Paper - Placental circulation
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Ramsey EM. Placental Circulation. (1972) MCV Quarterly, 8(1): 61-68.
| Online Editor |
|---|
| This historic 1972 paper by Ramsey (17 February 1906 - 2 July 1993) describes development of the placental circulation. She published extensively on placental and embryonic development and this paper uses both human and monkey placentas from the Carnegie Collection. Dr. Ramsey discovered the "Yale" embryo and was a member of the Carnegie Institute's embryology department research group from 1932 to 1971 when she retired. In addition to her research articles she published 2 books "The Placenta of Laboratory Animals and Man" (1975) and "Placental Vasculature and Circulation" (1982).
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Placental Circulation
Visiting Professor of Obstetrics and Gynecology, University of Virginia
School of Medicine, Charlottesville, Virginia
- Presented at the 43rd Annual McGuire Lecture Series, December 3, 1971, at the Medical College of Virginia, Richmond.
Introduction
One of the most important developments of recent years in the field of uterine physiology has been the recognition that the endometrial changes occurring during the menstrual cycle and those associated with pregnancy are interlocking, sequential events in an ordered progression from the first day of the cycle to parturition—and not separate phenomena as was formerly believed. No component of the endometrium illustrates this progression more strikingly than does the vasculature.
Much of the story of uterine vascular pattern and circulatory mechanism is based upon studies in the rhesus monkey, employing in vivo techniques inapplicable to clinical patients. (These studies were carried out in the Department of Embryology, Carnegie Institution of Washington at Baltimore.) Subsequent checking of the monkey findings against their human counterparts, in operative and necropsy specimens, etc., has shown the monkey to be a valid experimental model with reproductive system anatomy and physiology closely similar to the human (Ramsey and Harris, 1966).
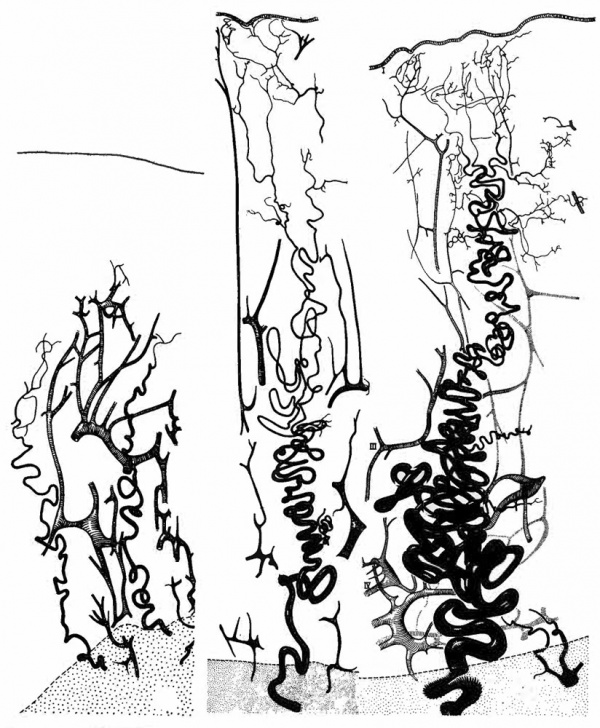
Following the menstrual slough the vasculature regenerates pari passu with the endometrial stroma and glands (Fig. 1). Initially a long capillary network forms between the stumps of spiral arteries in the basalis and the epithelial surface. Subsequently, muscular and elastic layers forming around the capillaries transform them into true arteries. It may be noted parenthetically that this is a more accurate description than the familiar statement that “spiral arteries grow toward the endometrial surface.” A rich capillary bed remains in the immediately subepithelial layer and connects the arteries with veins which run perpendicularly toward the myometrium.
Although the follicular phase of the cycle is frequently referred to as the “growth phase,” growth of spiral arteries continues unabated during the corpus luteum phase and even further on, as we will see. Indeed, vascular growth during the lutein phase outstrips stromal growth, so that the increasing length of the arteries must be accomodated within the endometrium by ever increasing coiling (Fig. 1).
Fig. 1. Camera lucida drawings of the vascular bed at three stages of the menstrual cycle in the rhesus monkey. Left. postmenstrual; Center. postovulatory; Right. late secretory. Myometrium stippled. (Reprinted with permission from Bartelmez. Contrib. Embryol. 361153-182, 1957.)
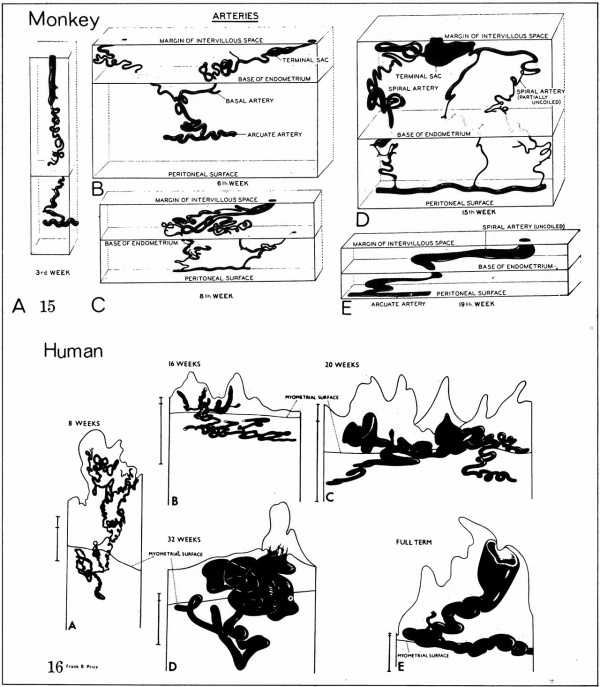
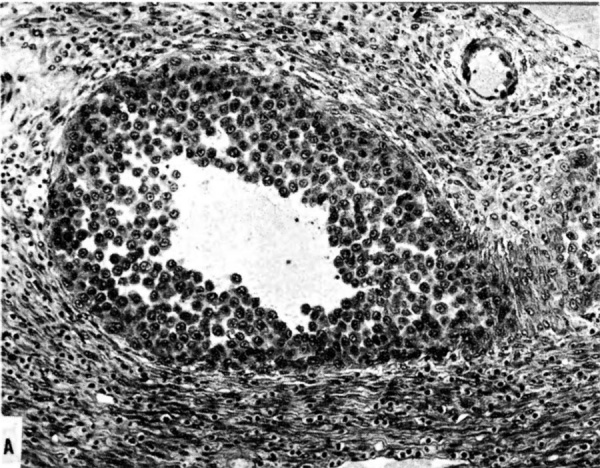
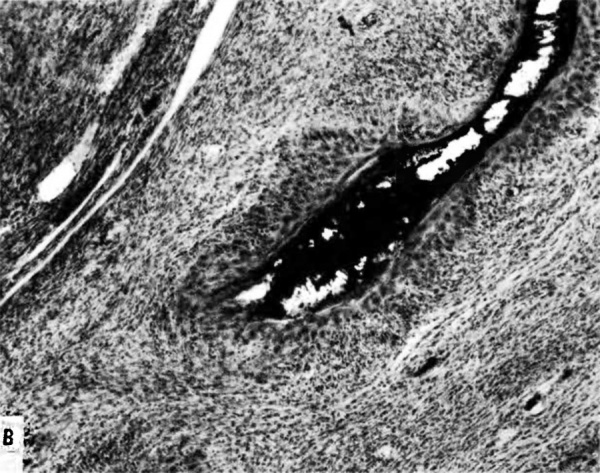
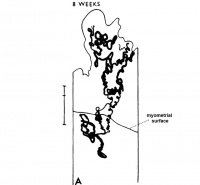
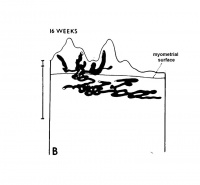
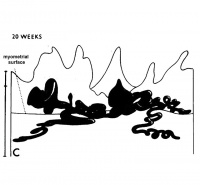
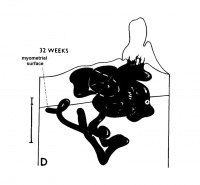
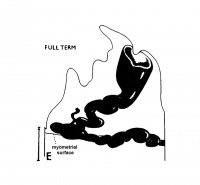
The implanting ovum achieves its first contact with the maternal blood supply when the penetrating trophoblast both taps and engulfs capillaries of the subepithelial network (Fig. 2), permitting maternal blood to seep, under very low pressure, into the lacunae of the trophoblastic shell. With progressive penetration by trophoblast, the terminal tips of spiral arteries are opened up and maternal arterial blood flows into the shell. This meanwhile has itself been enlarged and transformed by the development of chorionic villi (Fig. 3). It is around the villi, in the inter-villous space, that the maternal blood now flows and from now on we may speak of a placenta and placental circulation. Reconstructions of representative uteroplacental arteries, both human and monkey, at comparable stages of gestation (Fig. 4), show that there is very little qualitative change in growth pattern during the first weeks after implantation (Harris and Ramsey, 1966; Ramsey, 1949). The coiling of the arteries continues and there is just a slight indication of a new process at the arterial tips where trophoblast is beginning to replace normal wall structure. Soon however a change does become manifest. Arterial elongation (as determined by careful micromeasurements) is continuing, but the thickness of the endometrium is being diminished as the result of trophoblastic erosion combined with pressure of the overlying conceptus. Thus, the previously vertical arterial stems are diverted toward the margins of the implantation site, an increasingly sharp angulation developing. With the continuation of these processes in succeeding weeks, the increased coiling of the artery is no longer suflicient to effect its accomodation in the thinned endometrium, so back and forth and lateral looping is added. A terminal dilatation of the artery develops proximal to its point of entry into the intervillous space.
Fig. 2. Photomicrograph of an early human implantation. (a) trophoblastic lacunae; (b) maternal capillaries. Carnegie Collection 8004, 7th day of pregnancy, section 11-4-4. (Reprinted with permission from Hertig and Rock. Contrib. Embryol. 31: 65-84, 1945.)
At about mid-pregnancy when, as Reynolds has shown (Reynolds, 1947), the enlargement of the uterus by growth of its parts gives place to enlargement by stretching, the coils of the arteries are “paid out,” as coils of rope on the deck of a ship are paid out when the space between ship and anchorage is increased. The coils are more fully smoothed away in the monkey than in man, probably because monkey endometrium undergoes the greater stretching.
Fig. 3. Photomicrograph of a portion of a monkey placenta in situ. Chorionic plate above; entrance of an endometrial spiral artery into the intervillous space at the left. Carnegie Collection C-477, 29th day of pregnancy, section 47b.
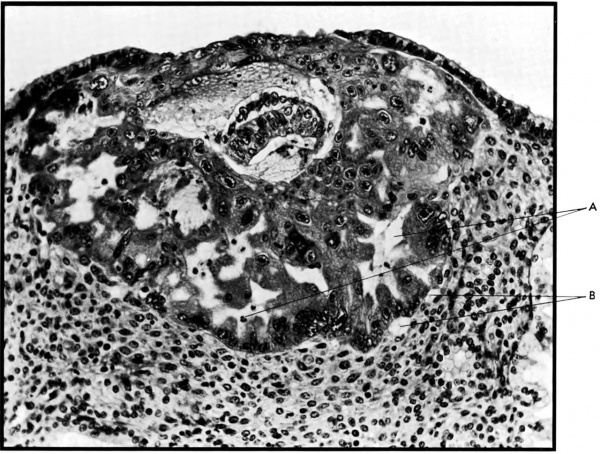
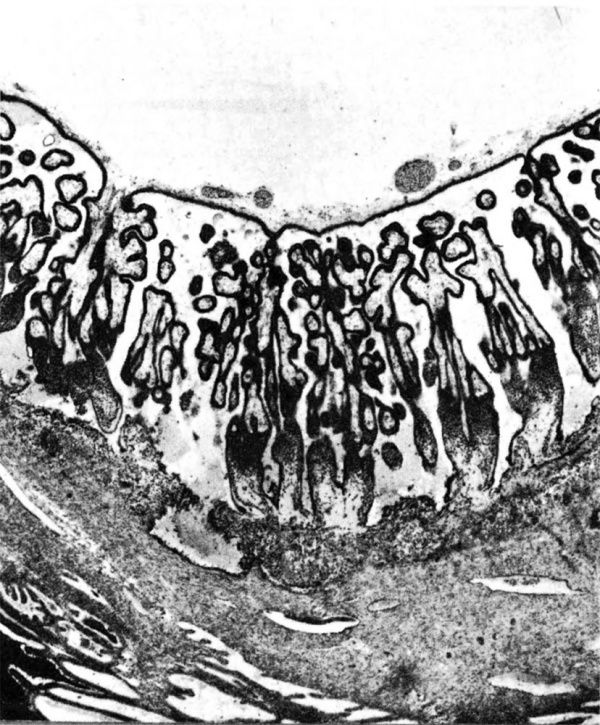
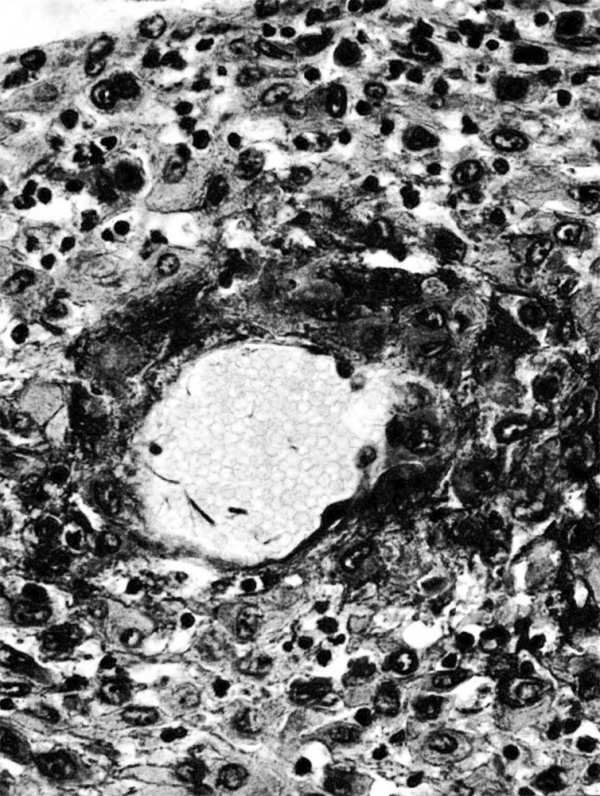
The terminal dilatations of arteries communicating with the intervillous space appear to be the result of the weakening of the vessel wall brought about by replacement of muscle and elastic tissue by trophoblast. Appearing first as an intraluminal accumulation (Fig. 5a), the trophoblastic cells gradually invade and replace the vessel wall (Fig. 5b). The invasion is earlier in the monkey and baboon than in the human, but it is deeper and more extensive in the latter. Human cytotrophoblast penetrates the endometrial stroma as well as entering the arterial lumen and invasion of the wall proceeds from without as well as from within (Fig. 6). The more drastic elimination of normal vascular wall resistance in man doubtless occasions the larger and more persistent terminal dilatations of human uteroplacental arteries. A further result of greater trophoblastic activity in the human is the erosion of arteries all the way to the midendometrium where branches arise from the main spiral stems. These branches then communicate with the intervillous space which explains why there is a proportionately greater number of arterial entries in humans than in monkeys. Upon occasion the trophoblastic action, in contrary fashion, may cause occlusion of branches or even main arterial stems.
Venous drainage, at all stages of the reproductive cycle, is a less dynamic aflair than arterial inflow. The basic venous pattern in the endometrium is a grid with dilatations into venous lakes at the junction of vertical and lateral limbs. These relationships continue into pregnancy with certain of the vertical channels increasingly distended as they are required to accommodate the ever increasing volume of placental blood. Other channels are passively obliterated by external compression.
On the physiological side there is again continuity between prepregnant and pregnant states. From the standpoint of circulation, this is most apparent in the persistence of an intrinsic contractile potential in the spiral arteries. This is manifested during the menstrual cycle by isolated contractions at the myoendometrial junction which produce ischemia leading to foci of endometrial necrosis and slough (Bartelmez, 1957), and in pregnancy by intermittency of flow through individual spiral arteries into the intervillous space (Martin, McGaughey, et al, 1964).
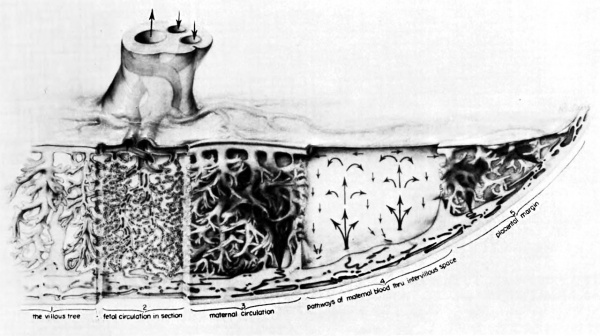
The opposite number to uteroplacental circulation is of course fetoplacental circulation. Propelled by the vis a tergo of fetal blood pressure, fetal blood courses through the umbilical arteries into the subdivisions which run laterally through the chorionic plate. Finally, the vessels dip into the substance of the placenta and travel through the arborizations of the fetal villous tree. -They proceed in comparable subdivisions to the terminal villi. There the fetal capillary bed, coming into its closest proximity to maternal blood in the intervillous space, forms the ultimate area of matemalfetal exchange. Oxygenated blood returns via vessels running through the same villous stems to the umbilical vein and thence to the fetal body (Martin and Ramsey, 1970).
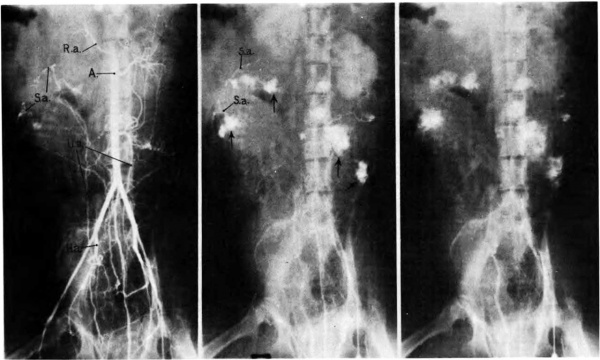
The mechanism of ‘circulation within the placenta, first hypothesized upon the basis of anatomical data, has been established by radioangiographic studies (Donner, et al, 1963). Especially with cineradioangiography, it is possible to visualize directly the inflow of arterial blood to the intervillous space (Fig. 7),. its circulation through the space, and finally its drainage back to uterine veins.
| Maternal Placental Artery Remodelling | ||||
|---|---|---|---|---|
|
|
|
|
|
| GA 8 weeks | GA 16 weeks | GA 20 weeks | GA 32 weeks | term |
Fig. 4. Diagrammatic representations of the course andlconfiguration of the uteroplacental arteries in the rhesus monkey and man, at comparable stages of gestation. (Reprinted with permission from Harris and Ramsey. Contrib. Embryol. 38: 43-58, 1566.)
The propulsive force throughout is the head of maternal blood pressure which drives blood into the intervillous space in discreet, fountain-like “spurts.” The incoming blood wafts aside the villi surrounding the orifices of entry, but once the propulsive force is reduced, in part by the, baffle action of the villi, the blood disperses laterally crowding the existing content of blood through the venous orifices in the basal plate into the uterine drainage channels (Fig. 8). During uterine contractions both inflow and outflow cease, in whole or in part, depending upon the strength of the contraction (Ramsey, Martin, McGaughey, et al, 1966). The volume of the placental pool, however, is maintained. That is to say, the old concept that “contractions squeeze the placenta like a sponge” is incorrect; rather blood is trapped in the placenta during contractions.
Fig. 5a. Photomicrograph of uteroplacental arteries in the monkey illustrating early accumulation of trophoblast within the lumen of the artery. Carnegie Collection C-477, 29th day of pregnancy. (Reprinted with permission from Wislocki and Streeter.‘ Contrib. Embryol. 27:1—66, 1933;.)
Fig. 5b. Photomicrograph of uteroplacental arteries in monkey illustrating subsequent replacement of the" arterial wall without trophoblastic penetration of stroma. Carnegie Collection C-629, 53rd day of pregnancy. (Reprinted with permission from Ramsey. Contrib. Embryol. 33:l13—147, 1949.)
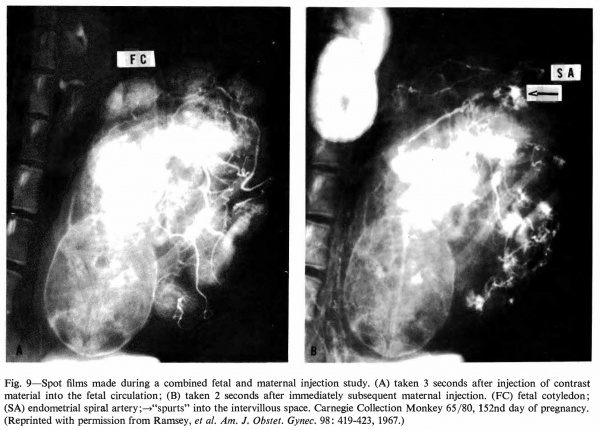
Radioangiography of the fetal side of placental circulation (Martin, Ramsey, and Donner, 1966) shows the progress of blood from the fetal body into the capillary network of the fetal cotyledons and back via the umbilical vein. Double injection of a radiopaque medium (Ramsey, Martin, and Donner, 1967 ), that is, into fetal and maternal circulations in rapid succession, permits visualization of the 1:1 relationship" between maternal spiral arteries and fetal cotyledons (Fig. 9).
Fig. 6. Photomicrograph of a human uteroplacental artery showing replacement of wall and penetration of stroma by trophoblast. Carnegie Collection 10117, 85th day of pregnancy. (Reprinted with permission from Ramsey. Prenatal Life. Wayne State University Press, 1970, pp. 37-53.)
Two points of clinical interest emerge from the foregoing. The first is that placental circulation ceases during strong contractions. That this may present the fetus with periods of anoxia is clear and should contractions be unduly prolonged, as the result of pathology or medication, it could indeed be critical. Second, and somewhat mitigating the implied threat of the cessation of flow, is the fact that the pool of placental blood is preserved throughout. Thus, under normal conditions, continued maternal-fetal exchange is made possible.
And that exchange, of course, is the whole purpose of the long and elaborate procession of vascular changes from Day 1 of the menstrual cycle to parturition.
Fig. 7. Photographs of X rays made at 2, 3, and 7% seconds respectively after injection of contrast material into a femoral artery of a monkey. (R.a.) renal artery; (S.a.) endometrial spiral artery;—>“spurts” into intervillous space. Carnegie Collection Monkey 60/14, 100th day of pregnancy. (Reprinted with permission from Ramsey, er al. Montanino Editore, Napoli. ll: 1779-1784, 1962.)
Fig. 8. Composite drawing of the primate placenta to show its structure and circulation. (Drawing by Ranice Davis Crosby for E. M. Ramsey. Courtesy of the Carnegie Institution of Washington.)
Fig. 9. Spot films made during a combined fetal and maternal injection study. (A) taken 3 seconds after injection of contrast material into the fetal circulation; (B) taken 2 seconds after immediately subsequent maternal injection. (FC) fetal cotyledon; (SA) endometrial spiral artery;—>“spurts” into the intervillous space. Carnegie Collection Monkey 65/80, 152nd day of pregnancy. (Reprinted with permission from Ramsey, et al. Am. J. Obstet. Gyrtec. 98: 419-423, 1967.)
References
Bartelmez GW. The form and the functions of the uterine blood vessels in the rhesus monkey. (1957) Contrib. Embryol., Carnegie Inst. Wash. 36:153—182.
BARTELMEZ, G. W. The form and the functions of the uterine blood vessels in the rhesus monkey. Carnegie Inst. Wash., Contrib. Embryol. 36:153—182, 1957.
DONNER, M. W., RAMSEY, E. M., AND CORNER, G. W., JR. Maternal circulation in the placenta of the rhesus monkey; A radioangiographic study. Amer. J. Radiol. and Roentgen. Therapy. 901638-649, 1963.
Harris JWS. and Ramsey EM. The morphology of human uteroplacental vasculature. (1966) Contrib. Embryol., Carnegie Inst. Wash. Publ. 625, 38: 43-58.
Hertig AT. and Rock J. Two human ova of the pre-villous stage, having a developmental age of about seven and nine days respectively. (1945) Contrib. Embryol., Carnegie Inst. Wash. Publ. 557, 31: 65-84.
MARTIN, C. B., JR., MCGAUGHEY, H. S., JR., KAISER, I. H., DONNER, M. W., AND RAMSEY, E. M. Intermittent functioning of the uteroplacental arteries. Am. J. Obstet, Gynec. 90:8l9—823, 1964.
MARTIN, C. B., JR. AND RAMSEY, E. M. Gross anatomy of the placenta of rhesus monkeys. Obstet. Gynecol 36:167 177, 1970.
MARTIN, C. B., JR., RAMSEY, E. M., AND DONNER, M. W. The fetal placental circulation in rhesus monkeys demonstrated by radioangiography. Am. J. Obstet. Gynec. 95:943947, 1966.
RAMSEY, E. M. The vascular pattern of the endometrium of the pregnant rhesus monkey (Macaca mulatta). Carnegie Inst. Wash., Contrib. Embryol. 33:113—147, 1949.
RAMSEY, E. M. Placental circulation in rhesus and man. Prenatal Life. Proceedings of the Third Annual Symposium on the Physiology and Pathology of Human Reproduction. Harold C. Mack (ed.). Detroit: Wayne State University Press. 1970, pp. 37-53.
RAMSEY, E. M., CORNER, G. W., JR., DONNER, M. W., AND STRAN, H. M. Visualization of maternal circulation in the monkey placenta by radioangiography. Scritti in onore del Prof. Giuseppe Tesauro nel XXV anno del Suo insegnamento. Montanino Editore, Napoli. II:1779—1784, 1962. 68
RAMSEY, E. M. AND HARRIS, J. W. S. Comparison of uteroplacental vasculature and circulation in the rhesus monkey and man. Carnegie Inst. Wash., Contrib. Embryol. 38:59 70, 1966.
Ramsey EM, Martin CB & Donner MW. (1967). Fetal and maternal placental circulations. Simultaneous visualization in monkeys by radiography. Am. J. Obstet. Gynecol. , 98, 419-23. PMID: 4986963
Ramsey EM, Martin CB, McGaughey HS, Kaiser IH & Donner MW. (1966). Venous drainage of the placenta in rhesus monkeys: radiographic studies. Am. J. Obstet. Gynecol. , 95, 948-55. PMID: 4958128
REYNOLDS SR. (1947). Uterine accommodation of the products of conception; physiologic considerations. Am. J. Obstet. Gynecol. , 53, 901-13. PMID: 20247845
Wislocki GB. and Streeter GL. On the placentation of the macaque (Macaca mulatta), from the time of implantation until the formation of the definitive placenta.. (1938) Contrib. Embryol., Carnegie Inst. Wash. 721-66.
© VCU. Licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported License. http://creativecommons.org/licenses/by-nc-sa/3.0 Acknowledgement of the Virginia Commonwealth University Libraries as a source is required.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Placental circulation. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Placental_circulation
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G