Preimplantation Genetic Diagnosis
| Embryology - 1 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
This current page is a general starting point for the topic of Preimplantation Genetic Screening (PGS, NIPT) also called Preimplantation Genetic Diagnosis (PGD) began during the 1990's as an alternative to other forms of prenatal diagnosis.
- "In the general population trisomies and sex chromosome aneuploidies account for approximately 70% of anomalies recognizable by conventional genetic analysis."[1]
Recently with the growth in Assisted Reproductive Technology (ART) or commonly known as In Vitro Fertilization (IVF), there is now a new form of prenatal diagnosis that involves genetic testing of the blastocyst before implantation. (More? Assisted Reproductive Technology)
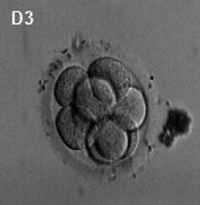
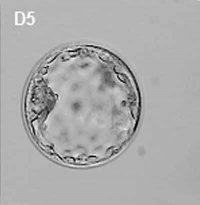
Generally, in vitro fertilised embryos are first cultured for up to three days. By this time the conceptus is composed of 6 to 10 cells (blastomeres) from which 1 or 2 cells are then removed by a laser for genetic testing. Some studies have also removed cells, or the polar body, at earlier days following fertilisation. While other studies have collected cells from later stage (day 5) blastocyst either the trophectoderm (trophoblast) or inner cell mass (embryoblast).
- This Embryology site is a developmental educational resource, it does not provide specific clinical details, you should always refer to a health professional.
| Assisted Reproductive Technology | In Vitro Fertilization
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Preimplantation Genetic Diagnosis <pubmed limit=5>Preimplantation Genetic Diagnosis</pubmed> |
| Older papers |
|---|
|
Genetic Testing
Trisomy
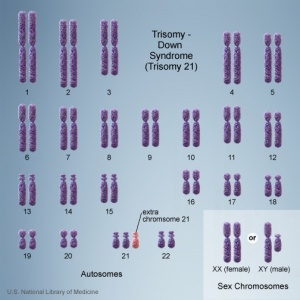
There are clinically more and more tests becoming available as we learn more about the genetic basis of some diseases. The most common diagnostic test relates to the current trend in an increasing maternal age, which has long been associated with an increase in genetic abnormalities, the most frequent of these is trisomy 21 or Down syndrome.
- Links: Genetic risk maternal age | Trisomy 21
Single Gene Disorders
- Cystic fibrosis
- beta-thalassaemia
- Spinal muscular atrophy
- Sickle-cell anaemia
- Huntington disease
- Myotonic dystrophy type 1
- Duchenne or Becker muscular dystrophy
- Haemophilia
- Fragile-X syndrome
Australia
A recent publication from NHMRC Medical Genetic Testing: information for health professionals (2010). This paper covers background information on all types of genetic tests, not just those associated with prenatal diagnosis.
Types of genetic tests
- Somatic cell genetic testing involves testing tissue (usually cancer) for non-heritable mutations. This may be for diagnostic purposes, or to assist in selecting treatment for a known cancer.
- Diagnostic testing for heritable mutations involves testing an affected person to identify the underlying mutation(s) responsible for the disease. This typically involves testing one or more genes for a heritable mutation.
- Predictive testing for heritable mutations involves testing an unaffected person for a germline mutation identified in genetic relatives. The risk of disease will vary according to the gene, the mutation and the family history.
- Carrier testing for heritable mutations involves testing for the presence of a mutation that does not place the person at increased risk of developing the disease, but does increase the risk of having an affected child developing the disease.
- Pharmacogenetic testing for a genetic variant that alters the way a drug is metabolised. These variants can involve somatic cells or germline changes. Even if these variants are heritable (that is germline changes), the tests are usually of relevance to genetic relatives only if they are being treated with the same type of medication.
USA
A new site developed by NIH "GeneTests" provides medical genetics information resources available at no cost to all interested persons. It contains educational information, a directory of genetic testing laboratories and links to other databases such as OMIM.
- Links: GeneTests | Medline Plus - Genetic Testing
Genetic Inheritance
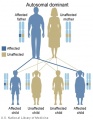
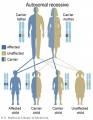
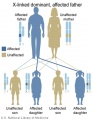
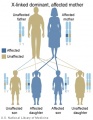
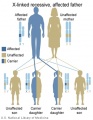
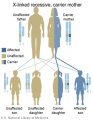
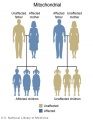
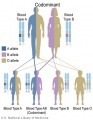
The figures below show the pattern of inheritance of a range of genetic disorders. In addition to these patterns are the known effects of increased maternal age and the effects of genetic mutations in the embryo and newborn.
- Inheritance Pattern images: Genetic Abnormalities | autosomal dominant | autosomal recessive | X-linked dominant (affected father) | X-Linked dominant (affected mother) | X-Linked recessive (affected father) | X-Linked recessive (carrier mother) | mitochondrial inheritance | Codominant inheritance | Genogram symbols | Genetics
Ethics of Testing
Major developmental abnormalities detected early enough can be resolved far more easily than those discovered late in a pregnancy.
What are the ethical questions that are raised by prenatal testing? Future individual rights or parents rights? But what about diseases, like Huntington's, where a diagnostic test can be made but there are no current treatments for the postnatal (95% of cases adult onset) disease?
Huntington's disease
Guidelines for the molecular genetics predictive test
- Recommendation 2.1 "the test is available only to individuals who have reached the age of majority."
- Recommendation 7.2 "the couple requesting antenatal testing must be clearly informed that if they intend to complete the pregnancy if the fetus is a carrier of the gene defect, there is no valid reason for performing the test."
References
- ↑ Kozlowski P, Burkhardt T, Gembruch U, Gonser M, Kähler C, Kagan KO, von Kaisenberg C, Klaritsch P, Merz E, Steiner H, Tercanli S, Vetter K & Schramm T. (2018). DEGUM, ÖGUM, SGUM and FMF Germany Recommendations for the Implementation of First-Trimester Screening, Detailed Ultrasound, Cell-Free DNA Screening and Diagnostic Procedures. Ultraschall Med , , . PMID: 30001568 DOI.
- ↑ Huang L, Bogale B, Tang Y, Lu S, Xie XS & Racowsky C. (2019). Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc. Natl. Acad. Sci. U.S.A. , 116, 14105-14112. PMID: 31235575 DOI.
- ↑ Wang A, Kort J, Behr B & Westphal LM. (2018). Euploidy in relation to blastocyst sex and morphology. J. Assist. Reprod. Genet. , , . PMID: 30030712 DOI.
- ↑ Genoff Garzon MC, Rubin LR, Lobel M, Stelling J & Pastore LM. (2018). Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin. Genet. , 94, 22-42. PMID: 29120067 DOI.
- ↑ Yahalom C, Macarov M, Lazer-Derbeko G, Altarescu G, Imbar T, Hyman JH, Eldar-Geva T & Blumenfeld A. (2018). Preimplantation genetic diagnosis as a strategy to prevent having a child born with an heritable eye disease. Ophthalmic Genet. , 39, 450-456. PMID: 29781739 DOI.
- ↑ Karimi Yazdi A, Davoudi-Dehaghani E, Rabbani Anari M, Fouladi P, Ebrahimi E, Sabeghi S, Eftekharian A, Fatemi KS, Emami H, Sharifi Z, Ramezanzadeh F, Tajdini A, Zeinali S & Amanpour S. (2018). The first successful application of preimplantation genetic diagnosis for hearing loss in Iran. Cell. Mol. Biol. (Noisy-le-grand) , 64, 1718. PMID: 30030956
- ↑ Garcia-Herrero S, Cervero A, Mateu E, Mir P, Póo ME, Rodrigo L, Vera M & Rubio C. (2016). Genetic Analysis of Human Preimplantation Embryos. Curr. Top. Dev. Biol. , 120, 421-47. PMID: 27475859 DOI.
- ↑ Gleicher N, Kushnir VA & Barad DH. (2014). Preimplantation genetic screening (PGS) still in search of a clinical application: a systematic review. Reprod. Biol. Endocrinol. , 12, 22. PMID: 24628895 DOI.
- ↑ Rabinowitz M, Ryan A, Gemelos G, Hill M, Baner J, Cinnioglu C, Banjevic M, Potter D, Petrov DA & Demko Z. (2012). Origins and rates of aneuploidy in human blastomeres. Fertil. Steril. , 97, 395-401. PMID: 22195772 DOI.
- ↑ Ramón Y Cajal T, Polo A, Martínez O, Giménez C, Arjona C, Llort G, Bassas L, Viscasillas P & Calaf J. (2012). Preimplantation genetic diagnosis for inherited breast cancer: first clinical application and live birth in Spain. Fam. Cancer , 11, 175-9. PMID: 22179695 DOI.
- ↑ Van Rij MC, De Rademaeker M, Moutou C, Dreesen JC, De Rycke M, Liebaers I, Geraedts JP, De Die-Smulders CE & Viville S. (2012). Preimplantation genetic diagnosis (PGD) for Huntington's disease: the experience of three European centres. Eur. J. Hum. Genet. , 20, 368-75. PMID: 22071896 DOI.
Reviews
Bodurtha J & Strauss JF. (2012). Genomics and perinatal care. N. Engl. J. Med. , 366, 64-73. PMID: 22216843 DOI.
Ly KD, Agarwal A & Nagy ZP. (2011). Preimplantation genetic screening: does it help or hinder IVF treatment and what is the role of the embryo?. J. Assist. Reprod. Genet. , 28, 833-49. PMID: 21743973 DOI.
Articles
Theodosiou AA & Johnson MH. (2011). The politics of human embryo research and the motivation to achieve PGD. Reprod. Biomed. Online , 22, 457-71. PMID: 21397558 DOI.
Harton GL, De Rycke M, Fiorentino F, Moutou C, SenGupta S, Traeger-Synodinos J & Harper JC. (2011). ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum. Reprod. , 26, 33-40. PMID: 20966462 DOI.
Harton GL, Harper JC, Coonen E, Pehlivan T, Vesela K & Wilton L. (2011). ESHRE PGD consortium best practice guidelines for fluorescence in situ hybridization-based PGD. Hum. Reprod. , 26, 25-32. PMID: 20966461 DOI.
Harton G, Braude P, Lashwood A, Schmutzler A, Traeger-Synodinos J, Wilton L & Harper JC. (2011). ESHRE PGD consortium best practice guidelines for organization of a PGD centre for PGD/preimplantation genetic screening. Hum. Reprod. , 26, 14-24. PMID: 20966460 DOI.
Harton GL, Magli MC, Lundin K, Montag M, Lemmen J & Harper JC. (2011). ESHRE PGD Consortium/Embryology Special Interest Group--best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum. Reprod. , 26, 41-6. PMID: 20966459 DOI.
Journals
Search PubMed
Search Pubmed: Preimplantation Genetic Screening | Preimplantation Genetic Diagnosis
- ART - Assisted Reproductive Technology a general term to describe all the clinical techniques used to aid fertility.
- blastomere biopsy - An ART preimplantation genetic diagnosis technique carried out at cleavage stage (day 3), excluding poor quality embryos, detects chromosomal abnormalities of both maternal and paternal origin. May not detect cellular mosaicism in the embryo.
- blastocyst biopsy - An ART preimplantation genetic diagnosis technique carried out at blastocyst stage (day 4-5), removes several trophoblast (trophoderm) cells, detects chromosomal abnormalities of both maternal and paternal origin and may detect cellular mosaicism.
- cell-free fetal deoxyribonucleic acid - (cfDNA) refers to fetal DNA circulating and isolated from the plasma portion of maternal blood. Can be performed from GA 10 weeks as a first-tier test or as a second-tier test, with women with increased probability on combined first trimester screening offered cfDNA or diagnostic testing.
- false negative rate - The proportion of pregnancies that will test negative given that the congenital anomaly is present.
- false positive rate - The proportion of pregnancies that will test positive given that the congenital anomaly is absent.
- free β human chorionic gonadotrophin - beta-hCG subunit of hCG used as a diagnostic marker for: early detection of pregnancy, Trisomy 21, spontaneous abortion, ectopic pregnancy, hydatidiform mole or choriocarcinoma.
- multiples of the median - (MoM) A multiple of the median is a measure of how far an individual test result deviates from the median and is used to report the results of medical screening tests, particularly where the results of the individual tests are highly variable.
- negative predictive value - The probability that a congenital anomaly is absent given that the prenatal screening test is negative.
- Non-Invasive Prenatal Testing - (NIPT) could refer to ultrasound or other imaging techniques, but more frequently used to describe analysis of cell-free fetal DNA circulating in maternal blood.
- polar body biopsy - (PB biopsy) An ART preimplantation genetic diagnosis technique that removes either the first or second polar body from the zygote. As these are generated by oocyte meiosis they detects chromosomal abnormalities only on the female genetics.
- positive predictive value - The probability that a congenital anomaly is present given that the prenatal screening test is positive.
- pre-implantation genetic diagnosis - (PGD, pre-implantation genetic screening) a diagnostic procedure for embryos produced through Assisted Reproductive Technology (ART, in vitro fertilisation, IVF) for genetic diseases that would generate developmental abnormalities or serious postnatal diseases.
- prenatal screening sensitivity - (detection rate) The probability of testing positive on a prenatal screening test if the congenital anomaly is present.
- prenatal screening specificity - The probability of testing negative on a prenatal screening test if the congenital anomaly is absent.
- quadruple test (maternal serum testing of a-fetoprotein Template:AFP, free B-hCG or total hCG, unconjugated estriol, and inhibin A) is a fetal chromosomal anomaly test usually carried out later in pregnancy (GA 14 to 20 weeks).
- single nucleotide polymorphisms - (SNPs) the variation in a single DNA nucleotide that occurs at a specific position in the genome.
- triple test - (maternal serum testing of a-fetoprotein Template:AFP, free B-hCG or total hCG, and unconjugated estriol) is a fetal chromosomal anomaly test usually carried out later in pregnancy (GA 14 to 20 weeks).
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 1) Embryology Preimplantation Genetic Diagnosis. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Preimplantation_Genetic_Diagnosis
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G