Sensory - Vision Abnormalities
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| ICD-11 Structural developmental anomalies of the eye, eyelid or lacrimal apparatus |
|---|
|
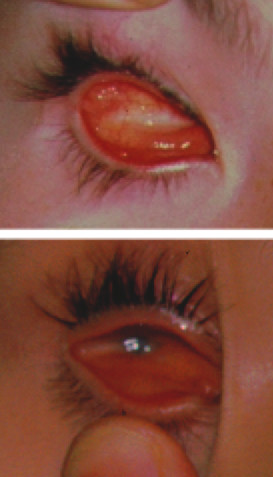
LA10.0 Microphthalmos - This is a developmental disorder of the eye that literally means small eye (micros = small; ophthalmos = eye). One (Unilateral Microphthalmia) or both (Bilateral Microphthalmia) eyes may be involved. LA10.1 Clinical anophthalmos - This refers to the clinical absence of one or both eyes. Both the globe (human eye) and the ocular tissue are missing from the orbit. The absence of the eye will cause a small bony orbit, a constricted mucosal socket, short eyelids, reduced palpebral fissure and malar prominence. Genetic mutations, chromosomal abnormalities, and prenatal environment can all cause anophthalmia. Anophthalmia is an extremely rare disease and is mostly rooted in genetic abnormalities. LA10.3 Congenital macrophthalmos - A condition caused by failure of the eye to develop correctly during the antenatal period. This condition is characterized by enlargement of the globe of the eye. LA11 Structural developmental anomalies of the anterior segment of eye - LA11.1 Structural developmental anomalies of cornea LA11.3 Aniridia LA11.4 Coloboma of iris LA11.5 Congenital corneal opacity LA12 Structural developmental anomalies of lens or zonula - LA12.0 Coloboma of lens LA12.1 Congenital cataract LA12.2 Congenital aphakia LA12.3 Spherophakia LA13 Structural developmental anomalies of the posterior segment of eye - LA13.0 Congenital anomalies of the vitreous LA13.1 Coloboma of choroid or retina LA13.2 Coloboma of macula LA13.3 Congenital vitreoretinal dysplasia LA13.4 Optic pit LA13.5 Congenital retinal aneurysm LA13.6 Congenital malformations of choroid LA13.7 Congenital malformation of optic disc LA14 Structural developmental anomalies of eyelid, lacrimal apparatus or orbit |
Introduction
These notes introduce the abnormal development of the eye and vision associated structures.
Anophthalmia (absence of an eye) and microphthalmia (small eye within the orbit) have a combined birth prevalence of approximately 30 per 100,000 population.[2]
Genetic factors include developmental transcription factors required for inductive/developmental events in the structure of the eye and retina development.
Many environmental factors during development can lead to vision abnormalities, including gestational-acquired infections, maternal vitamin A deficiency, smoking, X-ray exposure, solvent misuse and thalidomide exposure. A pregnancy Rubella viral infection example may cause blindness associated with congenital rubella syndrome.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Developmental Vision Abnormalities | Congenital Blindness | Anophthalmia | Microphthalmia | Bardet-Biedl Syndrome |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Neonatal Vision
Vision in the developing infant can be assessed by a number of tests for: central vision, stereoscopic (binocular) vision, refraction, colour vision, contrast vision, scotopic/photopic (dark/light) vision (retina/rods), and tracking (following and saccades), (retina, oculomotor muscles).
Preterm infants have been shown to develop a number of vision related abnormalities including: visual impairment, oculomotor abnormalities, and refractive error.[7]
|
|
| normal behaviour | cranial nerves |
LA10.1 Anophthalmos
| ICD-11 LA10.1 Clinical anophthalmos - This refers to the clinical absence of one or both eyes. Both the globe (human eye) and the ocular tissue are missing from the orbit. The absence of the eye will cause a small bony orbit, a constricted mucosal socket, short eyelids, reduced palpebral fissure and malar prominence. Genetic mutations, chromosomal abnormalities, and prenatal environment can all cause anophthalmia. Anophthalmia is an extremely rare disease and is mostly rooted in genetic abnormalities. |
| Anophthalmos (anophthalmia} is clinical description for the absence of an eye. Gene mutation of SOX2, a developmental transcription factor, has been associated with this condition. | 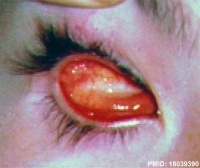
|
LA10.0 Microphthalmos
| ICD-11 LA10.0 Microphthalmos - This is a developmental disorder of the eye that literally means small eye (micros = small; ophthalmos = eye). One (Unilateral Microphthalmia) or both (Bilateral Microphthalmia) eyes may be involved. |
| Microphthalmos (microphthalmia} is clinical description for the presence of a small eye within the orbit and occurs in up to 11% of blind children. A human study has identified microphthalmia can be associated with mutations in the retinal homeobox gene (CHX10).[8] Syndromic microphthalmia-9 can be also be caused by mutations in the Stimulated by Retinoic Acid 6 (STRA6) gene. OMIM - MCOPS9 | 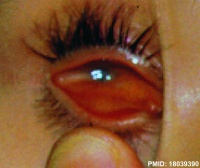
|
Maternal smoking in early pregnancy seemed to increase the risk for microphthalmia in the absence of a coloboma.[9]
LA10.3 Macrophthalmos
| ICD-11 LA10.3 Congenital macrophthalmos - caused by failure of the eye to develop correctly during the antenatal period. This condition is characterized by enlargement of the globe of the eye. |
Coloboma
Coloboma (Greek; koloboma = defect) is a missing pieces of tissue in the structures that form the eye. Occurs in occurs approximately 1 in 10,000 people and, depending on the eye tissue, has a different ICD-11 associated classification.
LA11.4 Coloboma of iris
| ICD-11LA11.4 Coloboma of iris - A disease of the eye, caused by trauma or congenital genetic mutation. This disease is characterized by notches or gaps in iris.
LA11 Structural developmental anomalies of the anterior segment of eye - LA11.1 Structural developmental anomalies of cornea |
LA12.0 Coloboma of lens
| ICD-11 - LA12.0 Coloboma of lens |
LA13.1 Coloboma of choroid or retina
| ICD-11 LA13.1 Coloboma of choroid or retina - A condition of the eye characterized by absence of the retina in the lower inside corner of the eye.
LA13 Structural developmental anomalies of the posterior segment of eye |
LA13.2 Coloboma of macula
| ICD-11 LA13.2 Coloboma of macula - A disease caused by malformation of the macula due to retinal inflammation during the antenatal period or by congenital genetic mutation. This disease is characterized by a clearly delineated defect in the macula. |
Bardet-Biedl Syndrome
(BBS) is an abnormality with triallelic inheritance and is characterized by a range of multisystem abnormalities incliuding postnatal developmental blindness.
- cone-rod dystrophy
- truncal obesity
- postaxial polydactyly
- cognitive impairment
- neural development
- male hypogonadotrophic hypogonadism
- female genitourinary malformations
- renal dysfunction
(More? OMIM - Bardet-Biedl syndrome | GeneReviews - Bardet-Biedl syndrome)
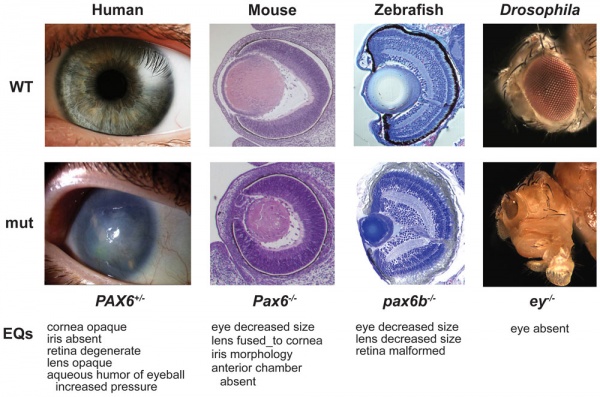
Pax6 Mutation
Phenotypes of wild-type (top) and PAX6 ortholog mutations (bottom) in human, mouse, zebrafish, and fly.[10]
Human mutations may result in aniridia (absence of iris), corneal opacity (aniridia-related keratopathy), cataract (lens clouding), glaucoma, and long-term retinal degeneration.
- Links: PAX
KA62.8 Congenital rubella syndrome
| ICD-11 KA62.8 Congenital rubella syndrome - A disease caused by an infection with the rubella virus in utero. This disease presents with symptoms depending on the timing of infection of the fetus and may present with birth defects (such as hearing loss), or intrauterine growth retardation. Transmission is by vertical transmission. Confirmation is by identification of rubella virus or detection of anti-rubella virus IgM antibodies in the neonate or infant. |
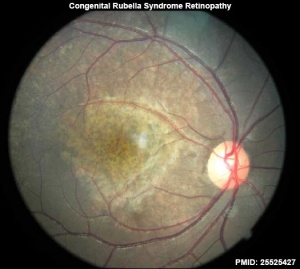
Congenital rubella syndrome (CRS) occurs as a result of a maternal rubella infection during the first trimester of pregnancy and is most commonly associated neural, cardiac and sensory abnormalities. Approximately 25% suffer from congenital cataracts and other eye abnormalities include pigmentary retinopathy and iris hypoplasia.
- Links: rubella virus
Retinopathy of Prematurity
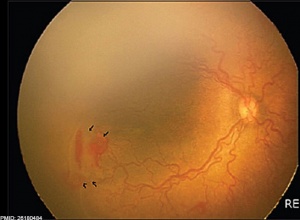
(ROP) A vascular proliferative disorder that affects the incompletely vascularized retina in premature neonates, birth weight 1250 grams or less and born before 31 weeks gestation GA are at highest risk. Classified as type 2 progressing to type 1, this is a primary cause of childhood blindness. Due to retinal immaturity, neovascularization occurs leading to retinal traction and retinal detachment, eventually affecting vision.
USA Statistics
- 14,000-16,000 of low birthweight (<1.25 kg) infants are affected by some degree of ROP.
- disease improves and leaves no permanent damage in milder cases of ROP.
- 90% of all infants with ROP are in the milder category and do not need treatment.
- About 1,100-1,500 infants annually develop ROP that is severe enough to require medical treatment.
- About 400-600 infants each year in the US become legally blind from ROP.
(Data NIH - National Eye Institute)
- Links: Vision Abnormalities | Birth - Preterm | Sensory - Vision Development | NIH - ROP | American Association for Pediatric Ophthalmology)
World Statistics
Sweden
Rate of anophthalmia decreased from the early 1970s from 0.4 to 0.2 per 10,000 births. Non-eye malformations were more common at anophthalmia (63%) than at microphthalmia (30%) Maternal smoking in early pregnancy seemed to increase the risk for anophthalmia or microphthalmia in the absence of a coloboma.[9]
United Kingdom
1988-94 prevalence of anophthalmia and microphthalmia was 1.0 per 10,000 births.[12]
USA California
1989-1997 prevalence per 10,000 livebirths and stillbirths for anophthalmia was 0.18 and for bilateral microphthalmia was 0.22. Risk of anophthalmia was approximately twofold among multiple births compared to singletons. (More? Shaw GM, etal., 2005)
Colour Blindness
Most common types of hereditary colour blindness are due to the loss or limited function of red cone (protan) or green cone (deutran) photopigments. This kind of colour blindness is commonly referred to as red-green colour blindness. (note the US spelling color)
- Deuteranomaly In males with deuteranomaly, the green cone photopigment is abnormal. Yellow and green appear redder and it is difficult to tell violet from blue. This condition is mild and doesn’t interfere with daily living. Deuteranomaly is the most common form of colour blindness and is an X-linked disorder affecting 5 percent of males.
- Deuteranopia In males with deuteranopia, there are no working green cone cells. They tend to see reds as brownish-yellow and greens as beige. Deuteranopia is an X-linked disorder that affects about 1 percent of males.
- Protanomaly In males with protanomaly, the red cone photopigment is abnormal. Red, orange, and yellow appear greener and colours are not as bright. This condition is mild and doesn’t usually interfere with daily living. Protanomaly is an X-linked disorder estimated to affect 1 percent of males.
- Protanopia In males with protanopia, there are no working red cone cells. Red appears as black. Certain shades of orange, yellow, and green all appear as yellow. Protanopia is an X-linked disorder that is estimated to affect 1 percent of males.
- Inheritance Pattern images: Genetic Abnormalities | autosomal dominant | autosomal recessive | X-linked dominant (affected father) | X-Linked dominant (affected mother) | X-Linked recessive (affected father) | X-Linked recessive (carrier mother) | mitochondrial inheritance | Codominant inheritance | Genogram symbols | Genetics
References
- ↑ Jivraj I, Rudnisky CJ, Tambe E, Tipple G & Tennant MT. (2014). Identification of ocular and auditory manifestations of congenital rubella syndrome in mbingo. Int J Telemed Appl , 2014, 981312. PMID: 25525427 DOI.
- ↑ Verma AS & Fitzpatrick DR. (2007). Anophthalmia and microphthalmia. Orphanet J Rare Dis , 2, 47. PMID: 18039390 DOI.
- ↑ Berry V, Georgiou M, Fujinami K, Quinlan R, Moore A & Michaelides M. (2020). Inherited cataracts: molecular genetics, clinical features, disease mechanisms and novel therapeutic approaches. Br J Ophthalmol , , . PMID: 32217542 DOI.
- ↑ Demontis GC, Aruta C, Comitato A, De Marzo A & Marigo V. (2012). Functional and molecular characterization of rod-like cells from retinal stem cells derived from the adult ciliary epithelium. PLoS ONE , 7, e33338. PMID: 22432014 DOI.
- ↑ Tibbetts MD, Samuel MA, Chang TS & Ho AC. (2012). Stem cell therapy for retinal disease. Curr Opin Ophthalmol , 23, 226-34. PMID: 22450217 DOI.
- ↑ Jimenez NL, Flannick J, Yahyavi M, Li J, Bardakjian T, Tonkin L, Schneider A, Sherr EH & Slavotinek AM. (2011). Targeted 'next-generation' sequencing in anophthalmia and microphthalmia patients confirms SOX2, OTX2 and FOXE3 mutations. BMC Med. Genet. , 12, 172. PMID: 22204637 DOI.
- ↑ Birch EE & O'Connor AR. (2001). Preterm birth and visual development. Semin Neonatol , 6, 487-97. PMID: 12014889 DOI.
- ↑ Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, Kocak-Altintas A, Sowden JC, Traboulsi E, Sarfarazi M & McInnes RR. (2000). Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat. Genet. , 25, 397-401. PMID: 10932181 DOI.
- ↑ 9.0 9.1 Källén B & Tornqvist K. (2005). The epidemiology of anophthalmia and microphthalmia in Sweden. Eur. J. Epidemiol. , 20, 345-50. PMID: 15971507
- ↑ Washington NL, Haendel MA, Mungall CJ, Ashburner M, Westerfield M & Lewis SE. (2009). Linking human diseases to animal models using ontology-based phenotype annotation. PLoS Biol. , 7, e1000247. PMID: 19956802 DOI.
- ↑ Gadkari SS, Kulkarni SR, Kamdar RR & Deshpande M. (2015). Successful Surgical Management of Retinopathy of Prematurity Showing Rapid Progression despite Extensive Retinal Photocoagulation. Middle East Afr J Ophthalmol , 22, 393-5. PMID: 26180484 DOI.
- ↑ Busby A, Dolk H, Collin R, Jones RB & Winter R. (1998). Compiling a national register of babies born with anophthalmia/microphthalmia in England 1988-94. Arch. Dis. Child. Fetal Neonatal Ed. , 79, F168-73. PMID: 10194985
Online Textbooks
- Developmental Biology (6th ed.) Gilbert, Scott F. Sunderland (MA): Sinauer Associates, Inc.; c2000. | Chick embryo rhombomere neural crest cells | Some derivatives of the pharyngeal arches | Formation of the Neural Tube | Differentiation of the Neural Tube | Tissue Architecture of the Central Nervous System | Neuronal Types | Snapshot Summary: Central Nervous System and Epidermis
- Neuroscience Purves, Dale; Augustine, George J.; Fitzpatrick, David; Katz, Lawrence C.; LaMantia, Anthony-Samuel; McNamara, James O.; Williams, S. Mark. Sunderland (MA): Sinauer Associates, Inc. ; c2001 Early Brain Development | Construction of Neural Circuits | Modification of Brain Circuits as a Result of Experience
- Molecular Biology of the Cell (4th Edn) Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter. New York: Garland Publishing; 2002. Neural Development | The three phases of neural development
- Clinical Methods 63. Cranial Nerves IX and X: The Glossopharyngeal and Vagus Nerves | The Tongue | 126. The Ear and Auditory System | An Overview of the Head and Neck - Ears and Hearing | Audiometry
- Health Services/Technology Assessment Text (HSTAT) Bethesda (MD): National Library of Medicine (US), 2003 Oct. Developmental Disorders Associated with Failure to Thrive
- Eurekah Bioscience CollectionCranial Neural Crest and Development of the Head Skeleton
Reviews
Bookshelf vision development
Articles
Search Pubmed
- Pubmed vision developmental abnormalities | Congenital Rubella Blindness | Anophthalmia | Microphthalmia | retinal stem cell therapy
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Terms
| Vision Terms | ||
|---|---|---|
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Sensory - Vision Abnormalities. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Sensory_-_Vision_Abnormalities
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G