derived the phallus and which includes the urethral and anal openings, arises as a broad conical eminence barely separated cranially from the umbilical cord, but with a well-defined caudal slope. Its rounded free end, as the primordium of the glans, is faintly separated from the basal portion. The lateral slopes, below the glans area, extend into the outlying basal tissue as the "lateral buttresses, " which I interpret as enlargements for the developing corpora cavernosa. The caudal slope is bisected by the shallow urethral groove whose margins are slightly elevated into the urethral folds. Distally, these margins merge into the glans area, while proximally they continue into the basal enlargements surrounding the anal pit. In the majority of embryos which have attained a length of 12 mm. the urethral membrane is ruptured to form the primitive urogenital opening, although a few cases have been found in which this perforation had not taken place at the stage of 16 to 17 mm. Practically from its first appearance the urethral groove shows a sex difference in its length, this difference being further accentuated upon the formation of the urogenital opening.
As development proceeds, the elongation of the tubercle and the medial migration of its lateral buttresses transform it into the somewhat cylindrical phallus, whose base is separated from the surrounding body areas by the newly formed labio-scrotal swellings (embryos 17 to 19 mm.). The latter, appearing first as broad, elevated areas extending laterally from the base of the phallus, are soon transformed into swollen ridges separated, in both sexes, from the base of the phallus by the lateral phallic grooves. It may be emphasized that in the majority of specimens the labio-scrotal swellings seem to be from the first separated from the phallus. In fact, only one embryo was found which showed these areas as definitely merging into the phallus, while in all of the others of about the same age the swellings were already separated from the phallus by pronounced grooves, the lateral phallic grooves.
Accompanying the elongation of the tubercle into the phallus, the urogenital opening becomes gradually more sharply outlined and the sex difference in its length correspondingly emphasized. The urethral folds which form the margins of the opening also increase in definiteness, partly through their own elevation and partly through their lateral demarcation from the cavernous portion of the shaft; the result of these combined factors being that the folds extend as plate-like caudal projections from the more cylindrical shaft. At the same time the terminal glans has increased in density so that it now forms an opaque white area in contrast to the translucent shaft, although the limiting coronary sulcus is not formed until the close of the phallus period.
As a result of these combined changes the male phallus at this time is markedly different from that of the female. The length of the urogenital opening is still, however, the chief diagnostic feature in the two sexes. In the male it consists of a long sUt extending from the base to the apex of the phallus, whereas in the female it extends only to the base of the glans. In the male it is more open distally, the apposition of the urethral folds reducing it proximally to a mere slit. This is in marked contrast to the condition in the female, where the opening (restricted to the shaft) has exactly the opposite shape, being broadest basally, while the terminal portion is narrowed into a slit.
No further pronounced morphological changes take place in the male genitalia until after the embryo has reached a CR length of 38 mm. At this time a series of changes begins which result in the transformation of the male genitalia into approximately their final form (about 45 mm.) and in the complete separation of the urogenital opening from the anus. The gutter-like urogenital sinus is transformed into the tubular urethra by the merging of the basal portions of the urethral folds into the raphe, reducing the primitive urogenital orifice to an irregularly shaped opening on the distal portion of the shaft. At the same time the glans becomes sharply defined by the formation of a wide coronary sulcus and the labio-scrotal swellings assume their final position caudal to the penis to form the scrotum, the halves of which become more or less closely approximated to the raphe in the midventral line, although they never entirely lose their bilateral character.
This period (45 mm. CR length) marks the completion of the definite' sex differentiation, characterized by the development of definite structural features in the male in sharp contrast to the lack of these characters in the female.
As growth continues, the gradual constriction of the urethral opening synchronous with the formation of the prepuce, to be described later, is accompanied by an outgrowth of its rim which, in embryos 60 to 85 mm. CR length, results in the formation of a decided cup in the bottom of which the opening is located. With the later stages of prepuce formation the urethral opening is shifted to a more terminal position in the frenular notch of the glans. It then becomes entirely closed and eventually there is formed the new, permanent terminal urethral opening on the apex of the glans.
The first evidence of the prepuce is found in embryos of about 65 mm. CR length, at which time it can be recognized as a pair of swellings on each side of the urethral opening. Gradually these swelUngs fuse together over the dorsum of the shaft to form a flattened ridge of skin whose distal margin has enveloped the proximal margin (corona glandis) of the glans (embryos 75 mm. CR length). Subsequent growth results in the progressive inclosure of the glans by this distally migrating fold of skin until at about 100 mm. CR length the originally naked glans is entirely covered. It must be emphasized that these observations upon the formation of the prepuce are based only upon the external examination of the genitalia of these older embryos and have not been confirmed by histological study. For this reason they must be considered merely as suggestions of what apparently takes place and may later be corroborated or contradicted when it becomes possible to make a study of sectioned embryos showing this development.
Development of the Female Genitalia
The entire process of development of the external genitalia in the female is accompanied by fewer pronounced morphological changes than occur in the male. It is noteworthy, however, that (in spite of this greater simplicity in structure) completion of development is more protracted, so that the final differentiation of the female genitalia, although brought about by comparatively minor changes, does not synchronize with the more complete transformation of the male (45 mm. CR length), but instead, beginning with a slight change at a length of about 50 mm., extends as a gradual process throughout a considerable period of early fetal life.
From its beginning until the stage of 21 to 25 mm. the genital tubercle of the female closely resembles that of the male except for the shorter urethral groove. About this period the female shows the beginnings of the caudal decurvation, which is apparently brought about by an excess in the growth of the cavernous over the urethral regions of the phallus. At the same time the urethral folds have become compressed into plate-like caudal projections supporting the slightly overhanging glans, which in this way is more clearly defined than in the male. As has already been pointed out, the coronary sulcus is not formed in either sex until the embryos reach a length of about 45 mm. From 25 mm. to 45 mm. this caudal decurvation becomes a more and more pronounced characteristic of the female phallus, and for this reason is a diagnostic feature of increasing importance as development proceeds.
In the female important changes occur at about the stage of 45 to 50 mm. CR length, and these likewise mark the termination of the phallus period. While much less extensive than the correlated changes in the male, they are nevertheless characteristic and indicate the appro.ximate assumption of the final form. The most pronounced modification occurring at this time is that the caudal ends of the labio-scrotal swellings grow towards each other and finally join in the midventral line to form the posterior commissure (50 mm. CR length). In this manner these originally paired swellings are transformed into a cranially open, horseshoe- shaped rim, inclosing the rest of the external genitalia and separating them from the anus.
The formation of the posterior commissure in the female thus synchronizes with the formation of the raphe in the male and may be considered as representing the advent of the final differentiation, and from now on we may refer to the genitalia by their adult terms. The labio-scrotal swellings form the labia majora. The glans and cavernous portion of the phallus may be considered as the chtoris, and the urethral folds as the labia minora. The inclosed primary urogenital opening may now be called the urethro-vaginal orifice. It must be pointed out, however, that the apphcation of these terms at this time is an arbitrary one, because the actual separation of the female phallus into these more definitely adult structures does not take place until some time after the close of the period included in the present study (100 mm. CR). Strict accuracy would demand that, until such division had been completed by the formation of the frenula clitoridis, the inclusive term phallus be retained.
Because of the persistence of the urethral folds (labia minora) in the female and their failure to fuse together as they do in the male, the female phallus retains a more conical outline than does the penis of the male.
In female embryos of 60 to 100 mm. CR length there is shown a partial development of the prepuce, although the complicated set of folds involved in its complete formation is not produced at this time and only partial growth of the glandular portion is completed at the close of this period. While the glandular prepuce is apparently formed in much the same way as it is in the male, its growth is markedly slower, and in embryos 100 mm. long the glans is not completely surrounded by it.
In these older embryos (60 to 100 mm. CR length) there is also some increase in the height of the labia majora, so that the inclosed portions are somewhat submerged in the rim thus formed, although this submergence is by no means as complete as it becomes in later fetal life. It should also be noted that in fetuses up to 100 mm. CR the labia majora are still cranially separated and there is no indication that they play any part in the formation of the mons veneris.
Conclusions
- There is a morphological sex difference in the external genitalia of the human embryo practically from their first appearance, characterized by marked difference in the length of the urethral groove upon the caudal slope of the genital tubercle. In some embryos the distal end of the groove extends onto the glans region (males), while in others it does not reach to this region (females).
- This should prove to be a fairly reliable character for the recognition of sex in human embryos at an earlier period than has heretofore been possible.
- From the first appearance of the genital tubercle, there apparently is no indifferent or undifferentiated period in the development of the external genitalia before they acquire their definite male or female characteristics.
- The caudal decurvation of the female phallus, first pointed out by Herzog, is a valid diagnostic characteristic and is applicable at an earlier period than was first indicated.
- The phallus period is definitely terminated in both sexes by the assumption of approximately the adult form of the genitalia. In the male this is brought about by the fusion of the urethral folds into the raphe and the accompanying caudal migration of the labio-scrotal swellings to form the scrotum. In the female it is characterized by the fusion of the caudal ends of the labio-scrotal swellings to form the posterior commissure.
Bibliography
Van Ackeren, F., 1888. Beitriige zur Entwicklunga geschichte der wciblichen Sexualorgane dea Menschen. Zeitschr. f. wiss. Zool., vol. 48.
Allen BM. The embryonic development of the ovary and testis of the mammals. (1904) J. Anat. 3(2): 89-154.
Bayer, 1903. Entnicklungsgeschichte des weibliehen Genitalapparates. Vorlesungen u. allg. Geburtshilfe, vol. 1', 1-104.
Bohm, J., 1905. Die ausseren Genitalien des Schafes. Morph. Jahr., vol. 34, 245-320.
Buchanan G. and Fraser EA. The development of the urogenital system in Marsupialia, with special reference to Trichosurus vulpecula. Part I. (1918) J. Anat. 53: 35-95. PMID 17103858
Dimpf, H., 1900. Die Teilung der Kloake bei Cavia cobaya. Morph. Jahrb., vol. 35, 17-05.
DisBE, J., 1905. Untersuchungen ilber die Umbildung der Kloake und die Entstehung des Kloakenhockers bei Talpa europea. Anat. Hefte, Abth. 1, vol. 27, 479-533.
DCrbeck, W.. 1907a. Die ausseren Genitalien des Schweines. Morph. Jahrb., vol. 36, 517-543. ,
1907b. Die ausseren Genitalien der Hauskatze. Morph. Jahrb., vol. 36. 544-565. ,
1907c. Tabellarische Ubersicht der Genitalentwicklung bei Saugetieren. Morph. Jahrb., vol. 36, 566-569.
Ecker, A., 1851-59. Icones Physiologicae. Erlauterungstafeln zur Physiologie und Entnicklungsgesehite. Leipzig.
Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979.
Fleischmann, A., and pupils, 1902-1907. Morphologische Studien iiber Kloake und Phallus der Amnioten. Morph. Jahrb.: IV. Die Saugetiere (Fleischmann), vol. 30, 653-060. V. Die Stilistik des Urodaum und Phallus bei den Amnioten (Fleischmann), vol. 30, 666-675. VI. Kloake und Phallus des Schafes und Schweines (Schwartztrauber), vol. 32, 23-57. VII. Historisch-kritische Betrachtungen (Fleibchmann), vol. 32, 58-96. VIII. Die Stilistik des Urodaums (Fleischmann), vol. 32, 97-103. IX. Die ausseren Genitalien des Schafes (Bohm), voi. 34, 248-320. X. Die Teilung der Kloake bei Cavia cobaya (Dimpfl), vol. 35, 17-64. XI. Das Analrohr des Schafes (Schwartztrauber), vol. 35, 65-74. XII. Bau und Entwicklung der ausseren Genitalien bei Cavia cobaya (Gruber), vol. 36, 1-26. XIII. Die ausseren Genitalien des Schweines (Dilrbeck), vol. 36, 517-543. XIV. Der ausseren Genitalien der Hauskatze (Dilrbeck), vol. 36, 544-565. XV. Tabellarische tJbersicht der Genitalentwicklung bei Saugetieren (Durbeck), vol. 36, 566-569. XVI. Die Stilcharactere am Urodaum und Phallus (Fleischmann), vol. 36. 570-601.
Fraber EA. Development of the urogenital system in the Marsupialia, with special reference to Trichosurus vulpecula. Part II. (1919) J. Anat. 53: 97-129. PMID 17103868
Frieuentual, H., 1911. Das epithelhbrnchen am Penis dea Meiischeufotus als Rest einea auruckgebildeten Stachelhornchens. Arbeiten aus dem Gebict der Experimcntellen Physiologie, vol. 2, p. 87. Jena.
Grcber. K.. 1907. Bau und Entwicklung der au.sseren Genitalien bei Cavia cobaya. Morph. Jahrb., vol. 40, 3-26.
Hennedero, B., 1914. Beitrag zur Entwicklung der iiusseren Gcnitalorgane beim Siiuger. Erster Teil. Anat. Hefte, vol. 50, Abth. 1, 423-497.
1917. Same; zweiter Teil. Ibid., vol. 55. Abth. 1, 227-414.
Herzoo, F., 1904. Beitriige zur Entwicklungsgeschichta und Histologic der miinnlichen Harnrohre. Arch. f. mikr. Anat., vol. 63, 710-747.
Keibel, Franz. 1896. Zur Entwicldungsgesohichte des menschlichen Urogenitalapparates. Arch. f. Anat. u. Physiol., Anat. Abth., 55-156.
Kollman, J.. 1907. Handatlas der Entwicklungsgesehichto des Menschen, 2 vols., zweiter Teil. Jena.
Krasa. F. C, 1917. Die Entwicklungsgeschichte des Urogenitalsystems beim Maulwurf (Talpa europea). Anat. Hefte, vol. 55, Abth. 1, 443-508.
Muller, JoH., 1830. Bildungsgeschichte der Genitalien. Diisseldorf.
Nagel, W, Ueber die Entwicklung des Urogenital systems beim Menschen. (1889) Arch. f. mik. Anat. 34: 269-384.
1892. Ueber die Entwicklung dor Urethra und des Dammea beim Menschen. Ibid., vol. 40, 264-287. ,
1894. Ueber die Entwicklung der inneren and ausseren Genitalien. Arch. f. Gyu., vol. 45, 453-477. ,
1897. Entwicklung und Entwicklungsfehler der weiblichen Genitalien. Veit'sHandb. d. Gyu., 1st ed., vol. 1,519-628. Wiesbaden.
Otis, W. J., 1906. Die Morphogenese und Histogenese des Analhockers nebst Beobachtungen iiber die Entwicklung des Sphincter ani externus beim Menschen. Anat. Hefte, vol. 30, Abth. 1, 199 258.
Pohlman AG. Has a persistence of the Müllerian ducts any relation to the conditions of cryptorchidism? (1904) Amer. Med. 8: 1003-1006.
Pohlman AG. The development of the cloaca in human embryos. (1911) Amer. J Anat. 12: 1-26.
Ratuke, H., 1832. Abhandlungen zur Bildungs- und Entwicklungsgeschichte des Menschen und der Tiere.
Retterer, E., 1890a. Sur I'origine et revolution de la region anogf'nitale des Mammiferes. Jour, de I'Anat. et de Physiol., vol. 26, 126-151. 153-216.
1890. Du developpement du pr6puce, de la couronne du gland et du col du pfenis chez I'embryon humain. C. R. Soc. de Biol.. No. 29, p. 528.
1905a. Du role de I'fepithfelium dans le dfeveloppe raent des organes genito-urinaires externes. C. R. Soc. Biol.. Paris, vol. 57, 1, 1040-1043.
1905b. Du dfiveloppement et de la structiu-e dearaphes dea organes gfenito-urinaires. C. R. Soc. Biol., vol. 57, 2, 22-25.
1914. Developement et histogenfese compare des organes gfenitaux externes. Jour, d'urol. m^d. et chir., vol. 6, 157-173.
Roschuletz, v.. 1890. Bcitrage zur Entwicklungsgeschichte des Genital-hockers beim Menschen und beim Schwein. Diss., Berlin.
Schwartztrauber, J., 1904. Kloake und Phallus des Schafes und Schweines. Morph. Jahrb., vol. vol. 32, 23-57.
1906. Das Analrohr des Schafes. Ibid., vol. 35, p. 65-74.
SpuLER, A.. 1910. Ueber die normale Entwicklung des weiblichen Genitalapparates. Veit's Handb. f. Gyn., 2d ed., vol. 5, 573-650.
Tiedemann, F., 1813. Anatomic der kopflosen Missge burten. Landshut.
Tourneux, F., 1889. Sur le developpement et revolution du tuberculo genital chez le fa-fu humain dans lea deux sexes, avec quelques remarques concernant le developpement des glandes pro statiquea. Jour, de I'Anat. et de la Physiol.. vol. 25. 229-263.
Wood-Jones F. Some points in the nomenclature of the external genitalia of the female. (1913) J. Anat. Physiol. 148: 73-80. PubMed 17232984
Wood-Jones F. The morphology of the external genitalia of the mammala. (1914) Lancet. 1017-1023.
Figures
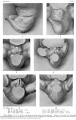
Plate 1
Fig. 1. Carnegie Embryo 467, 9 mm X 21.
Fig. 2. Carnegie Embryo 792, 8 mm
Fig. 3. Carnegie Embryo 2702, 8 mm X 18.5
Fig. 4. Carnegie Embryo 1784a. 12 mm X 21.
Fig. 5. Carnegie Embryo 1936, 14 mm, male. X 14.5.
Fig. 6. Carnegie Embryo 2023, 15 mm, female. X 14.
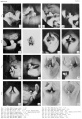
Plate 2
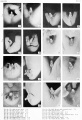
Plate 3
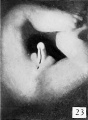
Fig. 25. No. 652K, 44 mm., Male X 4.
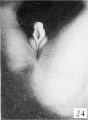
Fig. 27. No. 693, 45 mm., male. X 4.
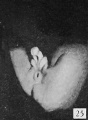
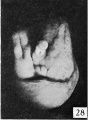
Fig. 28. No. 607, 38 mm., male. X 4.
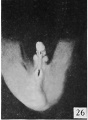
Fig. 29. No. 2250b, 36 mm., female. X 4.
Fig. 30. No. 2250a, 40 mm., female. X 4.
Fig. 31. No. 1686, 46 mm., male
Fig. 32. No. 1686, (lateral view). X 4.
Fig. 33. No. 1474b, 58 mm., male. X 4.
Fig. 34. No. 847, 58.8 mm., male. X 4.
Fig. 35. No. 1597b, 44 mm., female (perineal view). X 4.
Fig. 36. No. 1597b Same (lateral view). X 4.
Fig. 37. No. 1388, 51 mm., female. X 4.
Fig. 38. No. 1625b, 54 mm., female. X 4.
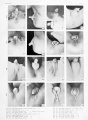
Plate 4
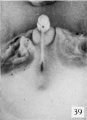
Fig. 39. No. 1183, 60 mm., male (perineal v-iew). X 4.
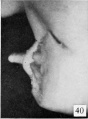
Fig. 40. No. 1183, Same (lateral view) . X 4.
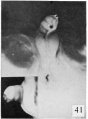
Fig. 41. No. 1163, 68 mm., male. X 4. (Insert, lateral
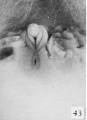
Fig. 43. No. 907, 60 mm., female (perineal view). X 4.
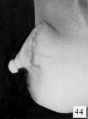
Fig. 44. No. 907, Same (lateral view). X 4.
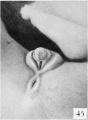
Fig. 45. No. 1282b, 65 mm., female. X 4
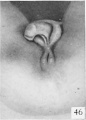
Fig. 46. No. 1542, 69 mm., female. X 4.
Fig. 47. No. 2026, 80-90 mm. (est.), male.
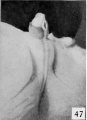
Fig. 48. No. 1705a, 83.2 mm., male. X 4.
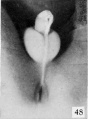
Fig. 49. No. 1852, 95 mm., male. X 3.
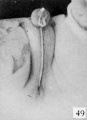
Fig. 50. No. 834, 98 mm., male. X 3.
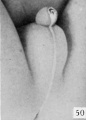
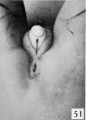
Fig. 51. No. 1455, 78 mm., female. X 4.
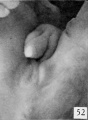
Fig. 52. No. 1474b 84 mm., female. X 4.
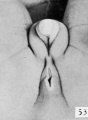
Fig. 53. No. 1831, 93.5 mm., female. X 4.
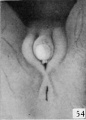
Fig. 54. No. 1476, 100 mm., female. X 4.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Contributions to Embryology Carnegie Institution No.61. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.61
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- Genital
- Human Fetus
- Historic Embryology
- Human
- Male
- Female
- Carnegie Embryo
- 1920's
- Carnegie Collection
- Carnegie Embryo 1163
- Carnegie Embryo 1183
- Carnegie Embryo 1206
- Carnegie Embryo 1282b
- Carnegie Embryo 1388
- Carnegie Embryo 1455
- Carnegie Embryo 1474b
- Carnegie Embryo 1476
- Carnegie Embryo 1542
- Carnegie Embryo 1597b
- Carnegie Embryo 1625b
- Carnegie Embryo 1686
- Carnegie Embryo 1705a
- Carnegie Embryo 1750
- Carnegie Embryo 1784a
- Carnegie Embryo 1831
- Carnegie Embryo 1852
- Carnegie Embryo 1936
- Carnegie Embryo 194
- Carnegie Embryo 2023
- Carnegie Embryo 2026
- Carnegie Embryo 217
- Carnegie Embryo 2250a
- Carnegie Embryo 2250b
- Carnegie Embryo 2393
- Carnegie Embryo 2702
- Carnegie Embryo 28
- Carnegie Embryo 389a
- Carnegie Embryo 467
- Carnegie Embryo 492
- Carnegie Embryo 590
- Carnegie Embryo 607
- Carnegie Embryo 684
- Carnegie Embryo 693
- Carnegie Embryo 792
- Carnegie Embryo 834
- Carnegie Embryo 847
- Carnegie Embryo 907
- Carnegie Embryo 948
- Carnegie Embryo 950
- Carnegie Embryo 955