Endocrine - Pineal Development
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The pineal gland (epiphysis cerebri) has an important role in the sleep/wake daily cycle (circadian), high melatonin plasma levels at nighttime and very low levels at daytime, and reproductive development. The gland is thought to evolutionarily to have been positioned as to be exposed to light, and hence remains a regulator of cyclic rhythms associated with day/night and day length. The pineal hormone (melatonin) has targets both in the nervous system and in many different peripheral tissues. Melatonin synchronises circadian rhythms acting through the two G-protein-coupled receptors (MT1, MT2) in the suprachiasmatic nucleus of the hypothalamus.
The embryo and fetus pineal does not produce significant amounts of melatonin, though has abundant tissue receptors. The maternal pineal gland produces melatonin in the normal circadian fashion and this melatonin can cross both the placenta and blood-brain barrier. In other species, maternal melatonin crosses the placenta into fetal circulation and may provide photoperiodic information during fetal development that influences later postnatal circadian (daily day/night) and seasonal (day length) rhythms. The pineals of non-mammalian vertebrates are photoreceptive, whereas those of mammals do not normally respond to directly light. Pineal blood supply is derived from the posterior cerebral artery choroidal branches and through the pineal recess is bathed in cerebrospinal fluid (CSF).
The melatonin levels in premature infants is lower and delayed, but not different when calculated from conception date. Other factors such as preeclampsia, growth restriction, and nursery lighting can cause altered rhythm development. The same study has also shown that full-term infants born at home and full-term twins born in the hospital had significantly lower metabolite excretion levels than hospital-born singleton infants at the same ages despite similar body weights.[1] Allopregnanolone, a pineal neurosteroid, acts on the cerebellum supporting survival Purkinje cell by suppressing caspase-3 expression.[2]
Postnatally in humans, the melatonin levels may act on clock gene expression in the pars tuberalis of the anterior pituitary for photoperiodic switching. Melatonin also has important functions related to the program timing of puberty. Note that there are many clinical studies investigating the possible role of melatonin in diverse health areas, from oxygen starvation at birth through to neural effects in old age.
Overview
- part of epithalmus - neurons, glia and pinealocytes
- pinealocytes secrete melatonin - cyclic nature of activity, melatonin lowest during daylight
- inhibit hypothalamic secretion of GnRH until puberty, pineal gland then rapidly regresses.
- other activities - possibly gamete maturation, antioxidant effect, protect neurons?
Links: pineal | Category:Pineal | Lecture - Endocrine Development | Lecture - Head Development
| Historic Pineal Papers |
|---|
| 1917 Pineal Region | 1932 Pineal Gland and Cysts | 1935 Pineal | 1937 Human Pineal | 1940 Nerve and Vascular Supply |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Pineal Embryology | Pineal Development | Melatonin Development | Melatonin Embryology | Melatonin Receptor | Pineal Hypoplasia | Pineal Tumour |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Development Overview
- Neuroectoderm - prosenecephalon then diencephalon
- caudal roof, median diverticulum, epiphysis
- Initially a hollow diverticulum, cell proliferation to solid, pinealocytes (neuroglia), cone-shaped gland innervated by epithalamus
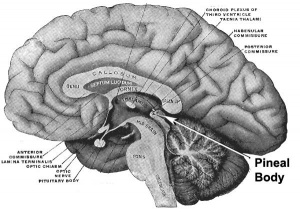
Epithalamus consists of the pineal gland and habenular nuclei
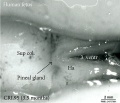
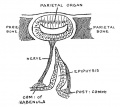
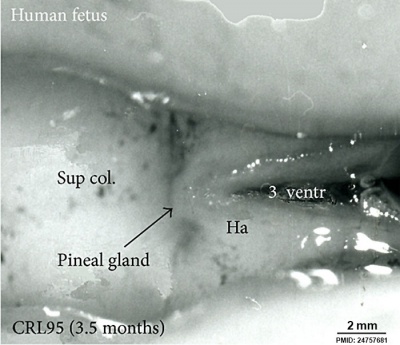
| Fetal Pineal Anatomy[9]
Superior (dorsal) view of the diencephalic-mesencephalic area of a 3.5-month-old human fetus. The third ventricle (3 ventr) without pial covering is seen to the right in the micrograph. The small pineal gland is a small protuberance (arrow) and merging via the broad stalk with the habenula (Ha). Sup col.: superior colliculus. Bar = 2 mm. |
|
Melatonin
Melatonin synchronises circadian rhythms through two G-protein-coupled receptors (MT1, MT2). Night release from the pineal gland activates melatonin receptors in the suprachiasmatic nucleus of the hypothalamus, this synchronises light–dark cycle of physiology and behaviour.
- Melatonin is synthesized from the amino acid tryptophan within the pinealocytes.
- Serotonin is first acetylated by aryl alkylamine N-acetyltransferase (AA-NAT), then converted to melatonin by acetyl serotonin methyl transferase (ASMT also known as hydroxyindole O-methyltransferase or HIOMT).
- Melatonin release is stimulated by darkness and inhibited by light and is said to have neurological "chronobiotic" properties for resynchronization of sleep and circadian rhythms disturbances. In the periphery, melatonin is also involved in the regulation of several complex cycles: seasonal reproduction, body weight and energy balance.
- Melatonin levels can be monitored by urinary excretion of the melatonin metabolite 6-sulfatoxymelatonin (aMT.6S).
Key Facts
- less than 30 min to 60 min - serum half-life of melatonin
- 70% - serum melatonin bound to albumin
- 30% - diffuses in surrounding tissues
- liver - primary metabolism
- kidney - secondary metabolism
Melatonin Receptors
The hormone melatonin acts through receptors (high affinity G protein-coupled) embedded in the cell membrane. Three different receptor subtypes have been identified in mammals: MT1 (MTNR1A) and MT2 (MTNR1B) and a putative binding site called MT3.
- MT1 - (4q35.2) expressed in humans in the pars tuberalis of the pituitary gland and the suprachiasmatic nuclei of the hypothalamus.
- MT2 - (11q14.3) expressed in the retina and brain.[10]
- MT3 - expressed in many non-mammalian vertebrates in a range of brain areas.
Innervation
The gland is connected to the hypothalamus suprachiasmatic nucleus (SCN) central rhythm generator through a multi-synaptic pathway.
Nerve fibers innervating the mammalian pineal gland originate from perikarya located in the sympathetic superior cervical ganglion, the parasympathetic sphenopalatine and otic ganglia, as well as by nerve fibers originating in the central nervous system.[11]
- sympathetic nerves - contain norepinephrine and neuropeptide Y as neurotransmitters
- parasympathetic nerves - contain vasoactive intestinal peptide and peptide histidine isoleucine
- trigeminal ganglion - containing substance P, calcitonin gene-related peptide, and pituitary adenylate cyclase-activating peptide
Molecular Development
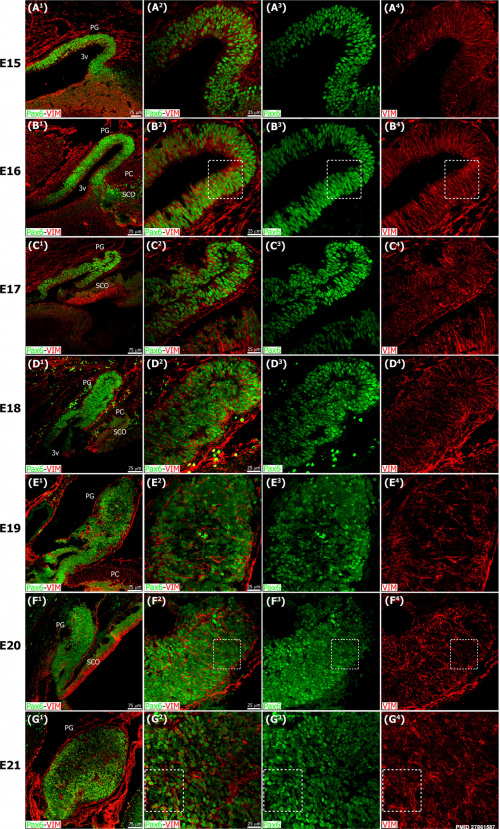
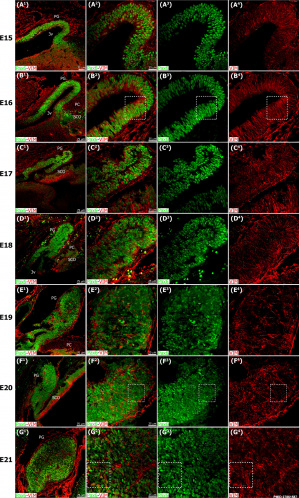
Pineal gland mouse (E15 to E21) |
The pineal gland develops from neuroepithelial cells that express the transcription factor Pax6 and the intermediate filament vimentin.
Panels display confocal microscopy of immunolabeled sagittal sections of rat pineal gland (PG) from embryonic day (E) 15 to E21.
Pineal organogenesis begins around E15 as an evagination of the neuroepithelium in the dorsal diencephalon that is densely populated by Pax6-expressing cells (green). The developing PG becomes a tubular extension at E16. The orientation of Pax6/VIM+ cells is radial at these stages. At E17 the pineal neuroepithelium begins to fold and fuses at the midline. After fusion of the neuroepithelium, double immunolabeled rosette-like structures are visible in the E18-E21 developing PG. At E21 the PG has developed into a recognizable globular structure. (A1-G1) 20x; scale bar: 75 μm. (A2-G4) 60x; scale bar: 25 μm. PC, posterior commissure. SCO, subcommissural organ. 3v, third ventricle. |
- Bsx - homeobox gene-encoded transcription factor[12]
- Nodal - zebrafish required for dorsal convergence of pineal precursors.[13]
- Pax6 - rat pineal gland from E16, peak expression around E18.[14]
- Fgf8a - zebrafish epithalamus acts permissively to promote parapineal fate.[15]
- DARPP-32 (Dopamine- and cAMP-regulated phosphoprotein of 32 kDa) is involved in the retinal pathway transmitting photic information that resets the circadian clock.
- OTX2, RAX, CRX, PAX4, TBX2B - also identified in development.
Links: molecular
Abnormalities
- Pineal Hypoplasia associated with retinal disease.
- Pineal Tumours in children are associated with abnormal puberty development.
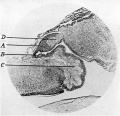
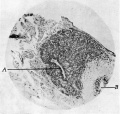
Histology
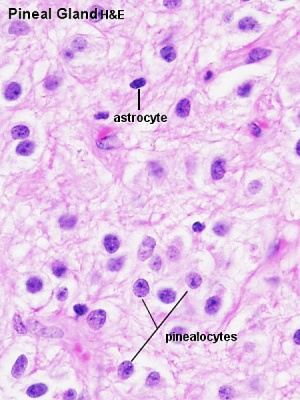
Adult Histology
- Astrocytes - small dark nuclei
- Pinealocytes - most nuclei present, larger lighter and round nuclei surrounded by a broad rim of light cytoplasm
- Endothelial cells - nuclei in association with the vessels and capillaries traversing the tissue.
- Cytoplasmic processes - "stringy" appearance from both pinealocytes and astrocytes
- Links: large histology image
References
- ↑ Kennaway DJ, Goble FC & Stamp GE. (1996). Factors influencing the development of melatonin rhythmicity in humans. J. Clin. Endocrinol. Metab. , 81, 1525-32. PMID: 8636362 DOI.
- ↑ Tsutsui K. (2019). Kobayashi award: Discovery of cerebellar and pineal neurosteroids and their biological actions on the growth and survival of Purkinje cells during development (review). Gen. Comp. Endocrinol. , 284, 113051. PMID: 30339808 DOI.
- ↑ 3.0 3.1 Ibañez Rodriguez MP, Noctor SC & Muñoz EM. (2016). Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator. PLoS ONE , 11, e0167063. PMID: 27861587 DOI.
- ↑ Bolat D, Kürüm A & Canpolat S. (2018). Morphology and quantification of sheep pineal glands at pre-pubertal, pubertal and post-pubertal periods. Anat Histol Embryol , 47, 338-345. PMID: 29774950 DOI.
- ↑ Gerasimov AV, Kostyuchenko VP, Potapov AV, Varakuta EY, Karpova MR, Sukhanova GA & Logvinov SV. (2018). Infradian Rhythm of the Content of Secretory Granules in Pinealocyte Cytoplasm in Mice and Rats. Bull. Exp. Biol. Med. , 165, 276-279. PMID: 29931631 DOI.
- ↑ Yamazaki F, Møller M, Fu C, Clokie SJ, Zykovich A, Coon SL, Klein DC & Rath MF. (2015). The Lhx9 homeobox gene controls pineal gland development and prevents postnatal hydrocephalus. Brain Struct Funct , 220, 1497-509. PMID: 24647753 DOI.
- ↑ Reiter RJ, Tan DX, Korkmaz A & Rosales-Corral SA. (2014). Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update , 20, 293-307. PMID: 24132226 DOI.
- ↑ de Faria Poloni J, Feltes BC & Bonatto D. (2011). Melatonin as a central molecule connecting neural development and calcium signaling. Funct. Integr. Genomics , 11, 383-8. PMID: 21465271 DOI.
- ↑ Møller M, Phansuwan-Pujito P & Badiu C. (2014). Neuropeptide Y in the adult and fetal human pineal gland. Biomed Res Int , 2014, 868567. PMID: 24757681 DOI.
- ↑ Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA & Gusella JF. (1995). Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Natl. Acad. Sci. U.S.A. , 92, 8734-8. PMID: 7568007 DOI.
- ↑ Møller M & Baeres FM. (2002). The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. , 309, 139-50. PMID: 12111544 DOI.
- ↑ Carstensen MB, Hertz H, Bering T, Møller M, Rohde K, Klein DC, Coon SL & Rath MF. (2020). Circadian regulation and molecular role of the Bsx homeobox gene in the adult pineal gland. J. Pineal Res. , 68, e12629. PMID: 31808568 DOI.
- ↑ Aquilina-Beck A, Ilagan K, Liu Q & Liang JO. (2007). Nodal signaling is required for closure of the anterior neural tube in zebrafish. BMC Dev. Biol. , 7, 126. PMID: 17996054 DOI.
- ↑ Rath MF, Rohde K, Klein DC & Møller M. (2013). Homeobox genes in the rodent pineal gland: roles in development and phenotype maintenance. Neurochem. Res. , 38, 1100-12. PMID: 23076630 DOI.
- ↑ Clanton JA, Hope KD & Gamse JT. (2013). Fgf signaling governs cell fate in the zebrafish pineal complex. Development , 140, 323-32. PMID: 23250206 DOI.
Online Textbooks
- Pineal Gland and Cancer-An Epigenetic Approach to the Control of Malignancy: Evaluation of the Role of Melatonin Eurekah Bioscience Collection - Neuropharmacology
- Endocrine changes in puberty Endocrinology -> The gonad
- Second Malignancies Cancer Medicine -> Section 24: The Eye -> 85. Neoplasms of the Eye -> Pediatric Ophthalmic Oncology: Ocular Diseases
- The Action of Melatonin on Experimental in-Vivo Tumors Eurekah Bioscience Collection -> Neuropharmacology -> Pineal Gland and Cancer-An Epigenetic Approach to the Control of Malignancy: Evaluation of the Role of Melatonin -> Effect of Melatonin on Tumor Growth
- Potential Significance of (Patho)Physiological Changes of Melatonin for the Aetiology of Cancer Eurekah Bioscience Collection -> Neuropharmacology -> Pineal Gland and Cancer-An Epigenetic Approach to the Control of Malignancy: Evaluation of the Role of Melatonin
- Effects of Exogenous Melatonin AHRQ Evidence reports and summaries -> AHRQ Evidence Reports, Numbers 61 - 119 -> 108. Mel
Journals
- Journal of Pineal Research Molecular, Biological, Physiological and Clinical Aspects of Melatonin
Reviews
{{#pmid31866317}}
Farias Altamirano LE, Freites CL, Vásquez E & Muñoz EM. (2018). Signaling within the pineal gland: A parallelism with the central nervous system. Semin. Cell Dev. Biol. , , . PMID: 30502386 DOI.
Ilahi S & Ilahi TB. (2018). Physiology, Pineal Gland. , , . PMID: 30247830
Chen YC, Sheen JM, Tiao MM, Tain YL & Huang LT. (2013). Roles of melatonin in fetal programming in compromised pregnancies. Int J Mol Sci , 14, 5380-401. PMID: 23466884 DOI.
Weinert D. (2005). Ontogenetic development of the mammalian circadian system. Chronobiol. Int. , 22, 179-205. PMID: 16021838
Macchi MM & Bruce JN. (2004). Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol , 25, 177-95. PMID: 15589268 DOI.
Barrenetxe J, Delagrange P & Martínez JA. (2004). Physiological and metabolic functions of melatonin. J. Physiol. Biochem. , 60, 61-72. PMID: 15352385
Ekström P & Meissl H. (2003). Evolution of photosensory pineal organs in new light: the fate of neuroendocrine photoreceptors. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 358, 1679-700. PMID: 14561326 DOI.
Thomas L, Drew JE, Abramovich DR & Williams LM. (1998). The role of melatonin in the human fetus (review). Int. J. Mol. Med. , 1, 539-43. PMID: 9852259
Articles
Barros VRP, Monte APO, Santos JMS, Lins TLBG, Cavalcante AYP, Gouveia BB, Müller MC, Oliveira JL, Donfack NJ, Araújo VR & Matos MHT. (2020). Melatonin improves development, mitochondrial function and promotes the meiotic resumption of sheep oocytes from in vitro grown secondary follicles. Theriogenology , 144, 67-73. PMID: 31918071 DOI.
Ibañez Rodriguez MP, Noctor SC & Muñoz EM. (2016). Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator. PLoS ONE , 11, e0167063. PMID: 27861587 DOI.
Shoja MM, Hoepfner LD, Agutter PS, Singh R & Tubbs RS. (2016). History of the pineal gland. Childs Nerv Syst , 32, 583-6. PMID: 25758643 DOI.
Sun B, Wang D, Tang Y, Fan L, Lin X, Yu T, Qi H, Li Z & Liu S. (2009). The pineal volume: a three-dimensional volumetric study in healthy young adults using 3.0 T MR data. Int. J. Dev. Neurosci. , 27, 655-60. PMID: 19665543 DOI.
Al-Hussain SM. (2006). The pinealocytes of the human pineal gland: A light and electron microscopic study. Folia Morphol. (Warsz) , 65, 181-7. PMID: 16988913
Saito S, Tachibana T, Choi YH, Denbow DM & Furuse M. (2005). ICV melatonin reduces acute stress responses in neonatal chicks. Behav. Brain Res. , 165, 197-203. PMID: 16182388 DOI.
Sumida M, Barkovich AJ & Newton TH. (1996). Development of the pineal gland: measurement with MR. AJNR Am J Neuroradiol , 17, 233-6. PMID: 8938291
Search PubMed
Search Pubmed: pineal development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- NIH The Julius Axelrod Papers | The Pineal Gland and the "Melatonin Hypothesis," 1959-1974
- NIH Child Health and Human Development (USA) Pineal Gland and Chronobiology: Regulation of Pineal Function
- University of Cincinnati SURVEY OF ENDOCRINE ORGANS
Additional Images
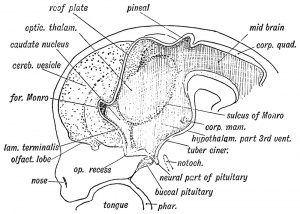
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
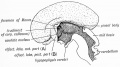
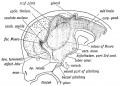
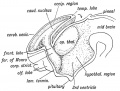
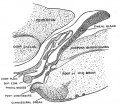
Cooper ERA. The human pineal gland and pineal cysts. (1932)
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
Terms
| Endocrine Terms (expand to view) |
|---|
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Endocrine - Pineal Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Endocrine_-_Pineal_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G