Paper - The cytological structure of the human chorionic villus and decidua parietalis
| Embryology - 2 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Baker BL. Hook SJ. and Severinghaus AE. The cytological structure of the human chorionic villus and decidua parietalis. (1944) Amer. J Anat. 73(3): 291-325.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Cytological Structure of the Human Chorionic Villus and Decidua Parietalis
Burton L. Baker, Sabra J. Hook and Aura E. Severinghaus
Departments of Anatomy, University of Michigan Medical School, Ann Arbor and College of Physicians and Surgeons, Columbia University, New York
(1944)
Four Plates (thirty figures)
Introduction
A large number of investigators have studied the general histology of the chorionic villus and decidua parietalis but few of them have been particularly concerned with the cellular components which may be associated with the process of secretion. In 1916, de Kervily published two papers in which he described in considerable detail the cytology of the human chorionic villus. Many of his observations, especially those relating to secretion by the trophoblast, have failed to receive general acceptance. Since that date, little attention has been given to the finer structure of the placenta. More extensive information concerning the cytology of the placenta is desirable in view of the current interest in the physiology of this organ. It has been shown to plaiyan important role in the regulation of the transfer of substances between the maternal and fetal blood streams and much accumulated evidence suggests that the placenta also acts as a gland of internal secretion. Hence, during the past several years We have carried on a cytological investigation of the human placenta, seeking to find structures within the villi and nearby decidua parietalis to which we might attribute an endocrine function.
Material and Methods
The source of the material studied is listed in table 1. The reasons for the interruption of pregnancy included tuberculosis, brain tumor, mammary carcinoma, uterine fibroma, glaucoma, anemia, mechanical injury to the fetus and throatened spontaneous, abortion. This pathology was considered in evaluating the cytological picture of each case and these specimens wliich showed evidence of infection or degeneration were used only as reference material. The operations in cases 1-34 (except nos. 14 and 25) were performed under general anesthesia.
Although the tissue obtained from therapeutic abortions was extremely disorganized, the zona compacta of the decidua parietalis could be differentiated easily from fetal fragments and villi by its pale color, compactness and glistening epithelial surfaee. Some of the zona spongiosa usually remained attached to it. When hysterectomies were performed, samples of the deeidua were obtained about 2 cm. from the edge of the placenta. The villi and deciduae were handled separately, small pieces of each being placed into the following fixatives: Bouin’s fluid, picric—alcohol-formol, Zenker,-formol followed by chromation in 3% potassium dichro— mate, and in Champy’s fluid followed by osmication and chromation according to the Severinghaus pituitary gland methods. In II_1aT.1}’-CHSBS, the tissues were handed directly to one of the authors bythe surgeon so that not more than 1 or 2 minutes transpired between the time of removal from the patientuand fixation. It was these specimens which exhibited the most delicate cytological detail in the trophoblast. In only a few cases did more than 5 minutes elapse before fixation. Zenkcr-formol—fiXed and Champy-fixed material was double embedded in celloidin and 60-62" C. paraffin. Best’s carmine stain with and without liematoxylin was employed for the demonstration of glycogen after fixation in picric—alcohol—formol and was controlled by saliva digested preparations. Atrichromc stain, described by Severinghaus ( ’39), was used after the other fixatives. Some Bouiri-fixed tissues were impregnated with silver according to the Bielschowsky technique (McClung). The preparations in the University of Michigan E-mb»ryol0gic.a.l Collection were stainedwith hematoxylin and eosin or Congo red.
Table 1
| Table 1 - Specimens studied | ||||
|---|---|---|---|---|
| Case | Type of Operation | Decidua (D) Villus (V) | Menstrual age (weeks) | Est. Menstrual Age (weeks) |
| 1 | Therapeutic abortion | D | 6—8 | |
| 2 | Hysterectomy | D V | 11 | 10.7 ? |
| 3 | Hysterectomy | D V | 20 | |
| 4 | Therapeutic abortion | D V | 12.5 | |
| 5 | Hysterectomy | D V | 17 ? | 15.7 |
| 6 | Therapeutic abortion | D V | 7 | |
| 7 | Therapeutic abortion | V | 13 | 14 ? |
| 8 | Spontaneous abortion | D | 14.2 | 14 ? |
| 9 | Spontaneous abortion | V | 13 | 14 ? |
| 10 | (Tubal pregnancy) | D V | 13 | ? |
| 11 | Spontaneous abortion | D V | 10 | 14 ? |
| 12 | Hysterectomy | D V | 15.1 | 13.5 |
| 13 | Hysterectomy | D V | 14.1 | |
| 14. | Subtotal Hysterectomy | D V | 14.2 | 13.5 |
| 15 | (Ectopic. pregnancy) | D V | 12.4 | |
| 16 | Therapeutic abortion | D V | 15.5 | |
| 17 | Hysterectomy | V | 10 | 9.7 |
| 18 | Therapeutic abortion | D V | 8 | |
| 19 | Delivery | V | full term | |
| 20 | Delivery | V | full term | |
| 21 | Delivery | V | full term | |
| 22 | Delivery | V | full term | |
| 23 | Therapeutic abortion | D V | ||
| 24 | Therapeutic abortion | D V | 8 (Dr.) | |
| 25 | Therapeutic abortion | D | 6 (Dr.) | |
| 26 | Therapeutic abortion | D V | 12.7 | |
| 27 | Therapeutic abortion | D V | 7 | |
| 28 | Therapeutic abortion | V | 15 ? | 14.7 |
| 29 | Therapeutic abortion | 13 | 13.2 | |
| 30 | Therapeutic abortion | V | 6-9 | |
| 31 | Therapeutic abortion | V | 12 | |
| 32 | Therapeutic abortion | V | 8 | |
| 33 | Delivery | V | 29 | |
| 34 | (Hydatidiform mole) | V | ||
| EH 47 4 | Hysterectomy | D V | 10.4 | |
| EH 78 | V | 9.6 | ||
| EH 82 | V | 11.1 | ||
| EH 102 | Hysterectomy | V | 9,0 | |
| EH 138 | Hysterectomy | V | 8.4 | |
| EH 1417 | Hysterectomy | V | 7 | 9.3 |
| EH 173 | Hysterectomy | D | 13.3 | |
| EH 181 | Spontaneous abortion | V | 15.8 | |
| EH 265 | (TubaI pregnancy) | V | 11.1 | |
- Except when estimated by the doctor during.the operation, “(Dr.)”, the menstrual age of the fetus was determined from the data of Mall on the hasie of the crown-rump length, Mall (’18) and Keibel and Mall(’10), p. 199.
- Following mechanical injury to fetus.
- Because of threatened spontaneous abortion.
- The Specimens designated by EH are from the University of Michigan Embryological Collection.
Observations
The chorionic villus (6-14 weeks)
Cytotrophoblast
The cells of the cytotrophoblast varied considerably in size, the larger ones appearing more vesicular after fixation in Champy’s fluid than in the other fixing solutions. This agrees with a previous observation by Langhans (’01) who used osmic acid fixatives also. The cytotroé phoblast cells showed frequent variations. in shape, being somewhat cylindrical when arranged in a continuous row (fig. 1) , and more ovoid with a convex syncytial surface when they were separated from each other by syncytium reacliing to thebasement membrane as was usually the case (fig. 3). The nucleus possessed a distinct membrane which was usually folded and which enclosed palely-stained nucleoplasm and one or more irregularly-shaped acidophilic nucleoli (figs. 2 and 6). If cytoplasmic structure was visible, it tended to be lightly stained and somewhat granular. The cytoplasm of the large, vesicular cells was most. dense in the region of the Golgi apparatus; from where finer strands extended toward the periphery (fig. 1).
The Golgi apparatus was found almost invariably on the side of the nucleus toward the syncytium (fig. 7). If the cytotro— phoblast consisted of a continuous layer of polygonal cells, this organelle was definitely polarized toward the surface of the villus. When the cells were separated by projections of the syncytium reaching to the basement membrane, especially in later stages, the Golgi apparatus was frequently located lateral to the nucleus. It was almost never foundbetween the nucleus and the thin but distinct reticular tissue basement membrane which supported the trophoblast. Usually the Golgi apparatus consisted of a meshwo1°k, with or with-— out vesicles (fig. 8), the looseness of arrangement varying directly with the size of the cell. In proximity to it, were found small granules and less regularly shaped material which stained with aniline blue in post~cl1romated Champyufixed preparations (fig. 1). These bodies occurred in no other parts of the cell and could not be demonstrated in all specimens. Mitochondria, were present throughout the cytoplasm but tended to be most concentrated at the edges of the Golgi body (figs. 1 and 3). In confirmation of de Kervily (’16) the mitochondria consisted chiefly of short rods or beads arranged in the form of chains of varying lengths. Some cells contained small quantities of glycogen.
The cytotrophoblast showed rather intense mitotic activity which, in localized areas, was responsible for the formation of cell columns and cell islands.
Cell columns
The cell columns of the cytotrophoblast were covered by syncytium and occurred at the dccidual ends of anchoring villi uniting the chorion to the decidua basalis (fig. 22). They have long been regarded as derivatives of the cytotrophoblast (v. Lenhossék, ’02; Rossi Doria, ’05) and since mitotic figures were most numerous at the fetal end of the cell column this origin can hardly be questioned. Sin:1i~ lar cell groups were found at the unattached ends of the branches of the villi. Since only very small areas of the placenta were shown in our cytological preparations, it was not always possible to determine the exact location of such proliferations. The cells of the cell columns and of the cell groups at the ends of villous branches differed from the cytotrophoblast la.yer in the following particulars, these differences being most pronounced in those cells farthest removed from the point of continuity with the cytotrophoblast layer. In preparations which did not preserve glycogen, the cells appeared to enlarge and to contain less stainable cytoplasm as they were pushed away from the site- of origin by the continued multiplication of cells in the cytotrophoblast layer of the villus (fig. 22). The empty cytoplasmic spaces in these cells is accounted for by the removal of glycogen since we, in agreement with Driessen (’O7), found them to contain large amounts of this substance. The mitochondria were fewer in number and more variable in size (fig. 21) and shape although tending to be globular or vesicular. They were less frequently arranged in beaded chains. Rarely could a distinct Golgi apparatus be discerned.
Cell islands
The intervillous cell islands are attached to villi (Keibel and Mall, ’10) and have been differentiated from the cell columns on the basis of their position between villi and lack of as continuous syncytial covering (fig. 25). in our preparations, the cell islands showed variable degrees of degeneration. This process was most advanced centrally, possibly due to the failure of tlie cell islands to become vascularized. Degeneration was accompanied, first, bythe appearance of small, irregularly—shaped formations of fib-rinoid (fig. 25). Later, these seemed to unite to form larger, denser masses (fig. 13) which spread toward the periphery, ultin1ately overtaking the cellular constituents.- The fibrinoid showed a strong affinity for eosin and a variable reaction to the acid fuchsin-aniline blue stain, appearing light. brown in many instances. In the region of fibrinoid and less frequently elsewhere, shrunken cells with pycnotie nuclei were observed in process of degeneration. Elsewhere, many cells possessed a lobated nucleus, osmiophilic nuclear membrane, small Golgi apparatus, few mitochondria and little cytoplasm. However, cells which seemed to be more active were observed, especially near the periphery of the islands next to the maternal blood. Both the cells and their nuclei were larger, the latter tending to be spherical. The Golgi apparatus was hypertrophied and encompassed dense cytoplasm and small blue granules, the latter increasing in size toward the periphery of the cell (fig. 13). Striking accumulations of mitoehondria occurred in the Golgi region (fig. 19). A separation of the cells at the edge of the island was quite evident since maternal erythrocytes were frequently observed between them. These cells with their numerous blue granules then appeared to be sloughed off into the blood of the intervillous space. Ghosts of these cells, depleted of grallules, were Observed (case 13) in the intervillous space a short distance from the islands. A few mitotic figures and large amounts of glycogen (fig. 16) were present in the islands.
Syncytium
The nuclei of the syncytium differed rather sharply from those of the cytotrophoblast after Bouin fixation by virtue of their smaller size, darkly-stained and wrinkled membrane and coarse chromatin masses (figs. 2, 3 and 6). The cytoplasm Was sometimes homogeneous and structure less, but frequently it had a granular or reticular appearance. This Variability even within the same villus may be attributed to the promptness of fixation, the fixing fluid used, and possibly to the age of the area of trophoblast in question. Staining of Champy—fixed material by the Severinghaus technique differentiated mitochondria, lipid droplets and granules, these being colored fuclisin, brown, and blue, respectively. The free surface of the syneytium was modified to form a brush border after fixation in (f3ha.mpy’(s fluid (fig. 5), Bouin’s solution (fig. 6) or Zenker-formol. In some areas it reached a height of about 1.6 p. In general, the brush border nonisisted of fine, Vertical, elose1y—approXima,ted projections which appeared coarser and more distinct after fixatioii in Bou=in’s fluid. The brush border Varied in height and in distinctness and regularity of arrangement of its individual elements in different parts of the same villus. Our techniques did not reveal definite basal granules such as those described by V. Lenhossék (’02). However, a fuehsinophilic line which may have been composed of Very minute granules was sometimes seen beneath a. regular brush border.
The Golgi apparatus of the syncytium was generally located in the subnuclear and nuclear zones but in thick syncy— tium, it occasionally extended into the area immediately above the nucleus. The Golgi apparatus consisted of threads of variable thickness as is well illustrated by a 16-week-old placenta (fig. 20). Most commonly they were curved to form loops and, in some locations, seemed to be united to form a network. The negative image of this structure was seen clearly in non-osmicated Champy and Bouin-fixed preparations as clear, curved channels similar in Width and shape to the blackened threads of the osmicated Golgi body 2). In some instances we liave observed areas of condensed cytoplasm in association with this negative image.
Nassonov (’23) and Bowen (’29) first reported that the Golgi apparatus plays an important role in the formation of secretory products in glandular cells. Their contention has been supported by subsequent investigators who have studied at Wide variety of glandular tissues. If this is true, it is significant that minute blue granules, singly and in clusters, were observed in the subnuclear zone of the syncytium (fig. 2). We could not demonstrate the relationship of the granules to the Golgi apparatus in osmicated preparations, for in these the granules could not be stained satisfactorily. However, in non-osmicate‘d Champy—fiXed preparations the granules were occasionally surrounded by at light zone which may have been the negative image of Golgi material. These granules stained similarly to the bluevgranules already described in the nearby Golgi region of the cytotrophoblast. Towards the free surface of the syncytium they increased in size, and become more perfectly spherical in the supranuclear zone and exhibited a homogeneous t.eXture (fig. 2). Occasionally, large bodies .as wide as 3.6 1.1 and apparently composed of the same substance, were observed in the region of, or adjacent to the nucleus. These granules were preserved by Champy’s fluid, Zenker—formol and, at least the larger ones, by Bouin’s fluid. Large numbers of ‘granules were found more commonly in thick than in thin syncytium and in these areas the general cytoplasm tended to stain more intensely with aniline blue. o
Liquefaction of the syncytical granules was suggested by their intimate relationship to clear superficial vacuoles which occurred most regularly immediately subjacont to the brush border. The largest granules measured about 1.8 p in diameter and were comparable with the average size of the superficial vacuoles. Some granules were surrounded by a clear areola (figs. 2 and 14); others appeared as a minute granule in the center of a clear vacuole. The composition and afltinity for aniline blue of other granules varied from a homogeneous, dark blue sphere to as delicate, non-stained co— agulant or clear fluid (fig..4) within a vacuole. In parts of some villi, especiallywhere the vacuoles were most numerous, large, and clear, the brush border was obscure and the outer— most boundary of the syncytium undulated (fig. 18), suggesting that the vacuoles had ruptured through the brush border leaving the innermost wall of the vacuoles as the outer boundary of the syncytium. Occasionally, the elements of the brush border were separated by clear vesicular spaces without disruption of the subjacent cytoplasm (fig. 18). How» ever, the vacuoles were found most frequently beneath an even and regular brush border. Blue granules occurred in the absence of any vacuoles but could never be found in the brush border or intervillous space.
The mitochondria of the syncytium possessed the shape of short, granular filaments or rods. Generally inform and size they were somewhat similar to those of the cytotrophoblast; in a few cases, they were definitely finer and more delicate. They could be found throughout the syncytium and were not oriented in respect to the surface. Individual mitochrondria were not smaller in the basal zone nor did they accumulate around the lipid droplets as described by de Kervily (’16).
However, accumulations of mitochondria occurred frequently in certain locations, the chief one being the hsupranuclear zone (fig. 2). These aggregations were most frequently seen in association with numerous blue granules and superficial vacuoles but either could be found in the absence of the other (fig. 3). VVe observed concentrations of mitochondria in the nuclear and subnuclear regions and in some instances these have seemed to be arranged about the negative image of the Golgi apparatus (fig. 2). T The lipid droplets, which have been described by many workers, appeared chiefly in the supranuclear and nuclear zones (figs. 5 and 14) but were found at other depths. They were readily difierentiated from the Golgi apparatus in os~ micated preparations by their form and brown color. The lipid droplets were present following Champy fixation without post-osmication in all cases includin g one of hydatidiform mole (no.s34). We observed the clear central space and eccentric shape of the droplets described by de Keryily (’16) in only one specimen but in this case the adequacy of fixation was open to question.
The syneytial buds did not present noticeable Variations in cytological structure as compared with the adjacent syneytial layer (fig. 12). A Golgi apparatus (fig. 17), blue granules and superficial vacuoles were demonstrable in them. The numerous mitochondria sometimes appeared to be less discrete. The brush border on at least the sides of the buds possessed a regular form but the terminal ends usually contained numerous superficial vacuoles and, over them, the brush border was frequently disrupted.
Origin of syncytium
We frequently observed cells adjacent to the basement membrane which seemed to be in the process of transformation from ciytotroplioblasts to syncytium. The cytoplasm in parts or all of such cells had become dark and indistinguishable from that of the syncytium (figs. 4 and 11). The nuclear membrane was wrinkled and, with the chromatin material, stained intensely, similar to that of the syncytial nuclei. Most significantly, the cell membrane was still clearly distinguishable throughout all or part of its extent (fig. 11). The mitochondria seemed to be rather variable in these cells, some being larger and intensely stained; others, faintly stained and clumped.
Connective tissue
The connective tissue in the branches of the Villous tree consisted of loosely arranged, connective tissue cells associated with delicate fibers which stained with aniline blue and blackened with silver. Some of these fibers blended into the basement membrane of the trophoblast. Numerous, Hofbauer cells were scattered. throughout the connective tissue. They possessed small, eccentrieally—placed nuclei, vacuoles of varied sizes, granules which stained with aniline blue, granular or rod-shaped mitochondria and a small Golgi apparatus located close to the nucleus. Some of these characteristics have been described by de Kervily (’16).
It seems that the Hofbauer cells should be classed as macrophages since they have been shown to take up suprayital dyes (Lewis, ’24) and to increase in number in sypliilitic infections (Hofbauer, ’25). The connective tissue was denser and Hofbauer cellsredueed in number in the trunks of the villous tree, as well as throughout the villus in late pregnancy. At term the basement membrane of the trophoblast was considerably thickened except over the blood—filled, subepithelial blood Vessels.
The decidua perietalis (7-17 weeks)
In the following description attention will be directed chiefly to the cellular elements of the stroma 1. The glands will be omitted since their epithelium has been reported it to show only minor changes during earlypregnancy (Bartelmez and Bensley, ’32).
Large decidual cell
The large decidual cell was the largest of the cellular elements of the decidua, being formed at all levels but reaching its greatest size in the zona -compacta. VVhen the large decidual cells weredensely packed they tended to be spherical in shape but in areas of loosely arranged connective tissue, such as occurred frequently beneath the surface epithelium, pseudopod—like processes of Variable length extended out from the cell body. The nucleus was large and contained pale nueleoplasm, a fine chromatin network and one or more irregularly shaped aeidophilie nucleoli (fig. 28). Some cells possessed multiple nuclei. The cytoplasm appeared finely granular following Zenker-formol fixation and delicately reticular or granular after fixation in Bouin’s or Champy’s fluids. Ulesko—Stroganofl’ (’()8) found the nucleus to lie in an uneolored field surrounded by darlter cytoplasm in deciduae fixed in Flemming’s fluid and stained with saffranin. In our material, if any portion of the cytoplasm stained more deeply, it was that area encompassed by the Golgi apparatus.
‘General histological descriptions of the decidua parietalis may be found in the works of March-and (_ ’04), TIlesko—Strogan.ofi" (’08), Stieve (’‘2-6) and Schroeder (’30).
The Golgi apparatus was a large, loose meshwork of spherical to oval shape and measured as much as 11.2 in in its greatest diameter (fig. 24). Vesicles occurred frequently Within its component parts. Generally, the nucleus fitted into an indentation at one side of the network. The negative image of this organelle was clearly defined after fixation in Zenker-formol, Boui11’s or Champy’s fluids. ‘Without entering into the discussion concerning the possible lipoproteid character of the Golgi apparatus (Kirl-tman and Severinghaus, ’38) it should be noted that We consistently observed dense cytoplasm alongside these clear channels which, in most preparations, definitely outlined the Golgi apparatus without need of osmication. Mitochondria were sparsely scattered, only occasionally seeming tobe more numerous in the region of the Golgi body (fig. 27). They consisted of faintly stained, delicate filaments. Clusters of small lipid droplets and sharply-outlined vesicles were infrequently present outside of the Golgi region. Variable quantities of glycogen were demonstrated.
The large decidual cell was usually surrounded by a dense connective tissue covering which was continuous with similar structures about other cells. It stained with aniline blue and reduced silver (fig. 30). Although frequently exhibiting a homogeneous appearance, in most cases its fundamental fibrous nature wasclearly discernible. Many cells appeared to contain homogeneous or fibrillar masses of connective tissue material Which exhibited the staining properties of reticular fibers (fig. 26). However, they were demonstrated regularly to be continuous with the extracellular connective tissue and seemed to have been secondarily enfolded by swelling or possibly movement of the processes of the cell. Therefore, they cannot be designated true intra-cytoplasmic structures. Mitochondria-laden processes of some cells it partially surrounded marked condensations of the pericellular covering apparently preparatory to more completely enfolding this material (fig. 27). Occasional fine argyrophilic fibers also appeared to be located within the cell but this position could not be verified definitely.
Smell decidual cell
This type was scattered throughout the zona compacta and spongiosa frequently seeming to be rather numerous beneath the basement membrane of glands. Bouin fixation revealed an intensely fuchsinophilic nuclear membrane enclosing pro_minent chromatin masses, in rareinstances the picture approaching one of pycnosis (fig. 28). In some instances the nucleus was definitely lobated while in others, it was drawn out into a dumb-bell shape which has been taken as an indication of rapid amitosis (UleskoStroganofi, ’08). In our material occasional cells contained mitotic figures. Glycogen filled most of the cytoplasmic space (fig. 29) so that after removal of this substance by the usual fixatives the cell appeared empty except for a dense mass of grallulated cytoplasm usually found in an indentation. at one side of the nucleus and from which fine strandspextended toward the periphery of the cell. The granules reached a rather large size, stained with either acid fuchsin or aniline blue in Champy preparations and were still present after fixation in Bouin’sfluid (fig. 28). Those which stained with acid fuchsin after Champy fixation frequently possessed a deep red rim and a pale. center. The mitochondria were concentrated inthis region and varied in shape from short curved rods to granules. The Golgi apparatus was not always demonstrable tcnding to be a small net (fig. 23) or fragmented. Post.-aosmication brought out black droplets in many of these cells, some of which possessed a. elea.r center. It was thought that these were the fuchsinophilic granules previously described. Bleaching of these osmium deposits proved to be extremely diflicult.
Histiocytes, lymphocytes, neutrophiles and eosinophiles were also present in the decidua. The histiocytes contained blue granules, small vacuoles, rod-shaped mitochondria and little glycogen. These cells were spherical in loose connective tissue but also assumed shapes which suggested ameboid motion. Their nuclear structure was somewhat similar to that of the sn1all decidual cell and lymphocyte. A.lthoug'l1 a specific stain was not used in this study we have never observed cells with the cytoplasmic characteristics of plasma cells. Plasma. cells have been described in the decidua (Wbderlhake, ’06) but 'Uilesko-Stroganoff (’O8) employed Unna’s stain and found few of them.
Discussion
Trophoblast
In attempting to interpret the cytological variations in the tropl1oblast one is soon impressed by the probability that other factors than the state of secretory activity influence the appearance of this epithelium. Regions of thick and thin trophoblast were commonly continuous with each other on the same villus. Fluctuations in intra-villous pressure might account for this thinning of the epithelium but such areas were frequently continuous with a thick syncytium covering polygonal cytotrophblast cells. These variations may be more satisfactorily explained on the basis of ameboid movement by the trophoblast, a deduction previously presented by Hofbauer (’03) and which is further supported by the occurrence of syncytial buds on the villi and syncytial giant cells in the myometrium. Failure of the cellular layer to pile up under these buds may indicate that syncytial movement, if present, occurs over the outer surface of the cytotrophoblast and is, to some extent, independent of that layer. Actual phagocytosis of maternal erythrocytes by the trophoblast has been reported in the human placenta by Boerma ( ’13), Brewer ( ’37’) and de Kervily (’16), the latter describing a preliminary active engulfment of the erythrocytes by the elements of the brush border. In our material red blood corpuscles seemed to have been held against the brush border in life by some viscous material (fig. 14). In agreement with v. Clauwenbeighe (’07) We did not observe erythrocytes in a position which would suggest that they were passing through the brush border. They did exhibit indications of histolysis and varied staining reactions when in the intervillous space which might indicate that they were in some way affected by a substance released from the trophoblast. It seems possible that the trophoblast may phagoeytize erythrocytes more actively during the period of active invasion of the endometrium than during the later stages which we have studied.
Some disagreement exists concerning the cytoplasmic constituents of the cytotrophoblast. Small grains of fat have been described by de Kervilyr (’16) and Ilofbauer (’05) after impregnation with osmium but according to Bondi (’11) neutral fat is not present in the cytotrophoblast. In our material all intra-cellular structures which were blackened by osmic acid could be classified asparts of the Golgi apparatus or mitochondria except in one case (case no. 14, fig. 8), in which brown bodies, possibly fatty pigment, were present in some of these cells after fixation i11 Champy’s fluid. Wlienbeck (’36) was not able to produce a blackening of granules in the cytotrophoblast of full-term placentae by means of the Da Fano technique. The finding of small quantities of glycogen in the cytotrophoblast agrees with the observations of Todyo (’12). Driessen (’()7) observed insignificant amounts.
The function of the cellcolumns and cell islands is not entirely clear. Obviously, the former play as part in fixation of the villi to the decidua basalis. Both cell columns and cell islands are characteristic of early pregnancy, the former having been reported to disappear by the end of the second month (Schroeder, ’30) or the fifth month (Terasaki, ’27). In our material they were observed as late as 3% months. The changes described in the mitochondria and Golgi apparatus of the cell columns were suggestive of loss of vitality. The large amount of glycogen in these cells might be given a similar interpretation since metabolic depression has been used to explai11 the accumulation of this material in the renal cells following obstruction of the renal vessels (Grierke, ’O5) and in the vaginal epithelium of the guinea pig (Tribby, ’43). A few cells in the cell islands sliovved some indications of secretory activity, but for the most part, they were degenerating to form fibrinoid. In fact, these islands have been thought to be an important source of the fibrinoid which oc~ curs commonly in the human placenta (Keibel and Mall, "10; Terasaki, ’27).
Many origins have been postulated for the syncytium (Schroeder, "30) but most Workers are now agreed that it arises from the cytotrophoblast, although little cytological evidence for such a change has been presented. Our observations support the view that the syncytium arises from the cytotrophoblast. We doubt that the dense cytoplasm of the “cytocentrum” of cytotrophoblast cells necessarily represents a stage in this transformation as has been described by Florian (’28); in our material the cytoplasm was typically most dense in the Golgi region. V. Lenhossék (’0.‘2), Rossi Doria (’O5) and v. Cauwenberghe ,(’07) have reported amitotic division of the syncytial nuclei and this is suggesetd by the frequent elongation of the nuclei and their accumulation in the syncytial buds. However, these formations are equally well explained on the basis of ameboid movement by the syncytium. Until more concrete evidence is supplied, the presence of amitosis in the syncytium must be viewed with skepticism. Neuweiler (’27) and Lewis (’24) have failed to observe proliferation of the syncytium in tissue culture. ,
The irregular appearance of the brush border raises the possibility that it might be at fixation artefact. Numerous workers have been impressed by the apparent lability of this structure. There can be no question that prompt fixation is prerequisite to satisfactory demonstration of it. Our findings indicated that in some locations, rupture of the superficial vacuoles accounted for the lack of a brush border, while in other places its absence could not be explained on this basis. Nevertheless, it is diflicult to understand how the act of fixation could induce the appearance of projections of such height and regularity of form as are shown in figure 6. In fact, v. Lenhossék (’02) reported their presence on fresh, non-fixed villi and called them “stereocilia.”
1 “Te interpret the blue granules Within the syncytium to be a formed product of cytoplasmic activity and a probable source of secretion. Judging from the illustrations of Brewer (_ ’37) there is no certain indication of identity between these granules and those which he described in the syncytiuin as phagocytized cells and which he hoinologized with those structures previously referred to by other workers as secretory products. V. Cauwenberglie (’07) observed an areola around some granules during late pregnancy and believed that the “basophilic granules” liquefy prior to secretion. He took the position thatthe syncytiuni is a gland and later received the support of de Kervily (’16) who further suggested that this secretion might be of a hormonal nature. The superficial vacuoles have been observed by these workers and illustrated but not described by Florian (’28) and Runge and Hartman (’29), yet their relationship to the blue granules has never been clearly set forth. Our observations strongly suggest that the superficial vacuoles are formed by liquefac~ tion of the blue granules. Bonnet ( ’O3) hinted at this finding but was probably considering larger, deep syncytial vacuoles in the following statement: “Ausserdern finder ich in den grossen.Vacuolen der Proliferationsknoten oft einem rnehr oder minder fiirbbaren wolkigen Inhalt, Grerinnsel einer noch unbekannten Fliissigkeit oder hornogene, gl‘¢inzendc, rundliche Korper voniebenfalls unbekannter Bedeutung. ”
It is probable that the contents of the vacuoles reachtliie maternal blood most frequently by diffusion. Some evidence of rupture was observed but since the act of fixation could conceivably cause an engorged vacuole to burst, a final conclusion in regard to the mode of emptying cannot be drawn from fixed material. The vesicular spaces Within thebrush border, a formation previously observed by V. Oauwenberghe ( ’07') and Hedenberg and Strindberg (’]6) and regarded by the former evidence for the rapid interchange of substances, Inay haveresulted from a sudden emptying of the vacuoles.
Vacuoles have been described in the syncytium of the monkey trophoblast by ‘Wisslocki and Streetor (’38) and related by them to pinocytosis. It seems to be more likely that the superficial vacuoles of the syncytium in man are concerned with a secretory rather than an absorptive process. The cytological evidence for this deduction is supported by the failure of Lewis (’24) to find a concentration of neutral red in the superficial zone after supranvital staining with this dye. Also, these vacuoles are associated with the thick syncytium of early pregnancy and are markedly reduced in the thin syncytium of late pregnancy. They are, then, least numerous at a time when the most rapid transfer of materials would occur as indicated by the finding of Flexner and Grellhorn (’42) that the rate of transfer of radioactive sodium from mother to fetus parallels the rate of fetal growth and is indirectly related to the thickness of the trophoblast. ‘We wish to emphasize that other clear vacuoles of varied shapes and sizes occurred less frequently during early pregnancy at all levels of the syncytium and that their presence could be due to pinocytosis. This may have been the origin of the large syncytial vacuoles of young embryos (13% days to 4 Weeks) reported by Friolet (’05) and Stieve (’26) to be as large as 50;; in diameter. Some of comparable size were observed occasionally in our specimens, especially in syncytical buds (fig. 25). Indeed, the general syncytial cytoplasm has been described by some investigators to be vacuolated. There are other factors, however, which may explain this appearance. fixation in Bouin’s fluid, for example, emphasizes the vacuolated appearance ofthe nuclear and supranuelear zones by removing fat. Also, delayed fixation may account for the finevacuoles reported by Herzog (’09). Since We, like Driessen (’07), have been unable to detect significantamounts of glycogen in the syncytium, vacuoles could not result from its removal.
De Kervily (’16) pointed out, onthe basis of the disposition of mitochondria and granules, that the syncytium is polarized toward the surface and added that the cytotrophoblast does not share in this orientation. Our observations on the distribution of superficial vacuoles and their relationship to the granules of the syncytium confirm his contention in regard to this layer. Although the position of the Golgi body cannot be taken as an indubitable indication of the direction of secretion (Kirkman and Severinghaus, ’38), the rather regular location of this structure on the syncytial side of the cytotrophoblast nucleus in association with mitochondria and occasional differentially-stlaiined granules, might indicate that the cytotrophoblast also is physiologically as Well as anatomically polarized toward the maternalr blood stream. The presence of differentially-stained granules with similar tinctorial properties in both the cytotrophoblast and syncytium, viewed in the light of the general cytological arrangement of both layers might also suggest that the syneytium merely continues the elaboration of a substance the formation of 'which is already begun in the cytotrophoblast. These cytological features are believed to be strong evidence that the trophoblast of early pregnancy performs a significant secretory function.
The decidua parietalis
It is well known that the connective tissue of the non-gravid uterine endometrium contains abundant argyrophilic fibers which are associated with cells of an embryonic type. In the opinion of Hitschman and Adler ( ’08) the decidual picture is to beregarded as an intensification of structural changes begun 6 to 7 days prior to the first missed menstrual period. The association of the large decidual cells Witli reticular fibers is, then, suggestive evidence of their origin from the embryonic type of connective tissue cell. I Costero (’3l) observed the dense covering of the large decidual cells following use of the Rio-Hortega technique and designated it a “membranoid peri-cellular condensation.” As this author has indicated, it appears to have been formed chiefly by compression of the inter—cellular stroma due to enlargement of the decidual cells. However, the presence of acculnulated, brightly—stained mitochondria in cytoplasmic processes which are adjacent to areas of dense connective tissue (fig. 27) might indicate that the large decidual cells continue to play an active role in the deposition of the matrix. The enfolding of homogeneous plaques of this material by these cells has lead to some confusion. They are probably identical "to the “intra—cellular erythrocytes” and the cytoplasmic “discs” which Ulesko-Stroganoff (’08) described in decidual cells. These bodies with their peripherally projecting fibers also correspond to the star-shaped argyrophilic network which Terasaki (’27) observed extending out from the center of decidual cells. However, the latter author recognized the possibility that they were located externally to the cell membrane.
In view of their size, the amount of glycogen contained by the large decidual cells was not great and not demonstrable in all cells. Driessen (’07), who used similar techniques, found the cells of the decidua parietalis positive in only six of eleven cases. It may be pertinent that most samples of deciduae were obtained from patients under general anesthesia.
The small decidual cell of our classification seems to correspond roughly with that described by Marchand (’04) and Ulesko—Stroganoff (’08) although the latter author further subdivided the small cells with hyperchromatic nuclei and granules. Our observations indicated that all of the small cells with dark nuclei, granules and large amounts of glycogen fall within the same category although the quantity of these cytoplasmic constituents varied considerably. The origin of these cells seems obscure. Both l\-flarchand and Ulesko-Stroganoff observed transitional forms between small and large decidual cells. There were indications in our material that they may have a common origin, namely, the embryonic type of connective tissue cell. The characteristics of the small decidual cell could be construed as being indicative of degeneration. p r T
Endocrine Significance
Browne and Venning (’36), Evans, Kohl and Wlonder (’37), and Smith and Smith (’37) have performed careful assays of human urine of pregnancy in order to determine the quantities of gonadotropin excreted at various times during the gestation period. From their data it is clear that the curve of excretion reaches a high peak at about the sixtieth day counting from tl1e last menstrual period and then descends by about the eighty—fifth day to a low level which is maintained until parturition. The data of Hamburger (’33) and of Smith and Smith (’37) showed that, in some cases, a high level of excretion might occur at, or be continued-into, the third month. This period of increased excretion corresponds rather well with the secretory phase of the trophoblast as shown by ourspecimens up to about the fourteenth week of pregnancy. It was not possible to observe a marked cytological difference between the villi at 7 to 9 weeks and those at about 14 weeks, although we did gain the impression that the cytophoblast cells from earlier cases contained more stainable cytoplasm and granules. At 16 weeks (110. 5) although the syncytial granules and mitochondria were still quite prominent, the latter were evenly distributed throughout the syncytium and rarely could a clear superficial vacuole be observed. The 20-week specimen showed a further reduction in secretory activity as evidenced by a thinner syncytium, low and irregularrbrush border, rarely a superficial vacuele, fewer and smaller mitochondria (fig. 15). This regression was still more marked at term when few granules and no vacuoles of a definitely secretory nature could be ,observed (fig. 10). Also, from about the fourteenth week on, the cytotrophoblast cells became continually more widely separated from each other. 1
Direct evidence for the production of gonatotropin by the placenta has been supplied by the implantation experiments of Phillipp and Huber (’36) which point more to the villi than to the decidua as its source. This view is supported by the induction of at positive AschheimwZondek test by injection of the mediumifrom placental cultures (Grey et al., ’38; Seegar Jones et al., ’43'). These findings show that the cytotrophoblast may be the original source of the hormone since the cytotrophoblast but not the syncytium proliferates in tissue culture (Lewis, ’24; Neuweiler, ’2<T; Grey et al., ’38). “Te have offered suggestive cytological evidence of secretion in these cells and possibly also in those of the related cell islands which are most prominent during, early pregnancy. If this is true one should expect that the cytotrophoblast would be present throughout gestation since gonadotropin is usually excreted in significant quantities until parturition. Cytotrophoblast cells were found at term and possessed a demonstrable Golgi apparatus (fig. 9) and mitochondria (fig. 10). On an average they were possibly larger than during early pregnancy, although fewer in number in a given length of trophoblast. The syncytium was frequently extremely thin over these cells (fig. 10).
In spite of this correlation one cannot yet conclude that the syncytial granules are a hormonal precursor. Kennedy (’33) assayed human blood of pregnancy and found the content in gonadotropin to increase steadily until the thirtiethweek which, if confirmed, would show that rate of excretion is not an index of secretion. However, Kennedy’s find» ings are cvontradicted by the evidence submitted by Smith and Smith (’37) who assayed both the blood serum and urine of twelve normal pregnant women for gonadotropin, and found the greatest concentration -of this substance to occur contemporaneously in these fluids. These latter findings tend to strengtlien the correlation which We have made between excretion of gonadotropin and trophoblast cytology. A further cause for hesitancyin assuming that the blue syncytial granules are a precursor of gonadotropin is the fact that only the tissue culture studies of Grey et al. (’38) and Seegar Joneset al. (’éL3) offer evidence that the trophoblast is capable of producing gonadotropin in the absence of the hypophysis. Indeed, the cytological picture of the hypophysis of pregnancy is one of intense activity (Severinghaus, ’.‘-39) which suggests that this gland might participate, at least, in the production of gonadotropin. Two of the ‘i.l1eO1‘l€!S-V\?'l1l(3i.1 have been postulated to explain the manner in which the urinary gonadotropin may stimulate the ovaries during pregnancy, involve the participation of the anterior llypophysiis . (Evans, Meyer and Simpson, ’32; Hamburger, ’33). Tt is also possible that the syncytical granules represent material in transit from the fetal to the maternal circulation or an histiolytic enzyme, although in the opinion of W/Vislocki and Streeter ("38) the importance of the secretion of such a substance by thetrophoblast has been over—emphasized.
An extra—ovarian source for estrogen and progestin during pregnancy seems to be established (Newton, ’39) and the placenta has been thought to be this source. Bennett and Vfislocki (’42) have madethe significant discovery that the syncytium but not the .c}*totr'ophoblasts renders a positive reaction to treatment with phenylliydrazine hydrochloride suggesting that the syncytium produces steroid substances. In View of the reported failure of the S}*‘11C}"‘[-l11II1 to pro1ife1'a.te, it is, then, of interest that Seegar Jones et al., (’43) failed todetect estrogens or progestin in assays of the media of placental cultures. The excretion of total estrogens (Cohen et al., ’35; Marrian et al., ’35; Smith et al., ’37) and of pregnanediol glucuronide (Browne et al., ’37; Smith et al., ’41) reachesa peak Within the 2 months prior to parturition. If one relates the syncytial lipid droplets to this period of rapid excretion, it is important! that t-lief,’ have been found to be least abundant during the latter part of pregnancy by Bondi (’11), Bennett and Wislocki U42) and by us.
It appears to be doubtful that the decidua parietalis performs an endocrine function. The vascular pattern» is one which does not suggest this activity since an intimate relationship between the capillaries and the lsarge decidual cells is non-existant. Thus, any substance produced by these cells would have to diffuse some distance through coniieetive tis— sue before reaching the blood stream. The large decidual cells were without differentially stained granules and the mitochondria, which are commonly related to the liberatio1'1 of secretion, were usually not promiiient in staining capacity, number, or size. Ulesko—Stroganoff .( ’08) reported cells similar in structure to our small decidual cells to be more common around the blood Vesselsbut the significance of their granules is at present not clear. The non-endocrine nature of the decidua. parietalis is further indicated by the failure of artificially induced deciduomata to prolong pseudo-pregnancy in the rat (Long and Evans, ’22) or to disturb the estrous cycle of guinea pigs (Dempsey, ’37).
Summary
Pieces of the chorionic villus and decidua parietalis were obtained from various stages of pregnancy (forty~three cases) and studied by a Variety of cytological techniques. Evidence was presented which indicated that the trophoblast of early pregnancy performs a significant secretory function, this period of activity being roughly contemporaneous with the time of greatest excretion of gonadotropin. Both cytotropho— blast and syncytium were polarized toward the surface of the villus. In some cases differentially-stained granules were present in the region of the Golgi apparatus of the cytotrophoblast cells. Similar minute granules occurred in the subnuclear and nuclear zones of the syncytium in association With the Golgi apparatus. These enlarged toward the surface of the syncytium assuming a spherical shape. The granules then appeared to liquefy forming vacuoles which liberated their contents through the brush border. Mitochondria were prominent about the Golgi apparatus of the cytotrophoblast cells and throughout the syncytium, frequently accumulating in the superficial zone of the latter layer. In late pregnancy, the cytotrophoblast cells were more sparsely scattered; the syncytium became thinner and possessed a low irregular brush border, fewer mitochondria and lipid droplets, and practically no blue granules or superficial vacuoles. These changes were interpreted to be indicative of reduced secretory activity. No convincing evidence of secretion was found in the small and large decidual cells of the decidua parietalis.
Acknowledgments
We gratefully acknowledge the assistance of Drs. E. T. Engle, A. R. Abarbanel, C. L. Buxton, B. M. Patten and N. F. Miller in the procurement of the material studied.
Literature Cited
BARTELMEZ, G. W., AND C. M. BEHSLEY 1932 Human uterine gland cells. Special Cytology, vol. 3, edited by E. V. Cowdry, Paul Eoeber Ine., New York.
BENNETT, H. 8., AN13 G. B. Wrsnoom 1942 The nature of the lipoid droplets in the syneytiel t1'ophobla.st. Anat. Rec.’ vol. 82, suppl., 1). 65.
BOERMA, N. J. 1913 Beitrag eur Kenntnis der Einbettung des menschliehen Eies. Monats. f. Greb. 11. 'Gy11Ie'1k., V01. 3'7, 11.. 723.
BONDI, J. 1911 Ueber dae Pett in der Place-nta. Arch. r. Gyn..k., vol. 93, p. 189.
BONNET, R. 1903 Ueber Syncytien, Plaernodielr und Syrnplasnia. in der Placenta der Siiugetiere und des Mensehen. Monats. f. Geb. 11. G}'I1.‘ik., vol. 18, p. 1. - '
BOWEN, R. H. 1929 The c-yt-ology of glandular secretion. Quart. Rev. 13101.. V0]. 4, p. 484.
BREWER. J . I. 1937 A normal human ovum in 3. stage preceding the primitive streak. (The Edwarde+Jones—Brewer ovum). Am. J". Anat, vol. 61, p. 429.
Bnownn, J. S. L., AND E. M. VEBTNING 1936 Excretion of gonadotropic substances in the urine during pregnancy. Lancet, vol. 231, p. 1507.
BROWNE. J. s. L., J. S. HENR-Y AND :1 M. vem-me 19137 The corpus luteum hormone in pregnancy. J. O‘1in.- Invest, vol. .16, p. 678.
Conner, S. L., G. F. Mneernn AND M. WATS-ON‘ 1935 EKG-1‘etiOn of oestrin during pregnancy. Lancet, vol. 1, p. 67-21.
COSTERO, I. 1931 Hietologisehe Untereuehungen iiber die feine Struktur der Placenta und dee eehwangeren Uterus. I. Mitteilung: T-.'Tber die Uterue—p1acen1;'2i.re Gritterfa.se1'struktu1'. Zeits. Anet. u. Ent-wick, vol. 96, p. 766.
De KERVILY, M. 1916 La. villosité do pla.centa_. Arch. mens. d’0bstet. et Gyn., vol. 8, pp. 93, 296.
DRIESS-EN, L. F. 1907 Ueber Glyliogen in der Plaeent-a.. A.rc.h. f. Gyn}.-i.k., vol. I 82, p. 278. ' '
DEMPSE:Y, E. W. 1937 The re1a.t,ion of deoiduoniete. to the reproductive cycle in the guinea pig. Anat. Rec, vol. 70. Suppl. 1, p. 119.
EVANS, H. M., K. MEYER AND M. E. SIMPSON‘ 1932 Relation of Prolen to the anterior hypophyse-a.l hormones. Am. J. Ph}'eio1., vol. 100, p. 14.1.
EVANS, H. M., C. L. KoHLs AND D. H. WONDER 193'? Gonadotropie hornrone in the blood and urine of early pregnariey; the normal occltrrence of transient extreinely high levels. J. Am. Med. 1313311., vol. 108, p. 287.
Fnnxrxnn, L. B.,_.5-.ND A. GrEL.LI-IORN” 1942 A comparative st_udy of place-nte permeability using radioactive sodium. Anat. Rec... vol. 82, suppl.. p. 19.
FLORIAN, J. 1928 Ueloer dz-is Sjmeytiurn im Trop]1ob1a.st jnnger menechlic.hen Emb-ryonen. Verhandl. d. a11-at-. Geee11eel1., vol. 66, eu_ppl.., p. 211.
FRIOLET, H. 1905 Beitrag zurn Studimn der meneehliehe-n Plaeentetion. Beitr. z. Geb. u. Gynieiln, V01. 9, p. 1. ' 316 BURTON L. BAKER. AND OTHERS
GEY, G. 0., G. SEEGAR. AND L. M. HELLMANN 1938 Production of gonadatropic substance (prolan) by ple.eental cells in tissue culture. Science, vol. 88, p. 306. 0
GIERKE, E. 1905 Des Glykogen in der Morphologie des Zellsto Rvcchsels. Beitr. z. path. .An'e.t. u. z. allge-m. Pa.th., vol. 37, p. 502.
HAMBURGER., C. 1933 Studies on gonadotropie _hormones_f1-0111 the hypophysis and chorionie tissue with special reference to their differences. Acts. Path. et Microb. Soand., Suppl. 17.
PIEDENBERG, M., AND L. STRINDB-ER-G 1916 Beitrag zur Kenntnis der A.na—tomie und der Funktion der mensehlichon 1-‘lazente. A.n_a.t. A112,, vol. 49, p. -11.
HER-ZOG, M. 1909 A contribution to our knowledg'e'0f._the e_a.rliest_ known st.a.ge.s of placentatioii and embryonic developme-nt in man. Am. _J. Anat., vol. .9, p. 361. .
HITSC.3HM_ANN, F., AND L._ -ADLER 1908 Der Ben der Uterusschleimheut des gesohleelitsreife-n Weibes mit bes’oIn:ler'er Beriieksic.ht.igu11g der Men~ struetion. Monats. f. Geb. u. (}ynii.]r., vol. 27, p ._1.
HOFEAUER, J. 1903 Die Fettresorption der Chorionzotte. Ein Beitrag zur 11-‘Jl‘_T1’1al8I1 A.11a—to1nie und Physio-logie der 1nensch1ich_en Placenta. Sit~ zungsber. cl. kaiserl. Akad..(1. W1SS8I1SC]). vol. 112, part -3, p. 204.
1905 G-1:-undziige einer Biologic der mensehliehen Pla.z'enta.. Leipzig.
--—-~——-—-—- 1925- "The fuI1ct._ion of the Hofbauer cells of the ehorionie villus particularly in I'ela.ti_on to acute infection and syphilis. _ Am. J. Obst. _ end Gym, "01 10, p. 1. I
KEIBEL, F._, AND F. P. M.«i1.I. _ 1910 I-Iuman Embryology, vol. 1., .1. B. Lippincott Co., P11i.la.r_lelpl1ia and .L0nd_on.
KENNEDY, VV. 1933 Quaiititetive variation of the anterior pituitary hormone, ' “APH-B”, inthe blood during pregnenc.y.Qua_rt. J. Exp. Physiol., V01. 23, p. 367.
KIRKLIAN, H., AND A. E. SEVERINGHAUS 1938 A review of the Golgi apparatus. Parts I and II. Axmt-. "R-eo._, V01. 70, pp. 413, 557.
LA1*?GH.-1}IS, T. 1901 Syncytium uud Zellsehicht P1ace'11te.rreste nach Aborte-n. ' Chorionepitholiome. Hydatidenmole. Beitr. z. Geb. u. G_'y'néi.k., vol. 5, p. 1. I '
LEWIS, W. H. "1924 Hofbauer cells (clasmatooyt-es) of the human chorionie villus. Bull. Johns Hopkins Hosp., vol. 35, p. 183. 0 9 9
LONG, J. A., AND H. M. EVANS 1922 The -oestrous cycle in the rat and its associated phenomena. Mem. Univ. Ca.1if., vol. 6, p. 1.‘
Mall FP. On the age of human embryos. (1918) Amer. J Anat. 23: 397-422.
MALL F. P. 1918 0n the age of human em.byros. A111. J. Ana.t., vol. 23, p. 397.
MARCHAND, F. 1904 Beitrag zur Kenntniss £181‘ normalen uud patho1.o-gisehen 1 Histologie der Deeidua. Am. f. G3;nii.k., vol. 72, p. 155.
MARRIAN, G. F., S. L. COHEN AND -M. VVATSON 19-35 Observations on the ex" cretion of estrin during pregnancy. J. Biol. Chem, V01. 109, p. lix. Nnssonov, D. 1923 Des Golgisehe Biiineiinetz und seine Beziehungen- zn dner Sekretion. Untersuchungen fiber einige Amphibiendriisen. Arch. f. Inikr. Ana-t., V01. 97, p. 136.
NEUWEILER, W. 1927 Ueber Explantetionsversuche mensehlicher P'laz-enter. Monats. f. "Gob. 11. Gyn.,'vol. 77. p. 437.
NEWTON, W. H. 1939 Some problems of endocrine function in p1‘egn3.ney. Sex -and Internal Seeretions, second edition, edited by E. Allen, C. 1-1. Danforth and E. A. D-oisy, Williams and Wilkins Go., Baltimore.
PHILLIP, E., AND H. HUBER 1936 Die Hornionale Rollo der D'eeiclua. Zentr. f. Gj_v11iik., vol. 60, p. 2706.
Ross: Donia, T. 1905 Ueber die Einbettung des rnensehlichen E163, studirt. an
_ einem kleinen Eie der zweiten Woche-. Arch. f. Gryn}:ik., vol. 76, p. 433.
RUNGE, H., .=\.Nn H. HIARTMANN. 1929 Beitreg znr Histologie der monsehliohen P'la.oentn.. Arch. f. .Gyn;*.':k., vol. 139, p. 5-1.
Soflnonnnn, R. 1930 Weibliehe Genitalorgene. v. Mollendorf’e Handb. der mikr. Anni. (lee Mensehen, vol. 7, part 1, Julius Springer, Berlin.
SEEGAB. Jorsnte, G. E., G. O. GEY AND M. K. GEY 1943' Hormone production by pl:3.c-.enta.l cells rnaintaineil in continuous culture. Bull. Johns Hopkins, Hosp., vol. 72, p. 26. 9 6
SEVERINGHAUS, A. E. 1939 Anterior hypophyeeal oytology in relation to the ' reproductive hormones. Sex and Internal Seeretions, second edition, edited by E. Allen, C. H. Danforth and E. A. Doisy, Williams and Wilkins 00., Baltimore. _ _
SEVERINGHAUS, A. EL, AND K. W. Tnolnrson 1939 Cyt_o1ogical changes induced in the liypophyeis by the prolonged a.d1ninistra.tion of pituitary extract-. Am. J. Path., vol. 15, p. 391.
SMITH, G. 17., 0. W. SMITH AND Gr. W. PINCUS 1937 Total urine:-y estrogen, estrone and estriol during a menstrual "cycle and a. pregnancy. Am. J. Phyeiol., vol. 12-1, p. 98.
SMITH, 0. W., AND G. V. S. SMITH 1937 Prolaizi and eetrin in the serum and urine of diabetic and non~<lio.betie Women during pr-egnaney, with especial reference to late pregnancy toxemia. -Am. J. Obst. and Gryn., Vol 33, p. 365.
SMITH, O. W., G. V. 8. SMITH AND S. SCHILLER 1941 Estrogen and progesterone metabolism in pregnancy. I. Spontaneous and induced labor. J. Clin. E11doer., Vol. 1, p. 461.
STIEVE, H. 1926 Ein 13% Tege altos, in der Gebiirmutter e._rha.ltene-3 und -dureh Eingrifi gewonnenes niensehliehoe Ei. Z-eits. f. mikr.-a-net. Forsch., vol. 7, p. 294. - '
TERASAKI, 0. 1927 fiber die Gitterfa_.serstrukturen in der Inenechliehen Plazentn. Zeits, f. G-eb. n. Gyn§.k., vol. 92, p. 94. _
Tooro, R. 1912 Em jungee me-nsehliches Ei. Arch. r. Gyn.iik., vol. 95, p. 425.
TRIBBY, C. L. "1943 The i11tro..cellula.r lipin, mucoid, and glycogen of the ‘F3.’ ginal epithelium of the guinea. pig. Anat. Ree, vol. 86, p. 425.
ULE:SKo-STROGANOFF, K. 1908 Zur Frags V011 (18111 feinsten Ban (lee Deei.duago— webee, seiner Histogeneee, Bedeutnng und dem O-rte seiner 'Entwie1~:~ elnng im Genita.la.pperat do-r Fran. Arch. f. Gyniiln, vol. 86, p. 542.
VAN CAUVVENBERGHE, A. 1907 Recherehee sur le role do syneytium dens la nutrition en'1bryonna.iro ehez la femme. Arch. de biol., vol. 23, p. 13.
VON LnNHossr':K, M._ 1902 Ueber dos Chorionepithel. Rev. in Zentrbl. f. G;\_2ni«ik., 1904., vol. 28, p. 229.
wEnEnH.u«m, K. J. 1906 fiber P1asma.— und Doeiduazellen. Monats. f. Geb. u. 'Gynéik., vol. 24, p". 316.
WIENBECK, J. 1936 Beitrag zur Histologie 11nd Plivsitalogie der Placentarzotte. Zeits £. mikr.-anat. Forsch., vol. 39, p-. 135.
WISLOCKI, G. B., AND G. L. STREETER 1938 On the plaeentation of the macaque (Macaca mulutte), from the time of implantation until the formation of the definitive placenta. Carnegie Contr. to Embryol., vol. 27, p. 3.
Explanation of Figures
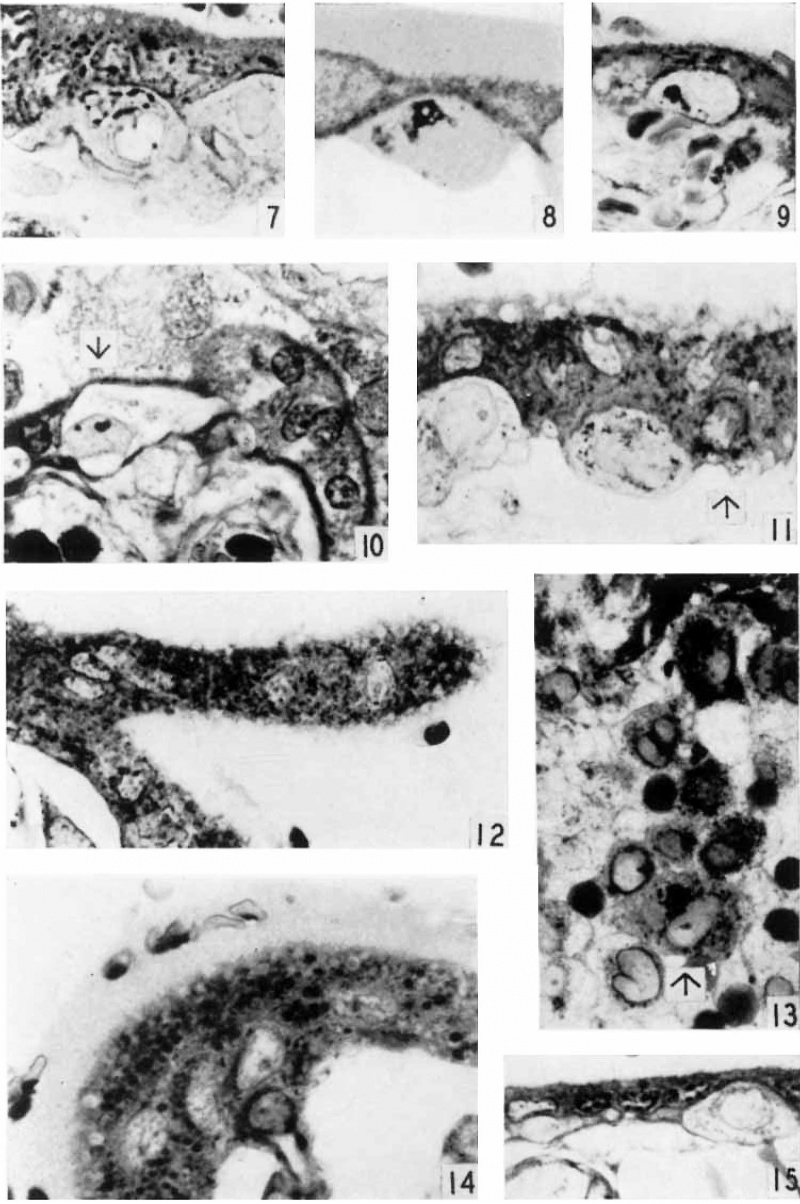
Unless otherwise stated, the figures are from 3 μm sections of Champy-fixed material stained by the Severinghaus Altmann—Masson technique; Golgi preparations were osmicated by the Sevoringliefus (Nassonov-Kolatschev) method before staining.
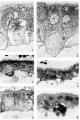
Plate 1
1 Drawing of cytotrophoblast cell showing two granules in the Golgi region with associated mitochondria. (black). A large vacuole is present at the right of the sgvncytial nucleus. Case 6. About x 4300.
2 Drawing of the syncytium showing minute granules in the subnuclear zone in association with clear curved channels, the negative image of the Golgi apparatus. The granules increase in Size towards the surface of the syneytium and show varied degrees of affinity for aniline blue. The majority are homogeneous; one large granule at upper right shows definite structure. Superficial vacuoles at upper left. Mitochondria. have accumulated in the supranuclear zone and about the negative image of the Golgi apparatus. Case 12. About x 3200.
3 Accumulation of mitochondria in the superficial zone of the sycytium in the absence of vacuoles and granules. Mitochondria (black) are shown in the cytotrophoblast cell, especially in the Golgi region at the left of the nucleus. The syncytial nuclei are more chromatic than those of the cytotrophoblast. Case 12. x 1160.
4 Secretory granules in the superficial zone apparently in various stages of liquefaction, with some eecuxnuluted mitochondria. A cytotrophoblast cell (arrow) appears above the capillary probably in process of transformation to syncytium. The cytoplasm is dark, and the mitochondria at left of nucleus, large and intensely stained. Contrast cytoplasm to that of cytotrophoblast cells in figures 3 and 5. Case 12. x1160.
5 Reguklar brush border and even distribution of mitochondria throughout syncytium. Some lipid droplets (black) in the syncytium. 01min of mitochondria above nucleus of middle cytotrophoblast cell. Case 12. x 1160.
6 Brush border after Bouin fixation. Case 13. x 1160.
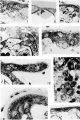
Plate 2
7 Loose Golgi apparatus of a large cytotrophoblast cell. Case 5. x1160.
8 Compact Golgi apparatus containing vesicles in a small cytotrophoblast cell. At left of nucleus are brown lipid bodies. Not stained. (Jase 14. X 1740. 9 Golgi apparatus of a full-term cytotrophoblast cell. Case 22. X 1160.
10 Full-term cytotrophoblast cell (arrow) with condensed cytoplasm in the Golgi region containing a few mitocliondria. Thin syncytium. covers this cell and on right side of villus above figure number is seen the low, scrubby brush border. The basement membrane is markedly thickened. Case 22. X 1.160.
11 0n the right (arrow) a cytotrophoblast cell in process of transformation to Sl}'I1(3__Ytl1.ll'l1 in which the cytoplasm is indistinguishable from that of the s_vnc.y'tium, but the ce.ll membrane is clearly visible above the nucleus. Mitochondria are clumped and briglitly stained. The syncytium shows some granules and superficial vacuoles with surrounding accumulation of niitochondria. Case 12. X 1400.
12 A syncytial bud with many mitochondria and a few small granules at the right of the most distal nucleus. Case 12. X 1160.
13 An area. from the edge of a cell island showing homogeneous fibrinoid at the top and a large granule-packed cell (arrow) with hypertrophied Golgi body near the bottom. Separation of the cells is indicated by the maternal erythrocytes scattered among them. Case 13. X 1160.
14 Seemingly viscous material on the surface of the villus holding maternal erythrocytes. Many of the latter stained partly red and partly yellow, or various shades of blue. A highly active syncytiiiin containing, in the supranuclear zone, many lipid droplets (black) and granules (gray), the latter appearilig to be in various stages of liquefaction. Case 12. X 1160.
15 Thin, inactive syncytium of 5 months, containing only one or two minute granules at the left. Case 3. X 1160.
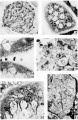
Plate 3
16 Glycogen in a cell island and some in the syncytium at the lower right. Picric—alcohol-formol. Best’s carmine. 5 μm Case 14. X 320.
17 Golgi apparzitusl in a syncytial bud. (lasso 18. X 1160.
18 The lower villus shows a vesiculated brush border, and accumulated mitochondria in the basal zone of the syncytium. The. brush border of the upper villus is more completely disorganized by the presence of superficial vacuoles. Case 12. X1160.
19 The edge of a cell island showing several eells with mitochondrial and granular accumulations in the Golgi region. Case 13. X .1280.
20 An oblique seetion through the trophoblast showing the extensive Golgi apparatus of the subnuclear and nuclear zones of the syncytium. Case 5. X 1160.
21 A. proliferation of the cytotrophoblast at the end of a villus. Basemrarlt membrane at. lower left. Point of continuity with cytotrophoblast layer at lower right. Some reduction in number of mitochondria. fibrinoirl degeneration of the syncytium at the right. Case 12. x 880.
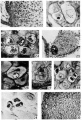
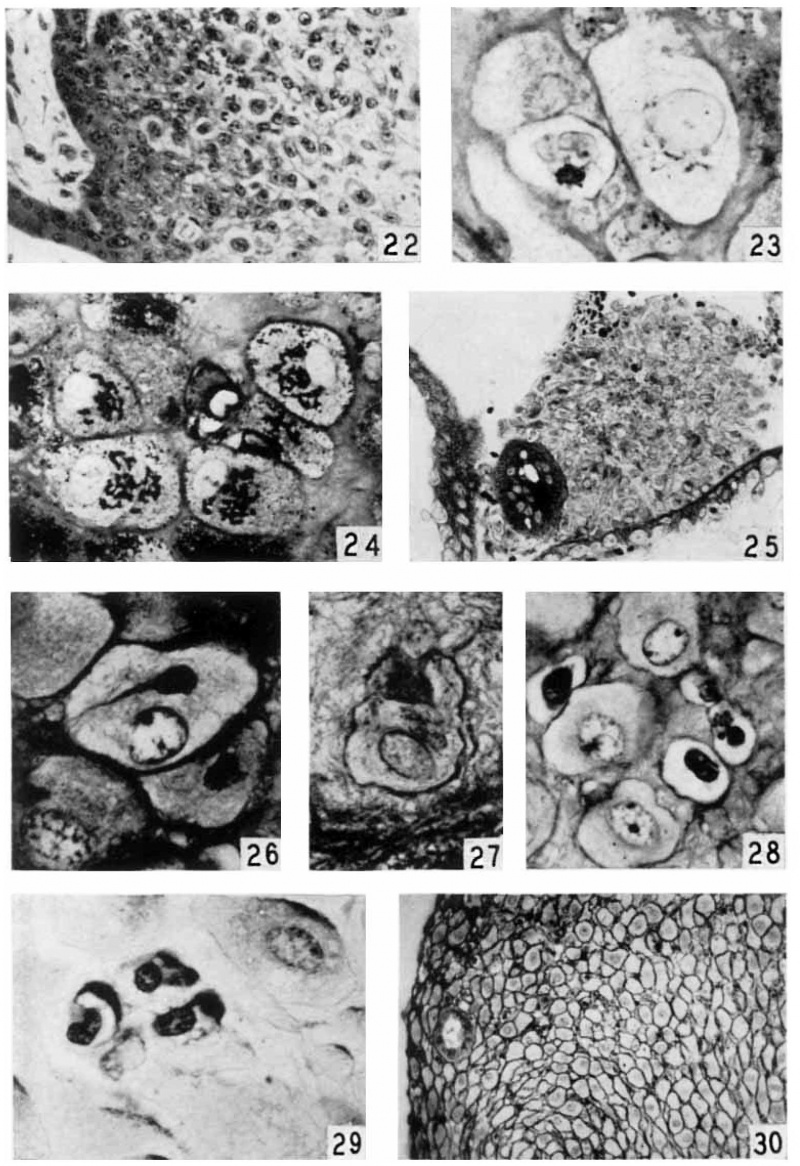
Plate 4
22 Cell column connecting a. villus at left to wall of Fallopian tube out of field at right. Three mitoses are present near cytotrophoblast of villus. Cells farther removed from villus are larger and enclose empty cytoplasmic spaces from which glycogen has been removed. EH 265. 10 μm Hernatexylin and eosin. X 230.
23 Golgi apparatus and lohated nucleus of a small decidual cell at lower left. Case 14. X 1360.
24 Golgi apparatus in four large decidual cells. Case 14. X 960.
25 A cell island near two villi, the tropliohlast of which is seen at lower right and at the left. The island is not covered with syncytiuni and shows an early stage of degeneration at the center with loss of cell structure and a.ppeara:nce of small masses of librinoid. At left of island is a syncytial bud containing large vacuoles. The small black dots in the syncytium and loud are lipid droplets Case 13. x 240.
26 Connective tissue material enfoldecl by a large decidual cell and centinuous with the peri-cellular condensation. Benin. Severinghaus Altn1annMassen. 532.. Case 5. ,)< 1160.
27 Large deeidual cell with two mitochondria—laden processes partially surrounding dense connective tissue matrix. Mitochondria also accuniulated in the Golgi region above the nucleus. Case 2. x 1160.
28 Three large decidual cells and three small decidual cells, the latter with intensely chromatic nuclei and empty cytoplasmic space. The one at the right shows a. clump of blue granules. “Benin. Severinghaus Altmann-lrlasson. fin. Case 17. X 960.
29 Glycogen deposits in three small decidual cells. Large decitlual cell at upper right with some glycogen at its upper border. Picric-alcohol-formel. Best’s earmine. 5;; Case 5. X 960.
30 Pericellular eendensatiens around large decidual cells of zone cornpacta. Benin. Bielschowsky and Harris. hematexylin. Case 12. x 176.
Cite this page: Hill, M.A. (2024, May 2) Embryology Paper - The cytological structure of the human chorionic villus and decidua parietalis. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_cytological_structure_of_the_human_chorionic_villus_and_decidua_parietalis
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G