Paper - A Human Ovum at the Previllous Stage
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Dible JH. and West CM. A human ovum at the previllous stage. (1941) J Anat. 75(3): 269–281. PMID 17104860
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Ovum at the Previllous Stage
Introduction
The specimen which is described in this communication consists of what is believed to be a normal ovum that has been not long imbedded in the uterus, and it represents the youngest stage of human development that has so far been recorded in this country. It was discovered by one of us (J.H.D.) during the course of a post-mortem examination of a woman, aged 28, who had died from internal hydrocephalus due to fibrous adhesions at the base of the brain which were probably the result of trauma. The post-mortem examination was made not more than ten hours after death, and there is no reason to suppose that the specimen is not normal.
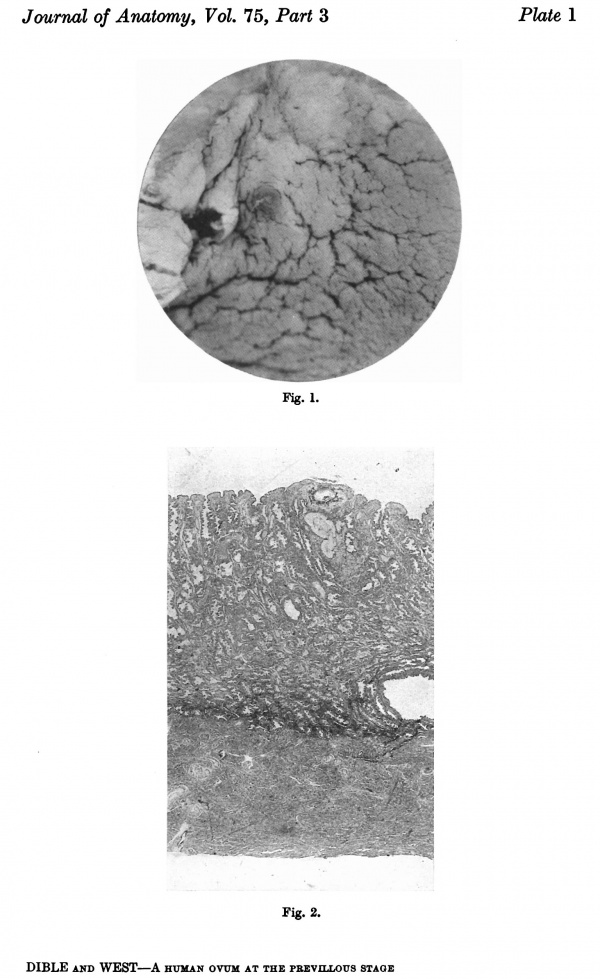
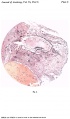
A large corpus luteum was seen in one ovary and this directed particular attention to the uterus; on opening this along the anterior wall the mucous membrane was found to be thick and velvety, and on the posterior wall, towards the fundus, there was seen a small vesicle (Fig.1) projecting above the surface which was thought to be an early ovum. A small wisp of fibrinous material was adherent to the summit of the vesicle. No menstrual history was available.
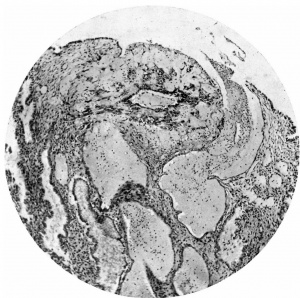
The opened uterus was fixed in formalin and, after being photographed, a block of tissue containing the vesicle was removed, cut at 4p,and stained with iron-haematoxylin and eosin; blocks of tissue from other parts of the uterus were also removed for study of the structure of the endometrium at a distance from the vesicle; this endometrium presents the characters typical of the premenstrual phase, with large, tortuous and active glands, and a rich blood supply; the sections show, too,that the fixation and preservation are good. In the sections of the block of tissue containing the vesicle the mucous membrane is5mm. thick and is divisible into a stratum compactum, 03 mm. thick, and a stratum spongiosum (Fig. 2).
The implantation site can be recognized in the sections with the unaided eye by the pink stain which has been taken up by some dilated glands lying atthebase of the area. Contrary to what has been found in several young specimens, and to what might have been expected from the appearance of the intact vesicle, the implantation site is not much raised above the surface in the sections. Implantation has occurred between the mouths of two glands which are bent round the ovum, and the site of implantation occupies the stratum compactum only. The ovum is not covered with a decidua capsularis, and the point of entry of the ovum into the endometrium is seen (Figs. 5, 7) as a crater-shaped area, from which the uterine epithelium is deficient, over-lying the summit of the ovum. The aperture of entry is 0-23 mm. wide; it is filed,as with a bung, by the 'operculum' (Teacher, 1924) and is capped by the 'closing coagulum' or 'Gewebspilz' (Peters, 1899). The latter appears as a loose mass of fibrinoid material containing some leucocytes and it was seen as the small wisp, referred to above, when the uterus was opened.
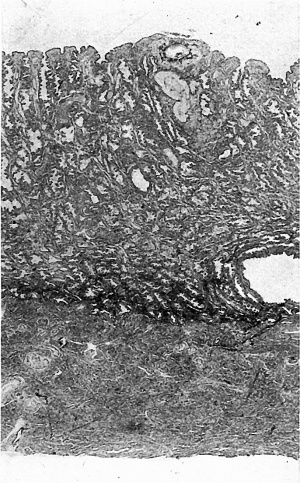
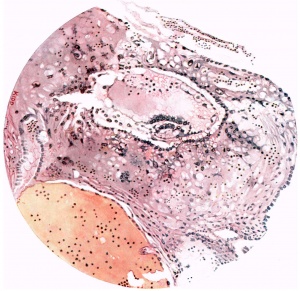
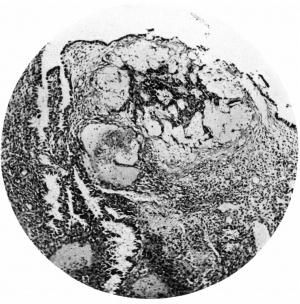
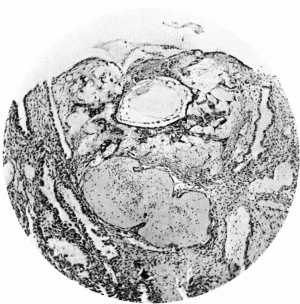
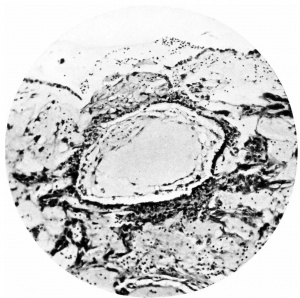
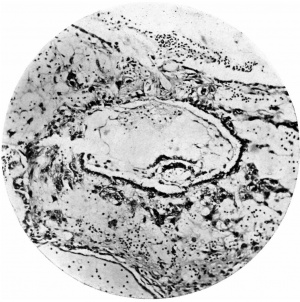
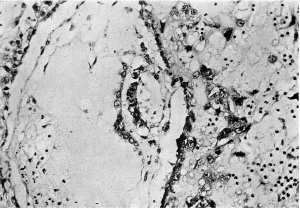
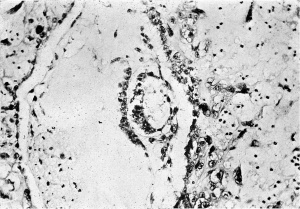
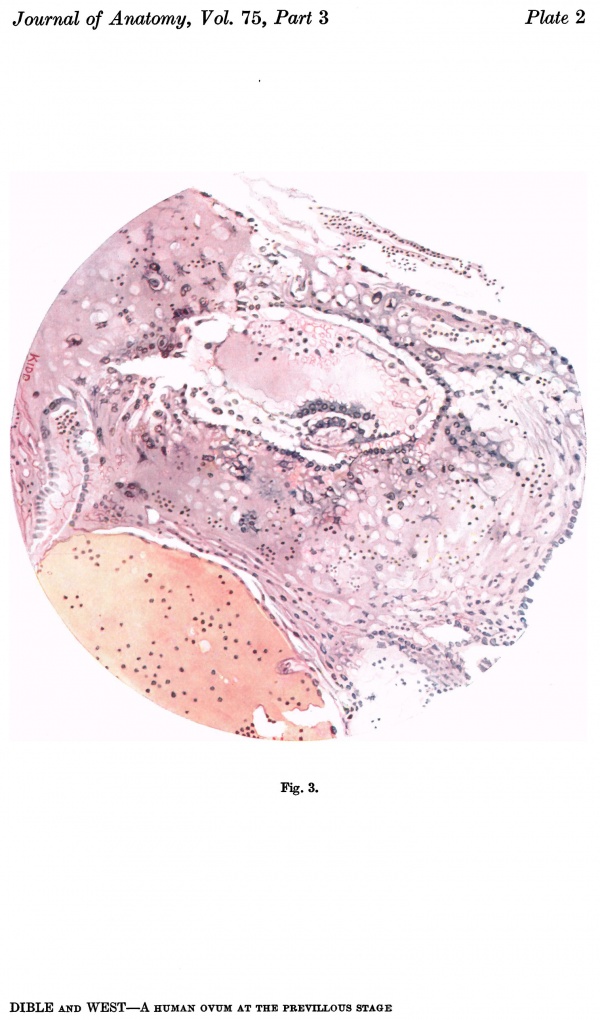
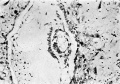
The ovum (Fig. 3) consists of a slightly flattened chorionic vesicle, the blastocyst, which has an internal measurement of 047mm. in the equatorial plane, i.e. from side to side, and of 0.28 mm. in the polar plane, i.e. from the surface inward. The inner surface of the blastocyst is smooth and has the embryonic rudiment attached at its base; the outer surface is irregular owing to cellular and syncytial processes growing out from it into the implantation cavity. These processes are stained deeply with haematoxylin and they stand out in sharp contrast with the clear cavity in which they lie (Fig.4).The smooth inner surface of the blastocyst is lined with primary mesoderm, and inside this there is the exocoelomic vesicle (Heuser, 1938) enclosed by the exocoelomic membrane (Heuser's membrane). The embryonic rudiment is composed of a thick ectodermal plate which forms the ventral wall of the amniotic cavity, and of a rather thinner entodermal plate which is continuous at its margins with the exocoelomic membrane. There is no yolk sac.
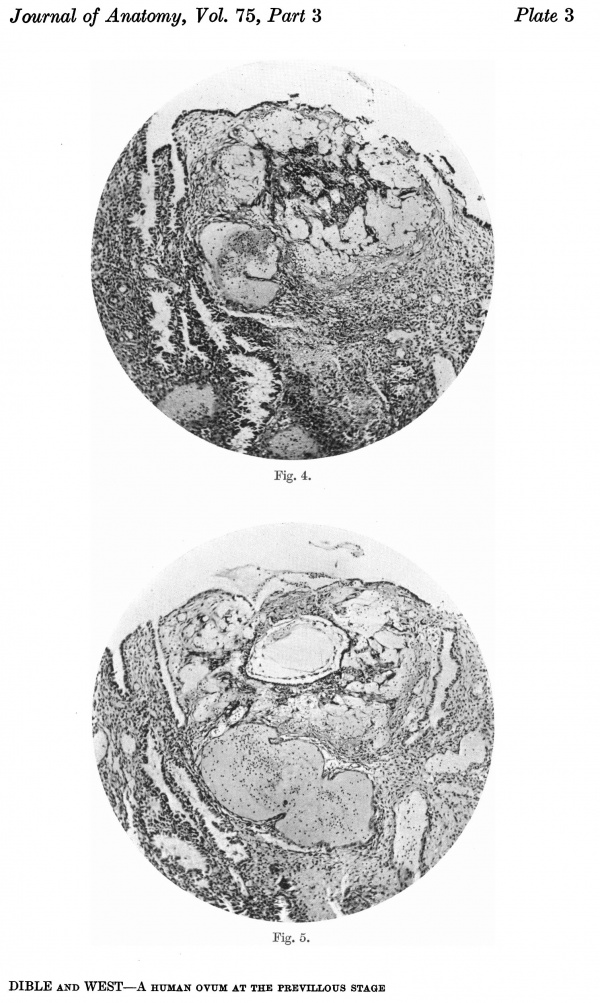
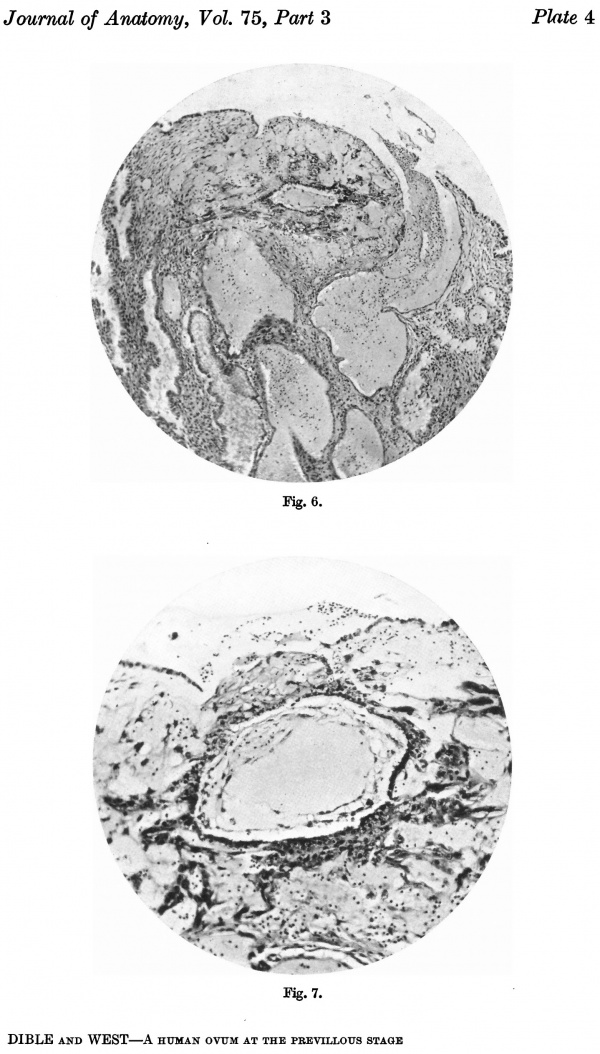
There are no chorionic viliand thus the specimen belongs to the group of ova at the previllous stage, which includes the Miller (1913, and Streeter, 1926), the Kleinhans (Grosser, 1922), the Muller (1930), and the two new Hertig ova of which a preliminary account only has so far appeared (Hertig & Rock, 1939). Further, it corresponds with Streeter & Wislocki's (1938) stage II for the macaque, which they term the 'stage of trophoblastic lacunae' and which they estimate to be long to the period from the 11th to the 14thday. The implantation cavity appears (Figs. 4-6) as an almost empty, clear and circumscribed area and thus stands out in sharp contrast with the adjacent endometrium on the one hand and the darkly stained trophoblast on the other; it is this character of the implantation cavity which gave to the specimen a cystic appearance when first seen in the uterus with the unaided eye.
Thetrophoblast. Growing from the outersurface of the blastocyst are the trophoblastic processes. In the account which follows, the terminology employed by Ramsey (1938) in her description of the Yale embryo will be used. Thus we are able to recognize central cytotrophoblast, peripheral cytotrophoblast, and plasmoditrophoblast or syncytium.
The central cytotrophoblast (Figs. 7, 8) forms the true wall of the blastocyst; it is composed of a single layer of lining cells which, for the most part, appear rather condensed and flattened and have darkly stained, round or oval nuclei, but poorly defined cel walls; in some sections the nuclei are packed closely together giving a dark border to the blastocyst, while in others, particularly in the region of the operculum, they are few and far between.
The peripheral cytotrophoblast (Figs. 4-8) appears as sheets, or columns, of cells growing from the blastocyst wall in to the implantation cavity; in other words, it is an extension outward of the central cytotrophoblast. The cels are larger and have more clearly defined walls than have those of the central cytotrophoblast, their nuclei generally are not so darkly stained, nor do they fill the cell to the same extent,so that there is often a clear space left between the nucleus and the cel wall. Beyond the peripheral cytotrophoblast, and also extending in amongst its cell columns, are grey, filmy masses and strands which may be nucleated or non-nucleated; this tissue is the plasmoditrophoblast, or syncytium (Figs.5-8). It is devoid of any cell boundaries,and in some places nuclei occur in clumps, like the 'heaped-up epithelial nuclei' describedbyStreeter& Wislocki(1938).
In the plasmoditrophoblast brown granules are seen in many places; similar granules have been described by Johnstone (1914) and Greenhill (1927). Johnstone suggested (p. 270) that they are composed of molecules of blood pigment adherent to a minute droplet of fat, the fat itself having disappeared in the course of the process of fixation and staining. We are of opinion that this pigment is a formalin-haemoglobin precipitate, and the fact that it is found mostly at the implantation site and in the cells of distended glands which form lacunae of blood rather supports this view, since these are sites of
haemorrhage, and it is well known that in slightly autolysed tissues such precipitates are common in histological material. The ovum, and the autolytic ferments present in the trophoblast, may well be responsible for some degree of local autolysis. Greenhill implies(p. 340) a similar origin of the granules in his specimen. It has not been possible to make any microchemical analysis of these granules as it would have involved an interruption in the series of slides,but it may be mentioned that they are anisotropic, as are the formalin precipitates. The frequent occurrence of these granules in association with leucocytes provides further support for their origin from blood.
Several authors have referred to the foam-like condition of the syncytium of early stages due to the presence of innumerable vacuoles of various sizes, and the general opinion seems to be that this vacuolated syncytium has been derived from a more solid plasmodium which has grown out from the blastocyst wall. Thus Bryce (1908) suggests (p. 43) that the spun-out condition of the plasmodium or syncytium is due to the formation in the vacuoles of a digestive fluid which, after rupture of the vacuoles, is replaced by maternal blood; the large amount of blood in the implantation cavity is a striking feature of the ovum T.B. 1. Streeter (1926), in his account of the Miller ovum, describes the lacunae as being small near the wall of the ovum and larger as the periphery is approached, and he refers to them (p. 37) as representing the 'histological picture of a process by which a solid mass of trophoblast becomes converted into a sponge-like syncytium'. The Miller ovum shows a less definite implantation cavity than the present specimen.
In our ovum the vacuolated network (Figs. 7, 8) seems to be made up largely of coagulated protein with branching cells scattered through it; these cells appear to be partly trophoblastic and partly stromal, and some are degenerate. This appearance is no doubt produced, so far as the protein is concerned, by fixation, and it seems probable that in life the contents were mainly fluid or gelatinous with strands of fibrin, amongst which the cells of the trophoblast were growing out from the blastocyst as in a tissueculture. We believethat our ovum, the Miller ovum, and the ovum T.B. 1, show progressive stages in the dissolution of the originally more solid plasmodium, the present specimen being at a stage intermediate between the Miller ovum, and the ovum T.B. 1, where the vacuoles have become filled with blood.The Muller ovum we believe to be at a stage similar to ours.The embryo Kleinhans, judged by the condition of the implantation cavity, seems to be at a stage intermediate between the present specimen and the ovum T.B. 1, for it shows a cavity which is fairly clear cut but which contains rather more blood than ispresentinourspecimen. By the same criteria, the two new Hertig ova are both at stages earlier than either the present specimen or the Miller ovum; Hertig's no. 7700 in particular shows an almost solid plasmodium surrounding the trophoblast wall.
Teacher (1924) refers to the two generations of syncytium, the destructive and temporary syncytium-the 'Implantations-syncytium' of Grosser-and the permanent syncytium of the chorionic villi-the 'Zotten-syncytium' of Grosser. It is this first generation of syncytium that we have before us,and we get the impression that itshows evidence of destructive action and also of beinginaprocessofdegeneration,for it is a degenerate tissue and yet it seems able to open into capillaries and, contrary to what Streeter found in the Miller ovum, into glands also. Streeter further stated that he was unable to find any evidence of destruction or ingestion of the stroma cells by the syncytial loops; in the presentspecimen,asserialsectionsareexamined from the non-implantation area towards the blastocyst, the first change seen is that the stroma becomes oedematous, the spaces between the cels are increased, and their nuclei become enlarged and paler. By the appearance of their nuclei these maternal stroma cels can be distinguished from the cels of the trophoblast, and in sections through the periphery of the implantation cavity they can be recognized without any doubt, though they are not numerous and their period of survival would appear to be short; here and there they can be seen lying in a mass of the filmy grey tissue that has been recognized as syncytium.
It may be suggested here that much of the lacy, network-like, appearance of the trophoblastseems to be duetodestructionofitscels,by autolysisor by extracellular digestion, or by a combination of both processes; the general picture suggests the presence of some rather active ferment, or of conditions causing autolysis. We believe that many of the spaces between the trophoblastic branches may contain autolysed blood, the more so because the red celsinmany of the capillaries in the vicinityareautolysed,thoughnot inthose fartheraway. Thisinterpretationisinconformitywiththesuggestionwe made above concerning the origin of the pigmentedgranules.Thus, there does seem to be evidence of the digestion of the stroma cels by the syncytium in this specimen, and we believe that such destruction must have been extensive, for there islitleevidence of the stroma having been simply pressed outward, as Streeter found to be the case in the Miller ovum, though in some places it is arranged more or less in concentric lamellae as if it had been pushed aside to someextent. It does seem that the main method of enlargement of the cavity, with the necessaryremoval of the stromacels,isbyaprocessofoedema of the stroma,degenerationofitscels and the irautolysisordigestion. Itisnoteworthy that there is a strip-the 'border-zone'-of degenerated and necrotic stroma alround the implantation cavity, except where itisin contact with a gland (Figs. 4, 5).
The implantation cavity
The implantation cavity measures 1-0 mm. in its equatorial diameter and 0575mm. in its polar. As has been mentioned above, it appears as an almost empty, clear and circumscribed area, and though this character of emptiness is an appearance only, yet in this character and in the sharpoutline of the cavity the specimen shows a condition different from what is found in other early ova, with the exception of that described by Muller, in that these have an implantation cavity more or less filedwith blood, and with an irregular and indefinite outline. The cavity rests on two glands which have been so greatly distended with blood that their epithelium has been quite flattened and resembles the endothelial lining of a blood vessel, for which it was at first mistaken (Figs. 3, 5 and 8). The distension affects mainly the deeper parts of the glands. Brewer (1937) states that in all human pregnancies reported there is an accumulation of blood in the uterine glands, and, in the Edwards-Jones-Brewer ovum which he describes, the glands are certainly very distended with blood, but the only specimen besides the present one that shows a comparable distension is that described by Herzog (1909). As to the cause of the distension in the present specimen, it is clear that in the case of one gland at least it is due to the fact that it does not open on the surface, its lumen being occluded with syncytium and its mouth incorporated in the implantationcavity. It is therefore distended in part with blood,andinpart with its own secretion which has noexit. In another case, the gland has burst on to the surface (Fig. 6) and its contents have been expelled into the uterine cavity where they form a wisp of fibrinous material impregnated with leucocytes.
The closing coagulum and the operculum
A wisp of fibrinous material has been alluded to already as the closing coagulum which caps the aperture of entry of the ovum in to the uterine mucous membrane (Figs.1, 3, 5, 7 and 8). The structure of this coagulum and of the contents of the implantation cavity is essentially similar, and it may be regarded as a part of the contents of the cavity which has become free on the surface owing to the absence of any decidua capsularis, and to the disappearance of the uterine epithelium over the point of entry of the ovum. The closing coagulum is also continuous in part with the contents of the gland which has burst on one side of the ovum. The very thin covering of the ovum was recognizable in the intact specimen, for theovum hadtheappearanceofatinycyst,likeablister in the thickmucous membrane,with its pole glistening throughthethincovering. Itisinteresting to note, in connexion with the origin of the closing coagulum, that Brewer (1937) showed a much larger and more organized 'blood clot' overlying the surfaceepitheliumattheimplantationsite,andhesuggestedthatitscharacter indicated previous bleeding into the uterine lumen; the implantation cavity in Brewer's ovum contains a good deal of blood, and this may explain the difference in the appearance of the closing coagulum in his specimen and in ours.
The implantation cavity does not surround the ovum completely, for in its superficial part it is interrupted by the presence of the operculum (Figs. 2, 5). As so well described by Schlagenhaufer & Verocay (1916), quoted by Teacher (1924, p. 179), the operculum, 'as the stopper of a bottle, as the keystone of a vault closes the entrance to the implantation cavity with its precious contents'. Itisclearlyanoutgrowth of the central cytotrophoblast at the superficial pole of the ovum, and it fils the space between this part of the ovum and the uterinecavity. Instructureitisliketheperipheralcytotrophoblast of the rest of the ovum, except that it is rather less cellular and more vacuolated; in it are some small masses of syncytium and a few degenerated maternal stroma cels.The whole mass has a degenerated appearance and gives the impression of being a tissue, derived from proliferation and outgrowth of the cytotrophoblast, which has nearly outlived its usefulness. In some sections(Fig.8)theoperculum and the closingcoagulumarecontinuous. Over the aperture of entry the uterine epithelium is deficient (Figs. 5, 7). Farther laterally it forms the sides of the crater-like area occupied by the operculum, butbothhereand in the adjacentglandsitisflattenedandhastheappearance ofhavingbeenstretched.Thisisquiteunliketheappearance of the epithelium lining the uterus and the glands generally, which is columnar.
The blood vesels
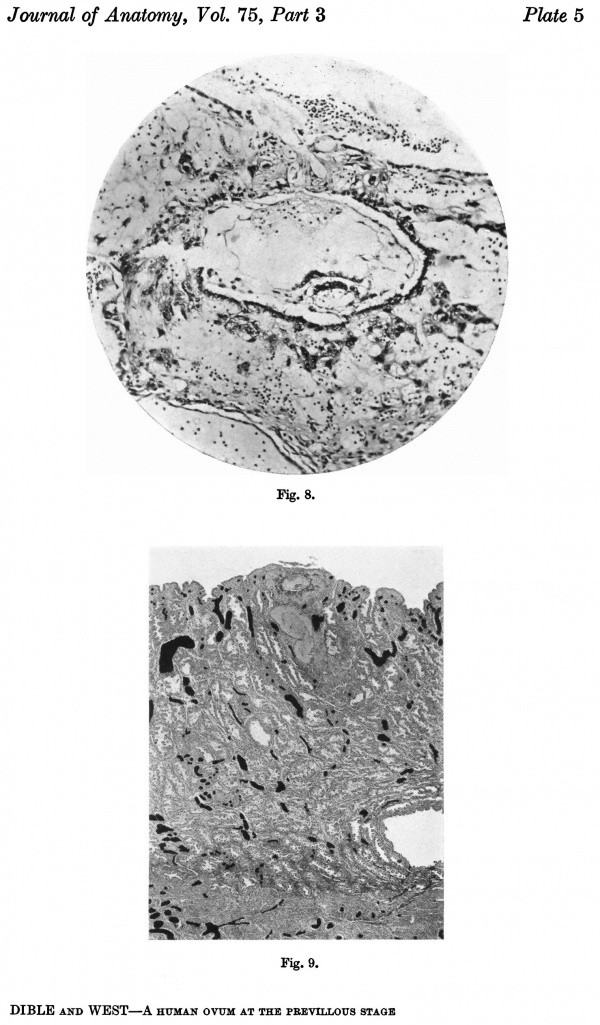
Fig. 9 is a photograph of a section through the middle of the ovum, and on it have been marked with ink all the blood vessels. It shows that the endometrium is well supplied with blood. Small,thick-walled,spiral arteries and larger and thinner veins are seen in the stratum spongiosum, but capillaries only are present in the stratum compactum. There is no definitive blood sinus at the base of the ovum, such as is described by von Mollendorff (1921a) in the embryo 'Sch.', and by Ramsey (1937, 1938) in the Yale and Lockyer embryos; instead, there are numerous small vessels round the implantation site which ultimately join into two main veins, one rather larger than the other. The capillaries of the stratum compactum are opened by the syncytium and become incorporated in the implantation cavity, giving rise, at least in part, to the clear spaces of the cavity.
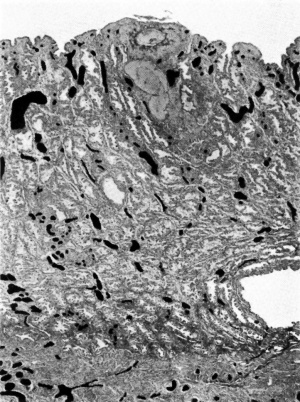
The embryonic rudiment
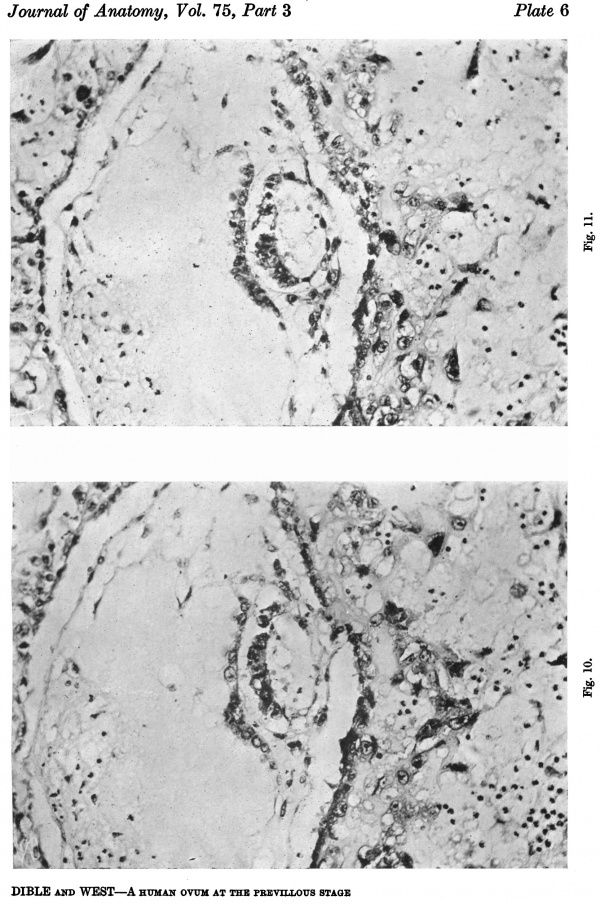
The embryo is found on the inner surface of the basal wall of the blastocyst. It consists of a thick ectodermal embryonic plate which forms the ventral wall of an otherwise thin-walled amniotic cavity, and of a rather thinner entodermal plate which has not yet differentiated into any partofadefinitiveyolksac. Spreadingout from the sides of the amniotic cavity are the cels of the primary mesoderm which, when traced in a lateral direction, form a continuous lining for the inside of the blastocyst (Figs. 7, 8, 10 and 11). Within this primary mesoderm there is an even thinner layer (Figs. 5, 7 and 8), the exocoelomic membrane (Heuser, 1938); this encloses a large, more or less spherical, cavity which we thought at firstwas the yolk sac, admittedly a good deal larger than we had expected to find in an ovum at this stage. This membrane will be referred to in more detail after the other parts of the embryohavebeendescribed. Itisunfortunatethatanumberofsections containing the embryo have been lost and it is thus not possible to make a completereconstruction,butthefollowingarethemeasurements of the embryo taken in the middle of the ten sections in which it occurs:
- Embryonic disc 0.1 x 002mm.
- Endodermal plate 0.14 x 0.016mm.
- Amniotic cavity 0.04 x 0.09 mm.
The cels of the ectodermal and entodermal plates do not differmuch; in each (Figs. 10, 11) the cels are closely packed, here and there piled up two deep, butstandinginacolumnararrangement in the ectodermandlyingendtoend in the entoderm.The cell boundaries are not well defined, butthenucleiare largeandgranular,exceedingthose of the centralcytotrophoblastbutequalled by some of those of the peripheral cytotrophoblast.
Inadiscussion of the formation of the human yolksacStieve(1931)shows a section through the embryo, Werner which resembles our embryo in having what he thought to be a large yolk sac. This was our interpretation also at first, but we have since had the opportunity and advantage of seeing Dr Streeter's beautiful photographs of the two new Hertig ova, of having his personal opinion about them, and of having read Heuser's (1938) paper on the 'Early development of the primitive mesoblast in embryos of the rhesus monkey'. The stages illustrated in Heuser's publication are of particular interest,forStreeter(1933)has shown that the difference in detail of form and in rate of differentiation and growth between man and monkey does not become appreciabletilthe end of the second month, and that known ages of macaques may be transferred to human embryos at least for the first month orsixweeks. It is therefore profitable to compare our ovum with the stages of the macaque described by Heuser, since, as we have suggested above, our specimen fals into the stage of development which Streeter & Wislocki attribute in the macaque to the period from the 11th to the 14th day, and Heuser gives photographs of embryos of the 11th, 12th and 13th day.
The most striking difference between each of these specimens and our ovum is seen in the condition of the entoderm. We have described thee ntoderm plate as being nearly equal in thickness to the ectoderm plate and have stated thatthereisnodefinitiveyolksac. Heuser's 11th day embryo shows no yolk sac,but in the specimen of the 12th day it is just beginning,and in the specimen of the 13th day it is seen clearly. In respect of the development of the yolk sac, then, our embryo corresponds with one of the 11th day in the macaque, the chief difference being in the thickness of the gut entoderm which, in the macaque, consists of a flattened layer of only one cell in thickness spread out on theinnersurface of the innercelmass.
Heuser states (p. 384) that 'Cells delaminating from the inner surface of the thickened trophoblast seem to be continuous with the primitive entoderm beneath the inner cell mass'. He finds that by the 11th day these cells become continuous with a layer that has split off from the ventral surface of the entoderm sheet so that a complete envelope, the exocoelomic membrane, isformed, havinganoriginpartlytrophoblasticandpartlyentodermal. Inourovum such a membrane is present, but its origin is not as clear as in the macaque; it appears (Figs. 3, 8, 10 and 11) to be continuous in the embryonic region not with a layer of cels split off from the entoderm but with the edges of the entoderm itself, nor is it certain (though it is probable) that the remainder has arisenbyasplittingoff from the innersurface of the trophoblast. Itthus appears that, as far as the development of the exocoelomic membrane is concerned, our ovum is at a stage earlier than that of the macaque at the 11th day.
We have referred above to the primary mesoderm as forming a continuous lining for the inside of the blastocyst. Heuser finds that till the 13th day, 'except in a small zone around the embryo itself, there is little if any additional mesoblastic tissue', that is, additional to the tissue that has formed the exocoelomic membrane. Our ovum has a trilaminar blastocyst (Fig. 7) composed of an outer trophoblast, a middle primary mesoderm, and an inner exocoelomic membrane; it thus corresponds, as regards the development of the mesoderm, with a macaque of some days later than the 13th.
The primary mesoderm looks as ifitmight well have been derived from a splitting off of cels from the inner surface of the trophoblast, and in the embryonicregionitiscontinuouswithcelslyingatthesides of the ectodermal plate and the amnion (Figs. 8, 10 and 11). These cells extend for some distance outward from the embryo and help to filthe space between the trophoblast and the exocoelomic membrane. This is seen particularly well in a glass-plate reconstruction in which the mesoderm cells are shown to be more numerous here than elsewhere.
With regard to the entodermal plate, we are unable to see more than one formofcel,though,insome sections,on theventralsurface of the platethere areafewcelswhicharelessdarklystainedthanthemajority,andwe can seeno filmofflattenedcelswhichmightgiverise to the basalportion of the exocoelomic membrane; nor can we seeany splittingoffofcellsfrom itsventral surfacetoformtheventralwall of the yolksac.We believeourspecimen to be at a stage antecedent to the completion of the exocoelomic vesicle.
For some years ithas been believed that the human yolk sac appears early in development and is already present in the youngest known specimens, that the yolk sac develops by a process of cavity formation in the ventral part of theinnercelmass,andthatalitswallsareofentodermalorigin. Ithasbeen believedthattheseconditionsholdgoodforotherprimatesalso. Forexample, J. P. Hill (1932), in his Croonian Lecture, described the condition of the yolk sac in the earliest catarrhine embryo then known, the 'Keim S' of Nasalis larvatus (Selenka); he believed it was at 'the stage when an originally solid endodermal yolk-sac primordium is in course of becoming converted into a hollow vesicle by a process of vacuolization' (p. 108).
More recent work on primate material by Heuser, Stieve, and Streeter has cast some doubt on the truth of these older theories, and Streeter (1937), in discussing the origin of the yolk sac in primates, states that its earliest cels are differentiated 'as a thin membrane between which and the gut endoderm there arises a narrow cleft. This cleft becomes rapidly distended and so forms the conjoint yolk sac cavity and gut cavity. This cavity is therefore dual in origin and is bordered on its dorsal part by cells that are to form the gut endoderm, an induced product or migratory element from the embryonic ectoderm. It is bordered on its ventral part by the yolk sac endoderm which is a derivative of the primitive mesoblast and is essentially trophoblastic.'
Through Dr Streeter's kindness we have had the pleasure of examining photographs of the two new Hertig ova and of an ovum of the macaque at about the same stage of development. The Hertig ova are at much the same stage as our own and, like it, show ectodermal and entodermal plates and an exocoelomic membrane which appears to be continuous with the edges of the entodermalplate.Themacaqueovum isataratherlaterstage,andinitthe exocoelomic membrane is separate from the entodermal plate and forms a closed exocoelomic vesicle on the ventral side of the entoderm. A yolk sac is present, and consists of a small vesicle with its dorsal wall formed by the entodermal plate and its ventral wall by cells which look different from those at the base; they are smaller, more flattened, and have more darkly stained nuclei, and they resemble the cels of the primary mesoderm and the dorsal wall of the amniotic cavity.
The study of our own specimen and of the photographs of the Hertig ova and the macaque lead us to believe that the older theories must be revised; for we find that the Hertig ova and our own, the youngest human specimens thus far known, have no yolk sac. The macaque ovum strongly suggests that the yolk sac does not arise by a process of cavity formation in the inner cel mass, and shows that the cels of the dorsal wall of the yolk sac are quite differentinappearancefromthoseofitsotherwalls. Streeter's(1937)suggestion, quoted above, at firstfiled us with astonishment and confusion, for it seemed that, in his view, there was no such thing as entoderm sui generis if the gut entoderm is a migratory element from the embryonic ectoderm, and if the yolk sac entoderm is derived from the primitive mesoblast and is trophoblastic in origin. The dificulty, however, seems to be one of terminology and of the distanceback in the irdevelopmentalhistorytowhichthedifferent layersaretraced.
After careful study, we agree with what we believe to be Streeter's views and the course of development seems to us to be as follows: The inner cell mass becomes differentiated into a thicker dorsal part, the embryonic ectodermal plate or embryonic disc, and a thinner ventral part, the embryonic entoderm. The exocoelomic membrane splits off from the inner surface of the trophoblast and becomes adherent to the edges of the embryonic entoderm, so that there is formed a temporary exocoelomic vesicle bounded dorsally by entoderm,andelsewherebycelssplitoff from the trophoblast.A layerofcells splits off from the ventral surface of the embryonic entoderm and joins with the cels of the exocoelomic membrane, or else the exocoelomic membrane grows round on the ventral surface of the entoderm and then becomes separated from it. Thus there is formed finally a complete exocoelomic vesicle, detached from the entoderm, occupying the interior of the blastocyst. The primary mesoderm, which has meanwhile been split off from the inner surface of the trophoblast outside the exocoelomic membrane, spreads as a thin layer over the ventral surface of the embryonic entoderm, between it and the exocoelomicvesicle.A cleft then appears between this film of primary mesoderm and the embryonic entoderm, so that there is formed a cavity bounded dorsallybythecels of the embryonicentoderm,andelsewherebycelsderived from the primary mesoderm. This cavity is the yolk sac, but, as Streeter pointed out, its dorsal wall will later be that of the gut, whilst its other walls will be those of the yolk sac proper. The alternative to this suggestion of the formation of the yolk sac is that the primary mesoderm does not spread over the ventral surface of the entoderm, and that the cleft is not between mesoderm and entoderm but within the entoderm itself. In opposition to this, however, it must not be overlooked that, at least in the macaque, the cells of the dorsal wall of the cleft are quite different from those of its other walls.
The amnion
As with the yolk sac, the cells of the ventral wall of the amnion are different from those of its other walls (Figs. 8,10 and 11). Its ventral wall isformedbycels of the embryonic ectoderm; the cells of its other walls are smaller, more widely spaced and more mesodermal in character. Streeter.& Heuser (1935) called attention to this sudden transition in the structure of thewalls of the amniotic cavity, and suggested that whilst the floor was formed by the ectoderm of the germ-disc proper the remaining walls were trophoblastic in origin.
It has been thought that the human amniotic cavity develops, like that of the bat, by a process of cavity formation in the more dorsal part of the inner cell mass, that the dorsal wall of the cavity is closely adherent to the inner surface of the blastocyst, and that the cells of all its walls are of ectodermal origin. Our ovum, the Hertig ova, and the macaque ovum referred to above, al show the same characters of the amnion (Fig. 11). The cavity is rather flattened in a ventro-dorsal plane, more so in the other specimens than in ours. The ventral wall is thick and formed by the embryonic ectoderm, the other walls are very much thinner and formed by cells that resemble those of the primary mesoderm. The dorsal wall is not continuous with the inner surface of the trophoblast, but is attached to it only indirectly through the intervention of a few scattered mesoderm-like cels. That the cavity has arisen by cleft formation seems clear, but it is not so certain whether the cleft has been formedwith in the dorsalpart of the innercelmass orbetweentheinnercell mass and the trophoblast. If the cleft arose with in the inner cell mass one would hardly expect to see such a difference between the cells of the ventral wall and the other walls of the cavity, and the amnion would be more closely attached to the trophoblast. Ifthecleftoccurredbetweentheinnercelmass and the trophoblast it would mean theseparation of the innercelmassfrom theinnersurface of the trophoblastand thefiling of the gap between thetwo with primary mesoderm, resulting in the formation of a cavity bounded everywhere, except ventrally, by cels of the primary mesoderm. The latter seems to be the more probable course and would explain the characters of the amnion described above, though it is more accurate to speak of a gradual separation of the dorsalpart of the innercelmass from theinnersurfaceof the trophoblast accompanied by a filingin of the space with mesoderm rather than of any actual cleft formation.
It thus appears that the yolk sac and the amniotic cavity have a rather similar method of development and that, as Heuser (1938) pointed out, the original inner cell mass is responsible for the formation of a small part only of the actual embryo, that is, the germ disc or embryonic ectoderm and a thin film of entoderm on its ventral surface.
Summary
A descriptionisgivenofahumanovum attheprevillousstageofdevelopment which was obtained at an autopsy.
The implantation cavity is unusually well defined and free from blood. It liesbetween and above greatly dilated glands which are almost filedwith partly haemolysed blood, and of which one has burst into the uterine cavity. The closing coagulum appears to be derived partly from the contents of this ruptured gland, and partly from the contents of the implantation cavity. The operculumfilstheapertureofentryin to the uterinemucous membrane; itis an outgrowth from the wall of the blastocyst, and gives the impression of havingalmostoutliveditsusefulness.
The blastocyst shows, growing from its outer surface, cytotrophoblast and syncytium; the syncytium is at the stage intermediate between the formation of the 'Implantations-syncytium' and the 'Zotten-syncytium' of Grosser. The blastocyst is a trilaminar vesicle, its outer wall being the trophoblast, its middle the primary mesoderm, and its inner the exocoelomic membrane. Theembryoisattached to the basalportion of the blastocyst,andiscomposed of an ectodermal embryonic plate, an entodermal plate, and an amniotic cavity. There is no yolk sac.
The origin of the exocoelomic membrane, the yolk sac, and the amnion isdiscussed. Itissuggestedthattheexocoelomicmembraneisderived,like the primary mesoderm, by splitting off from the inner surface of the trophoblast, that, in agreement with Streeter's suggestions, the yolk sac has its dorsal wall derived from embryonic entoderm and its ventral wall from primarymesoderm,thattheamnionhasitsventralwallderivedfromembryonic ectoderm and itsdorsal wall from primary mesoderm, and that the inner cel mass is responsible for only the embryonic ectoderm, or germ-disc, and a thin layer of entoderm on its ventral surface.
Acknowledgements
The authors are indebted to Mr W. Kiffin, of the Pathology Department, the University of Liverpool, for the preparation of the sections; to Mr C. Wilmott, of the British Postgraduate Medical School, University of London, and to Mr A. Welch, of the Anatomy Department, University College, Cardiff, for the photographs; and to Mr Douglas Kidd, of Liverpool, for the coloured drawing.Theyarealsoindebted to the JointAcademicCommitteeofUniversity College, Cardiff, for a grant towards the expenses of the publication of this paper, and to Dr George L. Streeter for his personal interest in the specimen and for photographs of the Hertig ova and of a macaque ovum.
References
The following papers have been consulted, but not alare referred to in the text:
Brewer JI. A normal human ovum in a stage preceding the primitive streak (The Edwards-Jones-Brewer ovum). (1937) Amer. J Anat., 61: 429-481.
BREWER, J. I. (1937). Amer. J. Anat. 61, 429. (1938). Contr.Embryol.Carneg.Instn,27,85.
Bryce TH. and Teacher JH. Contributions To The Study Of The Early Development And Imbedding Of The Human Ovum 1. An Early Ovum Imbedded In The Decidua. (1908) James Maclehose and Sons. Glasgow.
BRYCE, T. H. & TEACHER, J. H. (1908). Contribution to the Study of the Early Development and Imbedding of the HumanOvum. Glasgow:JamesMaclehoseandSons.
DARON, C. H. (1936). Amer. J. Anat. 58, 349.
GREENEmLL, J. P. (1927). Amer. J. Anat. 40, 315.
Grosser O. VII. The development of the egg membranes and the placenta; menstruation in Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia. GROSSER,0.( 1910). Thedevelopment of the eggmembranes and the placenta;menstruation. In Human Embryology, by Keibel, F.and Mall,F.P.1,91. (1922). Z.ge8.Anat.1.Z.Anat.EntwGesch.66,179.
HERTIG,A.T.(1935). Contr.Embryol.Carneg.Inetn,25,39.
HERTIG,A.T.& RoCK,J.C.(1939). Anat.Rec.73,Suppi.2,26.
HERZOG, M. (1909). Amer. J. Anat. 9, 361.
HEUSER, C. H. (1932). Anat. Rec. 52, Suppl. 15.
(1936). Anat.Bec.64,Suppl.3,20.
(1938). Publ.Carneg.Inmtn,No.501,383.
Hou, J. P. (1932). Philos. Tranm. B, 221, 45.
HIRAMATSU, K. (1936). Polia anat. japon. 14, 15.
JOHNSTONE, R. W. (1914). J. Obstet. Gynaec. May, p. 231.
Kiss,F.(1921). Z.gee.Anat.1.Z.Anat. Entw Geech.62,519.
LATTA, J. S. & TOLLMAN, J. P. (1937). Anat. Rec. 69, 443.
MULLENDORFF, W. VON (1921a). Z. ges. Anat. 1.Z. Anat. Entw~eech.62, 352.
(1921b). Z. ge.Anat. 1.Z. Anat. Entw Geech.62, 406.
MOSSMAN, H. W. (1937). Contr. Embryol. Carneg. Instn,26, 133.
MbLLER, S. (1930). Z. mikr.-anat. For-sch. 29, 175.
ODGERS, P. N. B. (1937). J. Anat., Lond., 71, 161.
PETERS, H. (1899). Die Einbettung des menschlichen Lies. Leipzig und Wien.
RAMSEY, E. M. (1937). Contr. Embryol. Carneg. In8tn, 26, 99.
(1938). Contr.Embryol.Carneg.Instn, 27, 67.
STIEVE,H.(1931). Verh.Anat.Ges.ErgAnzungsheft72,44.
STREETER,G.L.(1926). Contr. Embryol. Carneg. Instn,18,31.
(1933). Ann. Rep. Director Dep. Embryol. Carneg. Instn for 1932-3, p. 4. (1937). Anat.Rec.70,Suppl.1,53.
(1939). Ann. Rep. Director Dep. Embryol. Carneg. Instn for 1938-9, p. 147.
STREETER, G. L. & HEUSER, C. H. (1935). Anat. Rec. 61, Suppi. 44.
STREETER, G.L EWISLocnI, G.B. (1938). Contr.Embryol.Carneg. Instn,27,1.
TEACHER, J. H. (1924). J. Obstet. Gynaec 31, 166.
Explanation Of Plates
Plate 1
Fig. 1. The ovum, as seen when the uterus was opened. A wisp of fibrinous material on the summit of a small, dark, circular, congested and elevated area, just to the left of the centre of the photograph, marks the site o fimplantation. x1-6 approx.
Fig. 2. A section (slide 25-4) through the uterine wall at the level of the middle of the ovum: this is seen at the top of the section. x10.(There is an average of 5 sections on each slide.)
Plate 2
Fig. 3. Coloured drawing of a section (slide 25-2) through the middle of the ovum. x100.
Plate 3
Figs. 4, 5. Sections (slides 21-2 and 23-4) through the blastocyst. x33.
Plate 4
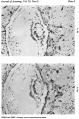
Fig. 6. Section (slide 27-3) through the blastocyst. x33.
Fig. 7. Section (slide 24-1) through the blastocyst. x72.
Plate 5
Fig. 8. Section (slide 25.2) through the blastocyst. x90.
Fig. 9. Section through the uterine wall (slide 25.3) on which the blood vessels have been drawn with India ink. x15-5.
Plate 6
Figs. 10, 11. Sections (slides 25-2 and 25.4) through the embryo. The amnion is below and the entoderm above. x200.
Gallery
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology Paper - A Human Ovum at the Previllous Stage. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_Human_Ovum_at_the_Previllous_Stage
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G