Paper - Description of a horseshoe kidney
| Embryology - 27 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Boyden EA. Description of a horseshoe kidney associated with left inferior vena cava and disc-shaped suprarenal glands, together with a note on the occurrence of horseshoe kidneys in human embryos. (1931) Anat. Rec. 51(2): 187-211.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Description of a Horseshoe Kidney Associated with Left Inferior Vena Cava and Disc-Shaped Suprarenal Glands, together with a Note on the Occurrence of Horseshoe Kidneys in Human Embryos
Edward A. Boyden
Institute of Anatomy, University of Minnesota
Five Text Figures and Two Plates (Five Figures)
Introduction
In reading the literature of any subject that goes so far back into the history of anatomy as the horseshoe kidney, one sometimes encounters references that may not be put aside until the flavor of the original dissertation has been duly sampled. Such a one is the “Isagogae Breves” of Berengarius, that pioneer research in the anatomy of separate parts of the body. In contrast to the enthusiastic, speculative writings of the early microscopists, here is recorded, in simplest fashion, an original observation made by one of the first systematic prosectors of the human body, in the year 1521, upon a subject “publiquely anatomized in our exercise at Bononia.”
In that individual the kidneys were continuous as if it were one kidney; and it had two veins, and two emulgent arteries, and two ureters (‘poros uritides’) with a single enveloping membrane; and it occupied the usual positions of the kidneys, likewise the middle of the back, which is in the place between the spleen and liver, a little below them.[1]
Since the time of Berengarius some 500 cases of fused kidney (ren unguliforrnis, arcuatus, soleiformis, etc.) have appeared in the literature, yet three recent comprehensive studies testify to the importance that is still being attributed to this striking anomaly.[2] To the clinician it presents an ever recurring problem in diagnosis for which more detailed information regarding the range and character of deviation is constantly being sought. To the anatomist it offers a fertile field for inquiry, since the critical stage in the development of this common anomaly has never been observed in the mammalian embryo.
As indicated by the title, the present specimen (a dissectingroom case, found in a white male, fifty-eight years of age) has two distinctive features (pl. 1). The first is its association with a left inferior vena cava — the only instance of this combination of anomalies that I have been able to find in the literature, although at least four cases of double postrenal Vena cava have been so reported (Schlesinger, ’24).[3]
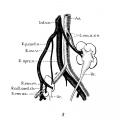
The second feature is the unusual form of the suprarenal glands — thin discs roughly quadrilateral in shape and lying against the muscles over the first lumbar vertebra, some distance above the upper poles of the horseshoe — as if they had been left to develop by themselves Without pressure from below (fig. 1). This, presumably, is a consequence of the unusually low position of the fused kidneys in this specimen, the left limb being coextensive with the second, third, and fourth lumbar vertebrae, the right not extending above the third lumbar vertebra. As formulated by Howden, in 1887, and previously noted by earlier observers, the suprarenal gland is not displaced when the kidney is ectopic, but is usually found in its normal position, “showing that its ordinary relation to the kidney is really one of accident”; and that when not “moulded on the top of the kidney,” the shape of the suprarenal gland is somewhat rounded in contour. In most cases of horseshoe kidney, however, the two poles are capped by characteristically modeled glands.
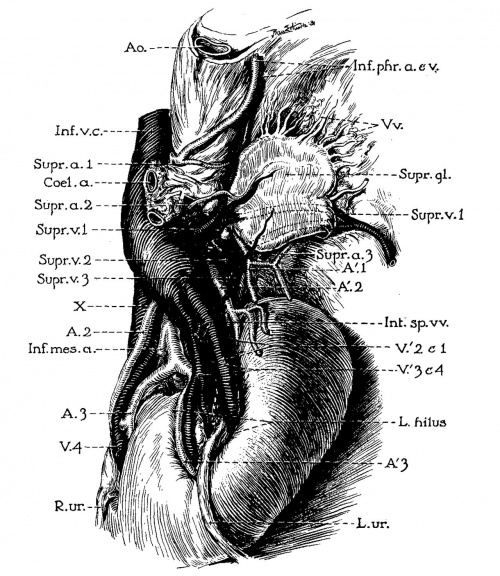
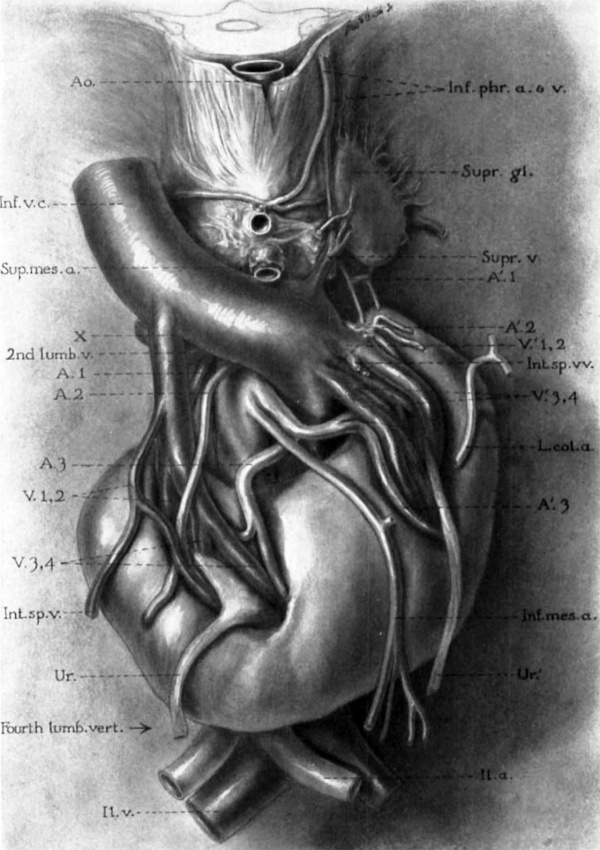
Fig. 1 Detail of same horseshoe kidney that is shown in plate 1; drawn from left side to display shape and relations of suprarenal gland. A’. 1, 2, 3, left renal arteries (1, dorsolateral branch of aorta in hiatus aorticus, arises at level of first lumbar vertebra; 2, lateral branch below superior mesenteric artery, at middle of second lumbar vertebra; 3, ventrolateral branch, from level of inferior mesenteric artery, between second and third lumbar vertebrae); A.Z,3, right renal arteries (see legend, pl. 1) ; Int.sp.m2., left internal spermatic veins (double); Supna. 1, 2, 3, left suprarenal arteries; Suprxv. 1, 2, 3, left suprarenal veins (note union of first two to form a vessel that resembles the adrenolumbar vein of quadrupeds) ; V’. 1, 2,3, 4, left renal veins; V. 4, fourth right renal vein: X, common trunk of right renal and internal spermatic veins.
Aside from these two distinctive features and the exceptionally broad area of fusion, this shield—shaped specimen conforms to the majority of horseshoe kidneys in that it is asymmetrical and located in the midline; that the axes of the two lobes converge downward; that its anterior surface shows traces of fetal lobulations; that its isthmus consists of parenchyma which unites the inferior poles of the two kidneys and passes anterior to the aorta and vena cava; that there are two ureters and pelves only; that each hilus is anterior and situated about midway between upper and lower poles; that the ventral lip of the hilus is poorly differentiated; that the ureters pass anterior to the isthmus; that each limb of the horseshoe is supplied by multiple renal vessels; and that the organ as a whole is placed somewhat lower in the lumbar region than the conventional position, but not as low as would be the case if the degree of fusion were greater (Rokitansky). So far as one could judge from cadaver material, it was not a movable kidney. Nor apparently was the decease of the patient due to the anomaly.[4]
Veins
Turning now to consideration of the venous system, the question arises as to whether the aberrant development of the kidneys has induced the formation of Vessels not ordinarily appearing in ontogeny,[5] or whether we are dealing merely with common variants, i.e., with the atypical persistence of veins normally present in the embryo. Quite likely a correct answer to this question would necessitate a study of the developing veins in embryonic cases of horseshoe kidney.
On the basis of the adult relations shown in plate 1, the venous pattern is probably best interpreted as a persistence of the left supracardinal vein of the embryo as defined by McClure and Huntington in their monograph of 1929.[6] This interpretation assumes that each kidney is drained by the definitive embryonic renal veins. On the left side this is quite obvious. The four renal veins (V’ 1, 2; V’ 3, 4) enter the vena cava at the level of the second lumbar vertebra and may be considered derivatives of renal veins I and II of the embryo. (Compare, also, with fig. 2, in which specimen both supracardinal and two left renal veins persist.)
On the right side, the modification due to the ectopic position of the kidney is so great as to raise doubt about the orthodoxy of the right renal veins (pl. 1). To be sure, there are four of them, which may be grouped into two pairs (V. 1, 2; V. 3, 4), the common stem of which (vein X) may represent a fusion of renal veins I and II of the embryo (compart with Fu, fig. 7).[7] The difiiculty with this interpretation is that it calls for an explanation of the origin of the right internal spermatic vein from primary renal outgrowths. Even if vein X be considered a drawn-out portion of the right renal collar to which both renal and sex veins are attached, it is still necessary to explain how one of the four renal Veins can drain into a sex vein. A third choice is to define the right internal spermatic vein as merely a branch of the first renal vein (V. 1) as indicated by the legend. With this in view, let us first consider the disposition of the right sex vein in those anomalous cases of left inferior vena cava in which the kidneys retain their normal position.
Three patterns seem to prevail. Either the right sex vein branches from the horizontal portion of the inferior vena cava (McClure and Butler, ’25, fig. 16), or from a small remnant of the right supracardinal Vein (V. Alten, ’13; Maxwell and Erwin, ’28), or from the proximal portion of the right renal Vein (Waring, ’94; Frankel, ’l0; Gladstone, ’12). These three types are easily interpreted on the basis of current diagrams explaining the origin of the sex Veins. But the attachment of the sex Vein to a distal portion of a renal Vein, as in plate 1, is obviously a result of some modifying factor, such as lack of rotation of the kidney in embryonic stages.[8] In searching the literature for pictures of unrotated kidneys, I have found that sometimes the sex vein branches from a renal vein as far distal as the hilus. Two such cases, found in unrotated but otherwise normally placed kidneys, are figured by McClure and Huntington (’29, pp. 105 and 109). More striking are the examples appearing in an ectopic, unrotated right kidney (fig. 7) and in a horseshoe kidney (fig. 9).
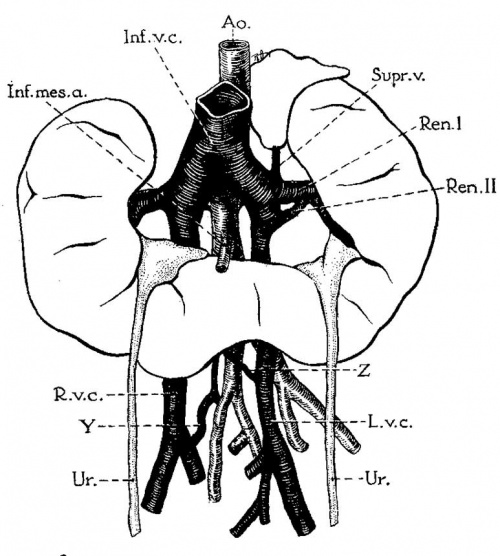
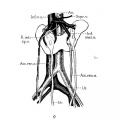
Fig. 2 Horseshoe kidney of seven-year-old girl, associated with double vena cava that lies posterior to kidneys; representing persistence of right and left supmoardinal veins of embryo. (After Marzynski, case 3; legends by E. A. B.). Note fetal lobulations of kidney. Ren. I and II, left renal veins representing persistence of primary embryonic renal veins (compare McClure and Butler, fig. 14); Y, remnant of intersupracardinal anastomosis which has retained a. cranial and caudal connection with the right supracardinal vein (McClure); Z, persistence of cardinal collateral channel (McClure).
Fig. 3 Multilobular fused kidneys of male cat, associated with double vena cava that lies anterior to kidneys; representing persistence of right and left posterior cardinal veins of embryo. (After 0. E. Johnson, case 2; legends by E. A. B.) Note division of kidney into four lobes. Only the two lowest are fused.
What is the explanation of this atypical pattern of the sex Veins in cases of unrotated kidney? Although I have searched without success for persisting cases in human embryos, nevertheless a study of sections of the normal 15—mm. embryo suggests that possibly the posterior cardinal vein may be concerned in this deviation. If the kidney remains unrotated, its hilus becomes applied to the posterior surface of the Wollfian body. Between the adjoining surfaces of these two organs will be located the posterior cardinal vein (laterally), the subcardinal Vein (medially), and frequent anastomotic channels connecting the two veins. What is more natural than that from an early period the capillaries of this anterior hilus should drain into the posterior cardinal vein or the channels connecting it with the subcardinal vein”! Several possibilities then remain. The posterior cardinal Vein may persist (as in fig. 8) and receive on its median side drainage from the kidney. If the subcardinal vein also persists throughout its length, then the sex vein may empty either into the Vena cava or into the upper portion of the posterior cardinal vein (fig. 8). If, however, the sex Vein loses both of its upper connections but retains the lower channels situated between the hilus of the kidney and the mesonephros, then the sex vein may drain into veins emerging from the anterior surface of the kidney, i.e., either into renal branches of the posterior cardinal vein, or, should the latter fail to persist, into branches of the definitive renal veins as they emerge from the hilus.
Obviously, there will be intermediate stages that can be interpreted either way. While Fu (fig. 7) probably represents a fusion of embryonic renal veins I and II, with low attachment of the sex vein (as explained in the preceding paragraph), it is not at all impossible that the renal vein that connects the sex vein with the vena cava represents a persistence of the upper segment of the right posterior cardinal vein. In figure 10 one or both of the upper pair of internal spermatic veins may in their upper parts be of posterior cardinal origin. Similarly, vein X of plate 1 is susceptible to interpretation as being either a posterior cardinal or a renal derivative, with a sex vein emptying into a renal branch at the hilus of an unrotated kidney.
On the other hand, the more medial position of vein X, which descends on the right side of the body as a continuation of the hepatic portion of the inferior vena cava, suggests that vein X may be a persistent right subcardinal vein. This interpretation is rendered the more plausible by a comparison with two drawings published by McClure and Butler. In their case of left inferior vena cava (fig. 16) the subcardinal vein has the same position as vein X of plate 1. It is not connected with either supracardinal or renal vein, and the posterior cardinal has dropped out. Assuming now that the right kidney is too inferior in position for the right renal vein to develop as a branch of the renal collar, then the subcardinal, by virtue of its mesonephric connections, is in position to take over the drainage of the kidney. To present it in a different way, if the right supracardinal vein and renal collar in figure 15 (of the paper cited) were suppressed, all the conditions for interpreting vein X as of subcardinal origin would be fulfilled. These several hypotheses, however, merely serve to emphasize the need for studying developmental stages of veins in persisting unrotated kidneys. Such embryonic specimens will doubtless appear; and then only, may one ascertain how arrested migration or rotation of the kidney has modified the venous pattern.[9]
Arteries
Less diificult to interpret are the three pairs of arteries that supply the kidney. These appear not to have arisen de novo — as in some cases of ectopic or horseshoe kidneys (fig. 8, 9) — but seem to represent merely a persistence of three of the nine pairs of mesonephric arteries from which the definitive renal pair is usually derived. According to Felix (Keibel and Mall, II, fig. 572), these nine arteries fall into three natural divisions: 1) a cranial group consisting of two pairs of arteries running dorsal to the suprarenal glands (these eventually shift their origin to the dorsal side of the aorta and form the group from which the phrenic arteries are usually derived); 2) a middle group of three arteries, passing through the glands but retaining a lateral attachment to the aorta—the group from which the suprarenal arteries are usually formed; and, 3) a caudal group of four arteries, passing ventral to the suprarenal glands and originating from the ventral side of the aorta—the group from which the sex arteries are usually selected. The definitive renal arteries are said to represent a persistence of either the lowest of the second or the highest of the third group.
Applying these criteria to the case at hand, it would seem that on the left side the first of the renal arteries (A’. 1, fig. 1) represents the fifth mesonephric artery—this, because it is the lowest artery to the suprarenal gland and because it also supplies the upper pole of the kidney. Its shift in origin to a dorsolateral position on the aorta and to the level of the first lumbar vertebra may be accounted for by the embryonic peri-aortic plexus described by Bremer. On the right side, the corresponding artery is apparently missing, owing perhaps to the much lower position of the right kidney.[10] The remaining arteries all reach the hilus of one kidney or the other and unquestionably are derived from group 3, since they come from the aorta at the level of the first two lumbar vertebrae and have no connection with the suprarenal glands. The lowest of the three pairs are of special interest in that the left one gives off a median branch to the isthmus and that both arise by a common stem which leaves the aorta at the lower margin of the second lumbar vertebra, just to the left of the root of the inferior mesenteric artery (pl. 1). This probably identifies it with the ninth pair of mesonephric arteries.
The suprarenal arteries are likewise three in number, at least on the left side (fig. 1). The lower two seem to correspond in level to group 2 of Felix’s classification, but quite likely the superior one, in view of its association with the inferior phrenic artery, is derived from group 1. The striking feature of the suprarenal circulation, however, is the presence of accessory veins on the left side, there being three suprarenal veins instead of the usual one. This, perhaps, is a consequence of the greater expansion of the glands, permitted by the failure of the kidney to reach their inferior poles. Another unique feature is the union of the first two veins lateral to the suprarenal gland (Supnv. 1, 2, fig. 1), suggesting the adrenolumbar vein of lower mammals. This does not, however, supply the body musculature, but is confined to the subperitoneal fat of the infraphrenic surface.
Embryonic Cases of Horseshoe Kidney
In concluding the description of cases, it seems desirable to summarize what is known about the occurrence of horseshoe kidneys in human embryos. At least three such instances have been described in the literature.
The first of these is the case briefly reported by Bonnet (’11) at the Leipzig meeting of the German Anatomical Association. Two years later, apparently the same case was described in detail by Budde, writing from Bonnet’s laboratory in Bonn, although no reference is made to the earlier account. This is by far the most interesting case, not only because it is the youngest, but because it is the only one that is illustrated or adequately described. Even so, one would wish that its topographical relations had been displayed by reconstructions of one sort or another. It was found in a 19-mm. embryo, six weeks of age. The isthmus consisted of parenchyma, containing collecting tubules of the first order not yet united with kidney tubules, which formed a bridge between the two kidneys immediately above their inferior poles. It lay anterior to the bifurcation of the aorta at the level of the fourth lumbar vertebra. Above the isthmus the limbs of the horseshoe sharply diverged. There were no anomalies of the blood vessels. The hilus of each kidney was anterior, indicating that fusion had preceded rotation. In the absence of any distinctive feature, Budde attributed the fusion of the kidneys to the low position of the stomach and to the enormous expansion of the liver.
The second case was briefly described by Felix in the Keibel and Mall Handbuch (German edition, 1911, p. 844). J azuta wrongly assigns this case to Keibel. Felix writes as follows:
I found it in an embryo of 30 mm. greatest length. The united parts form a transverse bar caught by the inferior mesenteric artery and are retained by this in their caudal position. Nevertheless, the right and left kidneys may develop further in a Wing—like manner and eventually almost reach the normal position of ununited kidneys. What the cause of union of the two blastemata may be is unknown, but even in the transverse bar the constituents of the two kidneys are sharply separated.
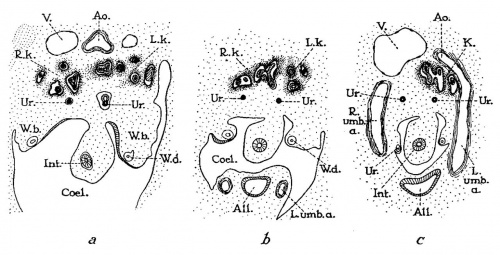
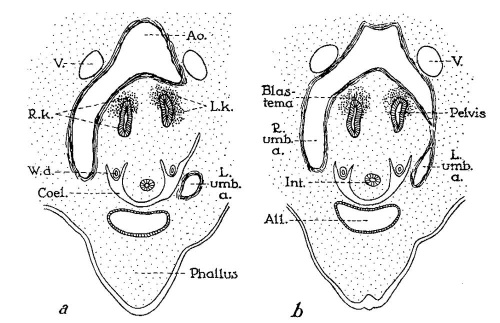
Fig. 4 Cross-sections through 14 mm human embryo (age, thirty-six to thirty seven days), illustrating what is presumably the youngest known stage in the formation of the horseshoe kidney. (a, b, c, from text figures 47, 48, 49 of embryo “Hg” of His collection (after Keibel, ’96). a is eighteen sections (each 6.6 /4) above b, and the latter is eighteen sections above c. Note increas ingly intimate fusion of right and left kidney masses (R.7c., L.k.) in passing from superior to inferior levels, until maximum fusion is reached at point where kidneys (K) are emerging from embrace of umbilical arteries (L. and R.umb.a.). Um, ureter; 17., posterior cardinal veins; W.b., Wolflian body; W.d., Wolflian duct.
The third case, by Jazuta (’24), appeared in a still older embryo of about 35 mm in length. Here, as in the above instances, each hilus faced anteriorly and the isthmus connected the inferior poles of the two kidneys. In contrast to Bonnet’s case, the isthmus consisted of kidney tubules only, no trace of straight tubules or medullary tissue being found. For this reason, J azuta interprets the anomaly as due to an excess of nephroblastema formed by the pro—, meso—, and metanephros as they succeed one another in development. What may, perhaps, be designated as a fourth case appears in Keibel’s classical account of the development of the human urogenital apparatus (’96). In figures 47, 48, and 49 (see fig. 4), he draws the blastemas of right and left kidneys of a 14-mm. human embryo as if continuous and as if the fusion were increasingly intimate as the sections reach that part of the kidney which lies in the crotch formed by the junction of the umbilical arteries (fig. 4, c). While Keibel does not make the application, three facts favor the interpretation that this is a horseshoe kidney in process of formation: First, Keibel was a most meticulous and precise worker. What he saw he reproduced with great accuracy. Secondly, in this long article of 101 pages and 153 figures, he was concerned with the portrayal of a series of complicated and everchanging processes in which details of the kidney blastema were distinctly secondary to his main purpose. Thirdly, he drew all sections of other embryos with the kidneys well separated (fig. 5). In any event, this embryo emphasizes the very close proximity of the blastemas at this early period, and indicates what little disturbance of normal relations is needed at this critical period in order to produce fusion of the kidneys.
Discussion
Numerous explanations have been advanced to account for the formation of horseshoe kidneys. In general, these theories fall into two groups—those dealing with extrinsic causes and those that attribute fusion to deviations that are inherent in the primordia of the kidneys.
Illustrative of the second group is an explanation advanced by certain Russian investigators — Jazuta, Jefremow, and others — namely, the theory that two ureters may grow into a common blastema.[11] The difficulty with this interpretation, as Ssokolow has pointed out, is that the two ureteric buds must also grow toward the midline in order to meet the fused blastemas. While this concurrent deviation of ureter and nephrogenic tissue is theoretically possible, there are certain observations that are unfavorable to this interpretation.
Among these is evidence regarding the position of the kidneys in marsupials. As Keibel pointed out long ago (p. 148, l.c.), the ureteric buds in embryos of these primitive mammals regularly grow out toward the midline, thereby preventing fusion of the Miillerian ducts and accounting for the persistence of two vaginae. Such a dorsomedial growth of the ureteric buds should therefore increase the likelihood of fusion, if growth of the ureter in this direction is essential to the formation of horseshoe kidneys. Yet this seems not to be the case; for, in the following personal communication (for which I am originally indebted to Doctor Grreenman, of The Wistar Institute) Dr. Charles S. Apgar writes as follows:
In a series of twenty-four opossum embryos, ranging in length from 5.56 mm. to 13.5 mm., not one showed evidence of fusion of the kidneys.
Out of a total of some sixty opossums of various ages, autopsied at the University of Pittsburgh over a period of three years, I do not recall any instance of horseshoe kidney.
Approaching the problem from a different angle, early investigators (e.g., Meckel, 1812) sought to explain the horseshoe kidney on the basis of arrested development—either restricted to the kidney or involving other congenital defects (vide Broesicke and Ognew). Yet, horseshoe kidneys are not characteristic of anencephalic or other teratological monsters, but commonly occur in otherwise normal individuals. This fact is substantiated by the experiments of Bagg, who subjected mice to light doses of unfiltered X-rays and thereby succeeded in breeding a race of descendants that perpetuated numerous defects of the kidney. Among such strains were embryos with polycystic kidneys and congenital hydronephrosis, indicating interference with the union of ureter and nephrogenic tissue and of Wolffian duct and cloaca. But although selection increased the variety and number of such defects, deviation was never in the direction of fusion of right and left kidney anlagen. (For complete description of these defects, see Brown, ’31.)
Fig. 5 Cross-sections through 11.5 mm human embryo (age, thirty-two to thirty-three days), illustrating normal position of kidneys in crotch of umbilical arteries. a,b (two sections apart) from text figures 29 and 30 of embryo “H. s. Bul.” (after Keibel, ’96). Note that pelvis of each kidney is directed anteriorly, an unrotated position that is retained in most cases of horseshoe kidney. All, allantois; Ao., aorta; Coel., body cavity; Int, intestine; L. and R.umb.a., left and right umbilical arteries; W.d., Wolffian duct.
It is not strange, therefore, that most authors have favored the interpretation that fusion takes place after each ureteric bud has penetrated the blastema of its own side, and that factors external to the kidney are responsible for union of these bilateral organs. Nevertheless, great confusion exists regarding these external factors. One reads about the expansive force of the duodenum; of the crowding downward of the kidney by the liver, stomach, and Wollfian body; the narrowing of the embryonic pelvis; of variations in lumbar and sacral vertebrae; of anomalies of the pelvic muscles, vessels, nerves, and urogenital organs; of excessive hair development; of tail formation; of presence of coccygeal fovea; of teratomata; of defective girdle migration, etc.— in fact, of everything that might exert mechanical pressure upon the emergent kidneys. From these speculations, which have largely emanated from contemplation of the adult anomaly, one turns with relief to the intuitive, yet concrete, embryological analysis of Lewis and Papez, the only published account of which is comprised in the following brief abstract:
In the summer of 1914, the collection of 165 series of 10 to 12 mm. pig embryos used for class instruction at the Harvard Medical School, was carefully examined to detect anomalies in the region of the developing kidneys. Although fused kidneys of the horseshoe type are said to occur not infrequently in adult hogs, the proportion of cases is not such that a kidney of this type might be expected in the number of series examined, and none was found. However, the normal relations of the kidneys to one another varied in such a way that we may offer a new explanation of this anomaly, namely, that it is due to the relation of the kidneys to the bifurcation of the aorta into the umbilical or common iliac arteries. This bifurcation forms a U—shaped crotch in which the kidneys are lodged, and from which they escape by migrating upward. The arteries, as a mechanical obstruction, tend to bring the right and left renal blastemas close together, so that fusion may readily take place. A fusion at the upper poles, making a horseshoe kidney convex superiorly, would probably arise earlier than the fusion at the lower poles, in which case the horseshoe would be convex inferiorly. The relations of the kidneys which seem to justify this interpretation have been demonstrated in a series of models.
Although the relations described by Lewis and Papez are better demonstrated by models, figure 5 indicates how small a shift in the position of the arteries is needed in order to bring the kidneys together.
Finally, if fusion occurs soon after the time when the kidneys enter the arterial girdle, then the critical stage in the formation of the horseshoe anomaly must be placed at an earlier period than has hitherto been supposed. As upward migration of the kidney does not begin before the 9 mm stage (Pohlman) and the kidneys of the His embryo (figured by Keibel) have already united at the 14 mm stage (thirty-six to thirty-seven days), the critical period may be located somewhere between thirty to forty days after conception — probably nearer the lower than the upper limit.
Summary
A description is presented of a fully dissected horseshoe kidney associated with left inferior vena cava—presumably the first report of this combination of anomalies in man.
Modification of the venous system is discussed in relation to arrested migration and rotation of the kidneys.
Confirmation of the Lewis and Papez theory of the cause of horseshoe kidney is found in a hitherto unrecognized embryonic case figured by Keibel, in which fusion of the kidneys has occurred previous to the 14 mm stage, a.t a time when the kidneys are migrating upward through the U-shaped crotch formed by the umbilical arteries.
Appendix
In connection with the above account, the writer undertook to find out whether or not there is any mention of the horseshoe kidney in the sixteenth-century codification of the Talmudic law known as the “Yoreh De‘ah.” Through the courtesy and generous assistance of Rabbi S. I. Levin, of Minneapolis, it is now possible to answer this question in the affirmative and to present a résumé of that section of the Yoreh De‘ah that deals with the anomalies and pathological modification of the kidney. For a general account of the rules governing the examination of animals by the ancient Hebrews, the reader is referred t.o a previous article (Boyden, ’26).
Section 44. The laws of terefah pertaining to the kidney.[12]
If the kidneys are absent, the animal is kosher [suitable for eating].[13]
If there are three or one kidney, the animal is kosher.[14]
If there is a hole in the kidney or hilus, or if the kidney is cut to the hilus by a sword, the animal is kosher; but if the hilus is swollen, then the animal is terefah [unfit for food].
If the meat of the kidney is like dead meat [i.e., falls to pieces if touched], which sickness reaches to the ‘White spot’ or even is restricted to the sinus of the kidney, the animal is terefah.
If it contains water that is turbid, even if the dead part has no smell, it is terefah provided? that it reaches the sinus. But if the water is clear and it tastes like salt, it is kosher even if it reaches the sinus. If the water is in a sac [cyst?], but is clear, then the animal is kosher. [Here, a difference of opinion is recorded: some rabbis say that if the kidney is sick and the water turbid or the meat deformed, then the animal should be declared terefah, even if such areas do not reach the sinus. But the decision of the law leaves it kosher.]
If blood is present, it is the same as if it were water. If the water is honey-red, the animal is still kosher. If muddy, it is terefah. (Some say it is terefah, if the honey—red reaches to the sinus.)
If stones are found in the kidney, even to the sinus, the animal is kosher.[15]
If the kidney is shrunken by sickness to the size of a bean [in calves or sheep] or to the size of a grape [in big cattle], the animal is terefah. [How may one tell that it is made small by sickness? The proof is in the capsule. If the capsule is shrunken, the kidney is sick; if it is smooth, then the kidney was that way from birth, and the animal is kosher. There are rabbis who say that if both kidneys are absent, i.e., reduced by sickness to nothing, then the animal is terefah. What shall be the proof? If the capsule of the kidney is filled With fat, it shows that this was its condition at birth; but if the capsule is empty of fat, it shows that the kidney has been present but has dissolved or melted away.]
If there are three or more kidneys and one has shrunken but the other two remain sizeable, the animal is kosher. [Some authorities are of the opinion that if the three kidneys are on one side and one of these is too small, then the animal is terefah, since the other two might be affected by being on the same side. Much depends on the color, although this must extend to the sinus. If the kidney is blue or green or red, the animal is kosher; if yellow, it is terefah. Kidneys of fowls are never terefah, because the kidney of birds is enclosed in a bone so that the intestines cannot reach it.]
If the kidneys are connected together, even at the pelvis, the animal is kosher.[16]
Footnotes
- ↑ This excerpt is taken from the edition of 1523 (p. 17 verso, opposite p. 18) from a volume deposited in the Boston Medical Library. For an exact copy of the title page and of the Latin text from which the above translation is made, the writer is greatly indebted to Prof. Frederic T. Lewis, of the Harvard Medical School.
- ↑ The most recent statistics, compiled by Ssokolow ('30), indicate that the horseshoe kidney occurs, on the average, once in 875 autopsies and four times as frequently in clinical cases.
- ↑ Two of these, from a. dissertation by Schiirholz, have not been available for study. The other two are reproduced as figures 2 and 3 of this article.
- ↑ Cause of death unknown, but wrongly attributed by coroner ’s report to coronary sclerosi.
- ↑ Compare with the modification in venous pattern caused by hydronephrosis of the Wolffian body in chick embryos (fig. 6, Boyden, ’24).
- ↑ In a personal communication, Professor McClure states that in his estimation, the cae represents a type C, as defined in the monograph jut cited (see fig. 50, p. 127), which has been modified in relation to an abnormal (pathological) fusion between the kidney anlagen, ventral to the aorta and left supracardinal vein.
- ↑ According to McClure and Butler (fig. 14 and text, ’25), the anterior renal vein (I) of thehuman embryo is a lateral branch of the subcardino-supracardinal anastomosis. The posterior one (11) is a branch of the ascending supracardinal vein, and usually disappears. (For persisting case, see fig. 10.) Both I and II divide dichotomously upon reaching the hilus, making a possible total of four veins that drain the kidney should the division reach back to the vena cava. In plate 1 the left anterior vein (V’.1,£’) ha 0 divided, but the branches of the posterior one (V’. 3, 4) are still united by a common stem.
- ↑ In accordance with current usage, the writer employs the term ‘rotation’ to indicate a change in the position of the hilus. For discussion as to whether there is an actual rotation of the kidney around its long axis or whether it is merely an apparent rotation due to a faster growth of the ventral lip of the hilus, see Priman, ’29.
- ↑ Since completing this article, the writer has found a dissecting-room specimen in which the anterior surface of a small unrotated, left ectopic kidney was literally covered by a venous plexus. This vascular network was drained by ix veins. One emptied into a. small left supracardinal vein; another, into the median side of a double left ovarian vein that encircled the kidney laterally; a. third, into a dilated remnant of a collateral cardinal channel lying in front of the bifurcation of the aorta; the fourth and fifth, into the left external iliac veins; the sixth, into the uterine vein, deep in the pelvis minor. In addition, there was a. complete renal collar encircling the. aorta at the level of the right kidney, but lacking the definitive renal veins on the left side. From this brief description it may be seen that the venous plexus of the hilus of this unrotated kidney retained connections with all three primary longitudinal venous systems of the embryo.
- ↑ The data for the right suprarenal gland are incomplete, since the gland was injured in removing the liver, to which it adhered. Nevertheless, no trace of a suprarenal artery to the upper pole of the right kidney could be found. This is all the more plausible, in view of the fact that the right sup:-arena] gland was separated from the kidney by the length of the second lumbar vertebra.
- ↑ While considering the irnprobability of primary fusion of nephrogenic tissue, the writer found such a case in a 10-mm. human embryo of the Minnesota collection. In this specimen (to be described in a subsequent paper), the right and left blastemas were definitely fused, yet each remained distinct, due to the fact that the right one (which surrounded the end of the right ureter) was differentiating, whereas the left one remained undifferentiated. Further search revealed the fact that on the left side the ureter and lower half of the Wolfiian duct were absent. Incidentally, this extends to the human embryo the observation originally made upon chick embryos, namely, that when the Wolffian duct is prevented from reaching the cloaca, experimentally, the renal blastema of that side develops up to a certain point, but fails to differentiate tubules in the absence of a. ureter (Boyden, ’24, ’27). More applicable to the point at issue is the new evidence that the blastema of one side cannot be stimulated to differentiate into tubules in the absence of its own ureter, even if it be in contact with an adjacent blastema that is undergoing normal development.
- ↑ At least three parts of the kidney were recognized by Talmudic authorities: 1) that part which ‘covers’ the organ (t11e capsule) ; 2) that part that is ‘bent’ (the hilus); and, 3) that part which is ‘white’ (the sinus). Of these, the last was considered most important and was referred to as the ‘life of the kidney.
- ↑ This rule is an exception to the Mosaic law which holds that total absence of an organ renders an animal unfit for food. Apparently this is based upon observation of newborn or stillborn animals. According to the Hebrew ritual, if an animal (other than bird) is born prematurely, or if there is doubt about the age, it may not be eaten until the end of the eighth day.
- ↑ This recognition of single and multiple kidneys apparently antedates the identification of similar congenital variations by European anatomists, although Aristotle mentions a unilateral kidney defect. According to rabbinical commentary, this observation dates back to a codification known as “Or Zarua,” composed about 1260 by Rabbi Isaac Ben Moses, of Vienna.
- ↑ Although the discovery of gallstones must be attributed to the ancient Hebrews (Boyden, ’26), this is not true for renal calculi, which were well known to the Hippocratic School. Apparently the first Hebrew record of stones in the kidney appears in the thirty-six chapters on the examination of slaughtered animals (Book “Rachaach”) written by Rabbi Eleazar Ben Judah, of Worms (1176-1238).
- ↑ This observation dates back to “Bayit Hadash” (1631-1640), written by Rabbi Joel Sirkes, of Cracow, and apparently represents the earliest Hebrew account of the horseshoe kidney.
Literature Cited
V. ALTEN, HANS 1913 Ueber linksseitige Lage der Vena. cava inferior. Anat. Anz., Bd. 43, s. 337-348.
BAGG, HALSEY J. 1925 Hereditary abnormalities of the viscera. I. A morphological study with special reference to abnormalities of the kidneys in the descendants of x-rayed mice. Am. J our. Anat., vol. 36, pp. 275312.
BERENGARIUS (GIACOMO BERENGARIO DA Curr) 1523 Isagogae B1-eves,pel1ucidae ac uberrimae in Anatomiam humani corporis a communi Medicorum Academia usitatae, a Carpo in Almo Bononiae, si Gymnasio ordinariae Chirurgiae Docente, ad suorum Scholasticorum preces in lucem datae. (Date in colophon.) (From copy in Boston Medical Library.)
BONNET, ROBERT 1911 Verh. der anat. Ges., Bd. 25, S. 211.
BOYDEN, EDWARD A. 1924 An experimental study of the development of the avian cloaca, with special reference to a mechanical factor in the growth of the allantois. Jour. Exp. 7.061., vol. 40, pp. 437-472.
- 1926 The accessory gall bladder. An embryological and comparative study of aberrant biliary vesicles occurring in man and the domestic mammals. Am. J our. Anat., vol. 38, pp. 177-232.
- 1927 Experimental obstruction of the mesonephric ducts. Proc. Soc. Exp. Biol. and Med., vol. 24, pp. 572-576.
Bremer JL. The origin of the renal artery in mammals and its anomalies. (1915) Amer. J Anat. 179-198.
BROESIKE, G. 1884 Ein Fall V011 congenitaler S-ffirmiger Verwachsung beider Nieren. Virchow’s Archiv., Bd. 98, S. 338-341.
BROWN, ALICE L. 1931 An analysis of the developing metanephros in mouse embryos with abnormal kidneys. Am. J our. Anat., vol. 47, pp. 117-172.
BUDDE, WERNER 1913 Ein sehr friihes Stadium von Hufeienniere. Anat. Hefte, Bd. 48, S. 297-306.
FRAENKEL, WALTER 1910 Linksseitige Vena cava inferior. Anat. Anz., Bd. 37, S. 240-241.
Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979.
GLADSTONE, REGINALD J. 1912 A case of left inferior vena cava, etc. Jour. Anat., vol. 46, pp. 220-227.
HOWDEN, ROBERT 1887 Case of misplaced kidney with Vundeseended testicle and rudimentary vas deferens on the same side. Jour. Anat. and Physiol., vol. 21, pp, 551-557.
JAZUTA, K. Z. 1924' Ein Fall Von der Hufeisenniere bei einem menschlichen Embryo, zirka 35 mm. Anat. Anz., Bd. 58, S. 398-400.
KEIBEL, FRANZ 1896 Zur Entwiekelungsgesehichte des menschlichen Urogenitalapparates. Archiv. f. Anat. n. Physiol. (Anat. Abth.), S. 55-156, Taf. III-VII.
KOLLMANN, J. 1893 Abnormitiiten im Bereieh der Vena cava inferior. Anat. Anz., Bd. 85, S. 75-80, 97-116.
LEWIS, FREDERJC T., AND JAMES W. PAPEZ 1915 Variations in the early development of the kidney in pig embryos, with special reference to the production of anomalies. (Abstract) Anat. Rec., vol. 9, p. 105.
McCLUR.E., C. F. W., AND Gnonen SUMNER HUNTINGTON 1929 The mammalian vena, cava posterior. Am. Anat. Mem., no. 15. Wistar Institute, Phila.
McClure CFW. and Butler EG. The development of the vena cava inferior in man. (1925) Amer. J Anat. 35(3): 331-383.
MARZYNSKI, GEORGE 1915 Zur Diagnostik der Hufeisenniere. Deutsehe Zeitschr. f. Chir., Bd. 133, S. 281-300.
MAXWELL, E. V., AND G. S. ERVIN 1928 Four cases of anomalous inferior vena eavavwith an explanation of their developmental origin. J our. Anat., vol. 62, pp. 184-197.
NICHOLSON, G. W. 1927 The kidneys and their development. Guy’s Hosp. Reports, vol. 77, pp. 362-385.
OGNEW, B. W. 1930 Die Hufeisenniere des Mensehen in Zusammenhang mit Varianten im Bau anderer Organe. Anat. Anz., Bd. 69, S. 330-341.
POHLMAN, A. G. 1905 A note on the developmental relations of the kidney and ureter in human embryos. Bull. Johns Hopkins Hosp., V01. 16, pp. 49-50.
- 1905 Abnormalities in the form of the kidney and ureter dependent on the development of the renal bud. Ibid., pp. 51-60.
PRIMAN, JACOB 1929 A consideration of normal and abnormal positions of the hilum of the kidney. Anat. Rec., vol. 42, pp. 355-364.
ROKITANSKY 1849 A manual of pathological anatomy. London. Syd. Soc. Publ., vol. 2 (l.c., p. 185). ("Quoted by L. R. Shore, Jour. Anat., 1930, vol. 64, p. 352.)
RUBASCHEVA, ANAsrAsIA 1930 Zur Morphologie der Hufeisenniere. Zoitschr. f. urol. Chir., Bd. 30, S. 246-255.
SCHLESINGER, B. 1924 Beitriige zu den Lage- und Bildungsanomalien der Niere, des weiblichen Genitales und der Vena renalis sinistra. Virchow’s Archiv., Bd. 248,8. 297-322.
$1soI<oI.ow, B. M. 1930 Ueber die Hufeisenfiirmige Niorc. Anat. Anz., Bd. 68, S. 449-495.
WAHING, H. J. 1894, Left vena cava inferior. Jour. Anat. and Physiol., Vol. 28, pp. 46-50.
Plates
Plate 1
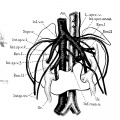
6 Horseshoe kidney dissected in situ. From white male, fifty-eight years of age. Note left inferior vena cava; disc-shaped suprarenal gland; multiple renal, suprarenal, and internal spermatic vessels.
Ao., thoracic aorta; A. 1, Z, 3, right renal arteries (1, from right lateral side of aorta at level of second lumbar vein; 2, from right anterior surface of aorta, 14 mm. above inferior mesenteric artery; 3, arises in common with AC3, from left anterior surface of aorta between second and third lumbar vertebrae); A’. 1, 2, 3, left renal arteries (see legend for fig. 1; and note median branch to isthmus of kidney); Fourthlumb/ucrt., bottom of fourth lumbar vertebra; Il.a. and Il.v., common iliac artery and vein; Inf.mes.a. and L.col.a., inferior mesenteric and left colic arteries (the accompanying branches of the portal vein are omitted from the drawing); Inf.phr.a.+12., left inferior phrenic vessels; Int.sp.'u.+m1., right and left internal spermatic veins ; End lumb.'u., second lumbar vein, branching from right inferior side of transverse portion of vena. cava (chief connection of latter with right ascending lumbar vein); Sup.mes.a., superior mesenteric artery; Supr.gl.+'u., left suprarenal gland and its principal vein; Ur. and Ur’., right and left ureters, passing anterior to isthmus of kidney; 17. and V’.1,2,3’, 4, right and left renal veins; X, common stern of right renal and internal spermatic veins (see text for interpretation).
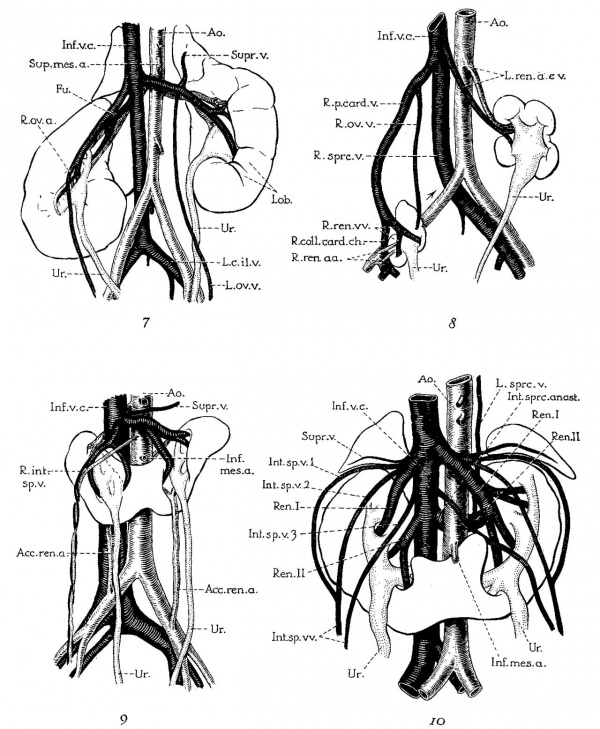
Plate 2
Figures of horseshoe and ectopic kidneys taken from the literature to illustrate Variations of renal and sex veins (legends by E. A. B.).
7 Unrotated, right ectopic kidney, from girl baby one year of age (after Schlesinger, ’24). Note fusion of two right renal veins (Fm), the distal origin of the right ovarian vein (R.ov.7;.), and fetal lobulations (Lob.).
8 Double ectopic, unrotated kidneys from a girl twenty-three years of age (after Melissinos, ’1l). Note position of diminutive right kidney on brim of pelvis, tl1e persistence of the right posterior cardinal vein (R.p.ea1'd.12.) and of the right collateral cardinal channel (I.’.coll.card.c7:..), the disappearance of the right common iliac vein (site indicated by arrow), and the origin of the right renal veins and arteries (R.ren.1m. and ac.) from the posterior cardinal vein and common iliac artery, respectively.
9 Horseshoe kidney, from a man forty-six years of age (after Ognew, ’30). Note distal origin of right internal spermatic vein (R.mI,.sp.v.) and the accessory renal branches of the iliac arteries (A(:e.ren.a.).
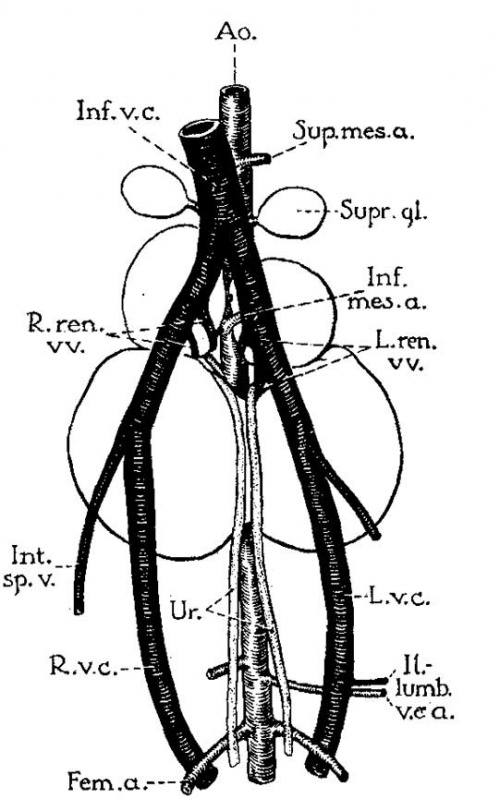
10 Horseshoe kidney from a man about forty years of age (after Ssokolow, ’30). Note arrangement of multiple internal spermatic veins (Int.sp.v. 1, 2,3), the persistence and Well—marked separation of both embryonic renal veins (Ren. I and II), the persistence of an intersnpracardinal anastomosis behind the aorta (1'nt.s;m*c.anast.) connecting the right supracardinal (Inf.o.c.) with the persisting left supracardinal (L.spr1;.) and renal (Re'rL.II) veins.
Cite this page: Hill, M.A. (2026, February 27) Embryology Paper - Description of a horseshoe kidney. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Description_of_a_horseshoe_kidney
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G