Paper - The Anatomy of Human Embryos with Seventeen to Twenty-three Pairs of Somites
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Wen IC. The anatomy of human embryos with seventeen to twenty-three pairs of somites (1928) J. Comp. Neural., 45: 301-376.
| Online Editor |
|---|
This 1928 paper includes several embryos from University of Chicago collection that were eventually contributed to the Carnegie Collection.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Anatomy of Human Embryos with Seventeen to Twenty-three Pairs of Somites
I Chuan Wen
Hull Laboratory of Anatomy, University of Chicago
Eight Heliotype Plates and Twelve Text Figures
Introduction
Many problems in early human development remain still unsolved. Studies of many more embryos from the period of somite formation will be necessary before accurate accounts of this stage of development can be written. It is important, accordingly, that every young specimen be described as fully as possible and its characteristic features be compared with those of the few similar embryos that are available for study, as well as those described in the literature. In the present paper an embryo with 17 somites (H951, Emb. Coll., Department. of Anatomy, University of Chicago) will be studied in detail and compared with embryos of 22 (H1903) and 23 (H984) somites of the same collection. The 17—somite specimen may also be compared with a 15—somite embryo of Giglio-Tos (’02), a 16-somite embryo of the Carnegie Embryological Collection (no. 470), twin embryos with 17—19 somites of Watt (’15), and the 20-somite embryo of Davis (’23). It differs, however, from all of the above mentioned in having certain features that throw light upon questions left unsettled in the others.
This work was carried on under the direction of Prof. G. W. Bartelmez. It is a pleasure to me to acknowledge my indebtedness to him for his constant aid, helpful criticism, and, above all, inspiring guidance. To Prof. R. R. Bensley and Prof. C. Judson Herrick my thanks are due for the training I received from them and for their courtesies shown to me during the course of this work. It is also a pleasure to me to express my appreciation of the courtesy of Prof. George L. Streeter for the privilege of studying the material belonging to the Carnegie Institution, Laboratory of Embryology. I have also received technical help from Miss Nixon, of Chicago, Mr. O. O. Heard and Mr. J. F. Didusch, of Baltimore, to all of them I wish hereby to express my thanks.
Material and Methods
The embryos herein studied are listed in table 1.
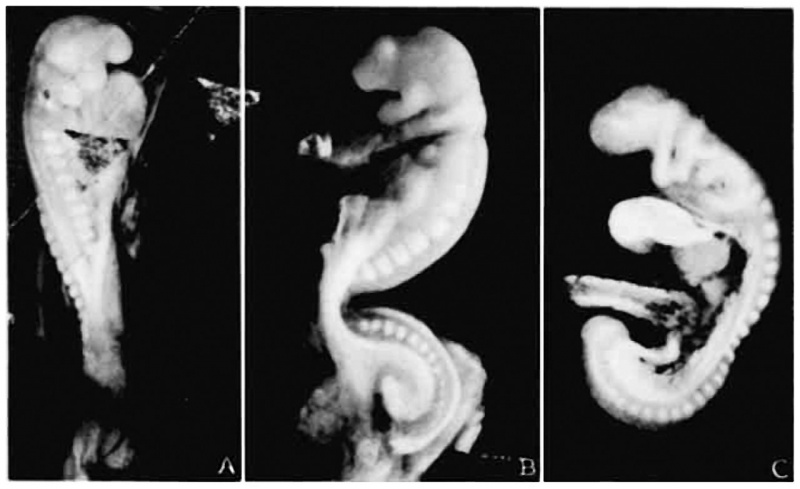
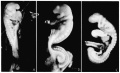
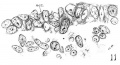
Fig. 1. Photographs of the embryos described, made under 80 per cent alcohol. A. The 17-somite embryo H951. X 16.6. B. The 22—somite embryo H1093. X 10. A. The 23-somite embryo H984. X 16.6.
Embryo H951 (fig. 1, A) was obtained from Drs. E. L. Whitney and J. T. Rocks, of Walla Walla, Washington. “The uterus was removed for fibrosis on the 13th day after the day on which menstruation was expected to begin,” opened and fixed in ‘4%’ formalin. No further clinical information could be obtained. The embryo was mordanted in aqueous chrom—sublimate, slowly dehydrated, double embedded in celloidin and paraffin, cut into transverse sections, and stained with iron hematoxylin (all but one slide being counterstained with eosin). During the mounting, five sections were slightly distorted and, unfortunately, three sections lost, so it is not easy to follow the series at the region between the 4th to 5th somites. A plaster model of the head region (figs. 2, 3), a wax model of the entire embryo, and one of the anterior portion of the central nervous system with the ventral pharyngeal wall were made from serial photomicrographs at the magnification of 150 times. Two other plaster models, one of the entire embryo and one of the head region alone showing the anatomy therewith, were made by O. O. Heard. Histologically, this specimen is in excellent condition.
Table 1
| Designation (University of Chicago Collection) |
Number of Somites | Greatest Length | Number and thickness of sections | Dimensions of Ovum (including villi) | Source of Specimen |
|---|---|---|---|---|---|
| H951 | 17 | 4.0 mm. in 80 per cent alcohol | 336 sections at 10 µ | 23.1 x 10.4 x circ.11 mm. | Hysterectomy |
| H1093 | 22 | 3.8 mm. (dors. flex.) | 323 sections at 10 µ | 14.5 x 11 x 7 mm. | Tubal pregnancy |
| H984 | 23 | 2.8~2.9 mm. in 95 per cent alcohol | 349 sections at 8 µ | 26.7 x 22.7 x 17.3 mm. | Abortion |
Embryo H1093 (fig. 1, B) was obtained by Dr. Garrette Van Sweringen and Dr. W. W. Duemling, of Fort Wayne, Indiana. The clinical record gives the following points: “The October period began on the 6th, 1924; next period from 10th to 14th or 15th. The expected period on December 10th did not appear. Flow supposed to be menstruation appeared on December 22nd, but bloody discharge continued until 31st.
Contraceptive measures were employed until after November period. Symptoms of ectopic pregnancy were observed on January 4, 1925, and operation was performed on the same day.” In cutting open the hematoma, the head of the embryo was severed from the trunk just caudal to the otocyst. Although no loss of tissue is apparently involved, yet the difference in the plane of sections of the two portions of the embryo makes it materially difficult to follow the series in the region of rhombomere VI. The specimen was fixed in neutral formalin, double embedded, cut transversely, and stained with iron hematoxylin. Histological differentiation is fair, but there is much shrinkage. Cells with coarse granules evidently representing necrobiosis are found abundant in the neural crest as well as the medullary tube. A wax model of the nervous system in front of the otocyst was made from serial photomicrographs at the magnification of 150 times.
Embryo H984 (fig. 1, C) was donated to the collection by Drs. E. W. Rawson and B. H. Foreman of Tacoma, Washington. It came from an abortion and after remaining in
weak formalin for several years it was mordanted in aqueous chrom-sublimate, embedded in paraffin, cut into transverse sections, and stained with iron hematoxylin. The material was macerated and considerable shrinkage and cracking occurred during dehydration. Histological differentiation is poor. Serial photomicrographs at the magnification of 125 times were made, but no model has been attempted.
External Form
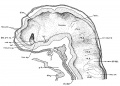
The external form of the 17-somite embryo may be ‘seen in figure 1, A. The features which are of chief value for seriation at this stage of development are the cranial flexure, optic vesicles, Visceral arches, otic pits, and the pronephric ridges. All of these except the last named are recognizable in figure 2, which presents the appearance of the left-side head region. In opening the uterus before fixation, the embryo was somewhat compressed and twisted, but this side of the head shows little evidence of distortion. The pronephric ridges are, however, not recognizable in figure 1, A, for they are obscured by the twisting of the embryo’s trunk in the region involved.
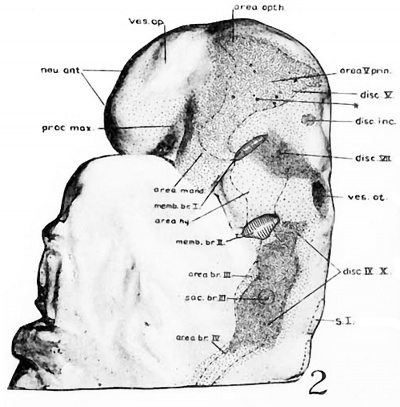
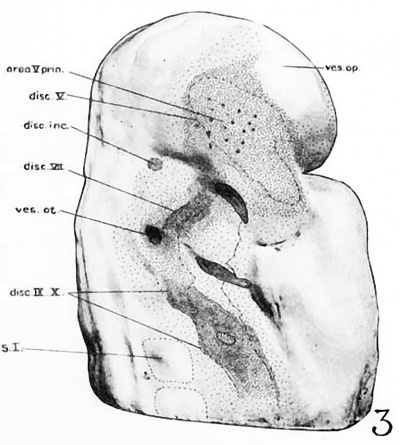
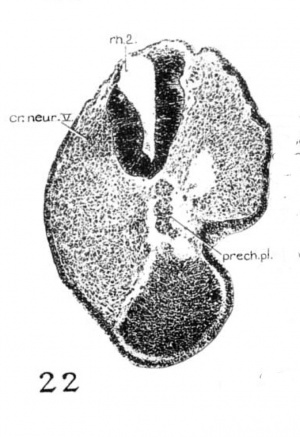
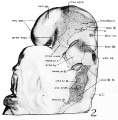
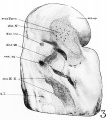
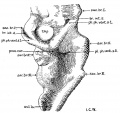
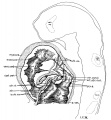
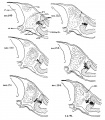
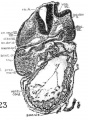
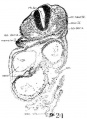
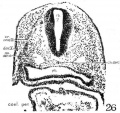
Fig 2 and 3 Photographs of the right and left sides of a model of the head of the 17—somite embryo, reduced in reproduction to 66% diameters. The ectodermal areas were projected from the model on which they had been plotted from measurements on the photomicrographs of the sections. The thickness of the ectoderm is indicated by the stippling, which is closer in the thicker parts. The coarse dots in area 17 pram. mark the location of cells emigrating from the placode; the spot marked * is illustrated in figure 11. The broken lines mark the areas over which deeply staining laminae of cells, probably of neural-crest origin, are closely applied to the overlying ectoderm. The visceral membranes are marked by parallel lines; sac.br.III indicates the position of the third pharyngeal pouch, which has not yet reached the ectoderm.
The cranial flexure appears to be an arc of a circle with a
very gentle curvature involving obviously much more than
the midbrain (fig. 1, A). According to the model (figs. 2,
3), which shows the shrinkage due to the technical procedure,
the midbrain stands out clearly at the extremity of the embryo
and the flexure upon the surface is represented by a slightly
elevated eminence. This relation of the midbrain forming
the knee of the cranial flexure was found by Bartelmez (’23)
in a series of younger human embryos. Cephalad to the
cranial flexure, the dorsal contour is gently curved until a
median flattened area is reached. This is the site of the
closing anterior neuropore. In the model the optic vesicle
forms a distinct eminence on the side of the head. Caudal
to the optic vesicle, we come to the branchial region. Two
distinctly marked branchial arches — mandibular and hyoid - are recognizable. At its proximal border the mandibular
arch shows an indication of the maxillary process, which in
this stage of development is but a narrow and small swelling
extending ventral to the optic vesicle from which it is marked
off by a shallow groove. The arches are elevated considerably above the gill membrane, so that we may speak of a
hyomandibular furrow. The mandibular arch is a bluntly
wedge—shaped eminence with its ventrally directed apex resting upon the heart region. The hyoid arch is similar in
shape, but much smaller and shorter. Its caudal border is
separated from the third arch by a shallow and broad fossa — the second gill furrow. Like the first arch, the extremity of
the second also reaches the cardiac eminence. As to the rest
of the arches, very little can be said of them, since no boundaries are visible on the surface. In the external view the third
arch is not delimited caudally. Although a clear-cut picture
of this arch is not present on the external form, yet the third
entodermal pouch can be recognized in the sections. Therefore, it would seem justified to warrant the assumption that
the third arch is in the process of formation. A little caudal
to this level but dorsally, the first somite makes its appearance
on each side, occupying a position relatively more posterior
than that of Wallin’s (’13) embryo (13 somites) and 0.0.
470 (16 somites; compare Bartelmez and Evans, ’26, figs.
16-17, pl. 7). The fourth arch, though appearing merely to
be a tiny rounded eminence on the surface, is without any
clear outline whatever. The entire branchial region is
bounded ventrally by a limiting sulcus of the cardiac eminence
marking the pericardial area from the rest of the body lying
dorsal to it. Starting with the site of the oral sinus, this
sulcus appears first as a deep groove curving on each side
of the embryo between the extremities of the mandibular and
hyoid arches and the heart swelling, then it gradually fades
out at the cephalic border of the third arch.
From the above description of the development of the branchial region, the 17—somite embryo H951 seems to fit well the gap between the 16—somite embryo 0.0.470 and the 20—somite one described by Davis (’23). The head region of
the former is sufficiently like that of H951; whereas in the
latter, Davis recognized three definite gill arches, the presence
of which undoubtedly marks a step further in development.
Furthermore, H951 is also perfectly in harmony with other
embryos of about the same age of development, so far as
the visceral arches are concerned. In J anosik’s (’87) embryo of 3 mm. in length and Watt ’s (’15) twin embryos of 17-19 somites, only two gill furrows are visible externally. In the
slightly older embryos, such as Van den Broek’s (’11) with
22—23, Thompson’s (’07) with 23, and Johnson’s (’17) with
24 somites, the presence of an additional gill groove, the third
gill furrow, has been recorded. In this respect, the 17-somite
embryo falls well in the first group rather than bridging over the gap between the two.
Turning to the dorsal aspect of the second visceral furrow, the otocyst in this stage of development is still wide open to the outside, with a definite ridge seen around the edge of the structure. A comparison of the two otocysts reveals that they are different from each other in size as well as in shape (figs. 2, 3). This different appearance was undoubtedly produced by the compression on the right side of the embryo at the time of fixation, as already pointed out.
Regarding the oral fossa, heart, and mesodermal somites a few words should be said here. Rostral to the pericardial swelling, the oral fossa appears as a deep depression bounded cephalad by the forebrain and laterally by the maxillary and mandibular‘ processes. The buccopharyngeal membrane cannot be seen from the external View on account of the apposition of the head extremity to the pericardial region. The heart forms a conspicuous prominence occupying the ventral body wall between the oral fossa and the yolk sac. Viewed from the front of the embryo, it is projected much farther on the right than on the left side. It is on the right side that the position of different chambers of the heart is indicated externally by shallow grooves. As to the somites, they can be clearly recognized in figures 1, 2, and 3.
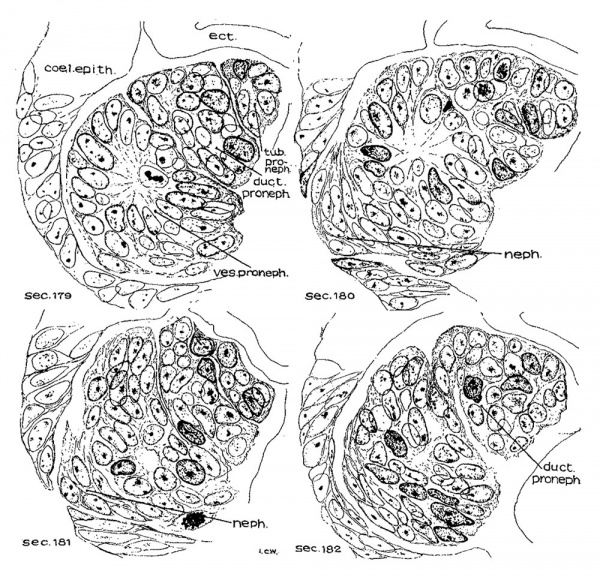
Turning from the head region of the embryo to its caudal portion about the level of the 9th to 15th somites, the pronephric ridges are visible on the external surface at this stage of development. This has not been noted hitherto. On the left side of the embryo the pronephros is recognizable on the surface just lateral to the mesodermic somites and extends from the cranial border of the 10th somite to that of the 15th, while on the other side, it appears from the middle of the 9th to the upper border of the 12th somite. Owing to the twisting of the embryo’s body in this region with a part of the somatopleure bending dorsally over the surface ectoderm (fig. 28), the right pronephric ridge is difficult to see on the model, but the elevation on the surface can be readily detected in sections. On the contrary, the left pronephric prominence presents a much clearer picture on the surface view. It appears as a slender longitudinal ridge limited by shallow sulci, the medial one marking the border from the somites and the lateral one from the rest of the surface ectoderm. This ridge gradually fades out caudal to the 15th somite, where the underlying nephric tissue becomes greatly diminished in size (p. 349, below). In the slightly older embryos H1093 (22 somites) and H984 (23 somites), no trace of the pronephric swelling on the external surface can be followed, and hence the whole structure no longer forms a surface ridge.
Nervous System
Neural Tube as a Whole
|
|
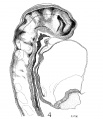
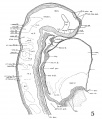
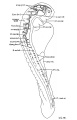
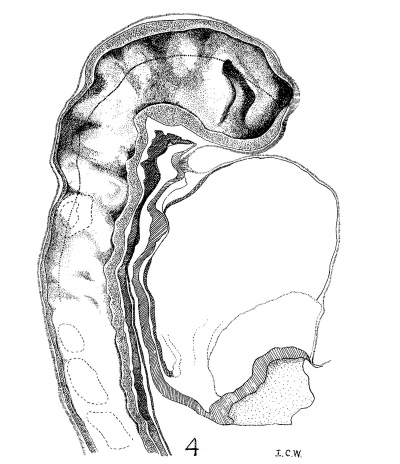
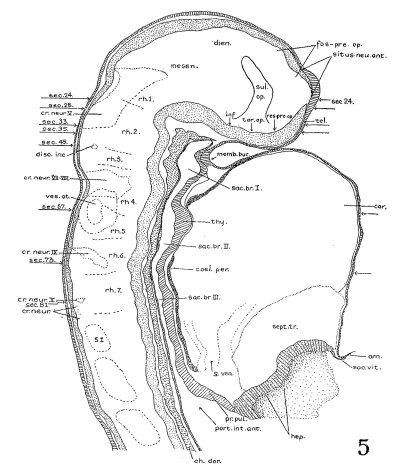
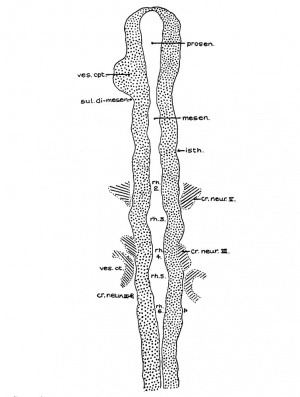
| Fig. 4. Head of the 17-somite embryo H951 from the medial view of a model of the right side prepared at 150 diameters and reduced in the illustration to 100 diameters. The dotted line indicates the plane of the projection reconstruction shown in figure 9. | Fig. 5. Key drawing to figure 4 prepared from a graphic reconstruction projected on the mid-sagittal plane. |
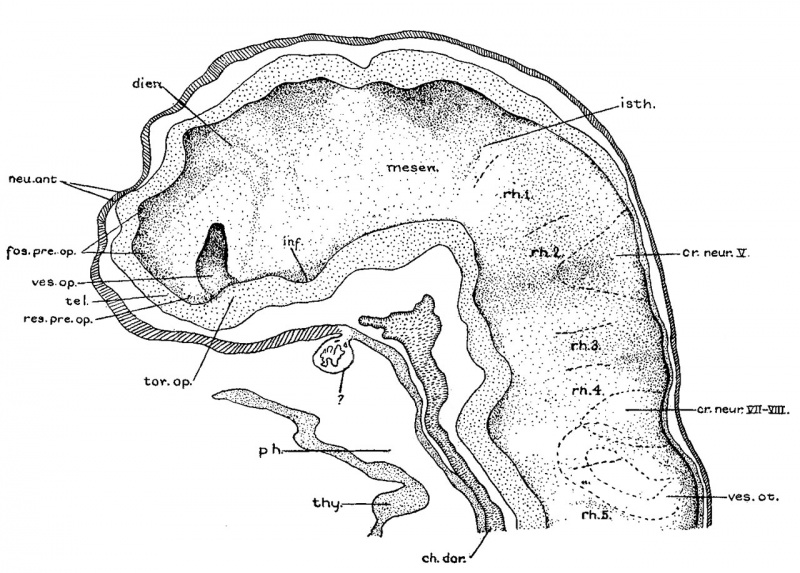
Before attempting any description of the different parts of the nervous system of embryo H951 (17 somites), it may be said that its neural tube as a whole (figs. 4, 5) presents an appearance similar to what Bartelmez (’23, ’26) found in a series of younger human embryos. When it is particularly compared with the 16-somite embryo C.C.470, it is distinctly more advanced in development. The closure of the anterior neuropore, the great thickening of the brain wall at the extreme cephalic end indicating a differentiation of the telencephalon, the wide expansion of the dorsal lamina. of the forebrain caudal to the neuropore so as to produce a ventral shifting of the optic vesicles, the lateral expansion of the first midbrain segment, and the appearance of the fourth ventricle are all strongly marked. These features, so far as we are aware, have not been fully considered in any of the specimens younger than this embryo with 17 somites. They are certainly demonstrable in one of our older embryos with 22 somites, H1093.
Subdivisions of the Nervous System
I. Prosencephalon
According to the model (figs. 4 and 7, B), the forebrain appears externally to be large and bulbous, with the optic vesicles bulging out from its wall. The cephalic end of the forebrain is indicated on the surface by a flattened area which is correlated with the observation made on the external form. From side to side, it reaches the broadest dimension in the region of the optic vesicles, becoming much narrower behind when the dimesencephalic boundary is approached. Dorsally, the forebrain has attained a great expansion be tween the optic vesicles and caudal to the site of the anterior neuropore. On the ventricular surface (fig. 4) it is a hollow cavity which communicates with the optic vesicles by the optic sulci. Rostral to the vesicles, the brain wall becomes expanded dorsally and thickened; the future thalamic wall will presumably be differentiated from this region. Due to this expansion, two recognizable dilatations of the brain wall are produced. The large anterior one (preoptic fossa) is more clearly marked" o11 the left than on the right side of the embryo, whereas the small posterior one is correlated with a slight outward bulging on the external surface, representing the cavity of the diencephalon dorsal to the optic vesicles in a later stage (fig. 7, C). At the lower border of the preoptic fossa, the brain wall gradually thins out until the neural epithelium becomes entirely lost to sight, this being the region where the closing of the anterior neuropore is not quite completed. Still lower to this site, the ‘preoptic recess’ of Johnston (’89) is indicated, where the great thick» ening of the brain wall is again marked so as to show the differentiation of the telencephalon. This thickening continues caudally to the whole floor of the forebrain and midbrain. Caudal to the recess just mentioned, a slight depression marked on the floor is seen to be connected with the optic ventricles. This marks the primitive optic chiasma, or torus opticus. Another depression, well marked and situated just behind the torus, probably represents the primitive infundibulum, which shows, however, no differentiation at this time nor can a corresponding differentiation of the ectoderm be made out.
Optic Vesicles
Externally, the optic vesicle first appears to spring from the greater part of the forebrain (fig. 7, A) and extends laterally, dorsally, and slightly caudad. Soon each vesicle is marked off from the forebrain wall by a deeper, caudally located groove and a shallow rostral sulcus (fig. 7, B). With the separation of the vesicle from the brain wall, these grooves become ventral and dorsal in position with reference to the forebrain, but not the embryo as a whole, as may be seen in figure 7, 0. They may accordingly be termed ventral and dorsal limiting sulci, respectively. In the 17 somite embryo the more caudal ventral sulcus, beginning almost at the torus, extends dorsally behind the optic vesicle and has begun to arch over it to become continuous with the dorsal sulcus which fades out at about the middle of the vesicle. From the ventricular surface we see (fig. 4) the optic ventricle opening into the third ventricle by a large oval orifice, bounded caudally (ventrally) by a strongly indented ridge which corresponds to the ventral limiting sulcus.
Fig. 6 A midsagittal projection reconstruction of the 22-somite embryo, H1093, showing most of the head. ‘?’ indicates the probable remains of the oral membrane.
It is longer than the less pronounced rostral ridge, which in turn corresponds to the dorsal limiting sulcus. The ventricular ridge is, however, longer than the sulcus of the external view, for it can be traced to the torus opticus, although it does begin to fade off at the level of the neuropore.
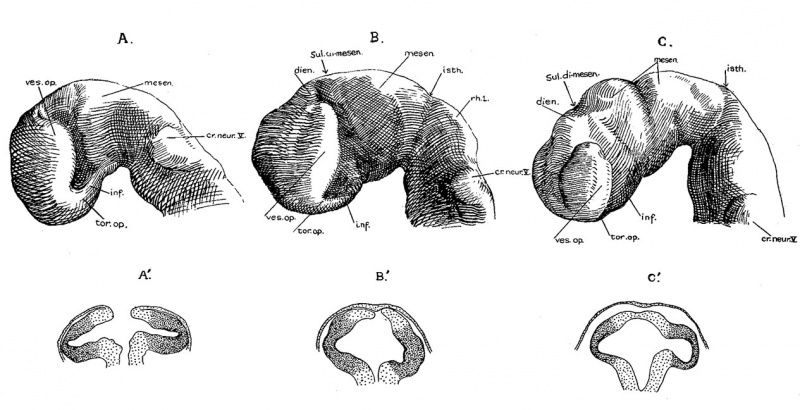
Fig. 7 Lateral views of the brain A, 16—somite C.C.470; B, 17~somite H951; 0, 22—somite H1093—all drawn from models and reduced to a magnification of 66% diameters. Note the absolute decrease in the size of the optic vesicle and the relations of the midbrain to the cranial fiexure. A’, B’. C’, are sections (nos. 28, 25, and 34, respectively) taken from the corresponding embryos and magnified 66% times. The region which in external view appears as optic vesicle is deeply stippled.
In studying the development of the optic vesicle in such a stage as represented by the 17-somite embryo, two important questions must be kept in mind. The first one is concerned with the factors that may be conceived of as possibly producing the changes during this and later stages. The second one deals with the part played by the brain wall rostral as well as dorsal to the optic vesicle in the formation of the future thalamic wall. Before answering these questions, it should be noted that the growth activity of the nervous system, before the anterior neuropore is closed, is chiefly concerned, from the midbrain forward, with a more rapid expansion laterally than. medially. As soon as the anterior neuropore is closed, a center of rapid growth is initiated in the midline, while the lateral expansion may still be progressing, but in a less marked way. It is in the former case that the optic vesicles expand laterally as if they were dragged out from a portion of the brain wall, and only a ventral optic limiting sulcus is produced at this time (fig. 7, A). As development proceeds, the anterior neuropore gradually closes and the brain wall dorsal as well as rostral to the optic vesicles grows so rapidly as to expand mainly in a dorsal direction, and so that the optic Vesicles are more distinctly separated off caudally, where their separation from the diencephalic wall first takes place. Thus the ventral optic limiting sulcus begins to creep around the caudal aspect of the optic vesicle producing the dorsal optic limiting sulcus; the vesicles themselves are thus displaced laterally and ventrally, diminished in size, and hence spherical in shape. A comparative study of the optic vesicles of other embryos will make the situation clearer. In the 16somite embryo (fig. 7, A) the anterior neuropore is still open and the expansion of the forebrain roof has not apparently begun as yet, so that the optic vesicles are lateral expansions, possessing only the ventral optic limiting sulcus, In the 17somite specimen, a further step of development is marked by a slight dorsal optic limiting sulcus, which is produced, as already pointed out, by a rapid forward as well as median growth of the dorsal lamina of the forebrain and at the same time the caudal growth of the vesicle itself. Should the growth activity be more rapid toward the dorsal brain wall, as in the case of the 22—somite embryo, it would be expected that a deeper dorsal optic limiting sulcus will be produced marking off the optic vesicle from the lateral brain wall more distinctly. Furthermore, this comparison shows that the optic vesicle actually, as well as relatively, decreases in size from the youngest embryo to the oldest, as Schulte and Tilney (’15) found in the cat (compare Bartelmez, ’23, p. 223).
When a section through the optic vesicles of the 17-somite embryo is superimposed upon a corresponding section of the 16-somite embryo, it is obvious that much of the cephalic limb of the latter has been incorporated into the cephalic wall of the former, which is actively expanding. In the 16—somite embryo, the two limbs of the V—shaped optic vesicle are uniform in thickness, except at the vertex (fig. 7, A’). In the 17-somite embryo, the vertex, i.e., lateralmost tip, of the optic vesicle, has three ‘rows of nuclei, while the cephalic limb adjoining the diencephalic wall is twice as thick (fig. 7, B’, on left). This is still more marked in the 22—somite embryo, where the arc of the C—shaped Vesicle is much thinner than the two limbs that are continuous with the thickened brain wall. From this it appears that the optic vesicle, during this stage of development (16 to 22 somites), has expanded by a. rearrangement of the neural epithelium at the outermost bulging portion, i.e., the vertex. It also indicates that a large portion of the original optic evagination, as represented by the two limbs of the V-, U~, and C-shaped vesicles in different stages, goes to the future thalamic wall, leaving a smaller portion of that evagination, i.e., at the vertex, for the development of the optic vesicle in later stages.
Anterior Neurpore
The description of the anterior neuropore of the 17-somite embryo is based chiefly upon the histological studies on sections, because the process of its closure can be better appreciated there than on a model or reconstruction.
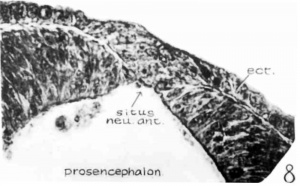
The section cut through about the middle of the site of the closing anterior neuropore is shown in figure 8. It is to be noted there that the surface epithelium is greatly thickened in the middle as a wedge-shaped plug of cell mass filling up the gap between the lips of the neural folds. The arrangement of cells there gives the impression that they are piled up one layer upon another, with exceedingly flattened cells on the outer surface, spindle—shaped in the intermediate layer, and two to three round cells in the innermost stratum. Under a higher magnification, the flattened cells of the outermost layer appear to be but cytoplasmic processes thrown across the midline to form a bridge between two sides; whereas the cells lateral to this region begin to assume definite forms arranging from elongated epithelial to low cuboidal cells. Next to this layer the cells, which are really not clearly demarcated from the layer just described and the one to be dealt with presently, but arbitrarily divided according to their shape for descriptive purposes, are arranged in an obliquely directed radiation toward the midline. This radial arrangement gives place to a few round cells with oval nuclei, bounding the ventricle. This type of cellular arrangement is responsible for the conical appearance of the cell mass on cross-sections. Now, turning to the neural epithelium, the neural folds have not yet fused and the arrangement of cells is clearly marked as contrasted with that of the head epithelium. But at the tip of the right neural lip (fig. 8), where the head ectoderm is continuous with the neural epithelium, the cells of the former bend inward upon the latter to form the brain wall. Tracing the sections forward (cephalic to fig. 8, i.e., ventral in fig. 5), it is seen that the lips of the neural fold gradually approach each other and then fuse. The plug of the head ectodermal cells in the meantime changes from its radial conical arrangement into an irregular cell mass between the flattened head epithelial cells and the epithelium of the neural tube, until it is completely separated from the nervous tissue. Now, let us proceed with the examination of sections immediately caudal to figure 8 (i.e., dorsal in fig. 5); the ectoderm and neural folds cannot be separated, the former still indicating an inward bending upon the latter to form the brain wall. Still caudal to these sections, the neural tube becomes closed by the joining of the two lips of the neural fold, and the head ectoderm is distinctly separated from the neural epithelium.
From the microscopic evidence, it is clear that the neural folds of the 17~somite embryo have not yet fused, but the ectoderm has thickened and grown over the small neuropore, so that a conical mass of ectodermal cells fills the gap between the neural folds. And the closing of this region in this case seems to involve two processes: one is by the proliferation of the overlying ectodermal cells to form the cell plug between the two lips of‘ neural fold, and the second is by the fusion of the two lips from below upward (i.e., cephalocaudally). In the first process, the ectodermal cells at first connect with the brain wall in a limited area; then, a little caudally, they form a thick plug which is intimately fused with the nervous tissue and fills the gap between the neural folds; finally, they leave the neural epithelium as a separate layer. This whole process takes place so as simply to give rise to an ectodermal membrane covering the site of the anterior neuropore, which closes later. In the second process, the lips of the neural fold become gradually fused Ventrodorsally, and this fusion seems to be made possible by the surface ectodermal cells liberated and bent upon the tips of the neural fold.
In the 22-somite embryo, as in Davis’ 20-somite specimen, the closure of the anterior neuropore is completed, but its site on the external surface can still be recognized. The head ectoderm at that region is attached to the underlying brain wall in a small area probably representing the last point where the tissues separate (fig. 6).
Recently, Sternberg (’27) gave a detailed description of the anterior neuropore of human embryos. In his 18-somite specimen, the anterior neuropore, which measured 98p in length, is still widely open. The histological picture of the site of neuropore showing the relationship between the head ectoderm and the brain wall is not unlike that of our 17—somite embryo, except that in the latter case the head ectoderm forms a complete membrane covering the small neuropore, which is in the process of closing. In his embryo ‘D’ with 25 somites (2.7 mm.), the anterior neuropore is closed altogether. In his 28-somite embryo ‘F’ (4 mm.), it is also closed, but its site on the surface can still be recognized as a furrow (‘sulcus neuropori anterioris’), wh.ich, with a corresponding ectodermal thickening, measures 250 u in length. In Watt’s (’15) twin embryos with 17-19 somites the anterior neuropore is still open. Judging from these findings as well as others in other human embryos, it seems that the time of closure of the anterior neuropore is a matter of individual variation. While Davis (’23) claimed that his 20-somite embryo indicates the ‘normal’ time of the closing of the anterior neuropore, we have here 17-, 18-, 19—somite specimens with a widely open neuropore and a 17—somite embryo (951) in the process of closing, and there is no reason for regarding any of them as abnormal. It seems, therefore, to indicate that the growth and differentiation of the nervous system at this time are not closely correlated with the number of somites; the latter criterion is only a convenient one for seriation of embryos at early stages.
II. Mesencephalon
Although a series of models of embryos between 16 and 28 somites (C.C/1529, 15—16—s.; 0.0.470, 16-s.; C.C.5072,.17—s.; C.C.2053,20—s.; C.C.836,28—s.) has been studied and the observations of Gage (’05), Johnson (’17), Davis (’23), Nilsson ( ’26), Girgis ( ’26), and Sternberg (’27 ) have been considered, we have not been able to identify the boundaries of the midbrain and the first rhombomere with any degree of certainty. This is chiefly due to several difficulties. first, there are no sure criteria for the identification of the subdivisions of the neural tube rostral to the second rhombomere, especially since the value of the dorsal cranial flexure as marking the midbrain in these stages has been questioned by Hochstetter and others. Secondly, there appears to be considerable variation in the length of the neural tube between the third rhombomere and optic vesicles, so that the portion of the neural tube which really belongs to the mesencephalic region is difficult to determine. Lastly, the series of stages of perfectly preserved embryos at our disposal is not large enough to enable us to say where the most rapid growth is occurring at any given time, and hence we cannot homologize the vaguely defined enlargements of the neural tube in this region. Because of these difficulties, we feel sure that any interpretation of the landmarks of the midbrain of embryos available at present must be simply a suggestion, which is to be revised or discarded when new facts come into View. 7
According to figures 4 and 7, B, the midbrain of the 17somite embryo appears to be wedge-shaped with a long arched roof and a short out-bulging floor. It is separated from the diencephalon by the dimesencephalic sulcus and from the hindbrain by the isthmus. As indicated on the model (fig. 7, B), the dimesencephalic border appears to be a shallow sulcus, while the isthmus is recognized on the external surface as a groove, which is correlated on the ventricular surface with a ridge extending from the roof of the neural tube ventrally and obliquely to a notch where the widely expanded portion of the chorda dorsalis lies. The floor of the midbrain has a constant relation to the rostral end of the chorda. as in many other human embryos (compare Watt, ’15, pl. 3, fig. 5; Davis, ’23, fig. 1; Johnson, ’17, fig. 1; Grirgis, ’26, pl. 1). Within these boundaries the midbrain appears to be incompletely divided into two segments, a wide anterior and a small posterior one. The anterior segment is conspicuous by its bulging out of the brain wall laterally, and this out—bulging correlates with the ventricular dilatation. Caudally, this segment is not clearly demarcated from the posterior segment, which is identified as a slight expansion of the dorsal lamina of the brain wall, situated just cephalic to the isthmus. A sulcus between the anterior and posterior segment comes halfway down on the side and disappears there, so that the midbrain is thus incompletely divided. For the midbrain of the 22-somite embryo it will be best understood by referring to figures 6 and 7, C. The question of the differentiation of the midbrain into two segments among human embryos is still an open one. In the 16- (C.G.470) and 17—-19- (Watt, ’15) somite embryos, all have been described with such a division, but Davis in his 20-somite embryo reported to the contrary, and Sternberg (’27) figured only one midbrain segment in his 18-somite specimen. In our 17-somite embryo it appears thatpthe posterior segment of the midbrain is greatly overshadowed by the anterior one in front and the first rhombomere behind, probably due to the fact that the differential growth in that region has not gone far enough to allow a clearer division. If a more active dorsal expansion of the forebrain and the lateral separation of optic vesicles would have occurred, so as to shift the anterior segment still farther forward, the second segment of the midbrain could be brought out into a more prominent feature. This seems to be the case with the midbrain of the 22—somite specimen. With this view in mind, it is conceivable that the differentiation of the midbrain into two segments is conditioned by the relative growth rate of its surrounding parts, i.e., by the dorsal expansion of the forebrain and the lateral separation of optic vesicles. Reaching such an important differential growth, it also seems possible that only the dorsal portion of the neural tube is involved, while the floor remains relatively unconcerned. This perhaps accounts for the fact that the boundary line between the two segments passes only halfway down on the side, not reaching the floor.
In the light of all features discussed above, it will be of interest to compare this portion of the nervous system concerned in other embryos. What Watt (’15) designated the ‘diencephalon’ of his embryo ‘VI’ (his fig. 5, pl. 3) seems to be the first segment of the midbrain of our identification; his ‘midbrain’ represents the second segment of the same; his ‘cerebral hemisphere’ corresponds to the portion of the diencephalon caudal to the optic vesicle (fig. 7, B, C). In the case of the 24.—somite embryo of Johnson (’17), both the ‘mesencephalon’ and a segment in front of it without any name (his fig. 1, pl. 2) probably represent the two divisions of the midbrain according to our terminology. In the 18—somite embryo described by Sternberg (’27, his fig. 1), he identified as the ‘Mammillar hocker’ of Hochstetter (’19) what we would consider as the floor of the midbrain. Another great confusion of the midbrain region in the literature is indicated by the fact that Nilsson (’26) in his 2.5—mm. embryo placed the very sharp cranial flexure between the first and second rhombomeres. It would be also of interest to compare the midbrain of the 17—somite embryo with that of the 14—somite cat embryo (Schulte and Tilney, ’15, pl. 34). The landmarks there seem to agree with those we have used, but designations these authors used are not without confusion. In terms of our terminology, their ‘mammillary region’ is really the floor of the midbrain; the ‘thalamencephalon’ cor? responds to our first segment of the midbrain; the so—called ‘anterior isthmian sulcus’ then represents the dividing line between the first and second segments. In rat embryos Adelmann (’25) recognized distinctly two segments of the midbrain as early as in the 10-somite stage (his fig. 16), although the neural folds at this time are still open everywhere cephalad of the anterior border of rh. 5. From all these comparisons it clearly appears that our knowledge concerning the midbrain region in its early development is still inadequate, and further careful investigations are to be hoped for when more specimens can be obtained.
III. Rhombencephalon
Rhombomeres
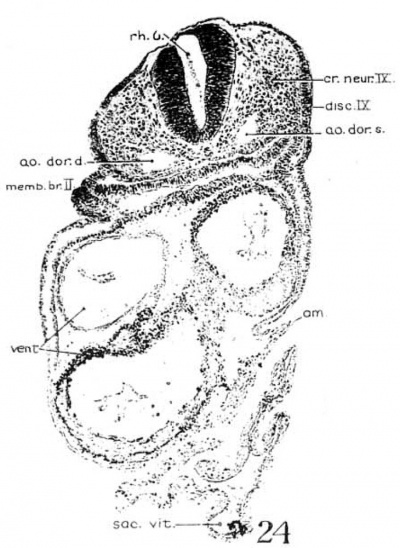
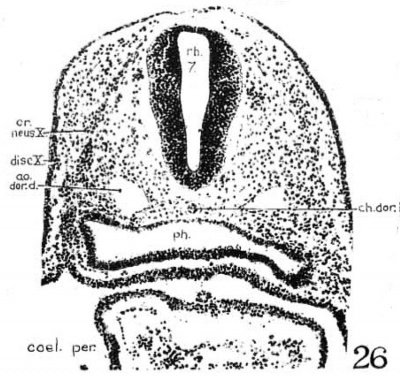
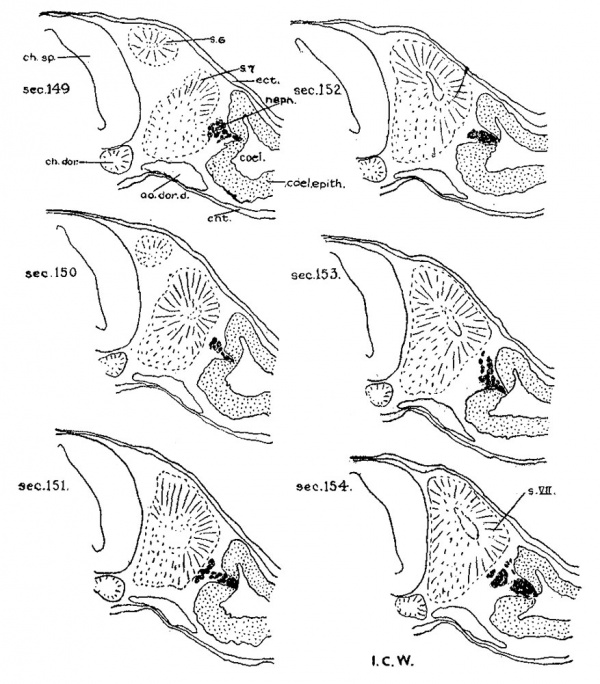
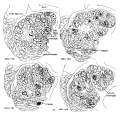
The portion of the nervous system extending caudally from the isthmus to the first mesodermic somite 'shows distinctly segmental appearance in the 17—somite embryo. The pattern of an alternate constriction and dilatation dividing the neural tube into transverse segments and their relations to the surrounding structures have been observed in various young embryos (Bartelmez and Evans, ’26) as well as in many older ones. In the period that we are dealing with here, there are, however, certain features which deserve special attention as to the significance in the differential growth activity of this part of the nervous system. figures 4, 5, and 9 show the typical pictures of the rhombomeres of the specimen with 17 somites. It may be said, in general, that the first, third, and fifth rhombomeres (rhs. 1, 3, and 5) show a similarly narrowed, though not identical, wedgeshape, with the relatively broadened base dorsal. Likewise, the second, fourth, and sixth rhombomeres (rhs. 2, 4, and 6) are all characterized by a thick lateral wall, so that the lumen is narrowed, except the dorsal part, where a dilatation is marked in each segment (figs. 20 to 24). The first rhombomere is difficult to identify on the external view, but from the ventricular surface is seen as a dilatation of the brain roof immediately caudal to isthmus. The second rhombomere is distinguished by its association with the trigeminal neural crest (cr.4zem*.V), the main trunk of which is attached to its rostral portion. The roof of this rhombomere is only one cell thick, while its lateral wall and floor consist of four to ten rows of nuclei. This thinning out of the roof plate begins at the region of rh. 1 and is one cell thick as far caudally as the level of the sixth rhombomere (rh. 6). A similar condition can be recognized in the 22-somite embryo (fig. 26). The same appearance can be identified in the figures of Giglio—Tos’ 15-somite embryo (’02), which is practically in the same stage of our H951, and also in Sternberg’s (’27) 18—somite specimen (his fig. 8). They all represent the earliest stages of development in which the characteristic expansion of the fourth ventricle --in man has been recognized. In the dorsal aspect of the third rhombomere (M. 3), there is associated a small round mass of cells, possibly representing a lost sensory ganglion (p. 333). The fourth rhombomere (rh. 4), which has its characteristic form in all earlier stages of human embryos, is associated with the acoustico—facial neural crest (cm/lem".VII—VIII) which is attached to its dorsolateral angle. The crest element here is only related to the cranial portion of this rhombomere, while its caudal part is overlapped by the otocyst, the posterior part of which overlaps the fifth rhombomere (rh. 5). The sixth rhombomere (rh. 6) has its relation to the glossopharyngeal neural crest (cr.nem".IX). Much less satisfactorily marked out than rhombomere 6, but about equal in size, comes the last rhombomere (rh. 7). It lies entirely in front of the 1st somite with its roof slightly dilated dorsally, but the floor remaining unchanged, and the vagal neural crest (0r.%em".X) is attached to its dorsolateral angle. In the oldest embryos described by Bartelmez and Evans (’26), the distance between the otic disc and the 1st somite is relatively shorter than in this 17 -somite specimen, and in the 16-somite embryo C.C.470 the region identified as rh. 6 lies just rostral to the first pair of somites. But in our H951 (17 somites) there is little doubt as to the division of the long segment between the otic pit and the 1st somite into rhs. 6 and 7, because the rapid growth in this region has brought about a segmental differential growth of an additional neuromere, the seventh rhombomere, which, however, has not as yet attained the characteristic form of other rhombomeres during this stage of development.
In comparing a large number of young human embryos, both on models in the Carnegie Collection as well as the mid-sagittal reconstruction figures in the literature, it is to be noted that the hindbrain is characterized by three typical dilatations of the neural tube, particularly ventrally bulged out. The middle one is invariably the most pronounced, while the ones preceding and following it are relatively smaller in size. They are unmistakably recognized by investigators as rhs. 2, 4, and 6, respectively. The other segments intervening between them, which appear to be definite in younger embryos, are not clearly marked, but rather reduced to be wedge—shaped in this or immediately following stages. It is significant that rhs. 2, 4, and 6 are functionally associated with the cranial neural—crest elements, so that their expansion in form may be considered as an expression of their relatively more rapid growth than other segments which have no definitive peripheral anlagen and the growth activity of which is comparatively arrested. For this reason it may be justified to designate the so—called ‘rh. 2’ as the trigeminal rhombomere; the ‘rh. 4,’ the acoustico-facial rhombomere, a.nd the ‘rh. 6’ (and probably ‘rh. 7’), the glossopharyngeal and vagal rhombomere in this or later stages.
Neural Crest
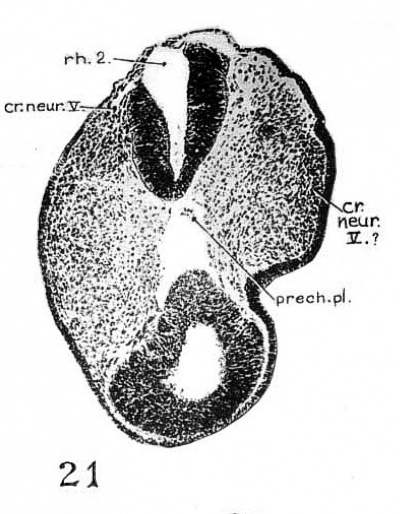
1. Trigeminal neural crest. As the ‘rostral neural crest’ has completely lost its connection with the midbrain, the crest element, which in this and later stages may be called the ‘trigeminal neural crest,’ is attached to the first and more obviously the second rhombomeres (fig. 5). It consists of a large compact mass of deeply staining cells attached to the dorsolateral angle of the neural tube by cell strands and reaching to the overlying ectoderm (figs. 20 to 22, especially ‘cr.~new.V,’ fig. 21), and a less deeply staining lamina of similar cells extending from it beneath an area of the head ectoderm (fig. 21, ‘cr.nem".V?,’ compare p. 331, below). The deeply staining part corresponds to the ‘proganglio neurale’ of Giglio-Tos (’02) and the ‘V ganglion’ of Watt (’15) and Davis (’23). The peripheral lamina corresponds to the ‘prenervi’ of Giglio-Tos (’02).
Fig. 21 Section 33 passing through the Vth neural crest and showing its relation to the arca V. The deeply staining mass of cells on the observer’s right (cr..neur.P 1) is continuous with the large mass of ‘Vth ganglion’ which adjoins the neural tube.
In interpreting the two parts of the trigeminal crest, there is no doubt as to the compact mass of cells attached to the brain wall being the neural—crest element, as it can readily be traced from its first appearance to the definitive V ganglion by its position and appearance (deep staining quality and clustering together of the cells). The situation regarding the peripheral subectodermal lamina of cells is rather puz~ zling. Are they neural crest or condensed mass of mesenchymal cells? We are unable to say with certainty. The chief difficulty is that the series of human embryos available is not adequate to trace its entire history. Continuity with the main mass of the V crest and a similar staining reaction are not adequate evidences for the identification of the crest element, especially since Adelmann (’25) found a peripheral mesenchymal condensation in the relatively precocious mandibular arch of the rat. The evidence for the crest origin of the peripheral lamina in 12- and 13—somite human embryos is given by Bartelmez and Evans (’26, p. 46).
A close examination of sections in series shows two concentrically arranged cores of cells in the mesenchyme recognizable on each side. They first appear at the level of rhombomere 1 and extend caudally and ventrally to the maxillary and mandibular processes. We venture to interpret these two whirls of cells as expressions of mesenchymal activity associated with the formation of the maxillary process and mandibular arch (fig. 20). T This might result in the crowding together of peripheral mesenchymal elements and produce the picture of the peripheral lamina, but it would not explain the intimate relation between the lamina and the overlying ectoderm (p. 331, below). It is altogether probable that a placodal-contribution toward the underlying condensation has occurred, as Gig1io—Tos (’02), Veit and Esch (’22), and Bartelmez and Evans (’26) described in other human embryos. To this point we shall come later in the discussion of the ectodermal areas.
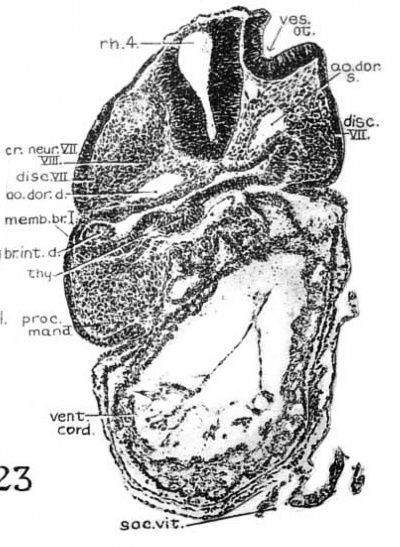
Fig. 23. Photomicrographs of sections from H951 (17 somites) Section 57, showing the VII-VIII crest, the VII epibranchial placode (disc. VII) the thyroid anlage, and the ‘gill rudiment.’
2. Acoustico—facial neural crest. This neural crest is in continuity with the dorsolateral angle of the fourth rhon1bomere, and spreads out laterally, underneath the otocyst, toward the VII epibranchial placode (fig. 23, dvz7sc.VII). It is easily recognized to be a sharply defined crescentic band of deeply staining cells, connected with the brain wall for nine 10 u sections on each side. Rostrally, the relations are as shown in figure 23 (observer ’s left). Caudally, it passes around the upper border of the otic pit and extends beneath it, through the hyoid arch, to the ectoderm (right side of fig. 23). In this region the mass of neural crest cannot be clearly separated from the mesenchyme, and the condensed cell mass is also fused with the ectodermal discus facialis. The histological picture of the fusion between the ectoderm and the underlying cell mass (neural crest) suggests a probable contribution from the placodal cells to the geniculate ganglion of the facialis, in the development of which in lower vertebrates (urodeles) the ectoderm plays an important part (Landacre, ’21, ’28; Stone, ’22, ’26).
The histological picture of the VII—VIII neural crest in the 22—somite embryo agrees, in general, with that of the 17 somite specimen, but a new feature which serves as a criterion to distinguish the crest cells from the surrounding mesenchyme comes in. This is the presence of a considerable number of deeply stained cells with coarse granules among the crest elements. This type of cell, which resembles the “cell degenerating as a" result of growth pressure or tension at the junction of the two tissues” (ganglion and mesenchyme), as Adelmann (’25) showed in the rat, is found to be present predominantly in the neural crest, but rarely in the mesonchyme. Tracing the sections serially, these degenerating cells stream out from the extreme dorsolateral angle of the neural tube, and then laterally and ventrally to the condensed mesenchyme of the hyoid arch, in which they become lost. The ectodermal thickening of this region will be considered later (p. 333, below).
Fig. 24 Photomicrograph similar to those of plate 1 from section 73 of H951 passing through the IX crest and placode.
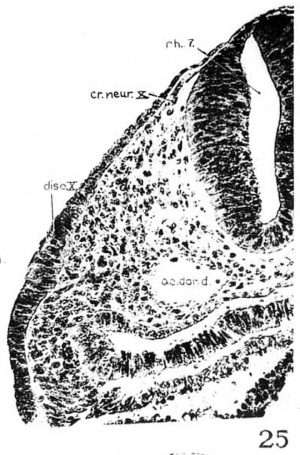
3. Glossopharyngeal and vagal neural crest. The glossepharyngeal neural crest (fig. 24) is represented by a clump of cells lying against the roof of rh. 6 and extending laterally and ventrally toward the ectoderm, which has a.lready become thickened in this region. Following the sections caudally from the otocyst, it is found that the neural-crest cells are coming out from the dorsolateral border of the neural tube. At the angle formed by the brain wall and the overlying ectoderm, there is first a spindle-shaped cell with oval nucleus and long cytoplasmic processes streaming out from the neural tube; then it is followed by a clump of similar cells, two or three in number, connected with each other; this clump in turn is in continuity with a big group of deeply staining cells extending ventrolaterally across the mesenchyme. toward, but not quite reaching, the thickened IX epibranchial placode. Caudal to the IX neural crest, there is a small strip of deeply staining cells connected with the dorsolateral Wall of the neural tube, which for a short distance can be differentiated from the mesenchyme (fig. 25). We recognize this as the beginning of the vagal neural crest‘ which occupies the middle of the seventh rhombomere. Between this level and the 1st somite, there are at ‘irregular intervals some isolated neural-crest cells, but no definite cellular clump resembling the one just described was observed. This part of the crest element will be profitably included in the discussion of the spinal neural crest. Separate origins of the glossopharyngeal and vagal neural crest tend to confirm the two ‘clumps of cells’ as representing the ganglia of the IX and X nerves, respectively, in the 20—somite embryo of Davis (’23) and in the 22-somite embryo of Girgis (’26). In our 22-somite specimen H1093, the X neural crest is clearly marked (fig. 26) and resembles closely that in the 15-somite embryo of GiglioTos (’02).
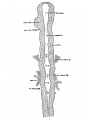
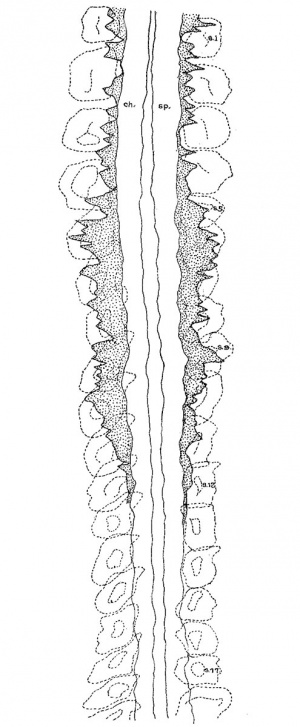
4. Spinal neural crest. For the sake of convenience in dealing with the neural crest of the 17—somite embryo caudal to rh. 7, we have arbitrarily included the crest element rostral to the third pair of somites in the spinal region, although anything in front of that level is to be regarded as belonging to the occipital region of the head. Histologically, only a few crest cells can be identified immediately caudal to the vagal neural crest. Definite crest swarms cannot be recognized until the upper border of the 1st somite is reached, whence they can be followed caudally as far as the intersegmental space of the 11th to 12th somites on the left and the cranial border of the 13th somite on the right side of the embryo. Beyond this point no crest cell can be found (fig. 10).
It has been reported by other investigators (Johnson, ’17; Davis, ’23) that analysis of the spinal crest has frequently met with difficulty because of its irregular character. As seen in sections, this structure is certainly indistinct in grouping and appearance, so that any clearly defined outline of its aggregation at any particular locality along the neural tube is impossible. However, an attempt has been made to count, under higher magnification, the crest cells migrating out from, or in continuity with, the dorsolateral border of the neural tube and bearing the characteristic feature of a closely packed cellular band with dark—staining nuclei. In doing this we have always kept in mind that only the nuclei should be counted, in order to avoid any duplication; that the number be made as conservative as possible; that cells doubtful in nature (presumably the mesenchyme) be left out of consideration. As the crest cells are thus presented on a numerical basis, they are then plotted in a graphic presentation which is finally transferred to the reconstruction of the neural tube in the spinal region.
For descriptive purposes, reference is made to the right side of the embryo (fig. 10). There the neural crest is shown to be irregularly aggregated along the neural tube from the level of the 1st somite to the 13th. Rostral to the 4th somite, crest cells appear to be much less developed than those immediately caudal to it. In the region of the first three somites, no definite pattern can be assigned to the crest cells as to their arrangement with reference to the somites§ In the 1st, 2nd, and 4th intersegmental spaces, they tend to be increased in number, but in the middle of the 1st and the caudal border of the 3rd somites they become greatly reduced. At the level of the 4th, 5th, and 8th to 11th segments, there is strongly indicated a segregation of crest cells opposite the somites, with the 8th— and 9th—somite regions more clearly marked than other places. It is also to be noted that in the 6th—s0mite level the crest cells attain their highest growth activity as manifested by the number of cells throughout almost the entire segment. Turning to the left side of the embryo, the cellular arrangement as a whole is essentially not different from that of the right.
In interpreting the spinal neural-crest cells of the 17-somite embryo, a few facts may be emphasized. first, the grouping of these cells with respect to the somites is by no means regular. The plotting is done accurately, and the picture of the ganglionic crest, as shown by Watt (’15) in the 17-19 somite embryos and Davis (’23) in the 20-somite embryo, cannot be demonstrated here. Secondly, the crest cells at the 5th—somite level reach the highest growth activity, which may be regarded as representing the differentiation of the first cervical nerve. If this be true, the first four and a half somites of this stage may enter into the formation of the occipital region. Lastly, the fact that crest cells tend to aggregate opposite the somites, as shown relatively clearly on the right side of the embryo, is important, according to Lenhossék’s ( ’91) finding in the embryo ‘Bulle,’ because the future spinal ganglia lie opposite the somites. For all these points, we need, of course, more materials for confirmation.
Ectodermal Areas
The areas of ectodermal thickening in the anterior end of the embryo with 17 somites are mapped out in figures 2 and 3. In the V area (area V), the epithelium measures 24 to 35 u in thickness, being intermediate in height between the tall epithelium of the otic pit and the thin integument covering the neural tube. Dorsal to the first visceral furrow at the level of rh. 3, there is a ‘discus incertus’ (Bartelmez) which measures 58 u on the right and 64;; on the left. Over the hyoid arch the epithelium becomes specially thickened, vary ing from 55 u to 71;: in height (area kg/.). The dorsal portion of this region is incorporated in the formation of the VII epibranchial placode, which caudally is continuous with the otic pit. Caudal to it, the epithelium of the extensive IX—X area is from 56 to 68 in thick. Dorsally, the ectoderm gradually thins out until it appears as a single layer of flattened cells 4 to 8 _u in thickness over the dorsum of the medullary tube (fig. 24).
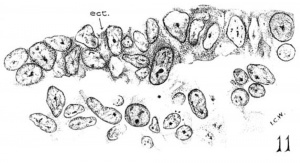
In the discussion of the trigeminal neural crest it has been pointed out that the area of contact between the ectoderm and the probable neural-crest elements is extensive, and that the outspreading of the latter coincides with the thickest region of the former, the boundary of which is indicated by broken lines in figures 2 and 3 (disc. V). Turning to a closer histological examination of the V area, we find that the epithelium of the ectoderm consists of two to three cells with papillary thickenings here and there on its inner surface. In these spots the cells are so irregularly arranged that migrations from the branchial ectodermal cells to the underlying cell elements are indicated. figure 11 illustrates this point. It should also be noted that the cells of the underlying tissue are, by cytoplasmic processes, united with the basal membrane of the ectoderm, so that a clear demarcation between the two tissue elements is impossible. Such pictures are found throughout the V area. and plotted on the model, as shown in figures 2 and 3 by the large circular spots. Comparing the two sides of the model, it is to be observed that these isolated centers of fusion with the neural crest(?) are more or less grouped together at the root of the mandibular arch, which we have designated as the ‘principal V area’ (area V prim). They are more abundant on the right side than on the left. Ventral to the ‘principal V area,’ the epithelium covering the mandibular arch is a single layer of cuboidal cells, but no indication of cell migration is observed. Dorsal to the ‘principal ‘V area,’ the ectodermal cells become gradually thinned out and the underlying cell lamina (neural crest?) is lost to sight.
Placodal contribution to the trigeminus in mammals has been recorded by Chiarugi (’94), Griglio-Tos (’02), Volker (’22), Veit and Esch (’22), Bartelmez (’24), and Bartelmez and Evans (’26). findings to the contrary have also been reported by others, among whom Adelmann (’25) in particular deserves consideration. This author concluded that there is no adequate evidence for placodal contribution to the trigeminus in the rat, and that the papillary thickenings on inner surfaces of the ectoderm suggesting possible proliferation were due to adhesions or oblique sections. He did not see any cells being actually pinched off from the ectoderm, nor did “many mitotic figures suggest the proliferation of cells into the subjacent tissue,” and hence any conclusion toward the placodal contribution to the trigeminus would be unwarranted.[1] Bearing these points in mind, we have carefully searched the broad V area of the 17-somite embryo, where no oblique sections are involved. We have seen that the cell migration seems to involve only a process of detachment of fully formed cells to the underlying cellular lamina, as Chiarugi (’94) observed in the guinea—pig. As in the case of neural-crest formation, there is no sign of mitotic activity or pinching off of cells from the ectoderm in these contributions to the subjacent tissue. We also believe that the close approximation between the two tissue elements cannot be considered merely as a phenomenon of adhesion, because the irregular arrangement of the epithelial cells and their relations to the underlying cell lamina (fig. 11) show something other than adhesion. Even if it be rega.rded as adhesions, it would be difficult to account for the distribution of such adhesions, limited onlywto the ‘principal V area.’ For these reasons we feel justified in assuming that the V area of this embryo with 17 somites shows some of the migratory activity which is indicated in human embryos of the 12—16-somite period (compare Bartelmez and Evans, ’26), and that this migration is presumably an expression of the ectodermal contribution to the future gasserian ganglion. The latter point is further supported by the fact that the clustering of migratory centers is at the confluence of the maxillary and man-dibular processes where the trigeminal eminence will appear in older embryos (compare the fischel embryo, Hochstetter, ’07).
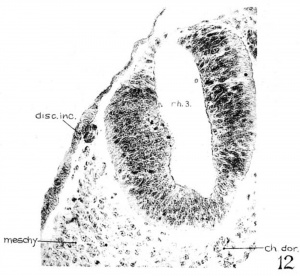
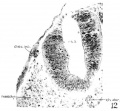
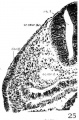
The appearance of the right ‘discus incertus’ in section is shown in figure12, and its relations appear in figures 3 and 5. A similar structure is present on the other side of the embryo. Each of them covers three 10 p sections, with epithelial cells greatly thickened and arranged as a small knob. In one section only (immediately preceding that of fig. 12) there are two cells at the dorsolateral angle of the neural tube connecta ing the intensely stained cell mass under the discus incertus by cytoplasmic processes. On the right side alone of the 23—somite embryo, a structure with similar cellular arrangement, but more prominent in appearance, yet related to rh. 3 as those just described in H951, is observed. This structure is not present at all in the 22-somite specimen.
Ventral to the discus incertus, the epithelium assumes a uniform thickening characterizing the ectoderm of the man dibular arch. At the dorsal end of the first visceral furrow the ectoderm is greatly thickened, extending from the dorsal angle of this furrow to the anterior border of the otic pit (figs. 2, 3). We recognize this area as the epibranchial placode of the VII ganglion, because the VII—VIII neural crest is in intimate contact with this portion of the ectoderm, as already pointed out in the discussion of the neural crest. Caudal but slightly dorsal to the VII epibranchial placode the thickened ectoderm gradually passes to the otic pit, which is still wide open. The histological appearance of the otic epithelium does not differ materially from that of the otic plate of younger embryos, as observed by Bartelmez (’22), except that the otic epithelium here is more compact and elongated in appearance, presumably due to a deeper invagination of the otic pit at this time. There is no brush border visible, but the processes extending out from the cells into the lumen are large. Ventral to the otic pit, the epithelium covering the second arch is even thinner than of the mandibular arch, but it becomes greatly thickened again caudal to the otic pit, where we come upon the IX—X epibranchial placode (figs. 2, 3).
The IX—X placode is represented by a thickened band of the ectoderm extending from the caudal border of the second gill membrane to the level of the second somite. The thickest part, consisting of two to three rows of oval nuclei, is in the region where the contact of the third pharyngeal pouch with the ectoderm will occur later. Dorsal a-s well as ventral to this thickened band, the epithelium gradually thins out Here again the cell mass underlying the placodal thickening is dense and a clear boundary between it and the basal mem "brane of the ectoderm is difficult to show (fig. 25). As already pointed out (p. 326), the IX neural crest is almost continuous with the subplacodal condensed cell mass. All that can be positively identified as the X neural crest is a cell clump in the mesenchyme (fig. 25, cr.%em*.X). It is then evident that the IX. neural crest of this 17—somite embryo is in the process of reaching its placode, but the X neural crest has not yet established its relations with the X placode, as shown in Griglio—Tos’ embryo (?02, his fig. 4). In the 22-somite embryo a similar condition is observed, but the cracking and shrinkage in that ‘region (fig. 26) make it difficult to see the true relation between the ectoderm and underlying cellular elements, particularly the papillary thickenings of the former on its inner surface. As to the 23—somite embryo, there is an extensive thickening corresponding to the IX—X area of embryo H951, and the epithelium there is about the same as that of the latter embryo in thickness. This undoubtedly represents the placodal thickening of the IX~X ganglia. Unfortunately, this specimen was also damaged by shrinkage, thus making a satisfactory analysis of the ectodermal areas impossible.
The first and second pharyngeal membranes, as indicated in figures 2 and 3, are covered with a single row of low cuboidal cells (fig. 23). No changes in the epithelium are noted as to show places of perforation of membranes, as Atwell (’26) observed in his embryo with 17 somites. The epithelium of the future saccus branchialis II is not thinned out as in the case of the two first gill membranes, although its site on the surface is indicated. As in earlier embryos the site of a gill membrane is thickened before the entoderm reaches the ectoderm.
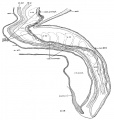
Chorda Dorsalis
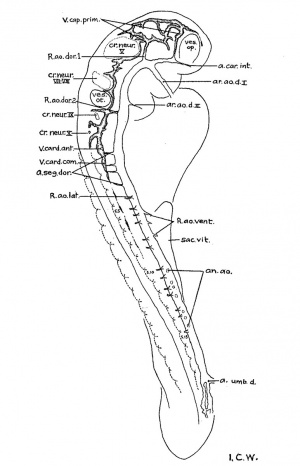
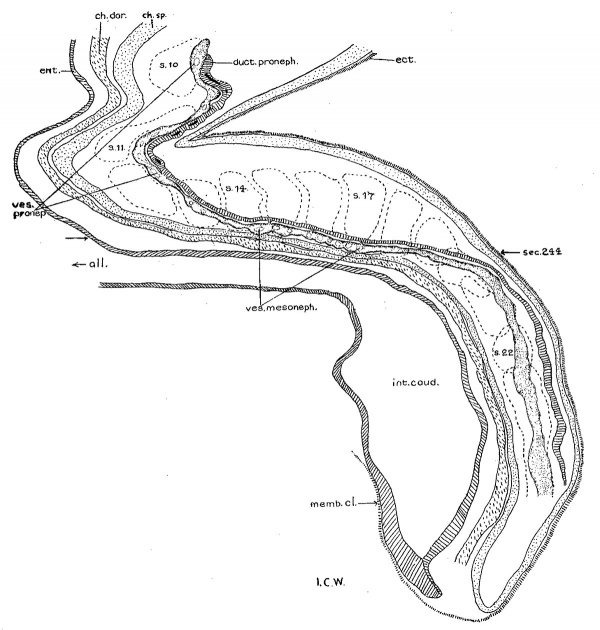
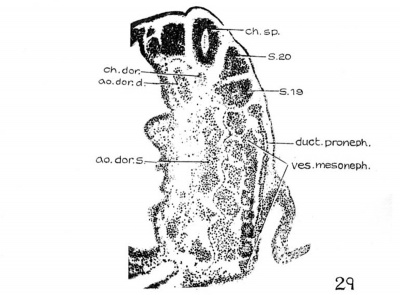
The chorda of the 17-somite embryo is shown in figure 5. I ts rostral end, which is slightly enlarged, lies under the floor of the midbrain and extends over the anterior end of the pharynx. In earlier embryos, the chorda appears to extend to the tip of the pharynx (Bartelmez and Evans, ’26, figs. 45 to 4.7), underlying the forebrain. In older embryos it is well known that the definitive chorda ends in the region of the midbrain, so that the rostral portion of the chordal tissue in the younger ones does not persist as such. In the light of the study of A.delmann (’22), we regard that tapered portion of the chordal tissue arching over the roof of the pharynx as the prechordal plate. Histologically, the cells are more irregularly arranged than those of the chorda proper. On the left side it is in continuity with the mesenchyme (fig. 22) and lacks the definite limiting membrane of the chorda, suggesting that chordal cells become the mesenchymal. In the 22- and 23-somite embryos the prechordal plate and its disintegration into mesenchymal cells are also recognized.
In the pharyngeal region the separation of the chorda from the roof of the pharynx has not yet been completed. While that process begins in the 12- to 14-somite stages, in the embryo of 17 somites there is no sign of the process beginning at the level of the first two pouches, but it is under way elsewhere caudal to the third pouch. Caudal-to the prechordal plate, the chorda assumes, in general, a cylindrical shape, though not of uniform diameter, intimately applied to the pharyngeal roof up to the level of the anterior part of the third visceral pouch. In this pharyngeal region the chorda has three marked constrictions: the first is found just caudal to the buccopharyngeal membrane; the second, at the junction between the first and second pouches; and the last, at the caudal portion of the second visceral pouch. At each of these constrictions the chordal tissue is not only actually reduced from about eighteen cells in a section to a few, but its investing membrane becomes less distinct and there is a corresponding groove in the pharyngeal wall. At the first point the gut wall is inseparable from the notochord as in. younger embryos. At the last two points a very faint limiting membrane of the notochord can be distinguished, but the relation is more intimate than other regions where the chorda becomes expanded and only approximates the dorsal wall of the pharynx. Caudal to the level of the third visceral pouch, the chorda is separated from the dorsal phar Ligeal wall by one or two mesenchymal cells. It C011tlI111£led'0 be circular in cross—section up to the level of the 4th thenite, where it becomes triangular in form as far as the 10th somite, its course and shape thereby being largely determined by the pressure of the dorsal aortae. As the aortae approximate each other in the midline, the chorda is shifted dorsally into close contact with the neural tube. At the level between the 10th and 14th somites the aortae become fused and the chorda is thus flattened ventrodorsally. ilaudal to the 14th somite the expansion of the hind gut begins, so that the chorda is brought in.to contact again with the gut wall. This situation persists for some distance until it becomes lost in the region of the primitive streak, where the tissue appears to be an undifferentiated cell mass.
The bearing of the contact points between the chorda and the pharyngeal wall upon the development of the future pharyngeal bursa was reported by Gage (’06), Huber (’12), Watt (’15), Johnson (’17), and Davis (’23). Gage, in the 3- to ]2—week—old embryos, and Johnson, in the 24—somite embryo, identified the site of the bursa at the posterior part of the pharynx (about the level of the third to fourth intersegmental space). In the pig and human embryos, Huber found the most caudal one of several contact points to be the region where, with a distinct thickening of the corresponding entoderm, the bursa will later develop. In the twin embryos, Watt recognized the least—developed area of the chorda opposite the second’ visceral pouch to be the site of the bursa, while Davis regarded that the last fusion point, i.e., the caudal part of the second pouch, with a corresponding thickening of mucosa, will probably give rise to the pharyngeal bursa. Comparing all these findings, it would seem that what has been called the site of the pharyngeal bursa in young human embryos is too far forward to be considered as that in the older ones, if it be regarded as having any morphological significance at all. The third constriction of the notochord in the 17—somite specimen is the same as that identified both by VVatt and Davis the bursa site, but no indication of the entodermal thickening at that level is marked. Nevertheless, the fact remains that the least—developed region of the chorda, as pointed out by Watt, is constantly present at the level of the second pharyngeal pouch of embryos with 14 to 20 somites.
Pharynx
Oral Sinus and Buccopharyngeal Membrane
The oral sinus and its membrane are obscured from View on the model by the apposition of the head to the heart swelling. The description of these structures is based upon the evidence from the sections. The oral sinus, which is out almost horizontally and hence renders the interpretation difficult here, covers only six 10 u sections. It appears to be shallow and broad toward the heart region, and then gradually tapers out cranially. It is separated from the pharynx by the buocopharyngeal membrane which seems to be ruptured along a narrow cleft on the right edge of the sinus at its attachment to the mandibular arch. In the middle part of the membrane there are two distinct layers of cells——the elongated ectodermal and shorter entodermal epithelium, In the former the nuclei are arranged in two rows, while in the latter only one layer of cells can be seen. The cells in both components are so crowded that the membrane appears to be very thick. The two tissues are distinct from each other everywhere, except along the line of the apparent rupture.
Grosser (’11) found the pharynx of embryo Pfannenstiel III (13-14 somites) to be still completely closed by the buceopharyngeal membrane; the membrane of a 15—somite embryo, Halg, where the epithelial cells are intimately fused so that the ectoderm and entoderm cannot be demarcated from each other, shows rupture in two places, probably produced by an artefact (p. 283); and in a still older embryo, Robert Meyer 300 (23 somites), the membrane is perforated, but in an irregular fashion. In the light of these observations, together with Davis’ (’23) in his 20—somite specimen where perforations occurred in the middle of the thinner out buccopharyngeal membrane, the apparent rupture in the 17-somite embryo is probably due to a tearing produced before fixation rather than a real perforation.
Ventral Pharyngeal Wall
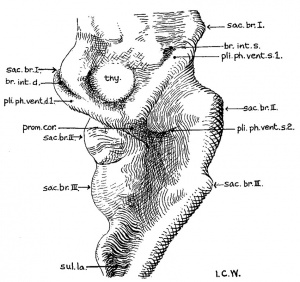
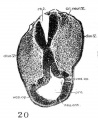
The external configuration of the ventral pharyngeal wall is shown in figure 13. In many respects it resembles the pharynx of Grosser’s 15—somite embryo, Halg ( ’11, his fig. 10, pl. 2), which is in turn similar to that of Davis’ 20-somite specimen. The thyroid anlage represented by a prominent swelling is situated a little to the right side rather than in the median plane. Caudal to it, but to the midline, there is another prominent swelling, which Davis has called the ‘heart swelling’, as produced by “the molding of the pharyngeal wall in the notch between the ventral aorta and the heart atrium” (’23, p. 25). In the 17—somite embryo we find that this swelling involves a dorsoventral expansion of the pharyngeal cavity at the level just caudal to the ventral aortic trunk. From it a pair of folds (ventral pharyngeal folds) proceeds obliquely cephalolaterally to the ventral angle of each first visceral pouch, marking off this clearly from the second pouch. Similarly, a second pair of folds, but much shorter than the first, can be traced from the heart swelling laterally toward the ventral angle of the second visceral pouch, more clearly marked on the left than on the right side of the embryo. Caudal to the second pair of folds, the heart swelling gradually fades out, and no more such folds can be found. At the cephalic end of each. first Ventral pharyngeal fold there is an invagination of the pharyngeal floor into the visceral pouch. This is the ‘Kiemenrudiment’ (br.mt.md.) of Grosser (’11, compare his figs. 4, 7, and 10), to which we shall come presently. Caudal to the level of the second Visceral pouch, the pharynx becomes narrowed down and compressed dorsoventrally with a concavity directed dorsally. Laterally, the first two pouches are in contact with the ectoderm, but the wall caudal to the second visceral pouch only indicates the impending extension of the third, which at this stage has not as yet reached its ectodermal area.
Internal Gill Rudiment
The ‘Kiemenrudiment’ of Grosser appears on the left firat pouch of the 17-somite specimen precisely as described by Grosser in his embryos (’11, his fig. 1). It is a bud—like projection from the ventral wall of the first visceral pouch adjoining the pharyngeal membrane and extends forward so that in sections (fig. 23) it appears free in the lumen. This rudiment is less prominent on the right side, although easily recognizable. In both of them, there is a central core of mesenchymal cells. The condition in the second pouch of the same embryo rather suggests an earlier stage of this rudiment. Here the lateral wall of the Visceral pouch on each side, which is already in ‘contact with the ectoderm, is thickened, so that a solid conical eminence of entodermal cells projects into the pharyngeal lumen. This resembles the structures described by Davis (’23) and Johnson (’17). Similar infoldings with mesodermal cores are present in the second Visceral pouches of the 23—somite embryo H984, but not in the first pair. In the left first pouch of the 22-somite specimen there is a minute elevation comparable to that rudiment at its characteristic position as in the embryo with 17 somites, but no sign of any in the second pair. This probably represents the later stage of the disappearance of the structure, whereas the rudiments have not yet appeared in the second pouch of this embryo.
Thyroid
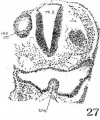
The thyroid, as shown in figures 5 and 13, is asymmetrically located between the first and second pouches. It lies immediately caudal to the bifurcation of the aorta. It is limited by a deep groove on all sides, except the cephalic margin, where it is at a same level with the surface of the pharyngeal floor, so that it appears as a spherical nodule, with a rostral attachment to the pharynx. Histologically (fig. 23), it is composed of epithelial cells which differ from those of the adjacent pharyngeal wall by their greater height and pseudostratified arrangement. There are two to three rows of nuclei with a‘ clear zone toward the pharyngeal cavity. The lumen is simply a shallow depression of the pharyngeal floor. But the thyroid anlage of the embryo with 22 somites presents a different picture (figs. 6, 27). Although this embryo was cut through just caudal to the otocyst (p. 304), the region of the pharynx where the thyroid should be is not damaged. There is no sign of a pharyngeal evagination whatever in this region, but just caudal to the first pharyngeal pouch and the bifurcation of the aorta there is a thick-walled medial invagination of the pharynx, which histologically resembles the thyroid anlage of embryos at this stage of development. It has a core of mesodermal tissue and an endothelial sprout from the ventral aortic trunk extending into it. While this condition may be artefact, the relations of this invaginated thyroid to the surrounding mesenchyme, the endothelium of blood vessels, and myocardial wall do not show any indication of it.
It is of interest to note here that the thyroid anlage has a definite relation to the ventral pharyngeal folds of the first visceral pouch, when we compare different young human embryos. In the Halg embryo (15 somites), Grosser identified the swelling at the confluence of the first pair of ventral pharyngeal folds as the thyroid (’_11, his fig. 10, pl. 2). But in Davis’ 20—somite embryo, Johnson’s 24-somite specimen, Robert Meyer’s 300 with 23 somites (Gros-ser, ’11, his fig. 12, pl. 2), and our 17—somite embryo, the thyroid is located eephalad to the swelling where the two folds meet each other. This disagreement seems to be accounted for by the fact that either Grosser might have beenmistakenin-interpreting the thyroid in his embryo Hal2, as Davis already pointed out (’23, p. 25), or that that specimen (Hal2) is Very different from others as mentioned above. It also seems probable that the thyroid anlage of the younger human embryo as Grosser’s Halg is incorporated in the point of the confluence of the first pharyngeal folds, and, as development proceeds, it is shifted cranially beyond the first pair of pharyngeal folds on account of the caudal prolongation of the ‘heart swelling’ of Davis in the production of a. second as well as a third pair of such folds (fig. 13). This latter condition is apparently true in embryos with 17 to 24 somites. The whole matter of identifying the thyroid anlage in young human embryos, however, can only be settled by further studies with certain essential criteria in mind. For instance, its relation to the first and second Visceral pouches, to the bifurcation of aorta (Kingsbury, ’l5), to the first pair of ventral pharyngeal folds, and the histological differentiation of its epithelium——all are main features. The last—named particularly is a picture not merely showing a much more thickened region than the adjacent pharyngeal wall (Grosser, ’11), but also indicating a distinct cytological differentiation in one case as early as the 12somite stage (Bartelmez, personal communication).
Lung and Liver
Caudal to the third pouch, the pharyngeal wall becomes abruptly narrowed from side to side. This region has a deep ventral groove in the midline which in the surface model (figs. 5 and 13) appears as a keel, continuing to the anterior intestinal portal. This is the laryngotracheal groove. Just rostral to the portal, its lateral wall is thickened especially on the right side. While the twisting of the embryo in this region makes the interpretation difficult, it is probable, in the light of Davis’ (’23) finding, that this thickening is the primordium of the lung.
The hepatic primordium is separated from the probable pulmonary anlage by a deep transverse groove (fig. 5) and is a thickening of the gut—epithelium of the anterior intestinal portal. It is already growing into the substance of the septum transversum. At the level of the upper border of the 3rd somite the laryngotracheal region on the gut wall is separated from the liver anlage by a great blood channel, the viteloumbilical trunk. As the series is followed forward, this channel increases in size, the intervening mesenchyme of the septum transversum ventral to it also expands, and the hepatic anlage decreases in thickness and is displaced farther ventrally. Taken as a whole, the liver anlage is a thickening of the dorsal and lateral gut wall, but the lumen is very narrow, so that at the first sight the primordium appears as a great dorsomedial thickening. It passes over very abruptly into a simple epithelium covering the adjacent septum transversum, and this in turn is continuous with the yolk—sac entoderm.
Vascular System
According to Davis’ ( ’27) recent studies on the development of the human heart, we can identify the different parts of the heart of the 17 —somite embryo as consisting of the sinus venosus, the atrium, the ventricle, and the bulbus cordis. As this organ is twisted i11 such a spiral way that its external form differs from the corresponding stages figured by Davis (compare his figs. 27 to 34), we believe it will be best understood by referring to figure 14.
The sinus venosus, which is attached to the pericardial floor and mainly embedded in the septum transversum, consists of a right and a left horn with an intermediate part between them. Each horn receives the vitello—umbilical trunk from its lateral aspect and the medial part of the sinus communicates cranially with the atrium. Due to the twist of the embryo’s trunk at this level, together with the fact that several sections of this region are missing, we are unable to find the anastomosis of the anterior cardinal vein with the plexus near the sinus venosus, but it is probably present in this stage of development. The atrium forms the most dorsal part of the heart and its bulk lies on the left side of the midline. Its cranial region is divided into the right and the left atrial cavity by the interatrial sulcus, which is marked as a notch on the myocardial mantle with a corresponding endocardial groove. The right atrium, being the larger of the two, appears to be a dome-like prominence fitting in the area dorsal to the bulbus cordis, while the left one narrows down gradually at the junction of the atrial canal. This canal is indicated as a constriction of both the myocardium and endocardium at the region between the atrium and the ventricle, and passes from the left atrium obliquely and cranially to reach the ventricle. The latter lies mainly on the right side of the embryo and forms a large prominence just caudal to the head on its Ventral aspect. It is in this region that the myocardial wall becomes Very thick, and the endocardial cavity small, so that a considerable space exists between the two. As series is followed forward, the Ventricular loop gradually moves to the left side of the embryo and then proceeds caudally to become the bulbus cordis, which is marked off from the ventricle by a slight constriction both on the myocardial and endocardial cavity. The bulbus cordis is directed obliquely and eranially toward the bulbus aortae.
Arteries and Veins
The blood vessels, particularly the veins, of the 17—somite embryo are not well adapted for detail studies, because their collapsed condition makes it materially difficult to follow their courses. We can, however, make out the important vessels, as shown in figure 15.
The ventral aortic trunk lies ventral to the thyroid. At the cephalic border of the latter the first pair of aortic arches springs from this trunk and proceeds cranially over the anterior extremity of the first visceral pouch into the dorsal aortae. Each arch, before bending dorsally and caudally, gives off a large branch, which is the primitive ‘internal carotid artery’ described by Congdon (’22) in somewhat older embryos. This artery (fig. 15) proceeds rostrally and gives rise to two small branches, one continuing with the main trunk and going ventrally to the optic vesicle, and the other going dorsally but caudal to it. This latter branch communicates with the deep venous plexuses (v.cap.med. of Evans, ’12) of the head, which is dorsal as well as caudal to the optic vesicle. The second pair of aortic arches is incomplete, but sprouts from dorsal aorta can berecognized. The first presegmental dorsal ramus from the aorta arises at the point where the first aortic arch joins it, going along the border of the trigeminal neural crest and anastomosing with the deep as well as the superficial veins in that region. The second recognizable dorsal branch is found at the level of the second visceral arch and communicates with the large vein which drains all the venous plexuses around the otic region and those cranial to it. Caudal to the second dorsal branch, the dorsal aorta gives off numerous dorsal segmental, lateral, and ventral branches at various levels, as table 2 will show. Some of them are traceable without difficulty, while others, due to their collapsed condition, are only partially represented. In figure 15 only the clearly recognizable branches are shown. Between the first and second somites dorsal segmental branches are given out and communicate in an extensive area with the anterior cardinal Vein—a feature constantly present in many other young human embryos. The lateral branches from the dorsal aorta are the most favorable ones to recognize, since they are distinctly traceable on sections. The ventral 1-ami of the dorsal aorta are only slightly indicated in the anterior portion of the yolk sac, where they are represented by ventral extensions of the endothelial cells from the aorta to the venous plexuses on the yolk sac. The two dorsal aortae come to fuse with each other in seven places between the levels of the 10th and 14th somites. This finding agrees well with thatiof Davis in his 20-somite embryo. In J ohnson’s (’17) 24-somite and Girgis’ (’26) 22-somite embryos, the fusion is more extensive than in either of these.
The venous plexuses in the head region are practically only recognizable in the deeper rather than on the superficial area, although the process of forming the latter may already be in progress, as blood cells can be seen scattered throughout the superficial mesenchyme. Traced caudally, all the tributaries around the trigeminal neural crest are combined to form a large venous trunk (1J.ca-p.pm’m.) which, upon receiving more tributaries from the vicinity of VII-VIII neural crest, otic pit, and 1X—X neural crest, may be called the anterior cardinal vein. Caudal to the level of the third somite there is a vessel much reduced in size and interrupted, which cannot be traced below the level of the 9th somite. This is all that can be recognized of the posterior cardinal vein. No ductus Cuveri could be found.
The arterial and venous system on the left side of the 17 somite embryo is essentially similar to that on the right, described above.
Nephric System
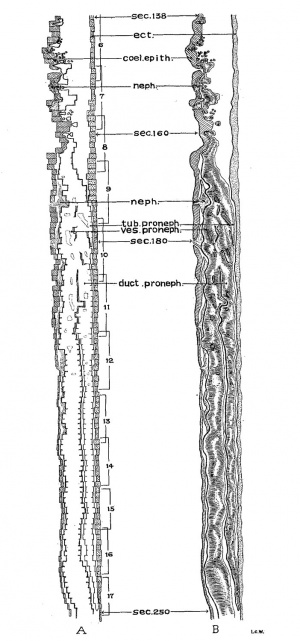
The nephric system of the 17-somite embryo is shown in figure 16. This represents only the nephric tissue of right side, which is more favorable for reconstruction. For descriptive purposes the entire system may be arbitrarily divided into three portions: 1) from the level of the 6th somite to the middle of the 8th; 2) thence to the middle of the 10th somite; 3) and the rest for the third portion. In the first portion we find isolated cell masses, which, lying closely in contact with the coelomic epithelium, are composed of irregularly arranged cells clearly differentiated from the cells of the coelomic epithelium in staining quality as Well as cellular arrangement. There are two places where the cells of such isolated masses appear to be wedged in between the cells of the coelomic epithelium, so that they are directly in contact with the coelomic cavity. figure 17 illustrates this condition. We recognize these as clearly the remains of nephrostomes. Beginning with the middle of the 8th somite, we find the second part of the nephric system as a definite and continuous longitudinal bar of cells extending caudally. This cell bar appears first to be small, and then progressively increases its bulk, until it reaches its maximum in size when the first pronephric vesicle is noticed at the level of the 9th somite. This is followed by at second pronephric vesicle in the next segment. As shown in figure 18 (section 182), cells of the dorsal aspect of these vesicles sprout dorsally to form the pronephric tubules; a structure appears on the reconstruction (fig. 16) to be also a longitudinal but short cell bar with a definite lumen inside. On the ventral side of the vesicles there are two distinctly recognizable nephrostomes in this region. It should be noted that cells of the nephrostome here (figs. 18 and 28) as well as at other levels indicated in figure 16 are arranged as cellular capsules which plug in between the coelomic epithelium, so that the picture is unmistakably different from the rest of the nephric tissue. Caudal to the second pronephric vesicle, the third portion of the nephric system, which we may speak of now as the nephrogenic bar, runs caudally as a continuous cord of cells with seven lumina enclosed at different regions and gives a dorsal sprout of a longitudinal cell bar as the primary excretory duct at the level of the middle of the 10th somite. This duct is completely separated from the underlying nephrogenic bar and shows its own independent caudal growth. Lumina are present at six places in the cranial region. Two of the nephrostomes in this part border on the coelomic cavity, and the other two appear merely as cellular capsules lying under neath the nephrogenic bar.
 Fig. 28. Photomicrograph from section 180 of H951 (17 somites) see figures 16 and 18. Zeiss. apo. imm. obj. ' X J JHomal III. X 800. This figure is turned at an angle of 90" as compared to figure 18.
Fig. 28. Photomicrograph from section 180 of H951 (17 somites) see figures 16 and 18. Zeiss. apo. imm. obj. ' X J JHomal III. X 800. This figure is turned at an angle of 90" as compared to figure 18.
Table 2
| Rami | ||
|---|---|---|
| Right side | Left side | |
| Dorsal segmental | ||
| 1. Rostral to somite 1 | 1. Between somites 1 and 2 | |
| 2. Between somites 1 and 2 | 2. Between somites 2 and 3 | |
| 3. Between somites 2 and 3 | 3. Between somites 3 and 4 | |
| 4. Between somites 3 and 4 | 4. Upper border of somite 5? | |
| 5. Between somites 5 and 6? | 5. Between somites 5 and 6? | |
| 6: Lower border of somite 6? | 6, Between somites 6 and 7? | |
| 7. Between somites 7 and 8? | 7. Between somites 7 and 8? | |
| 8. Between somites 8 and 9? | 8. Between somites 11 and 12? | |
| 9. Between sornites 9 and 10? | 9. Between somites 12 and 13? | |
| 10. Between somites 15 and 16? | 10. Between somites 13 and 14? | |
| 11. Between somites 14 and 15? | ||
| Lateral | ||
| 1. Middle of somite 4 | 1. Between somites 1 and 2? | |
| 2. Middle of somite 5 | 2. Between somites 3 and 4 | |
| 3. Between somites 5 and 6 | 3. Middle of sornite 4 | |
| 4. Middle of soinite 6 | 4. Middle of solnite 6 | |
| 5. Between sornites 7 and 8 | 5. Between somites 6 and 7 | |
| 6. Middle of solnite 8 | 6. Middle of somite 7 | |
| 7. Middle of somite 10 | 7. Middle of somite 8 | |
| 8. Between sornites 10 and 11 | 8. Middle of somite 9 | |
| 9. Between somites 11 and 12 | 9. Between somites 10 and 11 | |
| 10. Middle of somite 12 | 10. Between somites 11 and 12? | |
| 11. Middle of somite 13 | 11. Between somites 12 and 13? | |
| 12. Between somites 13 and 14 | ||
| 13. Between somites 14 and 15 | ||
| Ventral | ||
| 1. Middle of somite 5 | 1. Between somites 3 and 4 | |
| 2. Middle of somite 6 | 2. Middle of somite 6 | |
| 3. Lower border of somite 7 | 3. Between somites 7 and 8 | |
| 4. Middle of somite 9 | ||
| 5. Middle of somite 10? | ||
| Note. "?" in the table indicates vessels only partially represented on the section. | ||
Fig. 17 A series of projections from sections 149 to 154 of H951 (17 somites) to show the relations of the nephrostomal tissue at the level of somite 7 (compare fig. 16) in the region of the retrogressing pronephros.
Fig. 18 Camera-lucida drawings from sections 179 to 182 of H951, to show the differentiation of the nephric tissue at the level of somite 10 (compare fig. 16). Zeiss ape. imm. 3.0 mm., oc. K 12.5. Reduced. to 585 diameter.
Turning to the nephric system of the 22-somite embryo, it may be said that it differs from that of the 17-somite specimen only in degree of development. The whole system (fig. 19) consists of a longitudinal nephrogenic bar with a number of vesicles and a primary excretory duct. From the level of the 10th somite to the intersegmental space between the 12th and 13th somites, there are seven pronephric vesicles with definite lumen in each. At the upper border of the 13th somite, the nephrogenic bar is greatly decreased in size, appearing to be the least developed area. From thereon to the 19th somite, it appears to be bead—like, in the form of a series of vesicles, with cavities in each enlargement. We recognize this region as the mesonephric area (fig. 19). Caudal to it, the nephrogenic bar assumes a more or less uniform size without any lumen recognizable. This condition persists until it fuses with the intermediate cell mass. As to the primary excretory duct, which has lumina only in the cranial part, it is closely connected with the underlying nephrogenic bar at the level of the 10th to 12th somites.
Caudal to this region it is separated from the nephrogenic bar throughout the rest of its extent, except at three points in the mesonephric area, as indicated in figure 19. No nephrostome comparable to that in the 17-somite embryo is recognizable here.
Fig. 19 A graphic reconstruction of the caudal end of the 22-somite embryo, H1093. X 100.
In the light of the findings on the nephric system in other young human embryos (Felix, 312; Watt, ’15; Johnson, ’17; Davis, ’23; Grirgis, ’26; and others), the interpretation of the different portions of the system in the 17~somite embryo may be elucidated. The first part represents the degenerating area of the pronephros; the isolated cell masses are probably the remains of the nephric tissue being in the process of degenerating, while the nephrostomes, as suggested by Davis (’23), are therefore the last portion to degenerate. The second portion where the pronephric vesicles are present represents the pronephric area proper. It is the region where the pronephric vesicles give rise to pronephric tubules, by the union of the caudal ends of which the pronephric duct is formed. The third portion is really an ‘undifferentiated’ nephrogenic area, being thus designated because no definite features in this area can be fixed upon so as to characterize a typical nephric structure, except the duct. Undoubtedly, the mesonephros will develop from this region later, but in this stage of development (17-somite) We have no definite criterion to tell the landmark where the pronephros terminates and the mesonephros begins.
As to the formation of the primary excretory duct in the 17—somite embryo, a few words should be said here. Accord ing to the observation of Felix, that the pronephric duct is formed by the union of the caudal ends of the pronephric tubules and its independent growth caudally, our findings on the second portion of the nephric system as described in the above section certainly agree well with his. Probably the first tubule in our specimen has not as yet fused with the following one to become the duct. But at the level of the 10th somite we find a definite cord of cells growing out from the nephrogenic bar and going dorsally and caudally as the primary excretory duct. We are unable to find in this region any indication of individual tubules nor the union of their caudal ends to form this duct. Then the question which will arise here is: Does the definitive pronephric duct of man come from the fusion of caudal ends of the pronephric tubules, or does it grow independently at a different level where the tubules have nothing to do with its formation? This question certainly cannot be settled until a large number of well-preserved embryos are available.
Fig. 29 Photomicrograph of section 344 of H1093 (22 somites) which passes horizontally through the segmental duct and the mesonephric vesicles.
Besides, there is another point in our findings that differs from that given by Felix, Watt, and others. That is, are the pronephric vesicles separated as individual ones? In our specimens we find them to be continuous with each other and also with the nephrogenic bar, and that in the latter there are enlargements of the tissue with lumen inclosed at different regions. This latter phenomenon as seen in the 22-somite embryo (fig. 29) represents a series of vesicles without any interruption whatever. It is evident, then, that one has difficulty in following Felix and Watt in fixing any area along the nephrogenic tissue for the development of pro— and mesonephros with reference to the level of somites. It seems to us that the mode of differentiation of the nephric system is an expression of the potentiality of the organism as a whole rather than an isolation of its parts.
Summary
The differential growth of principal structures and systems of a 17—somite embryo, as compared with embryos with 22 and 23 somites, is described with the following results:
- The chief external features of the 17—somite embryo are the cranial flexure, optic vesicles, two visceral arches, and otic pits in the cranial region and the pronephric ridges at the 9th—15th—somite levels on the dorsal surface in the caudal part. The last—named landmark cannot be traced on the external surface of the 22- and 23—somite embryos.
- In the forebrain of the 17—somite embryo the anterior neuropore is found in the process of closure. Rostral to this site the brain wall becomes thickened, with a slight dilatation of the ventricle suggesting a telencephalon. The forebrain floor remains thick everywhere, except that the primitive optic chiasma and infundibulum are indicated as slight depressions. Each optic vesicle is marked off from the brain wall by a dorsal and a ventral optic limiting sulcus——a feature of differential growth that has not been hitherto observed in embryos younger than the 17—somite specimen.
- The wedge—shaped midbrain of the 17- and 22—somite embryos, marked off by dimesencephalic boundary in front and isthmus behind, appears to be divided into two segments.
- The hindbrain of the 1.7-somite embryo consists of seven typical rhombomeres. The neural crest of this region of the nervous system bears definite relations centrally with rhombomeres and peripherally with the thickened ectodermal areas. The V neural crest is centrally connected with rh. 1 and principally rh. 2, whereas its peripheral relation is to a placodal thickening, which apparently contributes cells to the formation of the future gasserian ganglion. The VII—VIII neural crest comes out from rh.4 and extends laterally to the facial epibranchial placode. The IX—X neural crests are centrally attached to rhs. 6 and 7, respectively, but their peripheral connection with the already thickened IX and X epibranchial placodes cannot be traced at this stage of development. In the 22-somite embryo this connection is demonstrable. In the region of rh.3 the ‘discus incertus’ is present bilaterally in the 17—somite embryo, but only on the left side of the 23-somite specimen. The spinal crest of the 17 -somite embryo tends to show aggregation opposite the somites on the right side, though more irregular groupings of crest cells are observed on the other. The fourth ventricle is just beginning to appear in the 17-somite embryo.
- The chorda of the 17—somite embryo is closely fused with the pharyngeal wall to the level of the third branchial pouch, but is separated caudally. In the fused region the chorda becomes greatly diminished in size at three points, suggesting the least developed areas, the last of which, according to some investigators, gives rise to the future pharyngeal bursa. This embryo, together with the 22 and 23—somite specimens, all possess a prechordal plate, which by disintegration becomes mesenchyme.
- The 17—somite embryo has its buccopharyngeal membrane apparently ruptured only on the right side. Its pharynx possesses two pairs of visceral pouches, reaching their corresponding branchial membranes, with the third one still in the process of formation. The thyroid anlage is asymmetrically located to the right side of the ventral pharyngeal wall. The ‘internal gill rudiment’ (Grosser) is present in the first and second visceral pouches of the 17—somite embryo, but only in the first left pouch of the 22-somite specimen and in both second pouches of the embryo with 23 somites. The pulmonary primordium of the 17—somite embryo is widely separated by a deep transverse groove from the hepatic anlage, which at this stage of development extends far cephalad into the septum transversum.
- The heart of the 17—somite embryo consists of the sinus venosus, the atrium, the ventricle, and the bulbus cordis. The first pair of aortic arches can be traced from the ventral aortic trunk to the dorsal aorta, but the second one is still in the process of formation. After giving off two branches to the region, of the optic vesicle, the dorsal aorta has several dorsal segmental, lateral, and ventral rami. The two dorsal aortae fuse at the level of the 10th—14th somites at seven places. Venous plexuses in the midbrain region form the primitive head vein, and those around the V, VII—VIII, IX—X neural crest and otic pit drain toward the anterior cardinal vein.
- The nephric system of the 17—somite embryo maybe divided according to histological pictures into the nephrostome region (6th—8th somites), which represents the degenerating area of pronephros, the pronephric—vesicle region (8th~10th somites), which is the pronephros proper, and the ‘undifferentiated’ nephrogenic region for the rest of the nephric tissue. The formation of the primary excretory duct in the second portion of the nephric system appears to be by the union of caudal ends of pronephric tubules, but that in the third portion is not in accordance with this observation. The pronephric vesicles are connected by irregular masses of cells with each other instead of being separated ones. The 22 somite embryo does not possess any nephrostome, but has mesonephric vesicles caudal to pronephric tubules.
Abbreviations for all Figures
|
|
| Abbreviations for all Figures | |
|---|---|
|
|
Bibliography
ADELMANN, II. B. 1922 The significance of the prechordal plate: an interpretative study. Am. Jour. Anat., vol. 31, pp. 55-91.
Adelman HB. The development of the neural folds and cranial ganglia of the rat. (1925) J. Comp. Neurol. 39(1): 19-171.
Atwell WJ. The prechordal plate in a human embryo with small neuropore. (1926) Anat. Rec. 32(3):200.
Bartelmez GW. The origin of the otic and optic primordia in man. (1922) J. Comp. Neural., 34: 201-232.
Bartelmez GW. The subdivisions of the neural folds in man. (1923) J. Comp. Neural., 35: 231-247.
Bartelmez GW. Ectodermal areas of the head in young human embryos. (1924) Proc. Amer. Soc. Zool., Anat. Rec. 29: 109.
Bartelmez GW. and Evans HM. Development of the human embryo during the period of somite formation, including embryos with 2 to 16 pairs of somites. (1926) Contrib. Embryol., Carnegie Inst. Wash. Publ. 362, 17: 1-67.
CHIARUGI, G. 1894 Contribuzioni allo studio dello sviluppo dei nervi eneefalici nei mammiferi: I-III. Publ. Inst. Stud. Sup. Sez. Med., firenze.
Congdon ED. Transformation of the aortic-arch system during the development of the human embryo. (1922) Contrib. Embryol., Carnegie Inst. Wash. Publ 277, 14:47-110.
CONGDON, E. D. 1922 Transformation of the aortic arch system during the development of the human embryo. Contributions to Embryology, vol. 14, Carnegie Inst. Wash., pub. no. 277, pp. 47-110.
Davis CL. Description of a human embryo having twenty paired somites. (1923) Carnegie Instn. Wash. Publ. 332, Contrib. Embryol., 15: 1-51.
Davis CL. Development of the human heart from its earliest appearance to the stage found in embryos of twenty paired somites. (1927) Carnegie Instn. Wash. Publ 380. 19: 245-284.
Evans HM. The development of the vascular system. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp570-708.
Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979.
Gage SP. A three weeks' human embryo, with especial reference to the brain and nephric system. (1905) Amer. J Anat. 4: 409-443.
Gage SP. The notochord of the head in human embryos of the third to the twelfth week and comparisons with other vertebrates. (1906) Science 24: 295.
GIGLIOT0S, E. 1902 Sugli organi branchiale e laterali di senso nell’uomo nei primordi del suo sviluppo. Monit. Z001. It., anno 13, pp. 105-119.
Girgis A. Description of a human embryo of twenty-two paired somites. (1926) J Anat. 60(4): 382-410.1. PMID 17104111
GIRGIS, A. 1926 A human embryo of twenty~two paired _somites. Jour. of Anat., vol. 60, pp. 382-410.
GROSSER, 0. 1911 Zur Entwicklung des Vorderdarmes mensehlicher Embryonen bis 5 mm. griisster Liinge. Sitzb. d. Kaiserl. Acad. Wiss. Wien, Math.naturw. K1., Bd. 120, Abt. 3, S. 271-310.
HOCHSTETTER, F. 1907 Bilder der iiusseren Kiirperform einiger menschlicher Embryonen aus den beiden Ersten Monaten der Entwicklung. Miinehen.
- 1919 Beitrage zur Entwieklungsgeschiehte des menschlichen Gehirns. I. Teil. Wien und Leipzig.
HUBER, G. C. 1912 On the relation of the chorda dorsalis to the anlage of the pharyngeal bursa or median pharyngeal recess. Anat. Rec., vol. 6, pp. 373-404.
JANOSIK, J. 1887 Zwei junge mensehliehe Embryonen. Arch. f. mikr. Anat., Bd. 30, S. 559-595.
Johnson FP. A human embryo of twenty-four pairs of somites. (1917) Carnegie Instn. Wash. Publ., Contrib. Embryol., 21: 125-168.
Johnston JB. The morphology of the forebrain vesicle in vertebrates. (1909) J. Comp. Neurol. and Psychol. 19: 457-539.
LANDACRE, F. L. 1921 The fate of the neural crest in the head of the urodeles. Jour. Comp. Neur., vol. 33, pp. 1-43.
- 1928 The epibranchial placode of the nervus facialis of the rat. Anat. Rec., vol. 38, p. 19.
V. LENHOSSEK, M. 1891 Die Entwiekelung der Ganglionanlagen bei dem mensehliehen Embryo. Arch. f. Anat. u. Phys., Anat. Abt., S. 1-25.
NILSSON, F. 1926 Die Segmentierung des Grehirns bei Mensehenembryonen. Zeit. f. mikrsanat. F0rschung., Bd. 7, S. 191-230.
SCHULTE, H. V. W., AND TILNEY, F. 1915 Development of neuraxis in the domestic cat to the stage of twenty~one somites. Ann. N. Y. Acad. Sci., vol. 24, pp. 319-346.
STEKNBERG, H. 1927 Beitrage zur Kenntnis des vorderen Neuroporus beim Ménschen. Zeit. f. Anat. u. Entwiek1ungsgesch., Bd. 82,.S. 747-780.
STONE, L. S. 1922 Experiments on the development of the cranial ganglia and the lateral-line sense organs in Amblystoma punctatum. Jour. Exp. Zool., vol. 35, pp. 421-496.
- 1926 Further experiments on the extirpation and transplantation of mesectoderm in Amblystoma punctatum. J our. Exp. Zool., vol. 44, pp. 95-131.
Thompson P. Description of a human embryo of twenty-three paired somites. (1907) J Anat Physiol, 41(3):159-71. PMID 17232726
VAN DEN BROEK, A. J. P. 1911 Zur Kasuistik junger menschlicher Embryonen. Anat. Hefte, Bd. 44, s. 273-304.
VEIT, 0., UND ESCH, P. 1922 Untersuehung eines in Situ flxierten, operativ gewonnenen mensehlichen Eies der vierten Woche. Zeit. f. Anat. u. Entwieklungsgeseh., Bd. 63, S. 343-414.
VGLKER, O. 1922 Normentafel zur Entwieklungsgeschiehte des Ziesels (Sper mophilus eitellus). Normentafeln zur Entwicklungsgesehiohte der Wirlbeltiere, Heft 13. Herausgegeben Von F. Keibel, Jena.
Wallin IE. A human embryo of thirteen somites. (1913) Amer. J Anat. 15(3): 319-331.
Watt JC. Description of two young twin embryos with 17-19 paired somites. (1915) Contrib. Embryol., Carnegie Inst. Wash. 2: 15-54.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The Anatomy of Human Embryos with Seventeen to Twenty-three Pairs of Somites. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Anatomy_of_Human_Embryos_with_Seventeen_to_Twenty-three_Pairs_of_Somites
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- ↑ At the recent meeting of the American Association of Anatomists Landacre (’28) presented evidence of contributions from the -VIII epibranchial placode to the underlying neural crest in the rat. He attributed Adelmann’s failure to find them to the fact that his series of embryos was altogether too small. Landacre showed that the sequence of events in the development of the cranial ganglia is not closely correlated with the differentiation of the somites and that a close series in the development of the latter cannot be regarded as a criterion of an adequate series for the study of ganglion formation. Landacre’s series comprises over 200 embryos from the period between 12 and 30 somites.