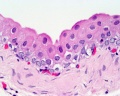
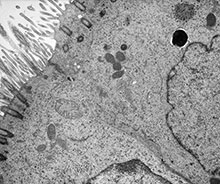
ANAT2241 Covering and Lining Epithelia
| ANAT2241 This practical support page content is not part of the virtual science practical class and provides additional information for student self-directed learning purposes. All practical class pages are located on Moodle - ANAT2241 |
General Objective
To learn the structure and function of covering and lining epithelia.
Associated lecture slides: Covering and Lining Epithelia (17.51 MB PDF) | 4 slides/page
Specific Objectives
- To identify covering and lining epithelia.
- To understand the terms endothelium, and mesothelium.
- To classify epithelia on the basis of cell morphology and cell arrangement (e.g. layering), noting the general absence of blood vessels in epithelia.
- To study the surface specializations of some epithelial cells: brush or striated border (microvilli), non-motile stereocilia and motile cilia and to recognise these specialisation in electron micrographs.
Learning Activities
To identify, draw and label the various types of epithelium (simple, stratified and pseudostratified) and to discuss some of the special functional features associated with their structure.
Virtual Slides: Covering and Lining Epithelia
|
|
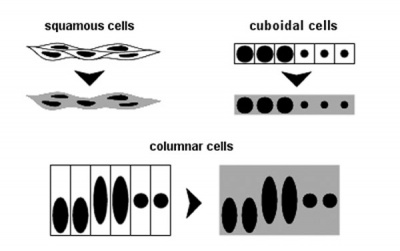
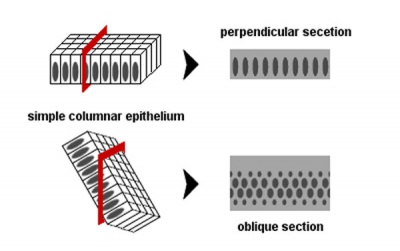
| Epithelia cell shape | Epithelia sectioning appearance |
Histology
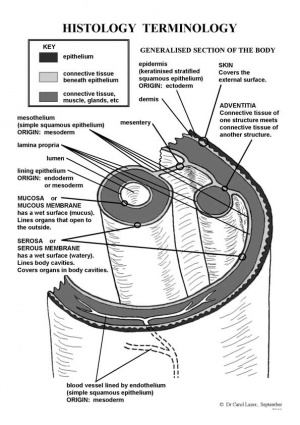
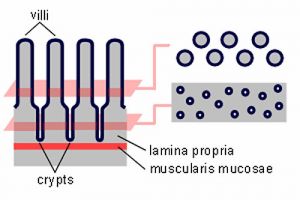
Epithelium forms continuous layers of cells that cover surfaces and line cavities of the body.
Simple epithelium

Venule with endothelium, a simple squamous epithelium lining. |
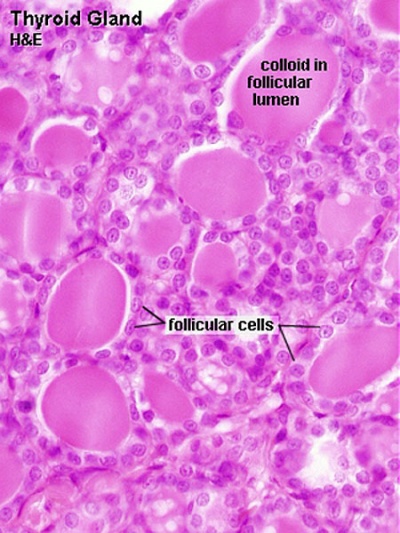
Thyroid follicles, with a simple cuboidal epithelium surrounding each follicle. |
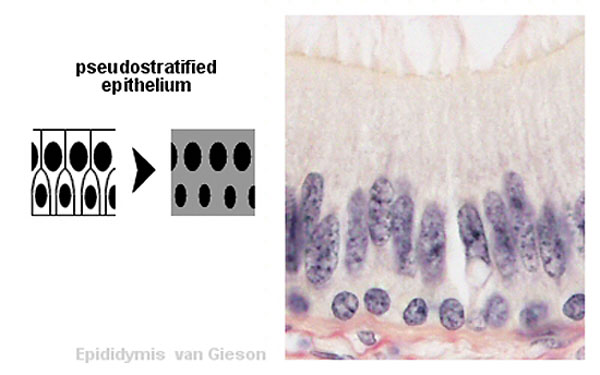
Pseudostratified epithelium
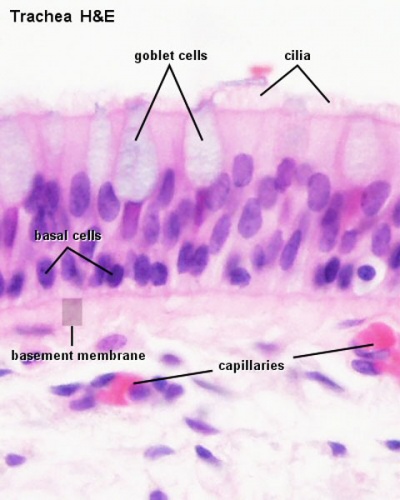
Trachea lined with a pseudostratified epithelium. |
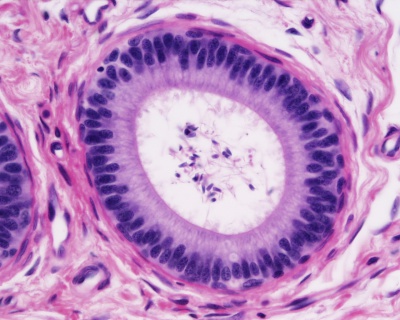
Testis ductus epididymidis lined with a pseudostratified epithelium. |
Testis ductus epididymidis lined with a pseudostratified epithelium.
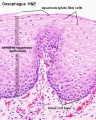
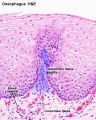
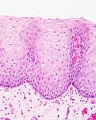
Stratified epithelium
Oesophagus stratified squamous non-keratinized epithelium.
Transitional epithelium
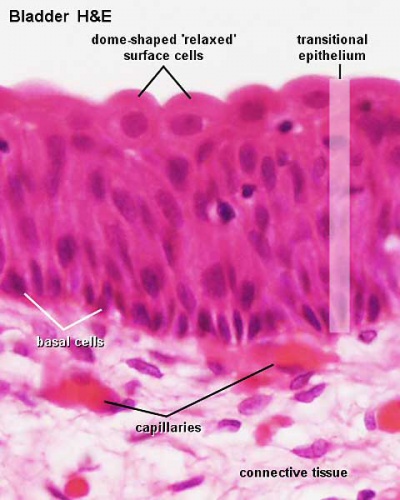
Urinary bladder transitional epithelium |
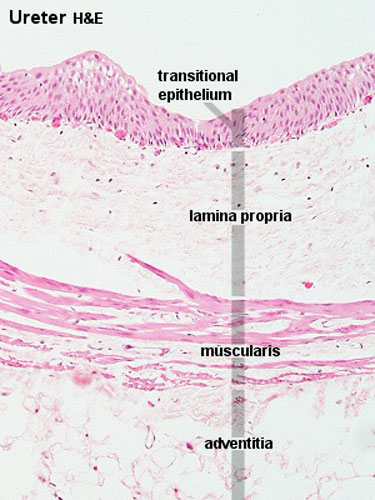
Ureter transitional epithelium |
Unlabeled Images
Electron Micrographs
Epithelia Specialisations - Cilia
|
| ||||||
| Scanning Electron micrograph
This respiratory epithelium surface view by scanning electron microscope (SEM) shows the 3 dimensional structure of the cilia protruding into the airspace. |
<html5media height="350" width="800">File:Mouse trachea 01.mp4</html5media> |
Epithelia Specialisations - Villi
Simple Squamous Epithelium
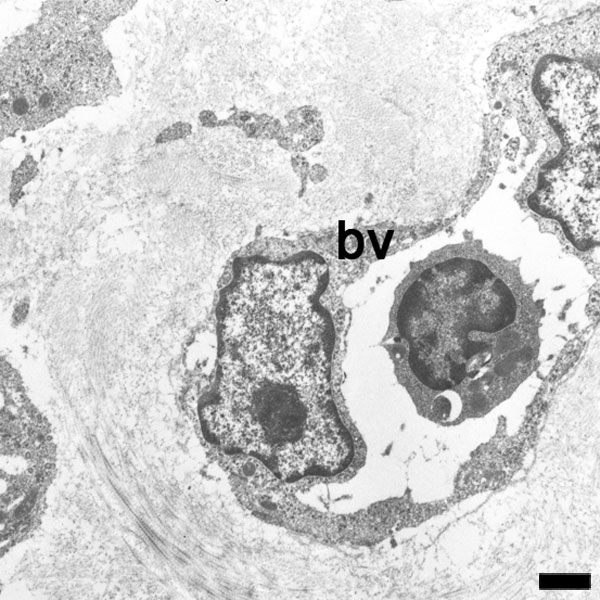
|
An electron micrograph of a capillary blood vessel with white blood cell located in the lumen.
The endothelium is an example of a simple squamous epithelium. Note the nuclei of the endothelial cells bulging into the vessel lumen.
Scale bar 1 μm (Stain - Osmium) |
Epithelia Specialisations - Junctions
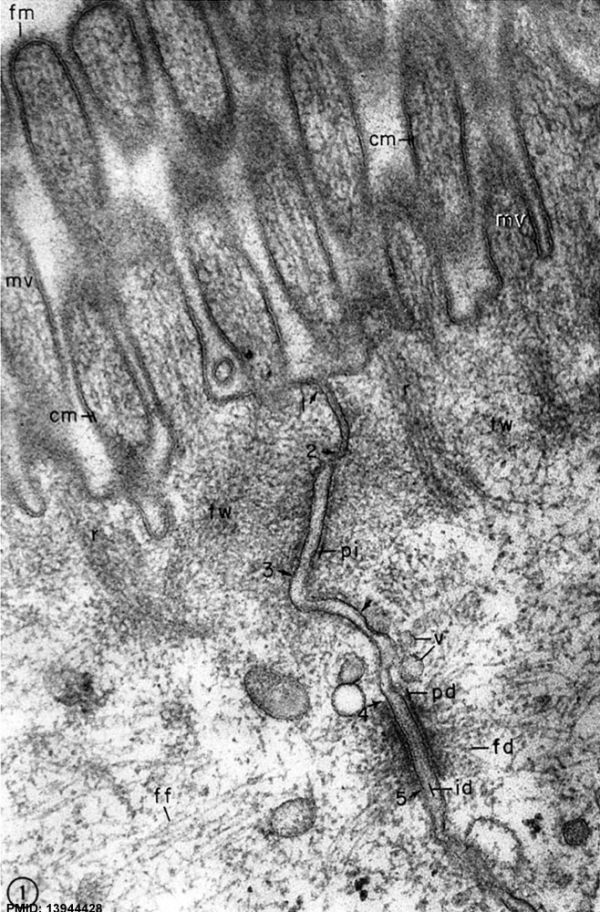
|
Junctional complex between two cells in the epithelium of the intestinal mucosa (rat), intestinal lumen is towards the top of image.
These 2 epithelial cells have a range of different junctional complexes along their lateral contact membranes. Half (hemi-) versions of these junctions also occur on the cell basal membrane where it attaches to the extracellular matrix (basal lamina, visible in EM), not shown in this image.
Note that the luminal membrane is nearly symmetrical, the outer leaflet being only slightly thinner and less dense than the inner leaflet, whereas the lateral membrane is definitely asymmetrical. The total thickness of all three layers is about 110 A along the apical surface of the cell but measures only about 70 to 80 A along the lateral intercellular spaces. Note also the fluffy dense material (fro) (probably mucus) associated with the tips and sides of the microvilli.
|
| 600px]] |
Course Links
- Histology Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ANAT2241 Support | Histology | Histology Stains | Embryology Glossary
| Common Histology Stains | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Practical Support
- Pages can be accessed from any internet connected computer.
ANAT2241 Support Links: The Virtual Microscope | Covering and Lining Epithelia | Glandular Epithelia | CT Components | CT Types | Bone, Bone Formation and Joints | Muscle | Nervous | Blood | Eye | Cardiovascular | Respiratory | Integumentary | Gastrointestinal | Gastrointestinal Organs | Lymphatic and Immune | Endocrine | Urinary | Female Reproductive | Male Reproductive | Histology Stains | Histology Drawings | Practicals Health and Safety 2013 | Moodle - 2019
ANAT2241 This practical support page content is not part of the science practical class and provides only background information for student self-directed learning purposes.
Cite this page: Hill, M.A. (2024, April 27) Embryology ANAT2241 Covering and Lining Epithelia. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/ANAT2241_Covering_and_Lining_Epithelia
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G