Paper - The Factors Involved in the Excavation of the Cavities in the Cartilaginous Capsule of the Ear in the Human Embryo
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. The development of the scala tympani, scala vestibuli and perioticular cistern in the human embryo. (1917) Amer. J Anat. 21: 300-320.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Factors Involved in the Excavation of the Cavities in the Cartilaginous Capsule of the Ear in the Human Embryo
Department of Embryology, Carnegie Institution of Washington, Baltimore, Maryland
Twelve figures
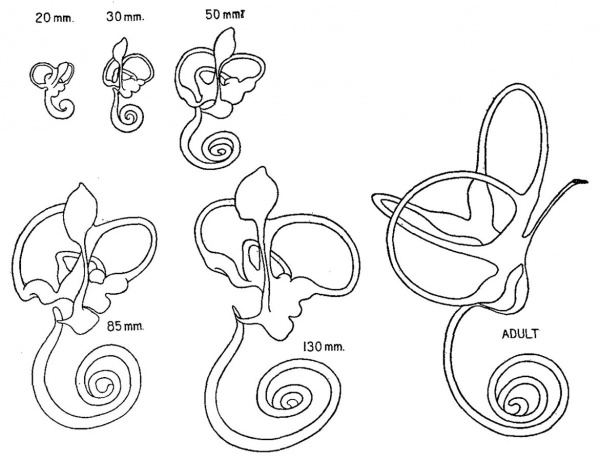
The main mass of the cartilaginous capsule of the ear matures into true cartilage when the human embryo reaches a length of 20 to 30 mm., at which time it has acquired what may be considered its adult form with characteristic chambers and openings. From this time on, throughout its whole cartilaginous period, and even after ossification has begun, it undergoes continuous growth, maintaining at the same time, however, its general form and proportions. Such a growth involves both an increase in the surface dimensions of the capsule and a gradual enlargement or excavation of its contained cavities. It is to the manner in which this excavation is accomplished that the writer wishes to call attention and particularly to the factors concerned in its progress whereby a suitable space is always provided for the enlarging membranous labyrinth. The actual amount of increase in size of the labyrinth is graphically pictured in figure 1. The outlines are made so that they show on the same scale of enlargement a series of wax-plate models of the left membranous labyrinth of human embryos having a crown-rump length of 20, 30, 50, 85 and 130 mm., as indicated in the figure. This covers the period during which the otic capsule is in a cartilaginous state. Ossification begins when the fetus has attained a crown-rump length of about 130 mm. The growth from then until the adult condition is reached may be judged by comparing the above with the final stage, labelled adult, which is taken from Schonemann’s reconstruction[1] and reproduced here so as to be on the same scale of enlargement as the younger stages. Since the cartilaginous labyrinth corresponds closely in form to the membranous labyrinth, particularly as regards the canals, one can see from figure 1 that there is a progressive increase in the size of the cartilaginous chambers throughout the whole embryonic period.
Fig. 1 Median views of wax-plate models of the left membranous labyrinth in human embryos having crown-rump lengths as indicated in the figure. The largest one is taken from Schonemann (’04) and represents the adult condition. They are all on the same scale of enlargement (4.4 diameters) and thus comparison of them shows graphically the amount of growth the labyrinth experiences during this period.
In addition to this increase in size, there is a change in the form of the cartilaginous labyrinth. The general proportions are maintained but there are alterations in the detailed form. As the canals become larger and longer they describe arcs of lesser curvature. If one compares the superior canal of an 80 mm. fetus with that of a 30 mm. fetus it will be found that in the former it has doubled its diameter and trebled its length. There is, moreover, a constant change in the relative position of the cartilaginous canals. The lateral canal, for instance, progressively recedes from the lateral wall of the vestibule. In studying this canal, therefore, one may know that it is steadily becoming larger by means of a process of excavation, but this is so managed that the canal as a whole moves in a lateral direction through the substance of the cartilaginous capsule. The topography of the cartilaginous labyrinth is so well provided with known landmarks that these changes in its size and form can be accurately followed. It is possible to determine deductively at what points new cartilage is being laid down and at what points it is being removed. On this account the cartilaginous capsule of the ear is a particularly favorable place for determining the histological features of the growth of cartilage.
As has been noted above the growth of the cartilaginous otic capsule resolves itself into an increase in its external dimensions with a simultaneous hollowing out and reshaping of its contained chambers. It at once becomes evident that this cannot be accounted for on the basis of a simple interstitial increase in the mass of cartilage together with its passive rearrangement to allow for the enlarging cavities, due for instance to a mechanical expansive pressure from the growing membranous labyrinth with its surrounding tissue and fluid. Such a passive rearrangement could only occur in a tissue that is very plastic, whereas cartilage is one of the least plastic of the embryonic tissues. Moreover the histological picture is not that of mechanical pressure. The cartilaginous chambers are always excavated slightly in advance of the space actually required by the membranous labyrinth, and there is no evidence of the labyrinth being cramped or of the creation of pressure grooves in the margins of the cartilage. Nor is the situation improved by the introduction of the conjectured activity of the perichondrium, either in explanation of the deposit of new cartilage or of the excavation of the old, since the perichondrium, as will be shown, does not make its appearance until after a considerable amount of the growth and hollowing-out of the labyrinth had been already completed. Therefore there is involved in the development of the cartilaginous capsule something more than interstitial and perichondrial growth, in the ordinary sense of the terms. On account of its bearing upon this problem, it is the purpose of the present paper to call attention to the occurrence of dedifferentiation of cartilage in the human embryo, and to point out the important part which this process normally plays in the hollowing out and reshaping of the otic capsule during its development.
The term dedifferentiation is applied here in the sense of a regression of certain areas of cartilaginous tissue to a more embryonic form, the same areas being subsequently rebuilt or redifferentiated into quite a different type of tissue. Dedifferentiation is defined by Child as “a process of loss of differentiation, of apparent simplification, of return or approach to the embryonic or undifferentiated condition.” In his noteworthy review of this subject he makes the assertion that the wide occurrence and significance of dedifferentiation in the lower animals and plants “must at least raise the question whether similar processes do not occur to some extent in higher forms.”[2] From the context it is evident that he refers to man as well as other mammals. The materialization of his prediction is here at hand in the development of the cartilaginous capsule of the ear. Before entering into this further it will be necessary to outline the earlier steps in the histogenesis of this particular tissue.
The Three Stages in the Development of Cartilage
The cartilage of the otic capsule in its transition from embryonic mesenchyme to true cartilage passes through three fairly definite phases: firstly, the condensation of mesenchyme around the otic vesicle; secondly, the differentiation of the condensed mesenchyme into precartilage; and thirdly, the conversion of precartilage into true cartilage. These three histogenetic stages merge more or less diifusely into one another and one‘ must bear in mind that such a subdivision is necessarily arbitrary and tends to result in an exaggeration of the distinctness of the lines of their demarcation. Their points of difference, however, are here emphasized because the reversal of one state of development into a previous state is the feature to which it is desired to call especial attention.
Stage of Condensed Mesenchyme
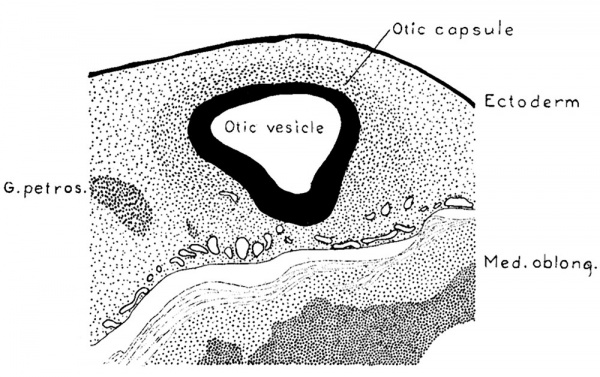
When a human embryo is 4 to 5 mm. long the mesenchymal tissue surrounding the otic Vesicle differs very little from that in other regions. The nuclei, however, are quite sparse in the regions ventral to the neural tube in the median line, and they become perceptibly more numerous as one explores laterally into the neighborhood of the otic vesicle. This slight increase in the number of nuclei around the vesicle marks the beginning of the mesenchymal condensation that is to form the otic vesicle. A definite layer of such nuclei is not found until the embryo reaches a length of about 9 mm.; it is then possible to recognize a fairly Well outlined zone of mesenchyme which represents the otic capsule in its first stage of development. In figure 2 is shown a sketch indicating the relations which exist at that time. It represents a transverse section through the otic vesicle at the level of the attachment of the endolymphatic appendage. The zone of condensed mesenchyme forming the primordium of the otic capsule abuts directly against the lateral wall of the vesicle and extends from there to a point about one-half the distance between the vesicle and the ectoderm. On the median side of the vesicle this zone is lacking, although there is a considerable number of mesenchyme cells clustered around the vascular plexus ensheathing the central nervous system, and among the nerve rootlets of the acoustic complex. When this zone is analyzed under higher magnification it is found that it still consists essentially of a mesenchymal syncytium. It differs morphologically from the adjacent mesenchyme, with which it is directly continuous, only in its more numerous and more compactly arranged nuclei and its somewhat richer network of internuclear processes. This is shown in figure 3 which is taken from an embryo a little larger than that in figure 2, but which in its general form is apparently in about the same stage of development.
Fig. 2. Section through the region of the otic vesicle in a human embryo 9 mm. long (Carnegie Collection, No. 721) enlarged 66.6 diameters. The primordial of the otic capsule, consisting of condensed mesenchyme, can be seen enclosing the vesicle on its lateral surface.
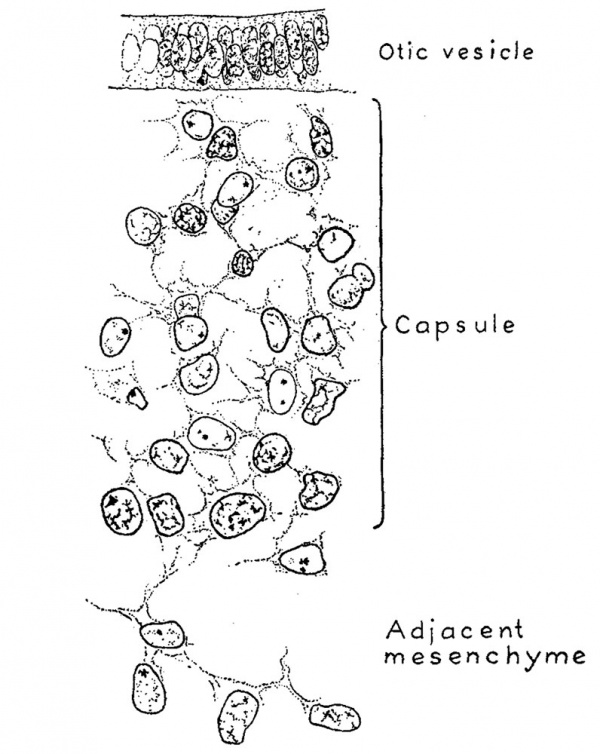
During the period of growth represented by embryos between 9 mm. and 13 mm. long, that is, up to the time when the semicircular ducts begin to separate from the main labyrinth through the apposition and absorption of the intervening membranous Wall, the zone of condensed mesenchyme around the otic vesicle increases in extent and compactness, thereby forming a sharply defined capsule which completely encases the labyrinth. This capsule of condensed mesenchyme has the same openings and corresponds closely in form to the cartilaginous capsule into which it is destined soon to be converted.
Fig. 3. Camera lucida drawing of a portion of the otic capsule while it is the state of condensed mesenchyme. It is taken from a human embryo 13.5 mm. long (Carnegie Collection, No. 695). The section is 10 microns thick and is enlarged 950 diameters. The syncytial character of the capsule can be seen and also its relation to the epithelial wall of the otic vesicle and to the surrounding mesenchyme.
Stage of Precartilage
The histogenetic changes which initiate the conversion of the capsule of condensed mesenchyme into a cartilage-like tissue make their first appearance just after the separation of the semicircular ducts from the main Vestibular pouch. This occurs when the embryo is about 14 mm. long. The conversion of the capsule into a true cartilage with a characteristic tinctorial reaction of its matrix is not completed until the embryo attains a length of 30 mm. Thus in embryos between 14 and 30 mm. long the otic capsule consists of a tissue in an intermediate condition between condensed mesenchyme and cartilage. This intermediate form is known as precartilage. It constitutes the second of our three stages of cartilaginous growth.
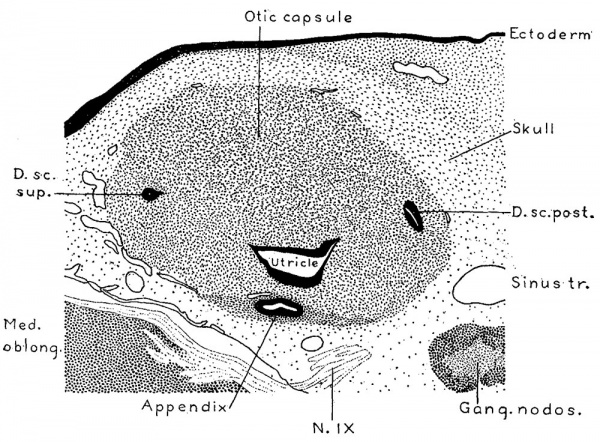
Fig. 4. Section through the region of the otic capsule in a human embryo 15 mm. long, (Carnegie Collection, No. 719). Enlarged 66.6 diameters. The epithelial portions of the labyrinth are shown in solid black and it will be noted that they are in direct contact with the substance of the capsule; there is as yet no periotic reticular tissue. The section passes through the superior and posterior semicircular ducts and through the utricle near its junction with the crus commune.
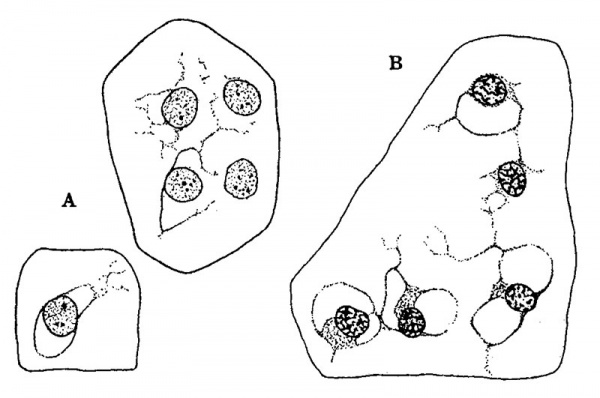
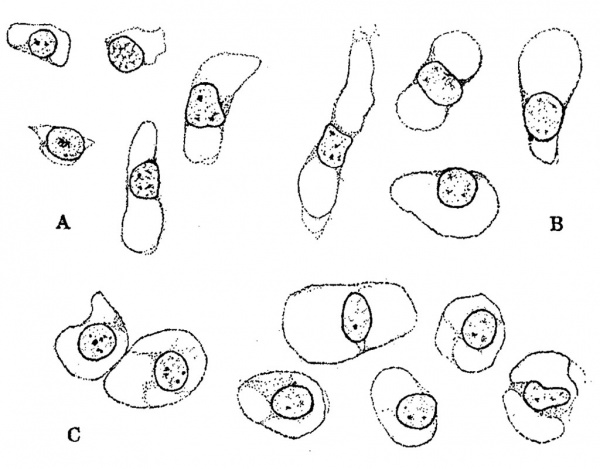
The general form and relations of the otic capsule at the beginning of its conversion from condensed mesenchyme into precartilage is shown in figure 4, which represents a horizontal section through this region in a human embryo 15 mm. long (Carnegie Collection, No. 719). It will be noted that the capsule abuts directly against the epithelial Wall on the labyrinth. Around the margins of the capsule there is a Vascular network the branches of which, however, do not penetrate into its substance. In its form it is essentially the same as its antecedent capsule of condensed mesenchyme, but in structure it can be seen to be undergoing certain characteristic alterations. These do not occur uniformly throughout its substance but appear earlier in some areas than in others. They consist of an increase in distance between the nuclei, together With an alteration in the internuclear protoplasmic network and its spaces. Whereas the capsule, as seen in prepared sections, has previously consisted of a mesenchymal syncytium, it now gradually loses its syncytial appearance. Most of the branching processes disappear and are replaced by a homogenous mass. Some of the processes, on the other hand, persist, and become thicker‘ and more sharply outlined. These persisting larger processes usually exhibit a characteristic relation to the nuclei. Two or more of them unite in the formation of a loop at one side or at one or both ends of a nucleus, thereby creating a perinuclear space which soon takes on a more transparent appearance than the surrounding homogeneous material that accumulates in the place of the disappearing processes. These changes can be seen in the sketches shown in figure 5, which represent characteristic areas in the otic capsule while in the precartilage stage in human embryos 17 and 18 mm. long. In the two sketches marked A the contrast beween the permanent and disappearing protoplasmic processes is already noticeable. In the sketch marked B the transition is more advanced although one can still recognize in the homogeneous matrix remnants of branching processes which have not yet disappeared. The persisting processes enclose characteristic capsular or perinuclear spaces. Similar spaces are shown in figure 6 which presents aiseries of isolated nuclei with their associated permanent processes such as are found in sections of maturing precartilage. In some of these (figure 6, C and figure 5, B,) there is a beginning accumulation of granular protoplasm at the margin of the nucleus which constitutes the so-called endoplasm and becomes enclosed with the nucleus in the capsule. After the formation of the spaces the endoplasm gradually accumulates and forms the cell body of the encapsulated nucleus. Thus in precartilage we find all stages in the transition, from a mesenchymal syncytium to a tissue consisting of partially encapsulated cell-islands separated from each other by a homogenous matrix.
Fig. 5. Camera lucida sketches showing characteristic fields in sections of the otic capsule while it is in the precartilage state. Enlarged 950 diameters. The groups labelled A are taken from an embryo 17 mm. long (Carnegie Collection, No. 576). Group B is taken from an embryo 18 mm. long (Carnegie Collection, No. 409).
Cartilage Stage
The transition from precartilage into cartilage gradually takes place in the otic capsule when the embryo is between 25 and 30 mm. long. This maturation is characterized by an increase in the amount of matrix combined with a more complete encapsulation of the nuclei, or cartilage-cells, as they may now be designated. With the increase in the amount of the matrix there is also a change in its chemical composition, so that it becomes possible to stain it differentially. This tinctorial reaction constitutes an arbitrary point at which it may be said that the precartilage becomes cartilage. In embryos 30 mm. long the greater portion of the otic capsule reacts tinctorially and has the histological character of young cartilage. With this stage we reach the third and final phase of the process with which we are dealing. The further changes from younger cartilage to older cartilage, and the conversion of cartilage into bone, are doubtless a continuation of the same general process but in the present paper they will not be taken into consideration.
Fig. 6. Characteristic precartilage cells showing the manner in which spaces become enclosed around them, eventually becoming encapsulated cells of true cartilage. Enlarged 950 diameters. Group A is from the otic capsule of an embryo 17 mm. long (Carnegie Collection, No. 296); Group B is from an embryo 24 mm. long (Carnegie Collection, No. 455); and Group C is from an embryo 23 mm. long (Carnegie Collection, No. 453).
Periotic Reticulum
It has been pointed out elsewhere by the Writer[3] that there is derived from the condensed mesenchyme surrounding the otic capsule not only the cartilaginous capsule but also the periotic reticulum which eventually intervenes between the capsule and the epithelial labyrinth. The relation existing between this reticulum and the three stages of cartilage that have just been defined must therefore now be referred to. The formation of the periotic reticulum is first indicated by a cluster of deeply stained nuclei that can be seen along the central edge of the semicircular ducts in embryos soon after the ducts are formed, and at about the time the otic capsule begins to change from condensed mesenchyme into precartilage. These nuclei constitute a focus at which the development of the reticulum and its blood vessels takes origin. Here the tissue of the capsule gradually takes on an appearance less like a cartilage-forming tissue and more like embryonic connective tissue. Spreading from this focus a narrow area is established which soon encircles the semicircular ducts and becomes the open-meshed vascular reticulum which in embryos 30 mm. long everywhere bridges the space existing between the epithelial labyrinth and the surrounding cartilage.
While in the stage of condensed mesenchyme and in the earlier part of its precartilage period the tissue of the otic capsule to all appearances abuts directly against the epithelial wall of the labyrinth as shown in figures 2, 3 and 4. It is possible, however, that some of the cells directly adjacent to the epithelium do not properly belong to the tissue of the otic capsule. It is conceivable that such cells may represent indifferent mesenchyme and perhaps angioblasts which were originally enclosed, along with the otic vesicle, by the condensed tissue of the capsule where they remain in contact with the epithelial wall in a resting condition until the embryo attains a length of 20 m. We might regard as an indication of their resumed activity the formation of the deeply stained foci along the central margins of the canals which have been described above. It might thus be maintained that the periotic reticulum is derived from a few predestined mesenchyme cells which after a latent period undergo proliferation and occupy the space vacated by the receding precartilage. On the other hand one may also maintain that the reticulum is derived from carti1age—forming tissue; that it is not a predetermined tissue but is simply precartilage that has undergone dedifferentiation. In the early stages when only a few cells are concerned this matter cannot be so well determined, the histological difference between early precartilage and indifferent mesenchyme cells not being sufficiently great for their certain recognition. In the later stages, however, it is quite evident that precartilage tissue is actually converted into a reticulum, and that the replacement of precartilage by a reticular connective tissue is accomplished by a process of dedifferentiation. By identifying a special area through its relation to a particular canal, and comparing this selected area in a series of stages, it is possible to observe the conversion of precartilage into reticulum, and to trace histologically step by step the manner in which a space occupied by precartilage in a younger stage is replaced by a reticulum in an older stage. This is the same procedure which occurs in the conversion of cartilage into pre~ cartilage and in the latter case, on account of the more highly specialized structure of the tissues, the picture is even more striking, as will be seen in the following outline in which the main features of the process will be pointed out.
Dedifferentiation of Cartilage
It has been noted that in embryos 30 mm. long the main capsular mass consists of true cartilage possessing encapsulated cartilage cells and an intervening matrix that is differentially stainable. A section passing transversely through the lateral semicircular canal of an otic capsule of this age is shown in figure 7. This, and figures 8 and 9, form a series showing at the same enlargement the same canal, i.e., lateral, cut in the same plane at three successive stages in its development. A direct comparison of these figures can thus be made and there is thereby seen the histological changes that occur with the growth of the canal. The successive figures may be superimposed upon each other and in this way the relative amount and position of the constituent tissues be determined. When this is done it is found that in the process of enlargement the true cartilage around the margin of the canal becomes replaced by precartilage and the precartilage in its turn becomes converted along its inner margin into the reticular mesenchyme which finally becomes the periotic reticulum. In other words, cartilage of the third stage as above described, reverts or is dedifferentiated into cartilage of the second stage and this in turn is dedifferentiated into a tissue approximating the first stage. It is this retrogressive adaptability of its tissues combined with their progressive development which render possible the enlargement of the otic capsule and the alteration in form and position of its contained cavities.
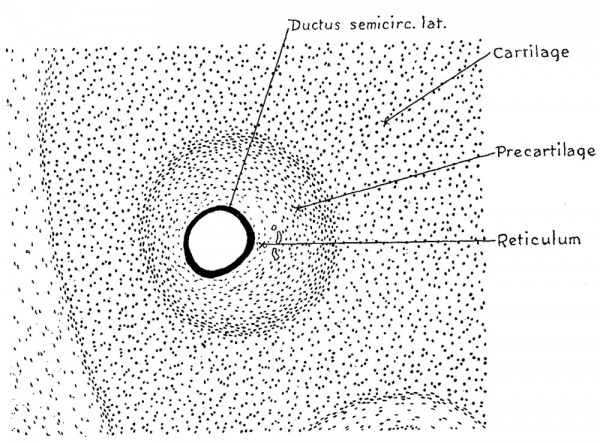
Fig. 7 Section passing transversely through the lateral semicircular canal in a human embryo 30 mm. long (Carnegie Collection, No. 86), enlarged 100 cliameters. The canal at this time is only slightly larger than the contained epithelial duct, but the zone of temporary precartilage marks out an area that is soon to be excavated by the process of dedifferentiation through which it becomes converted into a reticular connective tissue.
In the 30 mm. embryo shown in figure 7, the first of these three figures, it will be seen that the epithelial duct is separated from the main cartilaginous mass of the capsule by a surrounding zone of precartilage and intervening between the latterand the duct is a narrow zone of mesenchymal tissue which is somewhat reticular in character. This zone of reticulum has attained its greatest width on the median side of the duct, toward the right, being at this point about twice as wide as the thickness of the duct wall. It is characterized by its reticular arrangement and by the presence of small blood vessels which are not found in the precartilage, although they lie closely against its inner margin. The area of precartilage stands out conspicuously in material that has been intensely stained in hematoxylin without any counterstain. A series of this kind is represented by No. 199 in the Carnegie Collection. In that series the true cartilage is deep blue on account of the avidity with which its matrix takes the stain, whereas the precartilage shows only a nuclear stain and therefore is only faintly colored, as compared with the sharply demarcated and almost opaque cartilage surrounding it. The negative of this picture is presented in material where there has been an intense nuclear stain with subsequent decolorization of the cartilaginous matrix. Such a condition exists in figure 7 but is more marked in specimens where the stain is more intense, such as the series No. 972 of the Carnegie Collection. Under such circumstances the area of precartilage appears as a dark field in the midst of the faintly stained true cartilage. Depending upon the management of the technique it is thus possible in embryos about 30 mm. long to display the future cartilaginous canals; that is, the precartilaginous areas which approximately correspond to them, either as dark fields in a light background or as light fields in a dark background.
Fig. 8 Section passing transversely through the lateral semicircular canal in a human fetus 43 mm. long (Carnegie Collection, No. 886). Enlarged 100 diameters. The epithelial semicircular duct is larger in diameter than the one in figure 9, but that is the accidental result of its having been fixed While in a distended condition. The size of these ducts cannot be compared without taking account of this variation in their distension.
In the second figure of the series, figure 8, the area representing the future cartilaginous canal, is appreciably larger. Its perimeter, compared with that of the canal in figure 7, is in the proportion of 152 to 115, which are measurements in millimeters made on photographs taken at 100 diameters. By comparing the two figures it will be seen that the increase in size is obtained by the encroachment of the precartilaginous area upon the surrounding cartilage. The amount of this encroachment represents the amount of true cartilage which has reverted or dedifferentiated into precartilage. .In a similar manner the reticular zone surrounding the membranous duct has enlarged at the expense of the precartilage. The reticular zone as shown in this figure, taken from a human embryo 43 mm. long, forms a distinct and characteristic eccentric vascular field, but it undergoes its greatest expansion soon after this period.
In the 50 mm. embryo, as can be seen in the third figure of this series, figure 9, the area of the reticular zone is about the same in size as the Whole precartilage area in the 30 mm. embryo of figure 7. On comparing these two figures it becomes apparent that there is just as much, and even more, precartilage in the latter but it has moved outward into the area that was previously true cartilage. At this period the outer perimeter of the precartilage is 192 mm. as compared with 115 mm. in figure 7. As the old area of precartilage disappeared, a new and more peripheral area became established. Thus it may be seen surrounding cartilage. This more peripheral precartilage likewise in the end becomes reticulum.
Fig. 9 Section through the lateral semicircular canal in human fetus 50 mm. long (Carnegie Collection, No. 95). Enlarged 100 diameters. This section is taken at the same relative position and at the same enlargement as those in figures 7 and 8, so that they may be directly compared. It will be seen that the area of precartilage in figure 7 is now entirely replaced by reticulum, whereas a new and more peripheral area of precartilage has formed at the expense of that true cartilage has been dedifferentiated into precartilage and this in turn into the periotic reticulum. It is in this way that the enlargement of the canals is accomplished, a process of excavation based on the dedifferentiation of a specialized tissue into a more embryonic type, followed by a readjustment of re differentiation of this simpler form into a tissue adapted to the new conditions.
In addition to the excavation of cartilage there occurs, in connection with the growth and alteration of form of the otic capsule, the deposit of new cartilage. As the lateral cartilaginous canal, for instance, enlarges it also moves laterally, so that the distance between it and the cartilaginous vestibule increases, thereby producing a lateral- migration of the space as a whole. Such a migration must involve an excavation of the established cartilage on its lateral margin and the formation of new cartilage on its median margin. Therefore on the lateral margin we find true cartilage being dedifferentiated into precartilage and on the median margin precartilage being differentiated into true cartilage. The margins of the cartilaginous canals throughout the whole embryonic period are in an unstable condition and are constantly undergoing changes. These are either in the nature of a uniform excavation throughout their whole contour, resulting in a simple enlargement of the canal, or of an excavation in certain parts combined with a deposit of additional cartilage in others resulting in a change of form and position of the canal.
On account of the well defined landmarks that characterize the labyrinth, it is possible to orient points at which excavation and new deposit respectively are occurring. Thus one can follow the histological phenomena of these two processes with great accuracy. Where new cartilage is being deposited, the tissue shows all the stages of development from an embryonic connective tissue on its central margin through an area of precartilage to a true cartilage on its more peripheral margin. These different grades merging into one another repeat stages which characterized the whole capsule in embryos between 14 and 30 mm. Where the cartilage is undergoing excavation the same transitions exist, but the changes are more abrupt and there is a sharper line of transition between the different zones. The width, however, and the sharpness of the zones vary somewhat, being relatively wider and less abrupt in younger stages and becoming narrower and more abrupt in their transition in older fetuses. It is quite possible that these changes occur in waves and when the zones are wider and less abrupt it is due to the greater activity of this process of dedifferentiation and when the zones are narrower and more sharply outlined, as is common in older fetuses, the alteration is then proceeding more slowly.
The dedifferentiation of cartilage into precartilage involves first of all changes in its matrix including the loss of its tinctorial reaction, a decrease in its amount and an alteration in its structural appearance, in that it becomes less homogeneous and begins to show the presence of branching processes. As a result of these changes in the matrix, the encapsulated cartilage cells come to lie closer together, pressing to some extent directly against each other. The combined edges of the overlapping margins of flattened capsules give the appearance of wavy refractile lines running through the transition zone parallel to the margin of the canal. With these changes the capsules of the cartilage cells rapidly become incomplete and take on the appearance of branching processes. With the disappearance of the capsules the tissue assumes the appearance of a mesenchymal syncytium which then takes on a reticular character and becomes part of the general periotic reticulum. The question as to whether there is an active proliferation of the nuclei in the tissues subsequent to their alteration from cartilage to precartilage has not been definitely determined. The material at hand is inadequate for a satisfactory solution of this point, although in some specimens there seems to be an increase in the number of nuclei in the transition zones of precartilage, over and above the apparent increase associated with the absorption of the intervening matrix, which could only be explained in that way. It would seem very probable that with its dedifferentiation there should be associated a renewed proliferative vitality of a given embyronic tissue, sufficient at least for its reconstruction into the newer form.
Development of the Perichondrium
In studying the cartilaginous canals one must take into consideration the perichondrium and -its relation to the continual transformations occurring along their margins. Reasoning from the prevailing conceptions, concerning the activity of periosteum in bone growth, one might expect to find in the perichondrium an important factor in the growth and changes in the cartilage. In later periods its influence on cartilaginous changes cannot be easily determined, but fortunately for the solution of this point it happens that the perichondrium is late in making its appearance and therefore cannot take any part either in the deposit of new cartilage or in the excavation of the old until after a considerable part of this transformation is already completed.
The zone of precartilage surrounding the margins of the canals in embryos about 50 mm. long might be mistaken for perichondrium, such for instance as is shown in figure 9. If this area, however, is followed to a slightly older stage it will be found to be converted almost entirely into reticulum. The section shown in figure 10 is through the posterior semicircular canal of an embryo of the same length, 50 mm., but a little older in development. It is just at this age that precartilage very rapidly reverts to reticulum, much more rapidly than the surrounding cartilage in reverting to precartilage; and therefore in sections at this period we find only a thin rim of precartilage around the margins of the canals. The real perichondrium makes its first appearance when the fetus has reached a length of about 70 mm. A photograph of a section of the posterior semicircular canal of a fetus 73 mm. long (Carnegie Collection, No. 1373) is shown in figure 11. Examination of this section reveals along the outer margin of the periotic reticulum a condensation of its trabeculae resulting in the formation of a thin fibrous lamina or membrane near the margin of the cartilage. This is the perichondrium in its early form. It does not abut directly against the cartilage but is separated from it by a zone of transition tissue which consists partly of precartilage and partly of reticulum. This transitional precartilage-reticular zone, becomes narrower and more abrupt in later stages. In all of the specimens studied, however, it was found intervening between the perichondrium and the surrounding cartilage. It will thus be seen that the perichondrium is a derivative of the periotic reticulum. It. forms an outer limiting membrane along the cartilaginous margin of the latter in a manner somewhat similar to that in which the membrana propria forms an inner one along its epithelial margin. The relation of the perichondrium to the reticular tissue surrounding the labyrinth, as seen under higher magnification, is shown in figure 12. The section is a portion of the one shown in figure 11 and includes the successive strata from the epithelial wall of the labyrinth to the true cartilage. It will be seen that the rnembrana propria consists of a narrow meshed syncytium, such as is found in embryonic fibrous connective tissue, and constitutes a supporting coat for the epithelial wall of the semicircular duct. The main part of the periotic connective tissue consists of a wide-meshed reticulum and arborizing through it are the loops of small blood Vessels. The perichondrium forms in the outer part of this reticulum as a compact fibrous membrane. Peripheral to the perichondrium the tissue is still of a reticular type but passes in rapid transition into precartilage and then into a true cartilaginous tissue.
Fig. 10 Section through the posterior semicircular canals in a human fetus 52 mm. long (Carnegie Collection, No. 96). Enlarged 100 diameters. Here the replacement of precartilage by reticulum has been more active than that of cartilage by precartilage so there remains only a narrow zone of the latter. The reticulum begins to show an alteration in its trabeculae. Due to the retraction and rearrangement of the protoplasm of some of the trabeculae there results a coalescence of adjacent intertrabecular spaces. There are thus formed larger fluid spaces that are devoid of traversing trabeculae. As yet there is no perichondrium.
Fig. 11 Photograph of section through the posterior semicircular canal in a human fetus 73 mm. long (Carnegie Collection, No. 1373). Enlarged 100 diameters. It shows the porichondrium in its earliest form.
After making its first appearance, the perichondrium rapidly becomes more conspicuous. In fetuses 80 mm. CR length (Car negie Collection, No. 172) it consists of a dense fibrous coat more than twice as thick as that shown in figure 12. It is clearly separated from the cartilage by a narrow zone of transitional precartilage—reticular tissue. In slightly older fetuses, 85 mm. CR length, (Carnegie Collection, No. 1400-30) it has become a dense broad zone separated from the surrounding cartilage only by a narrow cleft of transitional tissue which still, however, can be recognized as reticular in character. In fetuses 130 mm. CR long (Carnegie Collection, No. 1018) the perichondrium presents a relatively mature appearance. As observed under lower magnifications, one is apt to conclude that the perichondrum is in direct Contact with the true cartilage. Under higher powers, however, a narrow zone of transitional precartilage can be seen intervening between them. In this dedifferentiating zone the matrix has largely disappeared and the cartilaginous capsules have collapsed and are flattened out. Thus the elongated endoplasm of adjacent cartilage cells is brought into contact, being separated only by the remnants of the capsular margins. The appearance of activity in this zone corresponds to the unstable condition of the margin of the cartilage which is still undergoing gradual excavation.
Fig. 12 Detailed drawing of a portion of the same section shown in figure 11. Enlarged 500 diameters. It can be seen here that the perichondrium is a condensation of the meshes in the peripheral part of the periotic reticulum and that it separated from the true cartilage by a transitional area of precartilage and reticulum.
Summary
From a study of the development of the cartilaginous capsule of the ear in human embryos it is found that the changes in size and form which it undergoes during its development are accomplished in part by a progressive and in part by a retrogressive differentiation of its constituent tissues. Throughout the entire period of growth, as far as material was available for study, it was found that the margins of the cartilaginous cavities undergo a process of continual transformation. They exhibit a state of unstable equilibrium, in respect to the opposing tendencies toward a deposit of new cartilage on the one hand and toward the excavation of the old on the other. The margins thereby are always either advancing or receding and in this way are produced the progressive alterations in their size, shape and position. In this manner a suitable suite of chambers is always provided for the enlarging membranous labyrinth.
The general tissue mass of the otic capsule during the period represented by embryos from 4 mm. to 30 mm. long passes through three consecutive histogenetic periods, namely, the stage of mesenchymal syncytium, the stage of precartilage and the stage of true cartilage. In the subsequent growth of the capsule it is found that in areas where new cartilage is being deposited the tissues of the areas concerned follow the same progressive order of development. In areas, however, where excavation occurs, where cartilage previously laid down is being removed, it is found that the process is reversed. The tissue in such areas returns to an earlier embryonic state, that is it undergoes dedifferentiation. Tissue that has acquired all the histological characteristics of true cartilage can thus be traced in its reversion to precartilage and from precartilage in turn to a mesenchymal syncytium. In the latter form it redifferentiates into some more specialized tissue«in this case for the most part into a vascular reticulum.
The perichondrium is a derivative of the periodic reticulum and forms an outer limiting membrane along its cartilaginous margin. During the foetal period the perichondrium does not rest directly against the true cartilage but is separated from it by a zone of transitional tissue consisting partly of precartilage and partly of reticulum. This transitional zone intervening between the perichondrium and the surrounding cartilage was observed in all of the specimens that were studied, which includes fetuses up to 130 mm. CR length. Owing to the fact that the perichondrium is late in making its appearance, being first seen in fetuses about 70 mm. long in can take no part in the early changes in the cartilaginous capsule either as regards deposit of new cartilage or the excavation of cartilage that had been previously laid down.
Literature
- ↑ Schoenemann, A. Die Topographic des menschlichen Gehiirorganes. Verlag Von Bergmann, Wiesbaden, 1904. Plate 2, figure 20.
- ↑ Child, O. M. Senescence and rejuvenescence. University of Chicago Press, 1915. Page 293.
- ↑ 3 Streeter, G. L. The development of the scala tympani, scala vestibuli and perioticular cistern in the human embryo. Am. Jour. Anat., vol. 21, 1917.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The Factors Involved in the Excavation of the Cavities in the Cartilaginous Capsule of the Ear in the Human Embryo. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Factors_Involved_in_the_Excavation_of_the_Cavities_in_the_Cartilaginous_Capsule_of_the_Ear_in_the_Human_Embryo
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G