Paper - A contribution to our knowledge of the earliest known stages of placentation and embryonic development in man
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Herzog MA. A contribution to our knowledge of the earliest known stages of placentation and embryonic development in man. (1909) Amer. J Anat., 9(3): 361-400.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Contribution to our Knowledge of the Earliest Known Stages of Placentation and Embryonic Development in Man
by
Maximilian Herzog.
From the Laboratory of Pathology of the Michael Reese Hospital, Chicago, Illinois. (1908)
With 30 Illustrations.
Introductory Remarks
The most important original contribution to our knowledge of the earliest stages of human placentation was made less than ten years ago by Peters, whose monograph, “Ueber die Einbettung des menschlichen Eies,” Wien, 1899, is well known to every student of the subject.
The present author, a number of years ago, became interested in human placentation, primarily in connection with the morbid anatomy and histopathology of ectopic gestation, and he has for years, as a matter of routine, searched for very young human ova in every uterus and Fallopian tube which fell into his hands, either as an operative or as a post—mortem specimen.[1][2][3][4] He has finally been fortunate enough to obtain a uterus from an absolutely unobjectionable case, containing an ovum, approximately like the Peters’ ovum, but including a well-preserved embryonic shield, younger, as it appears, than any human embryo heretofore satisfactorily described. The specimen was obtained in July, 1904, during his term of service as pathologist in the Government Bureau of Science, Manila, P. I.
The number of very young human ova unobjectionably adapted for a reliable study of the earliest stages of placentation is still exceedingly limited, and it may be said that We have not up to date a single specimen which is ideal and which will compare favorably with What can be obtained from the lower animals by timing conception accurately, removing its product from the living, and fixing it at once by the best methods at our disposal. Jung[5] in a recent monograph, to be mentioned more fully below, says in this connection: “The ideal specimen would be a uterus extirpated from a live woman, containing in situ an ovum of the first Week after conception. This ovum in situ, after removal from the uterus, should then be treated by aproved technical methods. The uterus itself should be free from those morbid changes which, according to common experience, pathologically alter the implantation of the ovum (myomata, chronic mctritis, etc.). We are yet far removed from this ideal. Not a single one of the specimens heretofore described satisfies even approximately these requirements, and it is at least doubtful whether we will ever have such a specimen at our disposal.”
The youngest and undoubtedly most valuable of these specimens is that of Peters. Without trying to detract from the great value and fundamental importance of the Peters’ specimen, it is proper to point out that it came from a case which strongly suggests the probability of pathologic changes.[6] Peter’s ovum was obtained from a woman who committed suicide by swallowing a large quantity of caustic potash solution, and who died three hours later. Such a rapid death after the ingestion of the fixed mineral alkalies is extremely rare, and must be connected with marked changes of the blood itself and of its circulation. I can find only the very incomplete record of a single similar case. that of a boy, said to have died three hours after swallowing lye.
The embryo in Peters’ specimen is said to be less well preserved than the chorion. It was described by Count von Spee as showing two very small epithelial cavities (the amnion and yolk sac), embedded in the chorionic mesoderm. The connection between the embryo and the chorion was thus so extensive that it was impossible to speak of an isolated body—stalk. Count von‘ Spec was uncertain whether “the first little anlage of an entodermal diverticulum (allantoic duct)” was present or not. The amniotic cavity was completely closed. It was lined in part by the very thin amniotic epithelium and in part by the tall columnar cells of the embryonic plate. A thin layer of mesoderm extended between the plate and the yolk sac, crossing the median line except toward one end of the embryo (thought to be the cranial end). Nothing is said of a neuronteric canal, or of blood vessels. Peters estimated the length of the embryo to be 190 microns.
A reconstruction of Peters’ ovum was made by Keibel, from nine teen outline drawings on 2 mm. wax plates prepared by Selenka, and found after his death. Nothing is reported concerning the model except the following.[7]
“Neither an allantoic duct nor an amniotic duct was found. The external surface of the yolk sac appeared uneven (hockerig), as if the ‘anlagen’ of vessels and blood had already been formed, but naturally this point could not be definitely decided from the wax plates and the model.”
After Peters’ ovum, the next youngest embryo enumerated by Keibel and Elze in the “Normentafeln” is the one described by Von Spee in 1896. It had a longest diameter of 1.84 mm., and a longest yolk sac diameter of 1.054 mm. Length of amnion and allantoic stalk, 0.76 mm. ; greatest width of the two latter, 0.76 mm. ; greatest width of yolk sac, 1.083 mm.; length of embryonic shield, 0.37 mm.; width of ectoblast plate, 0.23 mm.; length of allantoic duct, 0.35 mm. No amniotic duct was present. Between mesoblast and entoblast there were blood islands which caused the mesoblast to project strongly.
The authors of the “Normentafeln” believe that the embryo 1.74 mm. long described by Beneke, is older than Von Spee’s embryo. They consider that the description of Beneke’s specimen is unsatisfactory.
The Frassi embryo, the youngest examined in compiling the tables which accompany the “Normentafeln,” is described as follows: Length of embryonic shield, 1.17 mm.; width, 0.6 mm.; diameters of yolk sac, largest, 1.9 mm.; smallest, 0.9 mm. The embryonic shield is fiat, with a long primitive streak (0.5 mm.) and a shallow medullary groove. The medullary folds are not yet sharply defined At the anterior end of the primitive streak the canalis neurentericus is indicated (whether it is pervious is questionable) ; at the posterior end is the “anlage” of the cloacal membrane. There are no myotomes. “Anlagen” of vessels and blood are found on the yolk sac, and there are vessels in the allantoic stalk and adjacent chorion. There are no vessels in the embryonic shield. 'The amnion is closed, and there is no amniotic duct. An allantoic duct is present. The embryonic coeloin is not yet indicated.
Three embryos not considered in the “Normentafeln” are worthy of note; these have been described by Leopold, Teacher and Bryce, and Jung, respectively.
Leopold’s small ovum, which forms the basis of a monograph published in 1906, was obtained from a young woman who committed suicide by taking phosphorus. After a prolonged search of the interior of the uterus, Leopold found at the posterior wall of the corpus a small point, somewhat lighter than the surrounding tissue. A cubical piece of tissue containing this lighter point was properly fixed and cut into serial sections, each five microns thick. One hundred and sixty sections showed a very small cavity, which presented the following measurements: Length, 1.4 mm.; height, 0.9 mm.; thickness, 0.8 mm. In no section could Leopold find anything like an embryonic shield or an amnion, and hence he himself raises the question whether this ovum may not be a pathologic specimen.
We most decidedly believe that Leopold’s small ovum must be looked upon as pathologic. The absence of the embryonic shield must favor such a suspicion. We are either, it appears, dealing with some profound changes in an ovum, due to phosphorus poisoning, or possibly with the first stages of what would have been a hydatid mole.
Since Leopold published this monograph, it has generally been the tendency of those writing on the first stages of human placentation to discard his specimen from the list of young human ova. This is done by Jung, and we have one year previous to this author placed ourselves on record in the same sense.
Bryce and Teacher[8] have recently published an account of an ovum which they believe to be between thirteen and fourteen days old, and one day younger than Peters’ specimen. They received their specimen in “a mixture of urine and blood clot,” in which it had been immersed for twenty hours. It had been discharged by a healthy woman on November 5th, presumably as a result of the disturbance effected by coitus November 3d to 4th. The specimen was hardened in absolute alcohol and sectioned in paraffin.
Attached to the inner surface of the chorionic vesicle, two smaller vesicles were found. The larger, “torn and collapsed,” was attached to the chorion “definitely only at one point,” but the smaller was bound to the chorion by mesoblast strands, with which it was closely surrounded. The two vesicles were separate from one another. Bryce and Teacher consider that “after careful consideration of the sections and model the conclusion is inevitable that the larger vesicle represents the amnio—embryonic cavity and the smaller the entodermic vesicle or future yolk sac.” Page However, the embryo is so badly preserved that even this conclusion may be questioned, and on page 34 Bryce and Teacher discuss a different interpretation. In the very young embryo to be described in the present communication the yolk sac vesicle is larger than that of the amnion, and is attached to the chorion only at one point.
Jung’s ovum, fully and lucidly described in his recent monograph already quoted, is one of the very youngest and best preserved now on record. It was, however, not obtained in situ, but as the result of a curettage of the uterus, and was preserved in 80 per cent. alcohol, which, according to Jung’s own comparative studies, is not very favorable for the fixation of young human ova. In spite of this, its fixation has been very satisfactory, and karyokinetie figures have been preserved in the trophoblast, the decidua, and the embryo itself. According to the description given of the latter (p. 102, Jung, l.c.), the “Embryonalgebilde” was found in nineteen sections each on an average of 13 microns thick, making a total length of 247 microns. However, as Jung states, some of the sections containing the embryo had been lost. and an attempt at reconstruction had to be given up as futile. The whole “Embryonalgcbilde” is said to be enclosed within a thickening of the mesoblast in the interior of the ovum, attached to its basal surface. “Within the mass of mesoblast we see the ectoderm shield (‘Keimschcibe’ In transverse section it is a crescentic formation, with its concavity towards the basal side of the ovum. It is composed of high, cylindrical cells, some of which show mitoses. The extremities of the crescent are continuous with the closed amnion, which is composed of one layer of flat cells. The cavity formed by the embryonic shield and the amnion in transverse section has a lenticular shape, and it is filled with a finely granular coagulated mass. Externally the amnion and embryonic shield are surrounded by a thin layer of mesoblast, . . . which separates them from the yolk sac. The latter can only be seen distinctly in twelve sections. The yolk sac shows a simple layer of . flat entoderm cells. Toward the embryonic shield this entoblast rests directly upon the inesoblast which separates the ectodermalishield from the yolk sac; toward the other side the entoblast is strongly curved, and quite a distance removed from the mesoblast. The yolk sac, as a whole, has a somewhat hemispherical shape, with its base directed towards the embryonic shield. “The mesoblast, as already mentioned, extends from a broad base attached to the chorion towards the ‘Embryonalanlage,’ and forms ‘at its basal side the allantoic stalk. The latter is composed of mesoblast cells only, and shows no trace of an epithelial-lined duct. . . There are no distinct vessels. However, one sees here and there at the periphery of the mesoblast accumulations of cells, occasionally arranged in a circular manner, with a lumen in the middle. Similar formations are seen on the lateral portions of the allantoic stalk. I am unable to decide whether we are dealing with the first ‘Gefiissanlagen.’ The circular rings do not show in their lumen anything like blood corpuscles. Numerous mitoses are seen in the embryonic shield and in the allantoic stalk.”
History of the Case from which the Ovum Came
Our own ovum,[9] while not coming up fully to the most ideal requirements, approaches them so elosely that it must indeed be a very uncommon combination of circumstances which will furnish an investigator with a still better, equally young, human specimen. The ovum was obtained in situ from a healthy woman, with absolutely normal and healthy genitalia, who was almost instantly killed by a most peculiar accident.
The body of the woman was brought at once to the public morgue, and there placed in a large refrigerator. The post-mortem examination was made by the author, and the findings, as usual were recorded in the shape of a post—mortem protocol, which here follows, verbatim, as written down at the time.
Mrs. M. R, from Intramuros, Manilla; filipina, aged 25, died July 17, 1904. The post—mortem examination was made July 18, twelve hours after death. Immediate cause of death not known. It was stated that she had been struck by a small carriage (called carromata in Manila) shortly before she died.
Body of a well—developed young native woman, twenty—five to thirty years of age. Post—mortem rigidity strongly marked. Postmortem lividity quite noticeable. Abdomen somewhat distended. A repeated careful inspection fails to show any signs of external violence. No wounds, contusions or abrasions of any kind to be seen. On opening the thoracic cavity, the pericardium is found to be much distended, and shining through it there appears to be a firm, dark, blood coagulum. On opening the pericardium, it is found that it contains a large amount of dark, coagulated, gelatinous blood, and blood—tinged serum, distending the pericardium ad maximum and compressing the heart. A careful examination fails to show any perforation in the pericardium. The heart, which weighs 226 grams, presents a perforation, which begins two centimeters to the left of the anterior border of the interventricular septum. The perforation extends almost horizontally toward the left, being a little downwardly inclined. It forms a slit 2.2 centimeters below the sulcus of the heart. The edges of the perforation are almost clean—cut where they enter the myocardium, as if they had been produced by a dull, somewhat serrated knife. The cut takes a somewhat downward and inward course, traveling through the whole thickness of the myocardium. Whe1*e the cut enters the cavity of the heart, the margins are not very smooth, but rather irregular and ragged. The consistency of the myocardium is good. Its color is pinkish—brown, and all the serous surfaces are smooth. There are no atheromatous changes. The heart is covered with a very moderate amount of epicardial fat. In short, the whole organ, except for the wound, is absolutely normal. After the removal of the lungs (the apex of the right one showing a very few tubercles, and a little caseous nodule not larger than a lentil), it is seen that the second, fourth, and fifth ribs of the left side are fractured. The fracture of the second rib is found to be 7.5 centimeters posterior to the sternal articulation, that of the fourth one 9.0 cm., and that of the fifth one 9.5 cm. The anterior fragments are directed inwards. The fragments of the fourth and fifth ribs are very sharp and are surrounded by an area of subpleural blood extravasation. However, these fragments have not perforated the pleura costalis. The extravasated blood is strictly subpleural and no free blood is found on the surface of the pleura. The uterus appears somewhat enlarged, and the left ovary shows a fresh but already closed corpus luteum. On opening the uterine cavity a little hemorrhagic mass, about one—half centimeter or less in diameter, is found em~ bedded in the mucosa of the posterior wall, near the entrance of the left tube. This mass is carefully cut out and placed in Zenker’s solution, as it may contain a very young ovum. All the organs of the body, with the exception of the apex of the right lung, and the perforated heart, are found to be absolutely normal. They are all more or less congested with dark fluid blood. It appears clear that the woman must have been struck at the side of her body, or in the back, by a swiftly moving force. This force, however, did not produce any signs of external violence, particularly no contusions, abrasions, or wounds. The force traveled through the soft parts, and, meeting the resistance of the ribs, fractured them. The anterior sharp fragment of the fourth or of the fifth rib was evidently driven into the wall of the left ventricle, producing a complete perforation. A highly interesting point is that the sharp fragments neither perforated the pleura costalis nor the parietal layer of the pericardium. Only when the resistance of the firm wall of the ventricle was encountered did a rupture or perforation occur. A hemorrhage took place, and when the pericardium was completely filled, and the myoeardium much compressed, the heart’s action came to a sudden standstill. Death occurred from syncope at once.
Axnroiiicixn DIAGNOSIS.
' Fracture of the second, fourth, and fifth ribs of the left side. Complete perforation of the wall of the left ventricle. Hemorrhage into the pericardium. Compression of the myocardium. Beginning tuberculosis of the apex of the right lung.
Microscopic examination of the myocardium showed it to be perfectly normal.
To this history may be added the statement that it was later learned through the police reports that the woman had indeed been struck by the swiftly—moving shaft of a small carriage; that she fell forward, got up, staggered, fell again, and was dead within a very short time?
The small piece of tissue removed from the uterus was placed in Zenker’s solution, later washed in running water, and was then embedded in parafiin. Since our facilities for cutting serial sections were not the very best at this time (July, 1904) in l\'[anila, and since the writer was then engaged in the study of Bubonic plague, a few sections only were prepared, and the bulk of the block was preserved for future work. From the few sections examined, the firm impression was gained that the uterine mucosa presented the picture of a very early decidua, with cystic hemorrhagic gland spaces of the type of those of the Peters’ ovum. However, the first sections did not show either the cavity of the ovum or a trace of the trophoblast.
- The history of this case has previously been publislied by the author in a paper: “Peculiar (fases of '.l‘rz.uunatism of Internal Organs, Some Due to Tropical Conditions and Practices Surgery. Gynecolocy and Obstetrics, Vol. IV, No. 6, p. 741, Chicago, 1907; and Philippine Journal of Science, Vol. 2, 1907.
General Description of the Ovum
On June 15, 1907, the block of tissue was finally divided, and I am obliged to Dr. Day, Pathologist in the Chicago Laboratory of the Bureau of Animal Industry, for assistance in preparing a complete series of sections. The individual sections were all 7 microns thick; in all they numbered 303. About three sections were lost on the microtone; several subsequently floated off partly or entirely from the slides during the process of staining in hematoxylin and eosin, but fortunately none of the important sections containing the embryonic shield were lost.
The general outlines of the ovum are those of a bi—convex lens, a somewhat flattened elliptical body. The ovum was found in sections Nos. 63 to 235; the embryonic shield in sections 142 to 164. The measurements obtained by micrometer are the following:
Ovum (chorionic cavity-cXocoelom): Greatest length (section 153), 2.326 mm. Greatest width (section 153), 0.804 mm. Greatest thickness (17 2 sections at 7 microns each), 1.204 mm.
The trophoblast begins to show in section No. 47, and ends in soction>No. 264. Since the chorion mesoderm begins to show in section No. 63 and ends in section No. 236, the trophoblast on one pole is 16 sections or 112 microns thick, and on the other 29 sections, or 203 microns. In section 150 the trophoblast, towards the muscularis, is a little over one millimeter thick; towards the upper surface, 0.9 mm.
A list of the smallest human ova described, compiled from Peters’ and Jung’s collection, follows:
The author has iii the case under discussion and in a few cases of early tubal pregnancies attempted to obtain complete and perfect series of ova in situ by the paraffin enihellcling metliod. However, experience has taught him that young human ova in sitzi presenting‘ very heterogeneous tissue elements, including masses of maternal blood, are not best adapted for the paraffin method, but should preferably be embedded in celloidin.
Bryce and Teacher: 0.77, 0.63, 0.52 mm.
Peters: 1.6, 0.9,‘0.8 mm.
Herzog: 2.326, 0.804, 1.204 mm.
Graf von Spee: 2.5, 1.5 mm.
Jung: 2.5, 2.2 mm.
llertensz 3.0, 2.0 mm.
Beneke: 4.2, 2.2., 1.2 mm.
Leopold: 4.0, 3.7 mm.
Graf V. Spee: 4.0 mm.
von Heukelom: 4.5, 5.5.
Beigel—Loewe: 4.0, 5.0, 2.5-3.0 mm.
It appears from the above table that the author’s ovum is somewhat larger than Peters’ ovum. However, the real difference in the cubical contents of the two ova cannot be much, since the Manila ovum, while markedly larger in its longest diameter, is indeed not strictly elliptical, like the Peters’ ovum, but has the shape of a bico11veX lens. Tt is useless to speculate whether or not the Manila and the Peters’ ovum are of exactly the same age, or whether the former is a little older than the latter. We have in our case not made any efforts, at the time when the ovum was obtained, to get data as to menstruation and probable time of conception. Such an attempt would have been useless in the case of a full—blooded native from the lower walks of life. Even in those reported cases of very young ova, where these data were obtainable, the estimates as to the age of the embryo are not very correct. Peters estimated the age of his ovum as of three to four days; Leopold, an older ovum (4.0; 3.7 mm), as seven to eight days old. Jung thinks that the Peters’ ovum is considerably older than three to four days; Bryce and Teacher estimate its age at thirteen to fourteen days. Von Spee and Minot believe that seven to eight days intervene from the time of fertilization in the tube till implantation occurs in the uterus. The age of a human ovum, like that of the lower mammalian animals, whose early embryology has been sufficiently studied, can probably be best estimated from the stage of development of the embryo proper, but the data in this respect as to very young human embryos are insufficient.
Description Of The Embryo
The sections of the embryo have been accurately drawn under my direction by Miss Grace Amadon, and are shown in Plates I to IV, figs. 1 to The embryo is first seen in section 164 (fig. 1 of the embryo), and the ectoderm cells of the embryonic shield were last encountered in section 149. Hence the whole length of the embryonic shield proper is 16 by 7 microns equals 112 microns. However, the mesoderm extends as a thickened mass beyond the ectodermal limit of the embryonic shield from sections 150 to 143, or over a distance of 8 sections, equal in all to 56 microns. We have here what appears to be an extension of the shield—mesoderm beyond the shield ectoderm and entoderm—a mesoderm “Vorhof.” Including the mesoderm “Vorhof,” the embryonic shield extends through twenty—two sections; its whole length therefore is 22 by 7 microns equals 154 microns.
The first section (164) has hit the embryo in a tangential manner, and shows only a single layer of cells. The three following sections (figs. 2 to 4) consist of an inner layer of ectoderm and an outer layer of mesoderm. In the next section (fig. 5) a delicate strand of cells is clearly seen between the ectoderm and mesoderm. This must be interpreted as a subdivision of the mesoderm. It continues into the eighth section, in which the entoderrn first appears. The entoderm is represented by a small group of cells between the two layers of mesoderm, seen on the upper side of fig. 8. In fig. 9 the entoderm has expanded so that the cavity of the yolk sac now appears. It lies in the upper half of the figure.
The embryo is anchored to the chorion by an allantoic stalk, composed externally of mesoderm and traversed by a rather slender, somewhat curved canal of entoderm cells (figs. 9 to 14). The connection between the entoderm of the allantois and of the yolk sac presumably occurred in sections 156 and 155, but the continuity of the entoderm has been destroyed.
Around the allantoic stalk (figs. 10 to 13) where its mesoderm is continuous with the yolk sac mesoderm, there are found some solid and some open circular masses of mesoderm cells. The open rings are composed of three to four to five mesoderm cells; the solid round or oval cords contain a larger number of cells. These formations undoubtedly represent the earliest “anlage” of the yolk sac blood vessels. The chorion mesoderm and the mesoderm where it extends somewhat into the trophoblast do not yet show any traces of blood vessels.
The cavity of the yolk sac, which first appeared in section 156 (fig. 9) remains small and slit—like through sections 157 and 158. It then gradually increases and attains a transverse lateral diameter of about 176 microns in sections 152 and 150. In section 143, where the last of the “Vorhof” mesoderm is seen, the transverse diameter of the yolk sac is 192 microns. From here on it hangs down free for a considerable distance into the exocoelom. It can last be seen in section 122. Its entoderm and mesoderm layers are there very distinct. The yolk sac must have extended somewhat beyond section 122, but from 121 on it has been lost in the sections. It extended through at least thirty—four, and probably through forty sections, hence its greatest sagittal diameter was between 250 and 300 microns.
The cavity of the amnion, which has a diameter of from 100 to 160 microns in the first sections, becomes reduced to a canal in section 153, having a lateral diameter of 45 microns and a dorso—ventral diameter of 30 microns. This canal terminates in section 149 16). In the first sections of the embryo (figs. 2 to 8) the amniotic cavity is almost circular. Internally, it is bounded below by the thick embryonic shield, which is curved so as to form a deep crescent. Its concavity is toward the chorion. The ends of the crescent are continued as the very thin amniotic epithelium. However, the amnion is not complete in this region because its two lateral wings stop short and do not meet in the median sagittal plane. This seems clearly due to the artificial rupture of the very thin membrane. With equal certainty it may be said that the great concavity of the ectoderm shield is not artificial, but was present ante—mortem. So thick a layer, is not liable to distortion, and its cells show no evidence of disturbance.
Some embryologists have expected to find an inversion of the germ layers in early human embryos. The “Blattumkehr” in monkeys has been defined by Selenka as follows (Biol. Centralbl, Vol. 2, p. 552) :—
“Der Embryonalbezirk . . . ist gezwungen sich . . . im Inncrn der Keimblasse einzustiilpen, wobei das Entodcrm zur kappenartigen Hiille ausgeweitet wird, die Keimblatter sind daher an dieser Stelle umgelagert, unigekehrt, invertirt.”
It is now known that no true inversion of the layers occurs in man, but, as stated by Bryce and Teacher, “there is no doubt that the plate of embryonic ectoderm is inturned, and there is strong probability that the condition is a primary one and not due to a precocious formation of amnion folds.” (Page The primary infolding of the embryonic shield is strikingly shown in figs. 2 to 8.
Where the embryonic shield forms the floor of the amniotic cavity in sections 156 to 153 (figs. 9 to 12), there is an opening through the ectoderm in the median sagittal plane of the shield. No other indication of a neurotic canal was found. The opening, however, is presumably an artefact. Similar ruptures through ectoderm are seen in sections 13 and 15. Around the median aperture the ectoderm is not continuous with the entoderm, as would occur if the structure were a true neurenteric canal.
The cells of the three germ layers as seen in this embryo may be described as follows: The ectoderm of the shield is composed of more or less cuboidal or cylindrical cells, which are quite epithelial in character. The nuclei are round or oval, with a finely granular chromatin network, and with generally one, occasionally two, distinct, deeply~stained nucleoli. The cell protoplasm is generally very finely granular and stains moderately deeply with eosin. The chromatin network can be particularly well seen in the first sections of the ectoderm shield where the cells have been cut very favorably for this observation and where they are not so densely crowded as elsewhere. The karyokinetic figures are generally in the monaster stage. No distinct diasters were found. One cell was seen with two small vesicular nuclei containing densely but finely granular chromatin, and one pair of cells with the same kind of nuclei and an incomplete division of the protoplasm.
The ectoderm cells of the amnion are in general of the same description as the shield ectoderm cells; however, they have smaller
protoplasmic bodies, which are not cuboidal, but rather elongated.
The mesoderm cells of the embryonic shield have generally oval nuclei and exceedingly little protoplasm; they are connected with each other by very thin, filamentous, bipolar processes. The yolk sac and amnion mesoderm cells are elongated and connected with each other by bipolar processes. But where the mesoderm cells are most numerous, namely, at the allantoic stalk, their nuclei are larger and their protoplasm, which is fairly abundant, is generally irregularly polygonal.
The entoderm cells of the shield have nuclei very much like the ectoderm cells, but the protoplasmic bodies are smaller and often somewhat elongated. These cells are likewise more or less connected with each other by bipolar though shorter and coarser processes. The entoderm cells of the yolk sac have rather small nuclei, rich in chromatin, and, where they can be seen at their best, are almost geometrically cuboidal. They are rather small in size in comparison with the shield ectoderm cells. The entoderm cells of the allantois are like those of the yolk sac.
Description of Chorion and Decidua
Nomenclature
In order to facilitate the description of the chorion and of the decidua in which it was found embedded, as well as to avoid any misunderstanding on the part of the reader, it is well to outline shortly the nomenclature used.
That part of the uterine decidua on which the inner pole of the ovum rests and which is characterized in our case by the presence of large cystic gland spaces and lacunzc, densely crowded with blood, we will call the deciduct basalts. That thin strip of decidual tissue which separates the ovum from the uterine cavity will be designated as decidua capsularis, and that part of the decidua surrounding on all sides the equator of the ovum we will call the decidua vera.
In the latter at some distance from the ovum an inner spongiosa and an outer compacta can generally be easily distinguished. The mesoderm lining the interior of the chorionie cavity or exocoelom will be designated as the chorion mesoderm, while the chorion ectoderm will be called trophoblast. This term has been proposed by Hubrecht, and it is understood and used so generally that it is perhaps well to retain and not to replace it by other terms." The trophoblast is composed of two kinds of tissue, the inner cell masses and an outer covering of syncytium. We want to state here that the examination of our young ovum has confirmed our opinion, expressed a number of times previously, that both the cells proper of the trophoblast (the future Langhans cell layer) and the syncytium are derived from the fetal ectodcrm. VVe have never previously in hundreds of placentae in normal and in ectopic gestation, nor in our present case, found anything which would seriously suggest a derivation of the trophoblast syncytium from maternal cells. The ovum under discussion nowhere shows a possibility that the syncytium is derived either from surface or glandular uterine epithelium, from vascular endothelium, or from decidual cells. Hence the term ectoblast shell for the combined trophoblast cells and the syncytium is correct. We retain the well—known term trophoblast in spite of the fact that we consider it as to its etymology a misnomer. We have previously expressed ourselves on this subject, as follows: “The term trophoblast has been given by Hubrecht to the extraembryonic ectoblast shell under the impression that it had to do a good deal with the nutrition of the early embryo. We doubt, however, that
- 9Minot, in an address on “The Implantation of the Human Ovum in the Uterus,” delivered in 1904 before the Gynecological Society and printed in its
transactions, has proposed the term trophoderm, but he states himself: “In the address the term trophoblast was used in accordance with my understanding of Professor Hubrecht"s views and consent; but, as Professor Hubrecht has objected to this application of his term, it has been necessary to propose a new one. I regret that so good a name as trophoblast has to he dropped.” With this address the author only became familiar after the completion of the manuscript of this contribution. He now finds that his views of the physiology and the mechanism of the implantation of the human ovum given are in many respects identical with the previously published explanations of Minot this is the case. The mass of the trophoblast in our case is certainly many thousand times that of the embryo. It does not stand to reason to assume that nature in the phylogenetic development would provide, so to speak, at an enormous expense, a very large apparatus for the nutrition of a very small embryo. It appears more reasonable to assume that the trophoblast with its great proliferative energy, which We have likened to the growth of a malignant tumor, has more exclusively the function to provide the means for the embryo to safely implant itself at the very earliest date into the maternal tissues. The reaction of the maternal tissues in-contact with the proliferating trophoblast must not be looked upon as due to mechanical causes only, but to fermentative action of enzymes secreted by the trophoblast cells and diffused into the neighboring maternal tissues.”
The above statement we still hold to be correct on the whole. However, we agree with Bonnet” that the syncytium presents features, namely its property to stain very deeply with eosin, which suggest the possibility that it takes up hzemoglobin from the maternal blood for the benefit of the nutrition of the embryo.
The term syncytium in connection with placentation has been used very promiscuously and has been inaccurately applied to degenerating confluent cell masses of maternal origin. According to
Bonnet, the term syncytium has been introduced into histology by
Haeckel, who designated by it a nuclei—containing plasma, formed
by the confluence of previously separate and distinct cells. Taken
in this sense, the term syncytium as appied to the human trophoblast is probably a misnomer. It is very likely—though nothing
about this is known from actual observation—that the syncytium
of the human placenta is formed “ab origine,” as an outer strip
or capsule of protoplasm which is provided with expelled nuclei
from the cells of the inner cellular trophoblast. In a publication
“On the Pathology of Tubal Pregnancy,” quoted in a footnote above,
I have considered the syncytium of the human placenta as the homelogon of the periblast of transparent pelagic fish eggs, such as those of Fundulus, which I had a chance to study in the Summer of 1899, in the Woods Hole Marine Biological Laboratory, under the direction of Professor 0. O. Whitman. In these fish eggs the formation of the periblast—an outer capsule of plasma without cell boundaries, but with numerous nuclei——can, of course, be studied from stage to stage under the microscope, and the expulsion of nuclei into the plasma can be seen.
‘°Bonnet: Ueber Syncytium, Plasmodien und Symplasma in der Placenta der Siiugelhiere und des Meusehen. Mouatschrift fur Geb. u. Gyn., 1903, vol. 18, p. 1.
In spite of the fact that the syncytium of the human placenta does not deserve this name in the sense as originally applied, it is well to preserve its use, since it has been universally applied to the outer covering of the trophoblast, and of the later chorionic villi. For degenerative confluent cell masses in the placenta, whether they be of maternal or fetal origin, Bonnet has proposed the term symplasma, and he distinguishes between symplasma maternum and symplasma fetale. These terms have been accepted by Jung in the description of his ovum, and we will likewise introduce them into our considerations. Some of the German writers on placentation, following Bonnet, have come to use the terms “Grundschicht,” for the cellular part of the “Trophoblast” (the later Langhans layer), and “Deckschicht,” for the syncytium.
The General Position of the Ovum and its Mode of Entrance into the Decidua
At the time of the autopsy, as stated above, the ovum, or rather the small hemorrhagic spot, was found at the posterior Wall of the corpus uteri, comparatively high up in the fundus, and near the entrance of the left Fallopian tube; that is, on the same side where the corpus luteum ovarii was noticed. The dark, hemorrhagic spot which contained, as was later on found, the ovum was only very slightly prominent over the remainder of the thick, velvety mucosa. The uterus, after the careful removal of the dark spot, which was excised as a cubical mass, was preserved in the pathologic collection of the Government Laboratory, but I have not had a chance to reexamine it during my stay in Manila, and I do not know whether it has been preserved permanently or not.
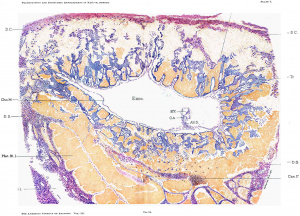
The mass removed was sectioned from above downward, and the ovum, which can best be seen as a whole in photomierograph (fig. 24), was found situated with it long axis parallel to a line drawn from one ostium internuin tubae to the other.
A glance at the photomicrograph (fig. 24) shows the following points as to the position and surroundings of the ovum in general: The ovum, including its trophoblast and the thin strip of decidua capsularis, protrudes very slightly above the surrounding surface. From the cavity of the uterus it is separated by a very thin decidua capsularis. This in the very center of the upper line of photomierograph (fig. 24, section 1125) is slightly deficient, and here we see a teat—like process of the chorion mesoderm extending almost to the surface. The inner pole of the ovum rests on a weclge-shaped mass of very large cystic gland spaces densely filled with blood. The decidua Vera near the ovum shows densely crowded hypertrophied gland spaces. They are somewhat cystic towards the muscularis, and towards the free surface present enlarged tortuous tubes, separated by intervening septa. The differentiation into a deeidua compacta and spongiosa, which is not well shown in fig. 24, appears more clearly in the photomicrograph fig. 26.
Large cystic glandular blood—filled spaces are found not only at the base of the ovum, but also towards one side. Such spaces were found also in the first set of sections examined, 1'. e., in sections which showed neither the trophoblast nor the chorionic cavity. If one studies sections 11 to 39 of the complete series, which likewise do not yet show any trophoblast, the following can be seen. Year‘ the surface, under a thin strip of decidual tissue composed of large, partly—degenerated, decidual cells, there is an opening or hole surrounded on all sides by profoundly degenerated decidua. The deeidua here is least degenerated at the upper stratum (capsularis), and most markedly degenerated at the inner (basal) aspect. The decidual cell masses are infiltrated with maternal blood, and are surrounded by large cystic gland spaces (fig. 27) filled either with blood, with hyaline, eosin—staining balls or masses, with degenerated, dropped-off glandular epithelium, or with networks of fibrin. The hole described is not empty, but more or less filled with blood and dropped—off degenerated decidual cells. In section 11 the canal or hole has no covering towards the uterine cavity, but the mass of blood and degenerated, chaotically distributed, decidual cells reaches to the very surface.
It is clear that this canal, which is more or less circular in outline, with diameters varying from 1.5 to 1.0 mm., indicates the route over which the ovum traveled to the place where it was found in the decidua. It is probable that the ovum after having been fertilized in the tube was brought to a spot indicated by the surface opening in section 11, or thereabouts. The ovum when less than one millimeter in diameter (this measurement, including, of course, the chorionic cavity and the whole then existing trophoblast) began to make its way into the decidua. It did, however, not eat its way deeply in at all, but traveled under the surface almost parallel to it, being separated from the uterine cavity only by a very thin strip of decidual tissue, in some places so thin that it became slightly defective, or at least apparently so. After the ovum had traveled through the canal described, of which there are about 200 to 250 micra in length left, it must have become stationary and must have begun to expand inwardly, towards the muscularis, and also laterally. It is clear that the ovum as found in situ in the decidua is much too large to have traveled through the canal, and it must have been much smaller, probably considerably smaller than one millimeter, at the time when this migration took place.
The canal, as stated, was found moderately well filled with blood corpuscles and degenerated decidual cells. We have, therefore, in our case, unlike Peters, who had in his specimen a mushroom coagulum, a still patent, though partly filled, small canal, running almost parallel to the uterine surface, through which the ovum by its own inherent destructive tendencies made its way to the place of final implantation. While the outer end of the canal described communicates with the surface and is practically open towards it, except as to the presence of maternal blood and degenerated decidual cells, the inner end is closed by the trophoblast of the ovum. On the opposite point of the ovum, which is found i11 section 264», where the trophoblast is last seen, we likewise have evidences of its great destructive tendencies. The next sections show numerous degenerating, droppcd—off decidual cells, mixed with blood. Thus, there is formed here also a kind of cavity filled with blood and detritus. However, it does not reach the surface, but remains separated from it by dccidual tissue. We see the ovum then everywhere more or less surrounded by cystic blood spaces or free blood. In this respect the early human ovum is very much like that of the hedgehog and mouse, as described by Hubrecht and Bonnet, surrounded on all sides by maternal blood, floating as it were in a lake of blood. The conditions in our ovum are more or less identical with what had been found by Peters in his case.
It can be seen in fig. 25 that the embryonic shield is anchored by its allantois to the ehorion mesoderm near the inner pole of the ovum and farthest away from the outer pole and the thin capsularis. The position of the embryo with reference to its outer and inner poles is thus identical with that of Peters’ ovum. In the Jung ovum likewise the embryonic shield appears to hold the same relations to the maternal organism, through orientation in this lastmentioned specimen, which was not obtained in situ, but by curettement, is not absolutely certain. The apparently identical position of the embryonic shield in the three young human ova is very probably not at all a matter of chance or accident, but is due to a very early automatic orientation of the ovum for the ‘benefit of the developing embryonic shield—from a biogenetic standpoint the most important part of the ovum.
Examining the sections which show the beginning and the course of the canal, the following conditions can be ascertained: The entrance of the canal is evidently not in a gland duct, but in an intcrglandular septum, through a decidua which here, in spite of profound degenerative processes, shows well the character of a compacta. On both sides of the entering canal we see the enlarged tortuous necks of uterine glands. Enormously enlarged capillaries reaching from the sides of the entering canal to the very surface can also be seen. Both at its entrance and along its course the canal is surrounded by enlarged gland spaces. The glandular epithelium everywhere shows profound degenerative changes, and the large cystic glands are partly or completely filled with blood and detritus, as already mentioned. There is no trace of glandular or surface epithelium left at the site where the canal takes its origin from the surface. Nor can one find any gland ducts opening into the canal, or into the cavity in which the ovum now is situated. All of the conditions point unmistakably to the fact that our own ovum, like that of Peters, penetrated into the docidua not through a gland space, but by eating its way through interglandular tissue of the compacta. It does not appear necessary to us to assume that the ovum can make its way into the decidua only through a spot denuded of the surface epithelium. Since the enlarged gland spaces, even where not in direct contact with the trophoblast, exhibit most marked degeneration of the lining epithelium, We may well assume that the trophoblast, in contact with the surface epithelium, can destroy it easily and make its way into the connective tissue of the decidua.
In our preliminary communication, read in August, 1907, before the Zoological Congress of Boston, we said: “We cannot conclude this preliminary report without pointing out what we might call the pathologic aspect of the early stages of placentation in man. The proliferation of the trophoblast, the manner in which it invades the maternal organism, pushing aside, destroying and changing maternal tissue elements, vascular and other structures, is the exact picture of malignant tumor proliferation, while the reaction of the maternal tissue, taken for itself alone, reminds one forcibly of a profound destructive hemorrhagic inflammation. It is very striking to the pathologist to behold in early placentation, in the apparatus and the phenomena which enable the young ovum to anchor and implant itself firmly into the maternal organism, the very paradigma of two such important pathologic processes as malignant tumor growth and hemorrhagic inflamation.”
A further study of the sections has only strengthened the impression gained previously. The destructive tendencies of the early trophoblast of the ovum are certainly very marked. If some hypothetical speculations may be here permitted, we would like to express our opinion that the trophoblast at a certain stage of its development secretes an enzyme which diffuses into the surrounding maternal tissues and here causes coagulation necrosis and complete degeneration of cells. The trophoblast cells, as represented by our specimen, are certainly not phagocytic in the ordinary sense of the word. VVe have in vain examined and re—examined our sections to find included in the trophoblast cells or the syncytium maternal blood corpuscles, fixed tissue cells or fragments or remnants of the same. VVe must, therefore, conclude that the cfi°ect of the tropho— blast upon the maternal tissue is brought about not by true phagocytosis, but through the action of an enzyme. If the latter destroys maternal cells to a large extent, and this destruction, of course, takes place, as can be seen, we have those conditions which under any circumstances would lead to violent inflammatory reaction, including enormous dilatation of veins and capillaries; free hemorrhages; and if the process take place in a glandular mucosa, with hypertrophy of the glandular apparatus. And, indeed, if we look upon the decidua in our specimen particularly, as seen in fig. 2%, to the left of the ovum, and in fig. 26, in the whole section, the resemblance between them and a typical, well—marked case of endonictritis glandularis hypertrophica is very striking. In fact, when the set of sections represented by fig. 26 was shown to a very competent pathologist with the statement that it was Very probably a very early decidua and that an ovum would be found in the block of tissue, he ridiculed the idea and firmly held that the section simply showed a typical strongly—marked case of endometritis glandularis hypertrophiea.
If we behold the great destructive tendencies of the early tronhoblast, the question presents itself, VVhy does this process evidently come almost to a standstill somewhat later in the course of gestation? Two possibilities present themselves. Either the secretion of the supposed destructive trophoblast—enzyme is limited as to time, or there is established a temporary immunity of the maternal tissues, These speculations, while at present entirely hypothetical, might perhaps be supported by experiments in which the effect of filtered extracts of animal placentze of various stages of development, and in repeated applications, upon the uterine mucosa, would have to be studied.
Peters, in his case, has shown how the ovum did not make its way into a gland duct, but had eaten its path into the decidua and he opposed the old theory as to the formation of the decidua refiexa. He, however, conceded the desirability of demonstrating the mode of entrance of the ovum in more than one case examined in situ.
Our own case, offering in some regards even better conditions, namely, almost absolute certainty of the absence of all pathologic deviations from the normal type, fully confirms the View of Peters of the mode of implantation of the ovum and of the erroneousness of the older theories. Both the Peters and our ovum show the correctness of Count von Spee’s“ hypothesis that the human ovum would be found to behave in its method of implantation into the uterine mucosa like the ovum of the guinea—pig. It was shown by von Spee that the dividing blastoderm of the guinea—pig eats its way through the uterine epithelium, into the connective tissue, causing here edema and hyperemia.
Exocoelom and Chorion Mesoderm
The ehorionic cavity or the exocoelom, as to its size and shape, has been sufiiciently described. The position of the embryonic shield, yolk sac and allantois have also been indicated. Aside from the “Keimanlage,” the exocoelom shows in its interior a finely granular, irregularly lumped material, which has stained intensely with eosin. This material can be well seen in photomicrographs, figs. 24 and 25. It is responsible for the fact that the sections of the embryo could not be photographed so that they appear on a clear homogeneous background. What is seen in the sections with reference to the eosin—staining granular material proves that the exocoelom “inter vitam” was filled with a watery fluid rich in coagulable proteids. The strongly eosin-staining properties of the granular material may perhaps be due to an absorption of hemoglobin indirectly derived from maternal sources. Towards the periphery, that is, towards the lining chorion mesoderm, and running parallel with it, are seen long slender fibers, more or less mixed with granular detritus. These fibers are evidently the remnants of degenerated, dropped—off lining mesoderm cells. The latter themselves are comparatively long and fusiform, with very gradually tapering long bipolar processes. Their protoplasm is finely and distinctly granular, well eosin-staining. The nuclei are oval, sometimes almost rod-like, with rounded ends like the nuclei of involuntary muscle cells. They have a fine but darkly—staining chromatin reticulum; often one or two nucleoli can be seen. The ehorion mesoderm in most sections has slightly retracted from the trophoblast ectoderm and we here can see distinctly along the outer margin of the mesodermal lining a fine, sharply—cut membrana limitans, as described by Bonnet, in a more advanced older ovum as separating the mesoderm of the villi from their eetoderm. The ehorion mesoderm forms teat—like or finger—like processes arising from the periphery and extending outwards into the ectodermal trophoblast. These processes likewise show the fine limiting membrane. Sometimes these processes arise near each other, but they do not yet show any dichotomous division. Here and there are seen floating in the exocoelom bands or filaments of mesoderm cells. They are interesting from the standpoint of the pathologist, because their occasional growth and persistence may lead to the formation of those so—ealled amniotic bands responsible for disturbance in the normal development of the embryo. These mesodermal bands and strands, crossing the chorionic cavity, have also been described for their respective specimens by Bryce and Teacher, Peters and Jung. They are important because they have been interpreted as the remnants of a once solid mass of mesoderm, existing before the formation of the coelom and exocoelom.
“Von Spee: Die Implantation des Meersc-11weincheneies. Zeitschr. f. Morph. u. Anthi-op., 1901, Vol. 111, p. 130; and Ifeber die 111€11SCl1l. Eikaminer, etc., Verh. der Anat. Ges. zu Kiel, 1898, p. 196.
Keibel (Normentafeln, Vol. 8, p. 12) in discussing the early mesoderm and the formation of the exocoelom says: “In man at a stage when a primitive streak cannot yet be demonstrated with certainty or even does not exist, we find the whole embryonic shield, yolk sac and amnion richly surrounded by mesoblast, as is also the internal surface of the ehorion. Spee, in describing his embyro H, says: ‘It appears almost inconceivable that the region of the still smaller primitive streak of an earlier period has furnished these masses (of mesoblast). Probably a small mass of mesoderm is produced from a primitive streak at a very early stage, and later this mass proliferates independently.’ ” “Although this view of Spec,” Keibel continues, “appears to be the most probable, it is well to point out that it is only an hypothesis, which encounters many difficulties. We cannot at present state anything more definite as to the origin of the mesoblast in man. Something more certain may be said of the origin of the coelom, although its earliest stages have also never been observed in man. It is certain, that, as in mammals generally, the extraembryonic coelom (the cavity of the ovum) is formed earlier than the embryonic ceclom, and we are probably correct if we assume that the extraembryonic coelom is. formed by cleavage (Spaltbildung) in a mesoblast which has already developed. This would be in accordance with what is known of the other mammals.
The Trophoblast and I is Syncytium
The early characteristic trophoblast of the human ovum, though first correctly described by Peters from his specimen, had previously been hypothetically constructed from the observation of an older ovum in situ, in which the peculiar ectoblast shell had been dilierentiatecl by the formation of the villi. We owe this description to van Heukelom”, who gives it in the following words: “One can best get a conception of these conditions if one imagines all villi connected at their periphery by beams of ectoblast, so that they form an ectoblast shell full of small and large holes. This shell is unevenly thick and rests directly on the maternal eompacta. . . .”
"von I-Ieukelom: Uelrer die menseliliehe 1'la('tental‘io11: Archiv f. Anat. u. Physiologic (Abth. Anatomie), 1898.
The trophoblast as found in our specimen surrounds the chorionic cavity or exoceclom of the ovum like a thick shell. It is, however, not equally powerful on all sides, as has been already indicated by the measurements of the trophoblast. Nor is it a solid mass of cells. It is, on the contrary, honeycombed by irregular communicating spaces contained in a network of irregular bands, strands and masses of trophoblast material. The cavities in the trophoblast are not empty, but well filled with maternal blood. There is a certain regularity of those trophoblast cavities which are situated next to the chorionic cavity. Here they are somewhat regularly cuboidal and they are placed around the chorion mesoderm like blocks of stone in a pavement. It is evident that this arrangement is brought about by two factors working in a certain sense against each other, namely, the pressure of the maternal blood and the growth energy of the chorion mesoderm. Towards the chorion mesoderm, these cavities are lined by a thin layer of trophoblast, composed of one cell layer of the “Grrundschicht” and one layer of “Deckschicht” or syncytium. Towards the periphery these cavities are lined by more powerful masses of trophoblast. In the middle stratum of the trophoblast there are irregular cavities of moderate size, and in the outer stratum the open, blood—containing spaces become very large and very irregular. In this outer zone we find the trophoblast material much diminished, and where it is in contact with the thin capsularis, it dwindles down to isolated thin threads or pillars of cells.
The cells of the “Grundschicht,” or that part of the trophoblast which later becomes the Langhans layer of the villi, are most characteristic in the middle zone where they are present in the shape of bands and beams and irregular masses, and where they have not been exposed to considerable pressure, as in the zone next to the chorionic cavity. In the middle as well as in the outer zone the cells have the following characters: The protoplasm is generally almost spherical, or in consequence of mutual compression of the cells irregularly polygonal. The cell boundaries are so very distinct that it appears as if the cells had membranes. The protoplasm has stained very lightly with eosin and gives the impression that the cells “inter vitam” must have been very “saftreich.” The nuclei are large, round and vesicular, with a finely granular chromatin network. Frequently one or occasionally two nucleoli can be seen; these likewise are more or less distinctly vesicular in appearance. The nuclei are about twice the diameter of the maternal red blood corpuscles, and the whole cells are three to four times as large in diameter as an erythrocyte. Karyokinetic figures are occasionally, though not very frequently, seen. (fig. 29.)
The trophoblast cells next to the chorionic cavity are cuboidal and compact. Their protoplasm is rather scanty, and it stains much deeper with eosin than that of the cells of the middle trophoblast stratum; the nuclei are somewhat smaller, more oval and slightly richer in chromatin. Towards the uterine cavity the trophoblast cells form thin bands which in sections present themselves as slender bridges connecting the trophoblast with the structure mentioned before as “the thin strip of the deeidua eapsularis.” Where they lead up to the surface the trophoblast cells have often broken through the syncytium. Here the cells become fusiform, bipolar and while not showing any marked features of degeneration, it becomes difficult to distinguish them from what appear to be decidual cells. It has previously been stated that the decidua capsularis separating the outer pole of the ovum from the uterine cavity is deficient in some portions, so that the ovum, in fact, is not yet entirely separated from the uterine cavity. This impression is very strongly conveyed by one of the sections which for some reason was cut much thinner than the others (it is certainly less than 5 micra). Here one can see that the trophoblast cells have proliferated outwardly, have broken through the syncytium and have become fusiform. They reach to and form the very surface. In this section decidual cells appear to be absent from the strip which borders upon the cavity of the uterus. However, even in this strip some undoubtedly maternal cell elements can be recognized, namely, mononuclear lymphocytes and polynuclear leucocytes, and also, of course, infiltrating erythrocytes.
The trophoblast cells, Whether they be present in a single layer, as towards the chorionic cavity, or whether they form larger or smaller irregular masses honeycombed by blood spaces, are covered by the syncytium. This consists of a layer of protoplasm in which cell boundaries are not demonstrable. In sections the protoplasmic layer is generally rather narrow, but there and there it is thickened, forming projections. These are seen particularly in the most peripheral parts. However, the numerous and large syncytial buds present in somewhat older placentze are not seen. The protoplasmic strip is deeply eosin-stained, but it shows a tinge as if it had also taken up some of the nuclear stain (hematoxylin). The very margin, however, is purely eosin-stained. The protoplasm is finely vacuolated. The distinctly eosin—stained outer strip consists of a cuticle and cilia. The cuticle can only be distinguished here and there in favorable places, but the cilia are almost everywhere easily seen. (fig. 30.) The syncytium in our specimen fully conforms to the description given by Bonnet as found in an early human ovum, fixed like ours in Zenker’s solution. This author says: “Towards the periphery the plasma of the syncytium is condensed into a stratum frequently staining very intensely with eosin, rubin or Heidenhain’s iron hernatoxylin. This outer strip, variable in thickness and distinctness, in fact appears like a cuticle. Its free surface in all sufliciently thin sections (3 to 5 micra) shows a very distinct and beautiful lining with cilia (“Biirstenbesatz”). This lining has also been described by Marchand” and Lenhossek. According to the latter, these cilia or rods are not motile (they Were studied by Lenhossek in a fresh preparation), and in specimens stained with iron hematoxylin they exhibit basal granules in the cuticle.”
Bonnet has not been able to see such basal granules, nor are they shown in our specimen, which, however, has not been stained with iron hematoxylin. The cilla in our sections present themselves as stifi”, fairly slender, moderately high rods, which form very regularly parallel rows. They are deeply eosin-stained and of the same color as the cuticle. As stated, they can be seen without the least difficulty almost everywhere in the sections where syncytium is found. These rods are unlike the cilia of the glandular epithelium, which can still be well seen in the innermost portion of the decidua spongiosa towards the muscularis.
”Marchand: Beobachtungen an juugen menschlichen Eiern. Anat. I-Ieft 1903, V01. 21, p. 217.
Since cells provided with a cuticle and cilia or rods generally have a secretory function, it is possible that this apparatus of the syncytium has something to do with the secretion of the hypothetical enzyme of the trophoblast mentioned above. The nuclei of the syneytium are generally oval, elongated and rather densely provided with chromatin. I have, like Bonnet. not been able to find any cuticle or basement membrane between the syneytium and the cells of the trophoblast. In speaking about the outermost processes of the trophoblast, we described above how the trophoblast cells have invaded the narrow strip of outer polar tissue designated as decidua capsularis. Such processes of proliferating trophoblast elements, including both cells and syncytial masses, are found extending into the maternal tissues around the whole circumference of the ovum. This zone directly surrounding the ovum, forming the soil into which such invading processes extend, has been called the “Umlagerungs—zone,” by Peters, a term which perhaps may be best translated by the “Border Zone.”
The Border Zone
The tissue which forms the bed of the ovum (Eilager) surrounding it more or less from all sides may be divided into three parts. the decidua basalis, the decidua capsularis, and the equatorial zone or decidua Vera. The border zone at the base and around the equa Torial planes of the ovum is characterized by the presence of large blood sinuses, originally formed from the capillaries and small veins of the uterine mucosa. Some of these blood lacunae have retained the outlines of vessels; others have become irregular spaces which have no resemblance to ordinary vessels. The largest of these blood sinuses in our specimen are found in the decidua basalis. near the inner pole of the ovum. But very large thin—walled blood spaces surround the ovum on all sides. They proceed from the basal decidua into the equatorial border zone, bend around the upper or outer hemisphere of the ovum, and Very nearly reach the thin polar cap of tissue, the decidua capsularis. No real blood spaces are, however, found in this thin cap of tissue, but only free blood corpuscles mixed with cells either of maternal origin (deeidual cells) or derived from the ectoblast shell. Very much enlarged capillaries and veins can also be seen at a distance from the border zone in the spongiosa and compacta of the decidua extending nearly to the surface. In the border zone of our specimen near the inner pole of the ovum there is a large irregular blood sinus about 1 to 1.5 mm. in diameter, which has been completely opened by the trophoblast and is in free communication with the blood—filled cavities of the trophoblast. On its inner side (towards the muscularis) this sinus is still lined by vascular endothelium. The wall towards the trophoblast has been extensively destroyed, so that the large blood space is lined on one side by much stretched but still fairly well preserved endothelium, and on the other by the irregular, ragged trophoblast. The border zone at the base of the ovum also shows many large cystic gland spaces filled with blood. Most of them can be recognized as derived from glands by remnants of dropped—ofT, degenerating epithelium, while the densely filled blood spaces, on the other hand, can be identified by their endothelial lining and the remnants or dropped—ofi floating portions of the same. But there are some cystic blood—filled spaces which cannot be readily identified as being originally glands or blood vessels. Nowhere in the border zone does the glandular epithelium or vascular endothelium show any proliferative processes; degenerative processes only are seen.
At the base of the ovum in the layer of the border zone nearest to the trophoblast a small, rather delicate strip of canalized fibrin is found. This strip consists of a network of fibrin in which are embedded decidual cells, red blood corpuscles and polynuclear leucoeytes. There is a more powerful mass of fibrin found in the equatorial tract of the border zone. This mass (fig. 28) contains rather coarse threads of fibrin and great numbers of red blood corpuscles. It appears that this mass has formed in and is filling out the lumen of an enlarged blood space.
We have described how the trophoblast in approaching the capsularis sends out bands and filaments of cells which become fused with and are lost in the thin capsular strip. The same process can be seen around the whole periphery of the trophoblast. Particularly around the equatorial plane the syncytium can be seen to take part extensively in this process of fusion. It appears that the syncytial masses after penetrating into the border zone have a tendency to break up into cells. Individual detached pieces of syncytium can often be recognized as such by the deep eosin stain of the protoplasm and by the rods lining the external surface. However, other portions of what appears to be detached syncytium in the border zone have lost their characteristics. It is in the border zone and only in it, in our sections, that marked degenerative processes are seen, and these appear to be mostly confined to cells and tissues of maternal origin. In the border zone are also seen larger protoplasmic masses containing several generally pyknotic nuclei. We think that these are detached degenerating portions of the trophoblast; whether they are portions of the “C‘rrundschicht” or the “.Deckschicht,” We are not able to decide. We believe that the large, irregularly round cells with vesicular nuclei are derived from the syncytium, since their protoplasm stains very deeply with eosin.
Peters describes numerous and profound changes in the trophoblast. These changes Marchand has already considered to be pathol.ogic and probably due to the fact that the woman from Whom this ovum came died from the effects of arapidly fatal dose of caustic potash. However, Marchand also believes that the extensive presence of blood in the trophoblast of Peters’ ovum is abnormal. In this respect he is mistaken, since our own ovum shows the identical condition.
It appears from Peters’ monograph (p. 50 and p. 51) that he found in plasmodial masses and in the syncytiumlmore or less normal and also much changed fragmentary red and white blood corpuscles. He describes this quite fully, and he draws from this observation the remarkable conclusion that the maternal blood with its own corpuscular elements contributes to the formation of the syncytium. Neither Bonnet nor Jung have found anything like this.
Not a trace of any such process or condition can be found in our own ovum. Nowhere were red blood corpuscles in toto or in fragments seen included in the trophoblast elements. It is quite probable that in Peters’ case the profound intoxication with fixed alkali had so changed the red blood corpuscles, had, as we would express it to—day, so “opsonized” them, that they became liable to be taken up by the phagocytic action of other cells. We know that red blood corpuscles, in consequence of the action of certain bacterial toxins, are so changed that this phagocytosis occurs. We want to mention in this respect what occurs in typhoid fever when numerous red blood corpuseles are taken up by the pulp endothelial cells of the spleen. That the elements of the trophoblast of the human ovum under absolutely normal conditions do exhibit towards the maternal blood corpuscles truly phagocytic properties is certainly not proven. Our own specimen, which we consider perfectly normal, shows absolutely nothing which would ustify such a conclusion.
We see in the border zone, particularly around the equatorial planes, cells which show already almost all of the characteristics of the later decidual cells. These cells exhibit a large vesicular nucleus, with rather scanty, finely granular chromatin and obtusely fusiform or irregularly polygonal protoplasm. Between them are found small mononuclear cells and polynuclear leucocytes. This is the picture seen in places a little distant from the ovum. Towards the very interior of the “Umlagerungszone,” the outlines of almost completely destroyed gland spaces with dropped—off degenerating epithelia, red blood corpuscles and fibrin are seen abundantly. Here also are seen these cells or cell remnants with pyknotic, irregular, shrunken nuclei, and a very dense, deeply eosin-staining protoplasm. The latter we consider as detached portions of the ectodermal trophoblast. Protoplasmic masses containing several nuclei, which We also take to come from the trophoblast, have already been mentioned. We have, however, not found larger masses of fused degenerating eells, either of maternal or fetal origin, hence we have no occasion in our case to make use of either one of the terms, symplasma maternum or foetale.
The Decidua
The character of the decidua as it exists in our ovum is well illustrated in figs. 24 and 26. In the “Umlagerungszone” and right next to its periphery the degenerative processes and the hemorrhages predominate. At some distance, however, we find a decidua well differentiated into a compacta and a spongiosa. How far distant from the ovum these characters have already been established We cannot say, since only the ovum and its next neighborhood have been sectioned and examined.
Jung, who, in his ovum, likwise found a distinct differentiation into a compacta and spongiosa quoted Hitchman and AdleI"s observation of the uterine mucosa before and during menstruation. These authors, even in the absence of gestation, found in the premenstrual period a temporary formation of a compacta and spongiosa. We have ourselves studied the menstrual changes on several specimens obtained per operationem and at once properly fixed, and we have previously (The Pathology of Tubal Pregnancy), summarily described them as follows: “The capillaries of the inter-tubular connective tissue are enormously dilated and densely filled with red blood corpuscles. Many of the latter are also found free, outside of the capillaries, between the connective tissue cells of the interglandular spaces. The whole mucosa is edematous and the connective tissue cells are pushed apart by the edematous and hemorrhagic infiltration. Some of the connective tissue cells, which in the intermenstrual periods are normally all of the type of small lymphoid cells, are enlarged, oval or fusiform. They assume a type found in certain forms of endometritis interstitialis and approach the type of decidual cells. It may really be said that the uterine mucosa in menstruation shows to a very slight extent the beginning stage of a decidua. Most of the surface epithelia of the mucosa are preserved; only a few are missing here and there. Changes similar to those described as characteristic for the menstruating uterine mucosa I have twice observed in the tubal mucosa during menstruation.”
The decidua spongiosa is considerably thicker than the compacta (fig. 26). In the former we find irregular gland spaces, much crowded, and separated from each other by small bridges of tissue. The proliferative energy of the glandular epithelium is shown by the fact that it is found inside of the gland space proper, in the shape of projecting papillary ridges, septa and digit—like processes. All of these masses of epithelia are lined up on a slender basis of connective tissue. The interglandular connective is composed of distinctly fusiform cells with elongated deeply-staining nuclei. In some places the gland spaces project considerably into the muscularis. Towards the latter there are occasionally found between the glands groups of small round lymphoid cells of the type of the cells seen in the interstitial tissue of the non-pregnant uterine mucosa. In the spongiosa at some distance from the ovum there are no very markedly enlarged veins or capillaries seen. But they appear towards the zone where the spongiosa goes over into the compacta. In the latter we see the more or less straight or decidedly tortuous ducts of the glands leading to the surface. Here also the epithelial lining projects in ridges and bands. Between the gland ducts solid septa are present. In the direct neighborhood of the ovum these septa contain enormously enlarged blood spaces (capillaries or veins); at some distance they show the tortuous cork-screw arteries, characteristic for the decidua compacta. The edema existing in the decidua is demonstrated in the spongiosa even at a distance from the ovum by a coagulated granular material found in the gland spaces. In the compacta the edema can be recognized in the solid septa. The cells here are distinctly pushed apart, they are embedded in a granular coagulated material. In the compacta are found cells which exhibit already quite well the characters of decidual cells. They possess a large oval nucleus, with distinct nuclear membrane, scanty chromatin and one or two nucleoli. They have a large protoplasmic body, oval, fusiform or irregularly polygonal in outlines, finely granular and well eosin-staining. Among these larger cells, small mononuclears of the type of lymphocytes or young connective tissue cells are quite numerous. "
The epithelium lining the glands is best preserved in the deeper layers of the more distant compacta. It is high columnar with nuclei near the basement membrane. The cilia in favorable locations are still preserved. The profound degenerative processes seen in the glandular and surface epithelium towards the ovum have several times been referred to. No karyokinetic figures were seen anywhere in the glandular epithelium. However, a few cells with two small densely stained round nuclei were found. I am, of course, well aware that the specimen described is one obtained post-mortem. However, the rapid cooling on ice and the subsequent proper fixation had Well preserved it. This, among other things, is attested to by the fact that mitotic figures were found in the embryonic shield and in the trophoblast.
None of the decidual cells, either large or small, show any karyokinetic figures.
Jung, who describes mitoses in the decidua of his specimen on page 29 of his monograph, says: “Marchand and Bonnet are the only authors who have heretofore described mitoses in the decidua. The present author in a paper published in July, 1898 (Superfetation in the Human Race), described among others an aborted specimen of superfetation. Both embryos were contained in the intact fetal membranes. One embryo was 8.6 cm. long; the other 16.5 mm. The superfetation was proven by a microscopic examination of the embryos and of their placentee, showing in both the different stages of development. In the description of the placentation of the small ovum the following passage occurs: “Large apparent islands of decidual cells. In_ some places the decidual cells present a very beautiful feature which I had not observed before in the deciduae of many other placentse examined. As is Wellknown, the large vesicular, round or oval nuclei of decidual cells are, as a rule, quite poor in chromatin, which is distributed in the form of small granules in a peripheral manner near the nuclear membrane. In some places of the young placenta of this case the nuclei are rich in chromatin, consistingvof coarse granules and masses, arranged in an aster—shaped manner, occasionally a disaster is fairly well recognizable. We have to deal with karyokinetie figures. That this is really the case, and that We are not dealing with a degenerative process of the nuclear chromatin is proven by the fact that the aster stage can be Well recognized, and, secondly, by the observation that leucocytes are absent at the place where the karyokinetic figures in decidual cells are found. I have previously pointed out that Where a degenerative process—coagulation necrosis—is going on in the decidua we find great numbers of polynuclear leucoeytes, many of which show nuclear fragmentation.”
The paper in which this statement was made shows a photomicrograph of these cells with karyokinetic figures. In re-examining the photograph now, I find that these cells are comparatively small cells. This would agree with the description of dividing decidual cell as given by Marchand.
The muscularis uteri, as far as it is included in the sections, appears to consist of muscle fibers already somewhat hypertrophied. Measurements by micrometer were not made.
Summary
From the object described in the preceding pages, an ovum almost identical in size and type with the Peters’ ovum, one may draw the conclusion that these two ova represent the normal type of the earliest known stage of human placentation. The mode of placentation and implantation in both are practically alike. Certain retrograde changes described in the cells and in the syncytium of the Peters ovum must be looked upon as histopathologic changes, probably due to the poisoning of the mother. This conclusion is justified not only from our own case, but alike from the studies of Marchand, Bonnet, Jung and others.
If we now attempt a summary description of the ovum and its manner of implantation and placentation, we have to make a few hypothetical statements, but, on the whole, we can give a resume based upon actual facts, as they are clearly represented by the specimen studied.
A human ovum at the earliest stage of normal development hitherto known, a stage which perhaps represents one to two Weeks after fertilization, is found interstitially embedded in the decidua. It is incompletely separated from the cavity of the uterus, because it is very superficially embedded and its outer pole is protected by a thin, incomplete decidua capsularis or a coagulum only. The ovum, after having been fertilized, has been transported to or near to the place Where it is found embedded. By the aid of an ectodermal trophoblast shell, which probably secretes an enzyme destructive to the epithelial cells and the connective tissue of the uterine mucosa, which is then in a premenstrual or menstrual condition of congestion and glandular hypertrophy, the ovum produces necrobiosis or coagulation necrosis in the structures of the mucosa. At the same time the trophoblast exhibiting great proliferative energy now penetrates through the necrotic tissue, into the connective tissue of the mucosa. Here the phenomena of edema and of a violent hemorrhagic inflammation are now established. Veins and capillaries become enormously dilated, the blood current becomes sluggish, edematous infiltration becomes pronounced. The ovum automatically orients itself, so that the embryo comes to be situated towards the muscularis. The proliferating trophoblast with its syncytium, provided with cilia or rods, at this time begins to break into and to open up dilated maternal capillaries. Maternal blood now makes its way into the trophoblast, whether it here finds preformed cavities or whether it forms these cavities in a loose protoplasm in consequence of hydrostatic pressure, we do not know. While the trophoblast opens up the enlarged maternal blood lacunae, the hypertrophy of the mucosa, as a whole, goes on. The gland spaces become large and cystic, their ducts lead to the surface in a tortuous manner. A separation into a spongiosa and compacta becomes early established, and in the latter some cells early assume a marked deeidual character.
The ovum is now interstitially embedded in the mucosa and surrounded by a border zone which is composed of an admixture of still attached or detached trophoblast elements, degenerating fixed maternal cells, both connective tissue deeidual cells and glandular epithelia. In this border zone (“Umlagerungszone”) are also contained enormously enlarged maternal blood vessels, cystic blood—filled gland spaces and free blood. The ovum almost floats as it were in a lake of blood partly contained in the trophoblast cavities, partly in the cystic maternal gland spaces, partly freely infiltrating more or less all of the tissue in the direct neighborhood of the growing germ.
When the preliminary paper was read in Boston, Mass., August 20, 1907, before the Section on Embryology of the Seventh International Zoological Congress, Professor A. A. W. Hubrecht, the Chairman of the Section, in the discussion of the paper, called attention to a misconception of the Writer as to certain relations of the amnion and yolk sac. It is needless to say that the conception of Professor Hubrecht proved to be the correct one. This necessitated on the part of the author a careful re-examination of the sections and some changes in the manuscript for the Transactions of the Congress.
Professor C. S. Minot then kindly placed at my disposal his laboratory and his library to enable me to make the necessary reexaminations and changes. To him, as also to Professor F. T. Lewis, I am under obligations for the great kindness shown on this occasion, and in the final revision of the manuscript.
The literature on human placentation is extensively given in Keibel’s Normentafeln; in Peters’ monograph; in Webster, “Human Placentation” ; in Pfannenstiel’s article in Winkel’s “Handbuch der Greburtshiilfe.” The most recent contributions are quoted in Jung’s monograph and Bryce and Teacher (l. c.). Stahl, in his article on “Die Embryonalhullen der Sanger und die Placenta,” in Vol. I, Part 2, Hertvvig’s “Handbuch der Entwickelungslehre der Wirbelthiere,” gives the literature on placentation among mammals in general.
Fig. 23. Colored plate drawn by Miss Katharina Hill, artist of the Department of Anatomy of the University of Chicago, from an enlargement of fig. 25, photomicrograph from section 153. The embryo shown in this plate is drawn after section No. 155, because in it the yolk sac, embryonic shield proper, allantois and allantoic duct are best seen.
All. S.-Allantoic stalk.
C. A.——Cavity of amnion.
Can. F.—F1ne fibrin threads and leucocytes (early canalized fibrin). Cho. M.—Chorion mesoderni.
D. C.-—Decidua capsularis.
Exoc.——Exocoelom.
Gl.—Gland space (epithelium partly preserved).
Mat. B1. S.~Large cystic gland spaces filled with blood. S. V.——Yolk sac.
Syn.-Syncytium.
Tr.—Trophoblast.
Literature Cited
- ↑ Herzog: Description of an Early Placenta in .siitu,. obtained from the living. The Am. Gym. and Obst. Journal, April, 1898, and Transactions Chicago Pathol. Soc, Vol. III, p. 317.
- ↑ Herzog: Superfetation in the Human Race. Chicago Medical Recorder, 1898, Vol. 15, p. 1.
- ↑ Herzog: The Pathology of Tubal Pregnancy. Am. Jour. of Ol'1stet., 1900, Vol. 42, No. 2.
- ↑ Herzog: Placentation in 21 Uterus Duplex Bicornis Gravis—Menses 1-2. Trans. Chicago Pathol. Soc., 1904, Vol. 6, p. 51,
- ↑ Jung: Beitriige zur friihesten I<li-Einbettung beim menschlichen Weibe: Berlin. 1908. This author has very fully gone into the recent literature of the subject, and I have repeatedly quoted from his monograph. * Jung then points out the great rarity of young human ova obtained in situ. He mentions those of Peters, von Spee, Leopold, van Heukelom and Frassi-Keibel, and he finds objectionable features in all of them.
- ↑ The conditions found, which are probably pathologic, will be pointed out below.
- ↑ Keibel and Elze, Vol. 8 of Keibel’s Normentafeln zur Entwicklungsgesch. d. Wirbe1th., 1908, p. 9.
- ↑ Bryce, Thomas H., and Teacher, John H.: Contributions to the Study of the Early Development and Embedding of the Human Ovum. Glasgow, 1908.
- ↑ The first more extensive connnunication concerning: our specimen was made before the Section of Embryology of the Seventh International Congress of Zoology, held in Boston, in August, 1007. It was then the privilege of the author to demonstrate the sections to such well-known European and American embryologists as Professors A. A. VV. Hubrecht, F. VV. van Wijhe, Charles Sedgwick Minot, Franklin P. Mall, A. Playfair McMurrich, T. G. Lee, and F. T. Lewis.
Previous to this communication, the specimens had been demonstrated in June, 1907, before the German Medical and the Gynecologic Societies of Chicago.
Plates
Plate I
—DC.
-—D.S.
Can.F‘. Ems. 1 to 22.—Drawings from twenty—two serial sections of the embryo. (Sections Nos. 164-143 of the entire ovum).
C. A.—Cavity of amnion. C. M.—Chorion mesoderm. D. A.—-Duct of allantois. Em. S.—Embryonic shield. Exo.—Exocoelom. Mes.—~Mesode1'm.
P. A.—AlIantoic stalk.
S. V.—Yo1k sac. V.—Anlage of blood vessels.
fiG. 4. fiG. 5 I fiG. 6.
Plate III
Plate IV
fiG. 13.
fiG. 12.
fiG. 15
fiG. 14.
Plate V
fiG. 18. fiG 19
fiG. 24. Photomicrograph from section No. 125. Zeiss, planar, X 15 diam. (approximately). This photomicrograph shows the relation of the ovum to the parts where it has implanted itself. It is separated from the surface by a ‘very thin capsularis and in the middle of the upper margin of the photograph may be seen a place where the chorion mesoderm is adherent in the form of a teat-like projection to the capsularis, which is defective at this place. The open ring seen a little to the right of the center of the chorion cavity (exocoelom) is a transverse section of the yolk sac which hangs down beyond the end of the embryonic shield. The chorionic mesoderm is surrounded by the trophoblast. The ovum rides as it were on a wedge-shaped group of enormously, cystically, enlarged gland spaces, and lacunae, filled densely with maternal blood. To the right and to the left are seen the enlarged glands of the uterine mucosa. Differentiation into a decidua spongiosa and compacta is not well seen in this section. At the left lower corner there is a strip of the muscularis.
fiG. 25.—Photomicrograph from section No. 153. Zeiss, planar, X 29 diam. Showing the chorionic cavity filled with a coagulated granular material. To the right is seen the embryo anchored by the allantoid stalk to the chorion mesoderm. In the upper corner a thin strip of degenerated decidua capsularis.
fiG. 26.—Photoinicrograph from a section outside of the implanted ovum (from first series of sections, unnumbered), Zeiss, planar, X 15 diam. Showing large cystic gland spaces partly filled with blood. To the left a differentiation into a decidua compacta and spongiosa is Well marked. The lower margin of the photomicrograph shows the muscularis uteri.
fiG. 27.—Photomicrograph from section No. 83. Zeiss Apochromat. 8 mm., proj. occ. No. 4, X 210 diam. Gland spaces near the ovum filled with spherical hyaline masses.
Plate VII
fiG. 26.
fiG. 27.
fiG. 28.—Photomicrograph from section No. 101. Zeiss Apochromat. 16 mm., proj. occ., No. 4, X 100 diam. A coagulated crescentic mass containing a coarse network of parallel fibrin threads stretches across the field.
fiG. 29.—Photomicrograph from section No. 210. Zeiss Apochromat. honi. oil. imm., 2 mm., proj. occ., No. 4, x 1000 diam. A group of trophoblast cells, in the center one with karyokinetic figure.
fiG. 30. —Photomicr0graph from section N0. 186. Zeiss Apochroniat, horn. oil. imm., 2 mm., proj. 0cc., N0. 4, X 1000 diam. Cuticle and rods of the syncytium shown on the free order running like a polar axis from A to B.
(The photomicrographs were prepared under the author’s direction by Mr. Frank T. Harmon.)
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - A contribution to our knowledge of the earliest known stages of placentation and embryonic development in man. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_contribution_to_our_knowledge_of_the_earliest_known_stages_of_placentation_and_embryonic_development_in_man
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G