Paper - The subdivisions of the neural folds in man
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bartelmez GW. The subdivisions of the neural folds in man. (1923) J. Comp. Neural., 35: 231-247.
| Online Editor |
|---|
| George Bartelmez (1885 -1967) was a Fellow in Zoology at the University of Chicago from 1907 and received his Ph.D. in embryology (bilaterality of the pigeon's egg) in 1910. Developed the human embryological collection in the Anatomy Department of the University of Chicago.
University of Chicago Embryo H279 was added to the Carnegie Collection as Embryo 3709. Carnegie Collection Embryos used in this study 836, 3709, 1878 and 1201a. These embryos cover the period week 3 to week 4.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Subdivisions of the Neural Folds in Man
Department of Anatomy, The University of Chicago, and the Laboratory of Embryology, Carnegie Institution of Washington
Six Figures
Veit and Esch have recently given us the most complete and detailed study of a vertebrate embryo during the period of somite formation that has ever appeared. All the labor and study expended upon it has been Well worth while, as human embryos of this period are very rare. The specimen is certainly normal and the preservation above reproach. The embryo has eight somites and belongs to the beginning of the third Week, a period which Prof. H. M. Evans and I have been studying for some years. Most of Veit and Esch’s findings fit well into the sequence of events as We have interpreted it from our series of embryos. There is, however, a radical disagreement in our interpretations of the nervous system, and in View of the great importance of the Veit embryo to human embryology, it would seem wise to call attention to the matter.
In his first paper based upon this embryo (’18), as well as in the complete description (’22) Veit has adopted a slight modification of the traditional interpretation of the nervous system in young human embryos. This seems to have originated with Kollmann (’89) in his description of the celebrated embryo ‘Bulle,’ which he had studied simply as a whole mount in balsam. The identification of the regions of the brain was, in the nature of the case, almost wholly subjective. The subsequent Writers who have ventured interpretations of the nervous system of embryos younger than ‘Bulle’ have followed Kollmann more or less closely. They have all made the forebrain relatively enormous and the hindbrain insignificant in size. None of these workers had a series of stages, such as is generally recognized as essential for the interpretation of the differentiations which can be seen in young embryos. It is not surprising that gross errors have crept into our text-books in spite of the correct interpretations of Giglio-Tos (’02; 15—somite embryo) and Low (’O8; 14-somite embryo).
We have now made a minute study of fourteen human embryos ranging from two to sixteen somites. In addition, the Davis (’20, ’23) embryo, of twenty somites, and the perfect 4-mm embryo no. 836 of the Carnegie Collection have been available for study. Of the latter there is a complete series of models in the Carnegie Laboratory, prepared (under the direction of Professor Evans) by Mr. O. O. Heard, who has made most of the other models I have used in this work. I am indebted to Doctor Davis for the use of his series of models. The identification of certain landmarks has made it possible to trace the history of the primary subdivisions of the brain to the 4-mm. stage, where there can be little doubt as to the three primary brain vesicles or the rhombomeres. Two of these landmarks are present from the youngest embryos on; others appear a little later, and together they afford a substantial body of evidence upon which our analysis is based. This evidence may be summarized as follows:
- A marked enlargement of the neural folds (or tube as the case may be) in the region of the otic plate can be recognized in every embryo. It may be termed the otic segment and can be shown to become the fourth rhombomere of the usual terminology. It could not escape notice even in a superficial study, and most writers have called it the second brain Vesicle, viz., Kollmann (’89), Dandy (’10), Bujard (’21, fig. 2), and the authors of various texts. This may also be the interpretation of Keibel and Elze (_’08, p. 18) for ‘Klb.’ Wallin (’13) and Veit and Esch (’2‘2) termed it the rostral end of the hindbrain. Giglio-Tos C02) and .Low (’08) have correctly analyzed the nervous system in their specimens, which were older than any described by the authors just referred to. The peculiarities by which the otic segment can be recognized are these:
- The form of the neural folds characteristic at this level (cf. p. 236).
- From the four—somite stage on, the acoustico-facial primordium is arising from its dorsal edge, and this division of the neural crest is distinctive in form and behavior (cf. p. 242).
- The adjacent ectoderm is thickened as the otic plate which can be readily recognized in the two- somite embryo (Ingalls, ’20), although it does not begin to invaginate until the eleven-somite period.
- The relation of the otic segment to such structures as the pharyngeal pouches, the heart, the aortic rami, etc., shift but slowly during the few hours of development we are studying, and these relations serve to confirm our identification.
- The second landmark is the midbrain which constitutes the knee of the cranial flexure. The latter can be recognized as the first abrupt bending of the neural axis which we encounter as We pass back from the rostral end.[1] It is obvious at least as early as the two-somite stage, but the midbrain thus located does not always have well-defined boundaries, unfortunately. Other differentiations appear, however, which help to delimit it. The optic primordium is such a one, since its caudal end serves to locate the di-mesencephalic boundary.
- The behavior of the neural crest of the trigeminal segment is distinctive so that it can be used to identify this part of the hindbrain in the older members of the series.
- The first somite is unmistakable because of its form and the behavior of its cells, and during the period involved there is but little shifting with reference to the nervous system.
The following figures are from midsagittal projection reconstructions which, with the exception of the first, were made at amagnification of 200 diameters. In the original charts every section was plotted and all were controlled by the study of themodels. The figures are from accurate copies by Mr. J . F. Didusch of my original charts.
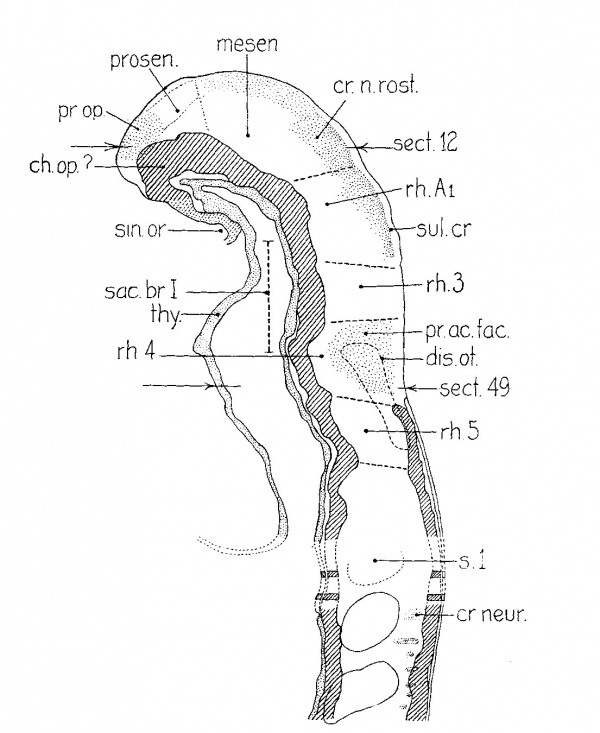
Figure 1 presents an analysis of a two—somite embryo (no. 1878 of the Carnegie Collection) which has been well described by Ingalls (’20). The neural folds can be divided into five segments which are separated either by constrictions or by sulci on the lateral surface of the folds. The midbrain (mesen.), defined by the cranial flexure, separates the forebrain (prosen.) from the hindbrain. The latter exhibits three subdivisions: the first (rh.A) is small but clearly defined, especially on the right side; the second, the otic (rh.B), is prominent, and the third (rh.C) involves the rest of the neural folds rostral to the first pair of somites. The otic segment in this specimen differs from those of all succeeding stages in that there is a general enlargement of the neural groove corresponding to it. The otic plate (disc.ot.) is practically coextensive with the otic segment and is well marked, as was pointed out by Ingalls (’20).
Figure 2 gives the relations in a four-somite embryo (H279, U. of C. Coll.) in which abundant mitoses are to be seen, although the specimen underwent great shrinkage during dehydration. It belongs to a group which includes that just described as well as Wilson’s ‘H3’ (’14) and ‘Klb’ (Keibel and Elze, ’O8, Normentafel No. 3). Forebrain, midbrain, and three hindbrain subdivisions were clearly visible in the gross specimen when studied in 80 per cent alcohol. It will be seen in the figure that the forebrain has begun to grow forward beyond the pharynx and that it is relatively longer than in the previous case. The midbrain is wedge—shaped. There are clear signs of differentiation in the hindbrain in that there are four segments and a ganglionic anlage. The first segment (rh.A) is still small and inconspicuous, whereas the second (rh.B) has acquired new characters which distinguish it for the rest of our period. The enlargement is sharply marked off by constrictions fore and aft, and corresponding to it there is a dip in the floor. Ventrally the neural groove is enlarged in the manner typical for neuromeres, but dorsally the folds approach one another only to diverge again at the dorsal edge where they are thickened. The thickening is the acoustico-facial primordium (pr.ac.fac.). These relations may be seen in figure 1 of my 1922 paper. The level of that section is indicated in the present figure 2, where it will be seen that the plane of section passes obliquely through the otic segment (rh.B) and the two succeeding segments. The latter two (rh.C1 and rh.C2) correspond in position to the third hindbrain segment of the previous specimen (rh.C). It may be that that segment has divided, but it is possible that H279 is an individual in which the segmentation of the nervous system is particularly pronounced.
| Fig. 1 A projection reconstruction on the midsagittal plane of a two-somite embryo (no. 1878, Carnegie Coll.). x100. The cut surfaces of the nervous system are hatched, those of the gut, stippled. The body ectoderm and the primitive streak are indicated by broken lines. The arrows show the plane of section. Section 44 is reproduced by Ingalls (’20) as figure A, page 81. disc.ot., otic disc or plate; lm.pr., primitive streak; mem.cl., cloacal membrane; mem.ph., pharyngeal membrane; mesen.,midbrain; n.pr., primitive node of Hansen; p.int.a., anterior intestinal portal; ph., pharynx; prosen., forebrain; rh.A,B, and C’, first, second and third hindbrain segments; 3.1, first somite with myocoale. | 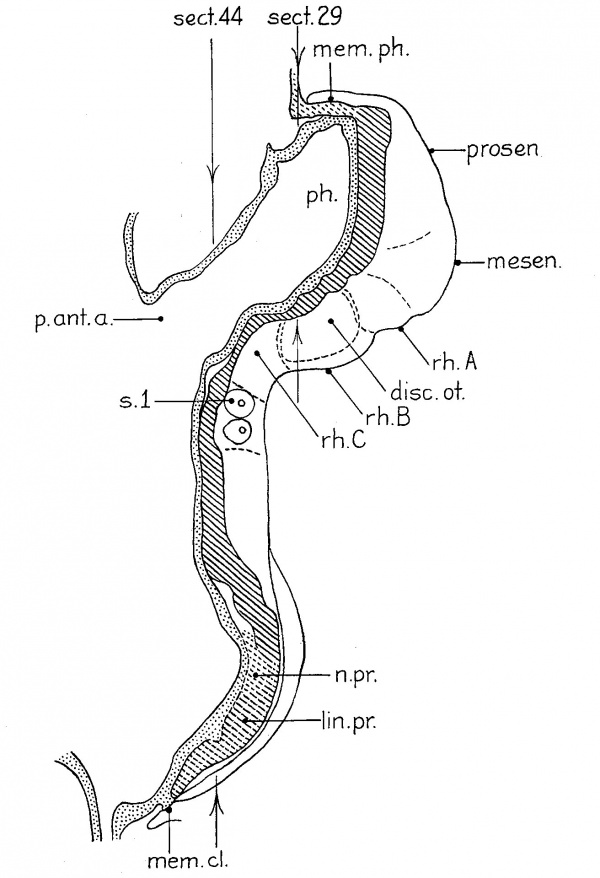
|
The eight-somite embryos, no. 391 of Mall (Dandy, ’10) and H87 (U. of C. Coll), are transitional to the next phase in the development of the nervous system. H87 has been briefly described and figured (Bartelmez, ’22; figs. 2b, 3, 4, 5, 7 , and 9a). We can recognize forebrain, characterized by the optic primordium, midbrain, and three hindbrain segments. The first hindbrain segment is larger than in any younger embryo and its crest primordium is proliferating mesectoderm as far caudal as the acoustico-facial anlage. The second segment is the otic from which the acoustico—facial primordium is beginning to separate. The third clearly corresponds to the first postotic of H279 (fig. 2, rh.C1), but the segment homologous to C2 is only vaguely indicated. The typical form of the otic segment with its hourglass-shaped lumen is shown in figure 2b of my paper of 1922. In d and e of the same figure it will be seen that the corresponding lateral fossa lodges the expanded crest primordium, but the presence of the fossa in H87, as well as in the younger embryos (e.g., ‘Klb’ and the Mall embryo no. 391), shows that it is not merely the result of the splitting off of the primordium, as would appear to be the case in the later stages.
| Fig. 2 A reconstruction like that of figure 1, of a four—somite embryo (H279, U. of C. Coll.); magnified 200 diameters and reduced one half in reproduction. The plane of section is indicated by the position of section 54 which was shown as figure 1 in my 1922 paper. camn-e., neurenteric canal; ch., chorda; disc.od., otic disc; mem. cl., cloacal membrane (the entoderm has shrunken away from the ectoderm) ; mem. ph., pharyngeal membrane; mesen., midbrain; ph., pharynx; pr.ac.fac., acoustico-facial primordium; prosen., forebrain; rh.A, rh.B, first and second hindbrain segments; rh..C'1 and 7'h.C'2 have resulted from the division of rh.C’ in the previous embryo; rh.B is identical with the definitive fourth rhombomere, rh.C'; with the fifth. | 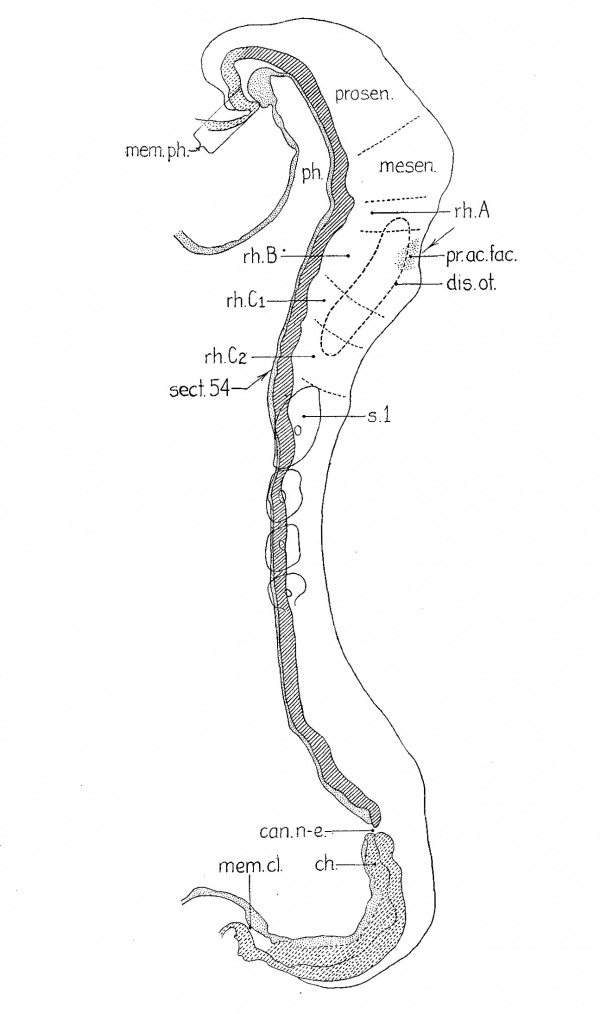
|
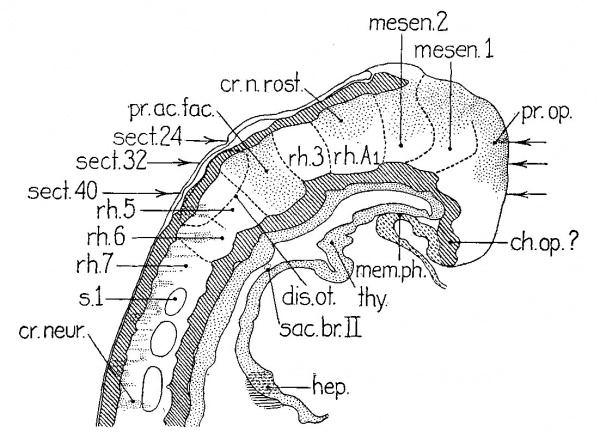
Our next stage in the development of the nervous system is represented by Veit’s embryo (eight somites), ‘DuGa’ of Eternod (nine somites), and H392 (U. of C. Coll., eleven somites). The analysis of the second of these is given in figure 3. VVhile I have not studied the sections of this beautiful specimen, I have had the series of accurate and detailed tracings of every section, magnified 200 diameters, made by Professor Evans in the laboratory of Professor Eternod, as well as the latter’s descriptions and the Ziegler model. These data, together with the study of the similiar H392, have left few uncertainties as to interpretation. The models and projection reconstructions were stacked from the profile given in figure 5 of Eternod’s (’99) paper. In this embryo (fig. 3) the continued growth of the forebrain around the end of the pharynx is associated with the great growth of the midbrain. The rostral end of this region has remained uncertain, as indicated in the figure by the dotted line. The other subdivisions can be recognized in the Ziegler model as well as in our own. The first hindbrain segment of the previous stage has not only increased in length, but has divided into two (rh./11 and rh.5’ in fig. 3), for there are now two segments between midbrain and the otic segment. The hindbrain subdivision A of figures 1 and 2 has split off a neuromere (rh.3) caudally, because later stages show that this new segment becomes the first preotic neuromere, i.e., the third rhombomere of the usually recognized series. Crest proliferation has ceased in this segment, but is still present rostrally as far as the forebrain. The cristal and optic primordia are probably continuous here as in other embryos of the same period, but this region is very difficult to interpret because it is cut tangentially. The otic rhombomere of ‘DuGa’ is typical in form, as may be seen in the Ziegler model, where it can be identified by the fact that the neural folds have closed as far as its caudal end. The first postotic neuromere (?'h.5) is, as usual, easily recognized and there is an indication of another behind it. Then we come to the break in the series which is indicated in the figure by the dotted lines. The lost sections, we are convinced, contained the first pair of somites, a matter which will be considered in detail in our full report of these embryos.
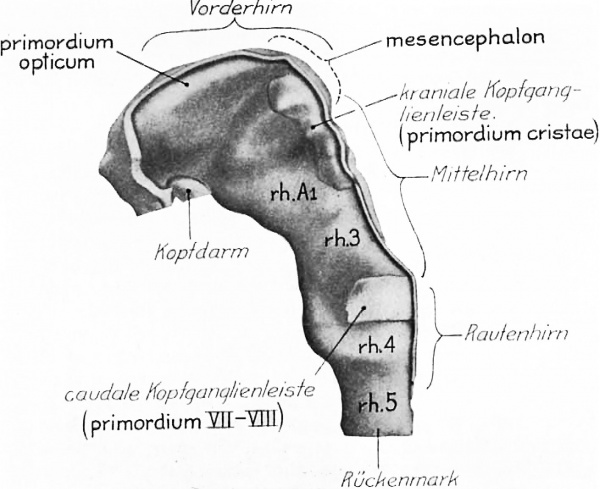
It is obvious from the excellent description and figures of Veit and Esch (’22) that their eight—somite embryo is but little younger than ‘DuGa.’ Their figure 9 is here reproduced as figure 4 with both the original labels and, in heavier type, the interpretation we place upon the enlargements of the neural folds. The otic segment rh.4 has the characteristic form; there is the great lateral fossa occupied in part by the acoustico-facial primordium (caudale Kopfganglienleiste) and the marked dip in the floor of the neural groove. figure 11 of Veit and Esch also shows this rhombomere clearly. The midbrain is readily identified through the cranial fiexure. Between these two we find the same divisions as in ‘DuGa.’ The figures of the nervous system show only one postotic neuromere, but the general reconstructions (Taf. X and XI) make it clear that there is at least room for another similar segment between it and the first pair of somites. The Veit embryo is, then, very like ‘DuGa’ so far as the subdivisions of the nervous system are concerned, and indeed in most other details of this system. The most striking difference between the two is the greater size of the midbrain in ‘DuGa.’ Associated, no doubt, with the rapid growth of the mesencephalon is the more obtuse cranial flexure in this specimen. figure 4 shows the neural crest (kraniale Kopfganglienleiste) extending to the rostral end of the midbrain. The forebrain folds are thickened laterally, so that a longitudinal ridge appears in the lateral View (of. fig. 4, primordium opticum). We are inclined to interpret this as the optic primordium, not only because of the general relations, but because Professor Veit’s photomicrographs show that its appearance in section is very like that of the eight-somite embryo H87, when one takes into consideration the difference in the plane of section in the two cases.[2]
Figure 5 presents the analysis of the thirteen-somite embryo briefly described by Wallin (’13). Doctor Senior has kindly placed this important specimen at our disposal and it is listed as no. 1201a of the Carnegie Collection. It represents a significant stage in the development of the nervous system, and in addition the boundaries between the neural segments stand out diagrammatically as a result of the marked shrinkage. It will be seen that the growth of the forebrain around the end of the pharynx has continued and in addition the lateral edges of the folds havebegun to concresce so that from now on the anterior neuropore is closing from both directions. The forebrain is longer than in the previous stages and it has a well-developed optic primordium as was recognized by Wallin. There are now two midbrain segments (mesen. 1 and 2) similiar in form to the differentiated rhombomeres—~a condition foreshadowed in the eleven-somite embryo H392 and the twelve—somite embryo H197 of Evans. As before, there are two preotic rhornborneric segments. The more rostral division of the neural crest (cr.n.rost.), which had previously been separated from that of the VII-VIII level by the disappearance of crest from the preotic rhombomere (M3), is still attached to the nervous system from the first hindbrain segment (rh./11), as far forward as the optic primordium. While the connection with the midbrain is soon lost, the con- tinuity with the dorsal edge of the hindbrain persists. This portion of the crest plays a part in the development of the semilunar ganglion, and we may designate the corresponding segment (rh./11) as ‘trigeminal,’ but the history of this ganglion is by no means clear from the material at present available. The acoustico—facial primordium extends as a dense cell column to the hyoidean epibranchial placode, and there can be no question but that cells migrate out from this ectodermal thickening. From what we know of other vertebrates it is probable that they contribute to the geniculate ganglion. The relations of crest and placodes in this embryo look forward to the conditions described by Giglio-Tos (’O2 a, b, c) in a fifteen-somite embryo. ‘Pfannenstiel III’ (NT. 6, fourteen somites) doubtless represents an intermediate stage. It is unfortunate that no one who has had the opportunity of studying this specimen carefully should have interpreted the relations there in the light of the illuminating studies of Giglio-Tos. His embryo well deserves a detailed description of the nervous system as a Whole. The ganglionic primordia are similar in the sixteen somite no. 470 of the Carnegie Collection, but the description of this embryo must be reserved for a later contribution.
Returning now to figure 5, it will be noted that the postotic (rh.5), as well as the preotic rhombomere (rh.3), shows no neural crest in this embryo. The sixth rhombomere (7*h.6) has a clump of crest cells migrating from its dorsal edge. From this level caudally there are strands of outwandering cells, but it has not been possible in this case to make out the clear—cut metamerism in the crest which Lenhossék (’91) described for Kollmann’s embryo ‘Bulle.’
Discussion
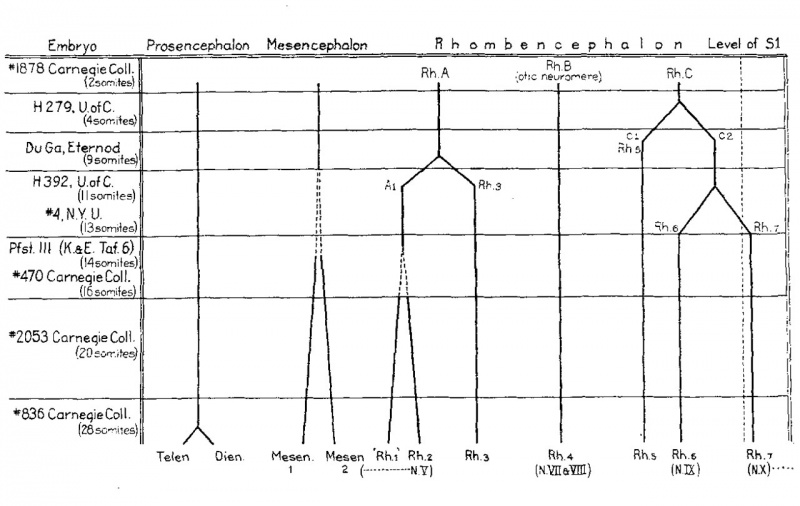
The findings with respect to the differentiation of the neuromeres are summarized in the diagram, figure 6. It will be noted that the otic segment is a primary as well as a secondary neuro- mere. Its form and relations make it possible to be sure that the second hindbrain segment (Rh.B) of the two—somite stage becomes the rhombomere usually termed the fourth (No.4). All of the other neural segments of this particular two—somite embryo undergo division subsequently. In the series at our disposal the division of the primary segments in general proceeds forward from the hindbrain. Next after the otic, the neuromere just caudal to it (rh.5) is cut off, and then the preotic (rh.3) makes its appearance. In the meantime the midbrain is growing, but two distinct segments cannot be recognized until after the sixth rhombomere has differentiated. In the sixteen-somite embryo (no. 470) the two mesencephalic segments resemble the hindbrain neuromeres in every respect. From the fourteen—somite stage on there are signs of growth in the first hindbrain segment (rh.A1) and some indications of a subdivision. Low (’08) recognized two rhombomeres here and most students of neuromeres have recognized three preotic rhombomeres. It does not seem evident in the human that the so—called first rhombomere is a complete neural segment. It might be interpreted as merely an enlarge- ment of the alar plate, viz., the early differentiation of the cerebellar anlage.
While it seems probable that in man the differentiation of secondary neuromeres usually begins caudally and proceeds forward, yet this is not necessarily always the case. If we may judge from the conditions in certain lower vertebrates (cf. Locy, ’95; B. Smith, ’12, et al.), it is possible that there may occasionally be human embryos in which all of the definitive neuromeres can be recognized from early neural-fold stages on. The presence of a definitive neuromere (rh.4) at so early a stage as two somites would indicate that the so—called ‘primary’ and the secondary or definitive neuromeres are expressions of the same fundamental metamerism. B. Smith has expressed a similar idea in saying that certain interneuromeric grooves have a marked tendency to appear earlier than others. Thus we find certain brain segments in most embryos which subsequently undergo subdivision into neuromeres. This phase of the problem richly deserves further study on such favorable material as Cryptobranchus.
Summary
The recognition of certain landmarks furnishes the basis for an analysis of the nervous system in neural fold stages. These are:
- The otic segment which has been traced through a series of human embryos by virtue of, (1) its characteristic form; 17) the behavior of the neural crest which arises from it, and, 0) its relation to other structures such as the otic plate.
- The midbrain which is located by the cranial flexure.
- The first hindbrain segment which, by its peculiar form and the relations of its neural crest, can be directly identified in the older members of the series.
- The relations of the nervous system to other structures, such as the somites and the pharynx, change gradually and they serve to confirm the interpretation based upon the differentiations of the nervous system.
Fig. 6 The embryos here included are selected as typical of definite stages in the differentiation of the brain segments. In the horizontal columns the segments present in each embryo are shown. The bifurcations of the heavy lines indicating the individual segments represent divisions of the original segments. This division is preceded in each case by localized growth at that particular level. Where this is gradual it is shown by the gradually diverging broken lines.
In a two-somite embryo there are five enlargements of the neural folds: forebrain, midbrain, and three hindbrain segments. The middle hindbrain segment (the otic) can be traced through the series and is identical with the fourth neuromere of the usual enumeration. The significance of this fact in the interpretation of neurorneres should not be overlooked. In the present series differentiation proceeds from the postotic region forward. After the rhombomeres have all appeared two mesencephalic neuromeres can be recognized.
According to this interpretation, the hindbrain is the dominant feature of the brain in early stages, and, contrary to the current conception, at the beginning of the fourth Week of pregnancy the forebrain is not relatively larger in man than in other vertebrates.
Footnotes
- ↑ Keibel and Elze (’08, p. 18) have called attention to a cranial flexure in the five- to six-somite embryo ‘Klb’ (Normentafel no. 3). It is not clear from the text whether they refer to the flexure as we have identified it or whether they mean the more caudal one. The latter is a more abrupt bend which appears in the Keibel model, but not in the figures of Kroemer which were reproduced by Pfannenstiel (’03). It is located at the rostral extremity of the otic segment which is well marked in this embryo.
- ↑ The basis for this is a series of excellent photomicrographs comprising every third section, made at a magnification of 75 diameters, which Professor Veit was so good as to send us.
Literature Cited
Bartelmez GW. The origin of the otic and optic primordia in man. (1922) J. Comp. Neural., 34: 201-232.
BUJARD, E. 1921 Modelage de la tete de l’embryon humain. Neuromérie et branchiornérie. Arch. Biol., T. 31, pp. 323-346.
Dandy WE. A human embryo with seven pairs of somites measuring about 2 mm in length. (1910) Amer. J Anat. 10: 85-109.
Davis CL. The relations of the somites of the head to the brain in a human embryo of 20 paired somites. (1920) Anat. Rec, 20: p. 232.
Davis CL. Description of a human embryo having twenty paired somites. (1923) Carnegie Instn. Wash. Publ. 332, Contrib. Embryol., 15: 1-51.
Eternod ACF. II y a un canal notochordal dans l'embryon humain. (There is a notochordal channel in the human embryo) (1899) Anat. Anz., 16: 131-143.
Gremo-Tos, E. 1902 a Sull’ origine embryonale del nervo trigemino nell’ uomo. Anat. Anz., Bd. 21, S. 85-105.
1902 b Sui primordi dello sviluppo del nervo acustico-faciale nell’ uomo. Ibid., S. 209-225.
1902 c Sugli orgarii branchiali e laterali di senso nell’ uomo nei primordi del suo sviluppo. Monit. Z001. Ita1., anno 13, pp. 105-119.
Ingalls NW. A human embryo at the beginning of segmentation, with special reference to the vascular system. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 274, 11: 61-90.
Knnmn, F., AND C. Enzn 1908 Normentafel zur Entwicklungsgeschichte des Menschen. Jena.
KOLLMANN, J. 1889 Die Korperform menschlicher normaler und pathologischer Embryonen. Arch. f. Anat. u. Physiol., Anat. Abth., Suppl. Bd., S. 105-138.
KROEMER, P. 1903 Wachsmodell eines jungen menschliehen Embryo. Verh. d. dent. Gess. f. Gynak., Wiirzburg, 1903. S. 537.
V. LENHOSSEK, M. 1891 Die Entwicklung der Ganglien beim menschlichen Embryo. Arch. f. Anat. u. Physiol., Anat. Abth., S. 1-24.
LOCY, W. A. 1895 Contributions to the structure and development of the vertebrate head. Jour. Morph., vol. 11, pp. 497-594.
Low A. Description of a human embryo of 13-14 mesodermic somites. (1908) J Anat Physiol. 42(3): 237-51. PMID 17232769 | PMC1289161
PFANNENSTIEL, J . 1903 Die Einbettung des Eies u. s. W. Kapitel III in Winckel, Handbuch der Geburtshilfe. Wiesbaden.
SMITH, BERTRAM G. 1912 The embryology of Cryptobranchus allegheniensis. Part II. Jour. Morph., vol. 23, pp. 455-580.
Veit O. Head ganglia of an embryo of eight somite pairs {Kopfganglienleisten Bei Einem Menschlichen Embryo Von 8 Somitenpaaren). (1918) Anat. Hefte, Bd. 56, S. 305-320.
1920 Ueber einen menschlichen Embryo aus dem Anfang der vierten Woche. Sitzngsb. Ges. (1. gas. Natwiss. zu Marburg, No. 6, S. 95-106.
Veit O. and Esch P. Untersuchung eines in situ fixierten, operativ gewonnenen menschlichen Eies der vierten Woche. (Examination of a fixed in situ, surgically obtained human egg the fourth week) (1922) Z Anat. Entw., 63: 343-414.
Wallin IE. A human embryo of thirteen somites. (1913) Amer. J Anat. 15(3): 319-331.
Wilson JT. Observations upon young human embryos. (1914) J Anat Physiol., 48(3): 315-51 PMID 17233002 PMC1288949
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The subdivisions of the neural folds in man. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_subdivisions_of_the_neural_folds_in_man
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G