Paper - An anencephalic embryo of 25 mm CRL
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hunter RH. An anencephalic embryo of 25 mm. C.R. length. (1934) Ulster Med J. 3(2):105-112.4. PMID: 20475996
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
An Anencephalic Embryo of 25 mm. C.R. Length
By Richard H. Hunter, M.D., M.CE., PH.D., M.R.I.A.
from the Department of Anatomy, Queen's University, Belfast
One of the commonest malformations of the human foetus is anencephalus. Descriptions have been given of it since earliest times, but the first worker to give a clear account of it as “an arrested closure of the primitive neural groove” was von Recklinghausenl in 1886. Since this time his description has been accepted by all writers, and Faldino2 in more recent times has confirmed his work. This description, however, makes no attempt to discuss the factors which inhibit the closure of the medullary groove, and many writers since von Recklinghausen have made tentative studies to discover the basic influence. Gaddi3 claimed that a constant feature was the absence or aplasia of the supra-renal body, a claim substantiated by other writers. Mandruzzatio,4 in a long description of an anencephalic human embryo of 48 mm. length “from vertex to the podalic extremity,” states that there were present “noticeably hyperplastic supra-renal glands,” the converse condition from that found by Gaddi. The other ductless glands were not discussed.
With this divergence of opinion on the subject of the condition of the suprarenals, and without any observations on the other glands, the appearance of a
human anencephalic embryo of 25 mm. C.R. length in my laboratory was hailed as
an opportunity to study, in this malformation, the condition of the supra-renal and
other ductless glands, and to learn if support could be found for either of the views
expressed by the many writers on the subject. It must be remembered that the
development of this embryo is greater than its total length would at first suggest,
owing to the absence of head development. If the head had been of normal size,
the total C.R. length of the embryo would have been increased by about 10 mm.
The stage of development of the structures of the embryo, apart from the abnormal
regions, also suggest that it should be compared to an embryo of 35 mm., and as a
consequence I have adapted this standard for purposes of comparison.
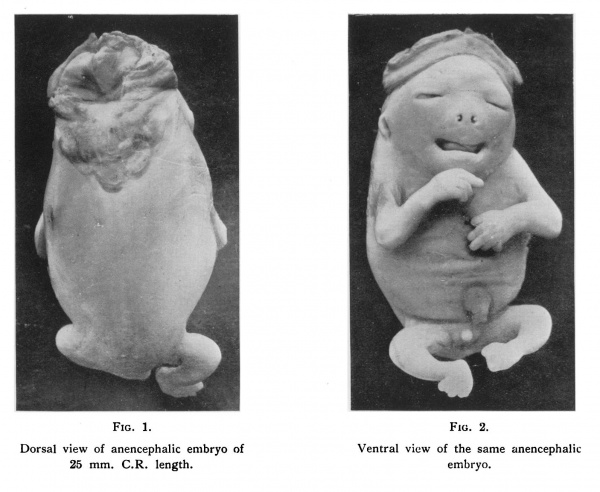
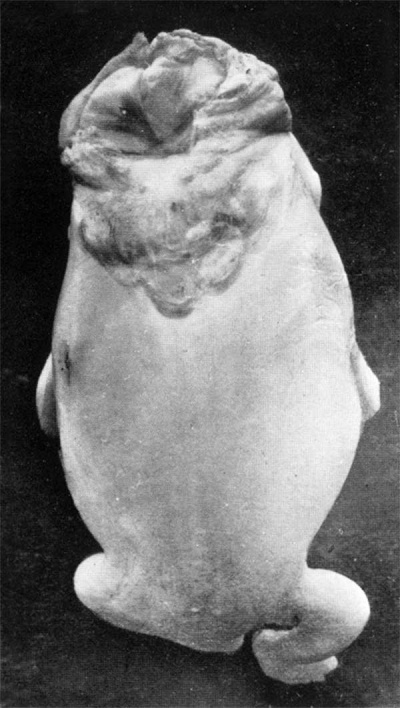
The embryo was first photographed (figs. 1 and 2), and then cut into transverse
sections of ten microns thickness. The sections were then stained in alternate slides
with haematoxylin and eosin, and with Mallory’s triple connective tissue stain.
These sections showed the supra-renal glands to be normal in development and
differentiation~ for an embryo of 35 mm. C.R. length.* They also showed the
- In a full-term human anencephalic foetus which I was enabled to study, marked hypoplasia
of both supra-renal glands was observed. This condition agrees with the description given of ‘all full-term anencephalics in the literature. It would seem, therefore, that taken in conjunction with the condition of the supra-renals of the embryo here described, and the embryo Vanencephalic embryo of 48 mm. C.R. length described by Mandruzzatio, the aplasia of the supra-renals is the result of the anencephalus, rather than the anencephalus the result of the failure of the supra-renal development.
thymus and the thyroid glands to be normal in size, shape, and position, as well
as in their histological differentiation. The condition of anencephalus therefore,
could not be the result of any lack of, or abnormal constitution of, the secretion of
any of these glands. The pituitary, however, did show changes from the normal.
There was complete lack of differentiation into the well-known sub-divisions of
pars anterior, pars intermediate, and pars posterior. It was present in the form of
a sparsely-filled sac, which most probably represented that part of the pituitary
developed from Rathke’s pouch, and it lay in the relationship to the body of the
sphenoid bone which it normally occupies. Histologically the tissue which composed
it showed a marked predominance of eosinophils over the basophils. This, with the
lack of development of the parts which normally arise from the infundibular
downgrowth from the floor of the mid-brain, were the only differences from the
normal which were found in this or any of the other ductless glands. This anomaly
of the pituitary, however, does not seem to have been the causative factor in
producing the condition, as Frazer5 in a description of an anencephalic embryo
states, “The pituitary body Was smaller than that in a 28 mm. specimen, but
almost as well developed.”
The open cranial fossa of the embryo was filled with a mass of fibrous tissue rich in blood-vessels. But it would appear that at one period in development, normal medullary tissue must have existed, as both optic bulbs were present. These showed the retinal tissue of each bulb to be thrown into folds of tissue consisting of differentiating ganglion cells, similar to those seen in a normally developing embryo of similar size to the one here under discussion, and as described by Mannfi in an encephalic embryo of 28 mm. C.R. length.
Nerve fibres, however, were not present leading from the retina backwards to
form nerve trunks. The retina, instead, extended postero-medially as a drawn-out,
cone-shaped prolongation lying within a space lined by cubical-shaped cells, which
were in continuity with the pigmented layer of the optic bulb, but which themselves
showed no evidence of pigmentation. Around this cone-shaped zone of retinal tissue
there was a mass of undifferentiated mesoderm continuous with that of the developing sclera. The optic muscles were present, and well developed. The inner ear was
also at a stage normal for an embryo of 35 mm. length, as were the circulatory,
respiratory, urogenital, and alimentary systems.
In the specimen which is here described, the skin of the neural tube area was
closed in the thoracic, lumbar, and sacral regions, although the cartilaginous neural
arches were ununited behind. But throughout these regions the ‘spinal cord was
represented merely by strands of fibrous tissue rich in blood-vessels, and closely
similar in appearance to the tissue which lay in the open base of the skull. The
latter condition would appear to correspond to the open form of spina bifida commonly found in the lumbar region of the body, as described originally by Kock,7 and in order to regularise terminology, it would seem better if it were termed
encephalo-bifida instead of the more usual term of anencephalus. The vertebral regions of this embryo, still following the terminology of Kock, corresponded to the
sub-cutaneous or cystic type of spina bifida.
Discussion
Although agreement has been reached in considering the open form of spina bifida as being due to a failure of the lips of the neural groove to meet, fuse, and differentiate from the ectoderm, the causative agent at work is unknown; neither has any agreement been found to explain the closed or subcutaneous form of spina bifida. Several explanations have been advanced, but none of them, unfortunately, has been substantiated by direct evidence. One suggestion, made by Wheeler,8 is that the developing neural tube after closure remains attached to the ectoderm, and that under such conditions the neural tube tissue is inhibited from differentiating. The two conditions, open and closed spina bifida, by accepting this explanation, would thus be the result of the same pathological agent, but acting at different stages of development, i.e., open spina bifida beginning before closure of the neural tube has occurred, and closed spina bifida after closure has occurred but before the neural tube has separated from the overlying skin.
Such an explanation, however, does not touch on the real problem, which is the discovery of the pathological agent which inhibits or prevents these changes. The problem of the causes underlying defective development in general has been discussed by Mall,9 who, as a result of a study of 163 pathological ova, suggested that malformations might be due to “injurious influences of atypical environmental factors,” due either to faulty nutrition of the embryo, or to imperfect elimination of the waste products of embryonic metabolism. Mall then makes the specific suggestion that the imperfect nutrition may be due to imperfect implantation of the ovum. Although such an explanation might be satisfactory for certain forms of malformations, it is not a satisfactory explanation for certain others, and the results obtained by experimental embryologists suggest that other agents are concerned with the production of spina bifida. These results point to a much earlier genesis : that organogenesis, normal and pathological, begins in the ovum itself, as the majority of defects produced experimentally are referrable to changes which can be induced physically on certain specific portions of the egg. The embryonal parts would appear to be pre-localized in the cytoplasm of the ovum, and that they make their appearance, in the words of Lankester, “as a sequel of differentiation already established and not visible.” VVorking on this assumption, Baldwin10 destroyed, by means of ultra-violet rays, specific areas on the equator of the white hemisphere of frogs’ eggs, and these eggs resulted in the formation of spina bifida embryos. These experiments of Baldwin, being specific in localization, are a refinement of, and give support to, the earlier work of Morganll and I-Iertwig,12 who found that by experimentally damaging frogs’ eggs, by means of chemicals, the closure of the blastopore was inhibited, and the process of closure of the medullary lips was as a consequence prevented. Many other experimental embryologists, working on the same lines, have obtained closely similar results. Stockard13 pointed to the chemical changes which are known to occur in the blood-stream of the mother in certain diseases, and he came to the conclusion that such abnormal chemical substances might possibly be the causative factors which produced the physical changes in the ovum, and which resulted in spina bifida. This view, however, was purely a hypothetical one, and was unsupported by any experimental evidence. VVerber,14 acting on the suggestions contained in Stockard’s paper, tried to prove its truth by direct experiment. He treated eggs of fundulus with chemical substances which occur in the blood of diseased persons, and he found that butyric acid and acetone produced “monsters analogous or homologous respectively to those found in human and mammalian foetuses.” These two substances are found in the blood of persons sufiering from diabetes, but before they can be accepted as the causative agents in the production of malformations, it would be necessary to show that such monsters appear in confinements of those who have had acetone and butyric acid present in the blood. Malpas15 touched on this point in a paper based on the study of a series of 294 foetal malformations which had occurred in a series of 13,964 deliveries. The statistics given by Malpas do not include the question of diabetes (acetone and butyric acid), although the observations made point to abnormal biochemical changes in the blood. His conclusions are summarized as follows: “Malformations are mostly attributable to the interplay of environmental factors, that among mothers there was very definite evidence of poor physique, and in many there was a low standard of living.”
Summary
It would appear the results obtained by experimental embryologists, taken in conjunction with the lack of evidence of any constant malformation of the ductless glands, or to constant malformations in cases of imperfect implantation of the ovum, that the condition of spina bifida must be due to some inherent defect in the egg itself, or to an injury produced physically or chemically to it before the primary divisions of cleavage have occurred. The possibility of biochemical changes in the blood-stream of the mother causing injury to the unsegmented egg cannot be excluded as being the pathological agent involved.
References
(1) VON RECKLINGHAUSEN, 1886, Virch. Arch., Bd. —, p.301.
(2) FALDINO, G., 1923, Chir. d. Organi di Mo-u. Bd. 7, p. 5.
(3) GADDI, P., 1885, Gas. Med. Ital. Milano, p. 21.
(4) MANDRUZZATIO, F. A., 1933, Amer. ]our. of Surg., Vol. 16, p. 107.
(5) FRAZER, ]._E., 1920, ]our. of Anat., Vol. 56, p. 14.
(6) MANN, IDA, 1928, “Development of the Human Eye,” London, p. 150.
(7) Kocx, W., 1881, Mitthen. fiber Fragen d. 'wissenschaft., Med. 1.
(8) WHEELER, T., 1918, Contrib. to Embry. Carnegie Inst. of Wash., Vol. 7, p. 91.
(9) MALL, F. P., 1908, ]our. of Morph” Vol. 19, p. 5.
(10) BALDWIN, W. M., 1915, Anat. Rec., Vol. 9, p. 366.
(11) MORGAN, T. H., 1894, Anat. Anzeiger, Bd. 9, p. 699.
(12) HERTWIG, 0., 1892, Arch. f. Mikr. Anat., Bd. 31, p. 353.
(13) STACKARD, C. R., 1909, ]ou1. Exp. Zool., Vol. 6, 530.
(14) WERBER, E. 1., 1915, Anat. Rec., Vol. 9, p. 560.
(15) MALPAS, P. W., 1933, Brit. Med. ]our., Vol 2, p. 699.
Figures
| Figure 1 | Figure 2 |
|---|---|
|

|
| Fig. 1. Dorsal view of anencephalic embryo of anencephalic 25 mm. C.R. length. embryo. | Fig. 2. Ventral View of the same anencephalic 25 mm. C.R. length. embryo. |
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - An anencephalic embryo of 25 mm CRL. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_An_anencephalic_embryo_of_25_mm_CRL
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G