Book - Contributions to Embryology Carnegie Institution No.43
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. A human embryo (Mateer) of the pre-somite period. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 272, 9: 389-424.
| Online Editor | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
This paper describes the Carnegie Collection Embryo No.1399, classified as Stage 8 occurring during Week 3.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Embryo (Mateer) Of The Presomite Period
Director of Department of Embryology, Carnegie Institution of Washington.
Volume IX. No. 43 pp389-424 (1920) With seven plates and four text-figures.
- Paper Links: Fig 1 | Fig 2 | Fig 3 | Fig 4 | Fig 5 | Fig 6 | Fig 7 | Fig 8 | Fig 9 | Fig 10 | Fig 11 | Fig 12 | Fig 13 | Fig 15 | Fig 16 | Table 1 | Chart 1 | Chart 2 | Chart 3 | Plate 1 | Plate 2 | Plate 3 | Plate 4 | Plate 5 | Plate 6 | Plate 7 | Paper | Carnegie Institution of Washington - Contributions to Embryology | George Streeter
Introduction
In the literature there may be found descriptions of 16 very young and apparently normal human ova, containing embryos in which the somites have not yet made their appearance. These specimens are all accompanied by authentic clinical data. There are, in addition, 4 presomite specimens which are probably normal, but in which chnical data are missing. When one arranges all of these specimens in their apparent order of development they will be found to fall into three clearly defined groups: First, those in which the primitive groove has not yet appeared; second, those in which there is a primitive groove but no neurenteric canal; and third, those in which the neurenteric canal and medullary groove can be definitely recognized. It has been my privilege to examine a well-preserved, normal specimen which would belong to the second of these groups, and in this paper I wish to present a survey of its main morphological features. The specimen has been temporarily deposited in the Carnegie collection for the purpose of this study, and has been listed in the catalogue as embryo No. 1399; it will be referred to, however, as the Mateer embryo, in recognition of the owner, who, appreciating the embryological importance of the specimen, brought it to our attention.
History of the Specimen
The ovum was obtained by Dr. Horace X. Mateer, of Wooster, Ohio, from a fibroid uterus which had been removed by Dr. H. J. Stoll, of Wooster, 11 days after the woman had missed her menstrual period. The patient, a white American, aged 39 years, had been married 17 years. This was her fourth pregnancy, and there had been no abortions or evidence of venereal disease. She is of a prolific family, the mother having had 17 children, and a sister 10 children. The followmg menstrual history of the case was obtained at the time of operation:
- Sept. 10, menstruation began.
- Sept. 12, menstruation ended.
- Sept. 19, coitus.
- Sept. 27, coitus.
- Oct. 8, menstruation expected but failed to appear.
- Oct. 19, hysterectomy.
It may be added that the patient had been informed by Dr. Stoll of the presence of the fibroid condition and warned that in case she became pregnant it would be necessary to remove the uterus. It is therefore probable that she made careful note of such matters and that the above clinical data may be relied upon.
The ovum was dissected out from the uterus and placed in 10 per cent formaUn (4 per cent formaldehyde) within an hour after the operation. It was placed upon a bed of cotton and appeared at first to be almost spherical. It flattened out, however, and at the time of cutting was ellipsoid in shape. The specimen was not carefully measured at that time. It was embedded in parafin and cut into serial sections through its equatorial plane by Dr. B. Harrison Winier. Dr. Mateer's laboratory assistant. In all, 277 sections were saved; 2 passing through the embryo and 6 through the extra-embryonic chorion were lost, making a total of 285 sections. Inasmuch as the microtome was set at lO^t, this, the shortest, diameter of the chorion may therefore be placed at approximately 3 mm. Forty-nine sections through the region containing the embryo were stained at once in carmine. The remaining sections were mounted on slides and, in February 1916, were forwarded to the Carnegie Laboratory of Embryology, where they were stained by various dyes, including hematoxylin and eosin, iron hematoxylin, eosin, aurantia, and orange g.
The 49 sections through the embryo were from the first kept in serial order. The order of the other sections through the extra-embryonic region was only partially preserved, but has since been restored as far as possible. This necessitated the renumbering of the entire series of sections, and throughout this paper the new serial numbers will be uniformly used.
Sections through the Embryo and Adnexa
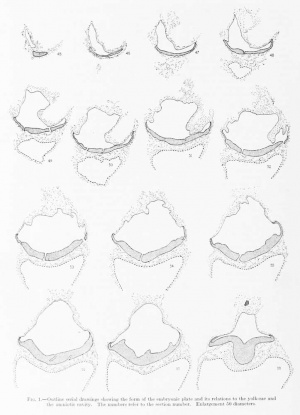
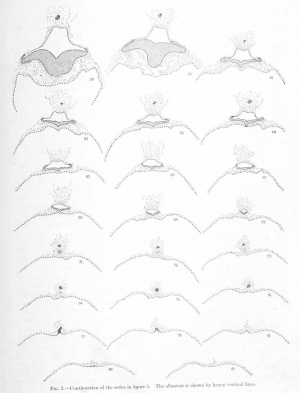
In order that the reader may trace the various structures making up the embryo and its adnexa, there will be given here a systematic description of the individual sections. The form of the structures is diagrammatically shown in figures 1 and 2. While the microtome was set at 10, measurements show that a few of the sections were cut irregularly, some being more, others less than lO/i. Where this occurs note will be made of it.
The chorion as a whole presents the form of a much flattened sphere, and the sections pass through its equatorial plane. The flattened surfaces may be regarded as the two poles; the one to which the embryo is attached we may call the placental or dorsal pole, and the opposite is the ventral pole. These poles are dorsal or ventral as regards the original position of the embryo; that is, the dorsal surface of the embryonic shield is towards the dorsal i)ole of the chorion, and the ventral surface towards the ventral pole.
Descriptions of the Sections
The first 26 sections of the series include only trophoblast and villi. The 27th, 28th and 29th sections traverse the chorionic membrane. In the 30th section one can see the chorionic cavity or exocoelom. The succeeding 13 sections include the narrow interval between the chorionic membrane and the embryo. This space is filled by a fine granular network of coagulum, in the meshes of which a few maternal red blood-cells can be seen. Since the chorionic membrane was not torn, it is probable that these red cells were displaced during the process of sectioning. The knife may have carried them from the intervillous spaces.
Sections 44 and 45 pass tangentially through the extreme rostral portion of the anmion. In section 45 the amniotic cavity makes its first appearance. Dorsally it is loosely adherent to the chorionic membrane.
Section 46 passes through the rostral end of the amniotic cavity and shows the obliquely cut rostral margin of the embryonic plate. An irregular strand of mesodermal cells can be seen everywhere investing the amniotic membrane and embryonic shield.
Sections 47 to 49 penetrate more deeply into the amniotic cavity showing the folds of the amniotic membrane. Ventral to the amniotic cavity the yolk-sac makes its appearance.
Section 50: A more detailed drawing of this section is shown in figure 16, plate 4. The amniotic cavity is still larger than the yolk cavity. The anmiotic membrane is folded so that it can be seen in both transverse and tangential sections. Where cut transversely it appears as a single layer of flattened ectodermal cells closely invested by an irregular layer of mesoderm. Dorsally, loose strands of mesoderm extend toward the chorionic membrane. Around its lateral margin the amniotic membrane bends sharply to become the embryonic plate. In its more lateral portions the embryonic plate consists of one or two layers of cylindrical ectodermal cells. The nuclei for the most part are toward the bases of the cells. More centrally the number of layers is increased to 3 or 4. Owing to the tangential direction of the sections, the middle portion of the embryonic plate appears thicker than it actually is. Ventral to the embryonic plate, strands of mesoderm extend between it and the yolk-sac. The mesoderm seems to be more adherent to the ectoderm of the embryonic plate than to the entoderm of the yolk-sac; between it and the latter there is a series of roomy clefts. The wall of the yolk-sac consists of a rather poorly defined strand of protoplasm with large, round, and oval nuclei, arranged irregularly in two layers. Some of these stam intensely, others are pale. Cell-boundaries can not be well made out. Within the yolk-sac there is a considerable amount of finely granular coagulum similar to that in the exoccelom. The amniotic cavity is perfectly clear. Apparently the outer row of nuclei represents the investment of mesoderm.
Sections 51 and 52: The space between the amnion and chorionic membrane is bridged by a mass of mesoderm more dense than in the previous sections, so that the sections are now definitely in the region of the body-stalk. Among these cells may occasionally be seen a group arranged in circular formation, so as to form a disconnected endothelial-like space. These spaces are for the most part empty, but now and then they inclose one or more cells. The amniotic membrane is much the same as in the previous sections. The embryonic plate is cut obliquely. The central part is uniform in appearance, showing no evidence of an neurenteric canal. The margin towards the amniotic cavity is covered on each side by a lateral sulcus which demarcates a transitional portion intervening between the embryonic plate and the amniotic membrane. This portion resembles the rhombic lip, to which is attached the tela choroidea in the hind-brain of the adult. About one-third of the distance between this lateral sulcus and the middle line is another groove, less marked, but which seems to be fairly constant throughout the successive sections. In the middle line there is no groove. Between the two sulci on each side the embryonic plate bulges into the lumen of the amniotic cavity, resulting in a longitudinal ridge which can be traced backward to about the region of the primitive groove. In the embedding of the specimen the tissue became brittle and an occasional crack is found crossing the embryonic plate. The mesoderm ventral to the embryonic plate shows pointy of intimate attachment to the latter, particularly in the lateral portions of the plate. Strands of mesoderm cross from the embryonic plate to the yolk-sac, forming trabeculae, between which is a series of clear, round spaces. Laterally, these spaces are continuous with the cleft that intervenes between the mesoderm and the wall of the yolk-sac, extending about one-quarter of the distance toward the ventral pole. The two layers of the wall of the yolk-sac, the endoderm and mesoderm, are more distinct than in the previous sections. No indication of blood islands is seen in this region. The content of the yolk-sac resembles the granular magma seen in the exocoelom and is perhaps slightly greater in amount.
Section 53 shows very well the attachment between the amnion and the chorionic membrane. The mesodermal cells are closely clustered around the anmion. The apex of the amnion is cut tangentially and so stands out in marked contrast to the mesoderm. The transition from the amnion to the embryonic plate is clearly shown on the left side of the section, the transitional portion being made up mostly of one layer of entodermal cells. The embryonic plate is everywhere clearly separated from the yolk-sac by the intervening mesoderm, which at several points seems adherent to it.
Section 54: In this section the amnion comes in contact with the chorionic membrane. The amniotic ectoderm does not show any connection with the chorionic epithelium.
Section 55: In the body-stalk there is seen an endothelial-like space within the lumen of which a cluster of 7 nuclei projects. The mesoderm lie between the embryonic plate and the yolk-sac is more closely attached to the former than to the latter.
Section 56: (Compare fig. 16, plate 4.) This section is particularly good for showing the relations between the amniotic membrane and the mesoderm. The former is nearly everywhere cvit in transverse section except at its extreme tip. The mesoderm is arranged as a membrane, closely investing the amnion and extending a short distance on the body-stalk. Ventrally it extends downward to inclose the yolk-sac, where it can be traced as a separate lamina for about one-half the distance to the ventral pole. Lying free in the exocoelomic space, at the; junction of the amnion with the body-stalk, is a small, empty, endothelial cavity. This can be traced only through two sections. It is surrounded solely by finely granular coagulum. The mesoderm between the embryonic plate and the yolk-sac is adherent at many points to both. The wall of the yolk-sac is cut obliquely for the most part. Its cavity now appears somewhat larger than the amniotic cavity. No blood islands are seen.
Section 57: A very intimate relation exists between the lateral wings of the embryonic plate and the subjacent mesoderm. In the body-stalk, near the tip of the amnion, there is a small mass of cells which apparently are ectodermal and may represent a bud from the amniotic ectoderm, which appears detacheil on account of the tangential direction of the sections.
Section 58: According to the memoranda obtained from Dr. Willier, two sections through the embryo were lost . On account of the abrupt transition between sections 57 and 58, it would seem probable that the sections are missing at this point. The abruptness is due partly also to the curve in the longitudinal axis of the embryonic plate, so that the plate is cut in this and the succeeding two sections in a markedly tangential direction. In this section the body-stalk is more condensed than heretofore and is fairly well inclosed by a membranous arrangement of the inesotlemi. It contains in its center the tip of the allantoic duct. At one point there is a slight indication of a lumen. The anmiotic cavity has become considerably contracted and conforms in a blunt manner to the form of the body-stalk. An intermediate plate still exists between the amniotic membrane and the embryonic plate. This is the first section in which a sharp groove appears in the median line of the embryonic plate — the primitive groove. At this point the ectoderm, mesoderm, and endoderm of the yolk-sac form one continuous mass, which corresponds to the primitive node of Hensen. The extent of this area is exaggerated, owing to the obliqueness of the section. In the ventral part of the yolk-sac a cluster of cells, apparently representing blood islands, can be recognized.
Section 59: (Compare fig. 14, plate 3.) The body-stalk consists of two portions — a round, more condensed poition surrounding the allantoic duct, and outside of this a triangular area of looser mesodermal tissue which extends up to unite with the chorionic membrane. In the more condensed portion several endothelial spaces can be seen. The allantoic duct contains a lumen. The formation of endoderm, mesoderm, and ectoderm is similar to that in the last section. What appear to be beginning blood islands can be seen in the ventral part of the yolk-sac.
Section 60: This section was cut 40 microns thick, otherwise it is much the same as section 59. The body-stalk appears as a very condensed mass and at its center can be seen the allantoic duct with a narrow lumen. The stalk is partly covered with a distinct mesodermal membrane. The amniotic cavity fits close against its ventral wall and is considerably contracted, due to the fact that the lateral intermediate plate lies against the main embryonic plate, thereby reducing the width of the cavity by nearly one half. ()wing to the thickness of the section the details of the fusion between the ectoderm, mesoderm, and entoderm can not be made out. As in the previous sections, however, there is no indication of a neurenteric canal.
Section 61: The transition from sections 60 to 61 appears to be very marked, but is due merely to the thickness of the preceding section, which conceals the change in form which the embryo undergoes at this point. By focusing up and down through the section one can recognize the change from a broad tangential section through the embryonic plate to a thin, narrower, transverse section, sufficient to account for the transition between these two sections. In section 61 the compact portion of the body stalk is separated from the chorionic membrane by the Icioser mesodemial tissue referred to in the description of previous sections. The attachment is maintained only by loose siraiuls of mesodermal cells. The allantoic stalk is very much constricted in this section and consists of only a few ceils which are much less compact than in the adjacent sections. Several endothelial spaces can be recognized in the compact portion of the body-stalk. A distinct mesodermal membrane incloses the ventral half of the stalk on each side, spreading over the amnion, whence it continues down over the yolk-sac, constituting the outer of the two layers of the wall of the latter. In the dorsal half of the wall it is distinct from the endodermal layer; ventral to this the two closely fuse and can no longer be distinguished as separate layers. The amniotic ectoderm fits closely against the round ventral surface of the body-stalk and laterally extends downward to a point where it becomes continuous with the transitional portion of the embryonic plate. The embryonic plate proper shows a sharply cut primitive groove, at which point the plate fuses with the endoderm of the yolk-sac. Whether any mesoderm is interposed in this section can not be definitely determined. Lateral to this point there is a considerable amount of mesodermal tissue intervening between the embryonic plate and the yolk-sac, being everywhere closely adherent to the former. It is connected with the yolk-sac by a few slender strands which mark off a series of clear, round spaces, the most lateral of which is continuous with a cleft separating the mesoderm and endoderm from the dorsal portion of the yolk sac. No endothelial spaces seem to be present in this region.
Section 63: The compact portion of the body-stalk is still farther removed from the chorionic membrane than in the previous section. The allantoic stalk is now somewhat larger, but no lumen can be recognized. A small, endothelial like ring of cells lies free in the exoccelom lateral to the mesodermal membrane covering the body-stalk. This section passes through the amniotic cavity in a transverse direction favorable for showing the structure of its ectodermal walls. Three distinct regions can be made out — the flattened amniotic ectoderm, the transitional lateral embryonic plate (consisting of one layer of cylindrical cells), and the embryonic plate proper (consisting of two or three layers of cylindrical epithehal cells). At the primitive groove the ectoderm is in contact with the endoderm of the yolk-sac. Lateral to this point there is a considerable amount of mesodermal tissue which has the appearance of flowing out from the lateral portions of the embryonic plate. Strands from this mesoderm extend out to the endoderm of the yolk-sac, outhning sppces similar to those described in the last section. The wall of the yolk-sac in its dorsal half consists of two distinct layers — mesoderm and endoderm. More vent rally the two layers fuse, and in the extreme ventral pole there would appear, in places, to be only one layer, endoderm. At a few points on the ventral portion of the yolksac there may be seen clusters of 3 or 4 nuclei, which possibly represent beginning angioblasts.
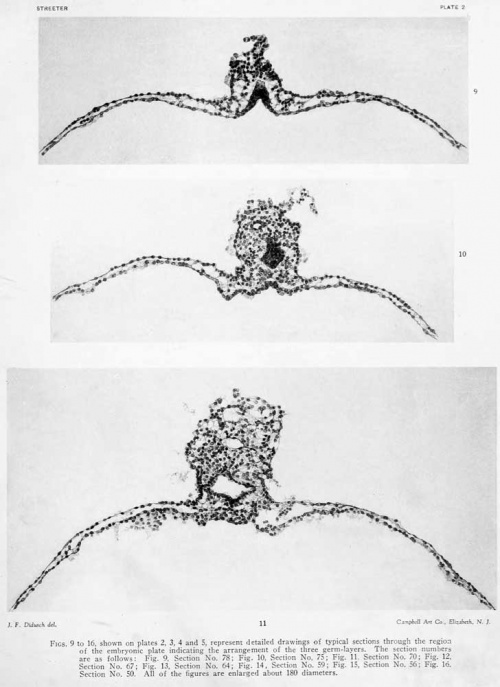
Sections 63 and 64: In the body-stalk and in the loose mesodermal tissue between it and the chorionic membrane are several endothelium lined spaces. In section 64, in the center of the body-stalk, can be seen a well-defined allantoic stalk containing a lumen. The form and structure of the amniotic cavity and its walls are much the same as in the previous section. The relation of the embryonic plate to the yolk-sac is very intimate in the region of the primitive groove. The embryonic plate shows the presence of numerous division figures. The mesoderm intervening between the plate and the endoderm of the yolk-sac is closely adherent to the former. A more detailed drawing of this section is shown in figure 13, plate 3.
Sections 65 and 66: In the loose tissue between the body-stalk and the chorionic membrane is an elongated space, in the lumen of which are a few cells. This is the largest space thus far encountered. The lateral surfaces of the compact portion of the body-stalk are entirely walled in by a mesodermal membrane. In the region of these sections the allantoic stalk is interrupted; at a point where it should be present one sees only the same mesodermal tissue found in other parts of the body-stalk. The amniotic cavity is rapidly contracting; its apex remains flattened in conformity to the ventral contour of the bodystalk. The embryonic plate is much narrower and the primitive groove is still sharply cut. The ectoderm at this point is not so closely adherent to the endoderm of the yolk-sac as in the previous sections. The ventral portion of the wall of the yolk-sac is very much thinned out, and one can not be sure that it consists of more than one layer. In the dorsal portions, however, an outer mesodermal membrane is sharply set off from the endoderm.
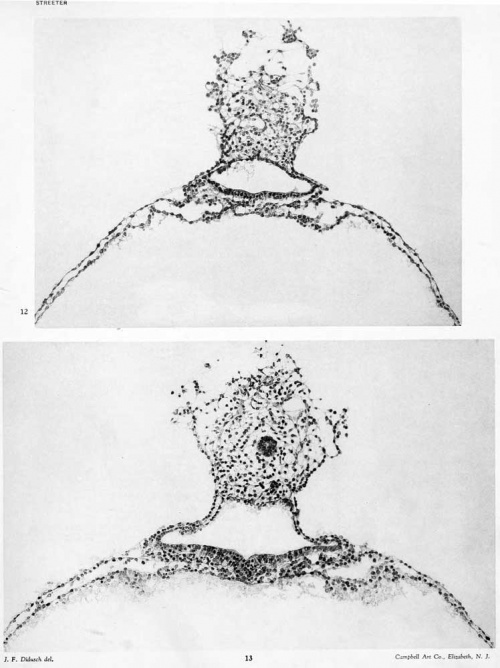
Sections 67 and 68: In the body-stalk is an open space in the area where one would expect to find the allantoic stalk, but otherwise there is no trace of that structure. The amniotic cavity is further contracted (see fig. 12, plate 2). The embryonic plate forming its floor still shows the characteristic outlines of a primitive groove at the center. It is thinner than in the preceding sections, consisting of one to two layers of cells, and the middle is no longer in contact with the endoderm of the yolk-sac. The mesoderm between the embryonic plate and the yolk-sac is predominantly adherent to the former, being separated from the latter by a series of spaces similar to those described in the previous sections. In the mesoderm of this region there is no evidence of blood-vessel formation. To the left of the loose tissue, in section 68, intervening between the body-stalk and the chorionic membrane is a group of mesodermal cells which take part in the formation of a structure that will be followed in the next eleven sections. There is so trace of the allantoic stalk. The primitive groove can still he recognized. The ectoderm at this point, however, is not in contact with the endoderm of the yolk-sac. As in previous sections, the ventral portion of the yolk-sac shows very little evidence of being blood-vessel formation.
Section 69: The amniotic cavity is more contracted and still shows the presence of a primitive groove. The relation of the embryonic
plate to the mesoderm intervening between it .'uui the yolk-sac is less closely maintained than in the foregoing sections. In the ventral portion of the yolk-sac is a distinct group of angioblasts, consisting of a strand of about 12 cells. On each side of the strand the wall of the yolk-sac is very thin.
Section 70: The group of mesodermal cells referred to in the last section can now be recognized as arranged in the form of a membrane,
cut tangentially. The compact portion of the body-stalk is much smaller and the only evidence it shows of an allantoic stalk is a doubtful open space. The amniotic cavity is now very small; its ventral floor still ha.s the characteristics of the embryonic plate in contra.st to the thin amniotic ectoderm of its roof, as can be seen in figure 11, plate 2. Several angiogenetic areas can be recognized in the ventral portion of the yolk-sac.
Section 71: The body-stalk is now somewhat detached from the loose mesodermal tissue intervening between it and the chorionic membrane. It contains the beginning of the main portion of the allantoic stalk and the tip of the amniotic cavity, the floor of which consists of a small group of ectodermal cells projecting ventrally. The ventral portion of the yolk-sac shows a continuation of the angiogenesis referred to in the last section.
Section 72: Dorsal to the body-stalk can be seen two separate masses, each of which has an average diameter of about the thickness of the chorionic membrane. The one to the left is a contiimation of the mesodermic membrane seen in the previous section, and here it can be seen that the mesoderm incloses a solid mass of ectodermal cells of two kinds; a dorsal, paler group, and a ventral, deeply staining group, the two being sharply marked off from one another. The other mass is somewhat less compact and consists partly of mesoderm and partly of cells whose form is better seen in section 73. In the center of the abdominal stalk is the allantoic stalk, sharply marked off and with a clearly defined lumen. Ventral to this is the tip of the amniotic cavity, whose walls are still differontiated in the dorsal amniotic ectodenii and the ventral embryonic jjlate. The yolk-sac can now be seen at about its greatest diameter, and is spherical in outline. Its dorsal third is composed of two separate layers, mesoderm and ectoderm. In the ventral two-thirds the layers are so intimately fused that they can not be distinguished; at the extreme ventral pole they have the appearance of a single layer, although the existence of angioblasts in this region indicates the presence of mesodermal elements. One group of angioblasts consists of a round, compact clump of 5 nuclei. The largest group gives the appearance of an elongated oval endothelial space, compactly filled with about 15 nuclei.
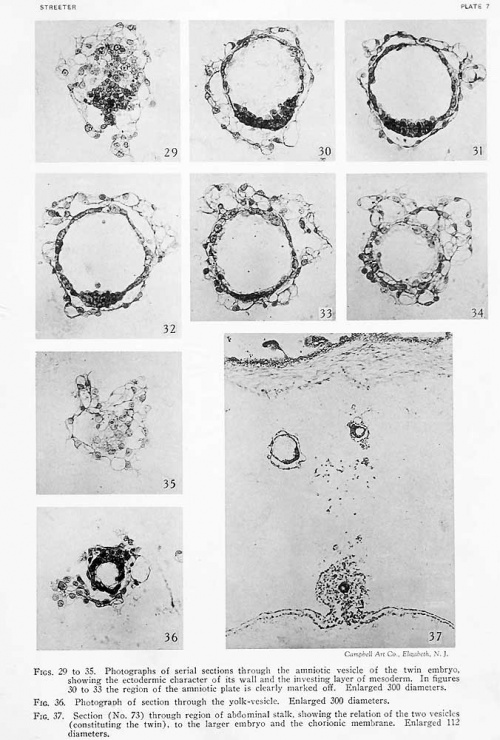
Section 73: The character of the two small masses seen in the previous sections, in the space intervening between the body-stalk and the chorionic membrane, can now be clearly made out. The larger one (to the left) consists of an ectodermic vesicle with an average diameter of 0.1 mm. The dorsal two-thirds of its wall consists of a single layer of flattened cells resembling the amniotic membrane seen in the main
part of the specimen. The ventral third consists of two or three layers of closely packed cuboidal or cylindrical ectodermal cells. Within the lumen is seen a scant amount of colorless, finely granular coagulum. The whole yolk-sac is surrountled by a more or less membranous and loosely attached layer of mesoderm. The other mass is likewise an ectodermic vesicle surrounded by a membranous layer of mesoderm. It is completely detached from the larger vesicle and differs from it in that its wall consists of a single uniform layer of cuboidal cells. Not including the mesoderm surrounding it, its largest diameter is 0.05 mm. The diameter of its lumen is not quite half that of the larger vesicle. Proceeding to the main part of the specimen we find no trace left of the amniotic cavity in the body-stalk. The allantoic stalk, cut shghtly oblique, can be seen with its lumen. The body-stalk is fairly well closed in by a membranous layer of mesoderm. Near its junction with the yolk-sac is a constriction, at the level of which the endoderm of the yolk-sac extends dorsally about half the distance to the allantoic stalk. Upon studying the wall of the yolk-sac one finds the angioblast-fonnation to be most active at its ventral pole.
Section 74: The larger ectodermal vesicle seen between the body-stalk and the chorionic membrane is cut in a very favorable plane and its structure can Ik; clearly recognized. It apparently represents an amniotic vesicle with a single layer of thin, liattened amniotic ectoderm, and a thick floor-plate of cylindrical embryonic ectoderm, the whole being inclosed by a layer of mesoderm. There is no eviilence of a primitive streak. The smaller mass, which is probably a definitive yolk-sac, shows an incomplete luiiicii in this section. The bodystalk of the principal embryo shows the allantoic stalk, together with an inverted V-shaped mass of obliquely-cut endoderm extending from the yolk-sac to unite with the allantoic stalk.
Section 75: The floor-plate of the small ectodermic vesicle lying between the body-stalk and the chorionic membrane is narrower, now occupying only one-fifth of the perimeter; otherwise the vesicle is about the same as in section 74. The smaller adjacent vesicle has disappeared except for a small area of its investing mesoderm. In the body-stalk of the main specimen the allantoic stalk is nearer the V-shaped evagination of the endoderm of the yolk-sac (fig. 10, plate 2). The endoderm appears to be a little thicker in the area of evagination, which is perhaps due to the oblique direction of the section. The ventral portion of the yolk-sac was mechanically injured, as was the case also in the two succeeding sections.
Section 76: The small ectodermic vesicle which we have followed in the preceding sections differs here, in that it consists entirely of thin, obhque cut ectoderm, owing to the fact that the cavity is now contracted. The ectoderm is completely surrounded by mesoderm, which shows a vacuolization-process but no blood-vessel formation. The smaller vesicle has now entirely disappeared. In the main specimen the endoderm has not quite united with the allantoic stalk.
Section 77: The ectodermic vesicle is rapidly contracting and shows an obliquely cut wall surrounded by an irregularly vacuolated layer of mesoderm. In the body-stalk of the main specimen the allantoic stalk is directly continuous with the evaginated endoderm.
Sections 78 and 79: The ectodermic vesicle has now disappeared and there are left only portions of the investing mesoderm. In the main specimen the thickened V-shaped extension of the endoderm of the yolk-sac represents in a clear manner the way in which it evaginates to become continuous with the allantoic stalk, as shown in figure 9, plate 2. In the ventral pole of the yolk-sac numerous foci of angiogenesis can be recognized, the most advanced of which show the presence of completed blood-vessels packed with blood-cells.
Section 80: This section was cut 20m thick. There is nothing left of the body-stalk except its point of attachment to the yolk-sac. The thickened area of the evaginated endoderm and the small amount of mesoderm in the place of the body-stalk can still be made out.
Section 81: Traces of the body-stalk can still be recognized. The ventral part of the yolk sac shows very good examples of early angiogenetic foci.
Section 82: All trace of the body-stalk has disappeared. The dorsal pole of the yolk-sac, however, can be readily distinguished from the ventral pole by its distinct endodermal and mesodermal layers, which are separated by a cleft. Also the principal angiogenetic activity is found in the ventral half.
Sections 83 to 91: Ventral and dorsal poles of the yolk-sac can be distinguished. Numerous blood-vessels, partly filled with blood-cells, are found.
Sections 92 to 96: These sections are stained with cresylecht violet, which differentiates very well the cells contained in the early blood-vessels of the yolk-sac. In many of these vessel-forming masses the endothelium-lined lumen and its contained cells are very well differentiated.
Sections 97 to 101: Heavily stained with hematoxyhn, eosin, aurantia, and orange g. A considerable amount of granular coagulum is present in the yolk-sac and resembles very closely the coaglum existing in the exoccelom.
Sections 102 to 105: Stained in hematoxylin and eosin. The yolk-sac is becoming smaller and the sections through its wall are therefore somewhat obhque. This facilitates the study of the young blood-vessels, some of which exist in the form of a small plexus. In addition to the granular coagulum there is found in the lumen of the yolk-sac a few cells resembling small, mononuclear leucocytes. It is possible that these are displaced cells, as these sections are somewhat broken.
Sections 106 to 110: Deeply stained with hematoxjdin, eosin, aurantia, and orange g. It is possible that these sections are out of their order and should perhaps have been placed before the preceding four sections. They show considerable angiogenetic activity.
Sections 111 to 115: Stained by the BiondiEhrlich method. These sections show very clearly the process of differentiation of the mesoderm of the yolk-sac into endothelium and contained blood-cells.
Sections 116 to 124: The first 4 of these are stained in safranin and light green; the last 4 in hematoxylin, eosin, aurantia, and orange g. In these sections the yolk-sac rapidly rounds off and disappears. The coat of mesoderm is thicker in this region and shows well-developed vessels. This point corresponds to the ventral pole of the yolk-sac.
Sections 125 to 277 contain onlv the chorion.
Chorion
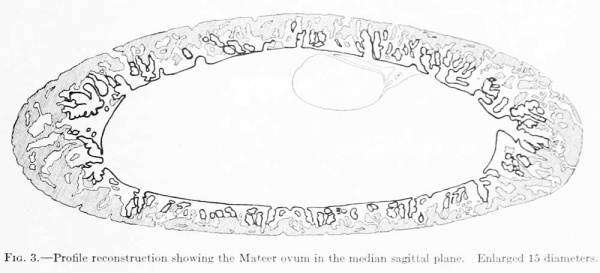
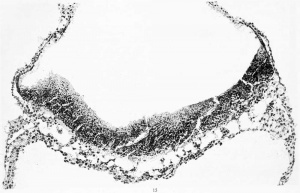
When the ovum was dissected out from the uterus no drawings or measurements were made of the chorion. It was noted, however, that after being in the fixing fluid, and just before embedding, the ovum formed a flattened sphere. The sections are cut in the equatorial plane and are nearly circular in outline. When reproduced by means of a profile reconstruction they present the form shown in figure 3, which would correspond to a median sagittal section through the whole ovum. This reconstruction was shown to Dr. Willier and it was his impression that the flattening of the ovum, as there represented, is more extreme than the ovum itself actually showed. In case more sections were lost at the time of cutting than the report stated this error would be accounted for. We must therefore introduce a reservation as to the accuracy of the length of the polar axis of the ovum ; instead of 3.5mm., as given below, it may be nearer 6mm.
Measurements taken from the reconstruction and from the individual sections jdeld the following dimensions :
- Outside dimension of entire specimen - 9.0 by 8.0 by 3.5 mm.
- Inside dimensions of chorionic sac - 6.1 by 5.6 by 2.5 mm.
- Length of longest villus (not including cell-column) - 0.8 mm.
- Average length of villi - 0.4 mm.
In the process of dissecting out the ovum practically the whole trophodermic shell was included, some of the areas showing transition into decidua. The arrangement and form of the villi and of the encrusting trophoderm are shown in figure 3. The chorionic membrane seems to be everywhere intact. The cavity contains a coagulum (magma reticule), which in some places takes the form of a compact, finely granular mass, and in others is arranged in finely granular, reticular strands, irregularly meshed. In the sections stained with carmine the magma is barely perceptible, whereas in sections stained with hematoxylin and counterstained with eosin, aurantia, and orange g, this substance is quite conspicuous.
The chorionic membrane is made up of a mesodermal and an ectodermal layer. The former presents an entirely different picture from the magma just
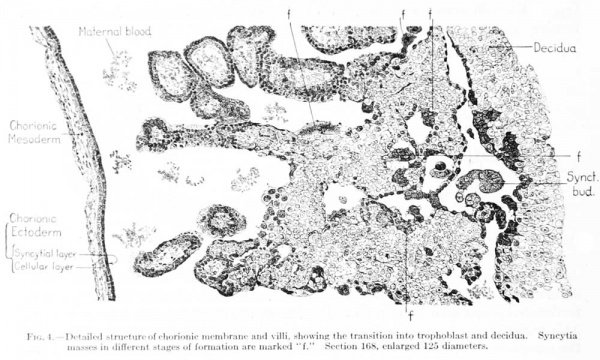
referred to and there is no evident transition between the two. Owing to the distension of the chorionic cavity the mesoderm is everywhere stretched out as a thin layer covered in by the double-layered ectoderm. The mesodermal and ectodermal layers are for the most part of about the same thickness; in some places, however, the mesodermal layer is thicker. The mesoderm can be traced up into the villi, where it forms their stroma, which is in various stages of vascularization. On examination of the ectodermal layer of the chorionic membrane under higher magnification it can be seen to be made up of an inner, cellular layer (Langhans layer), and an outer syncytial layer, the cell-boundaries of which are less distinct and the cytoplasm much more compact and granular. This picture varies somewhat in different portions of the chorion. In some places the two layers are much the same; in others the contrast is quite striking. In some areas the outer syncytial layer shows active vacuolization. Occasionally small syncytial buds are found projecting from the chorionic; membrane. The surface of the chorionic membrane is bathed in maternal blood, as is evidenced by the presence of mature blood corpuscles.
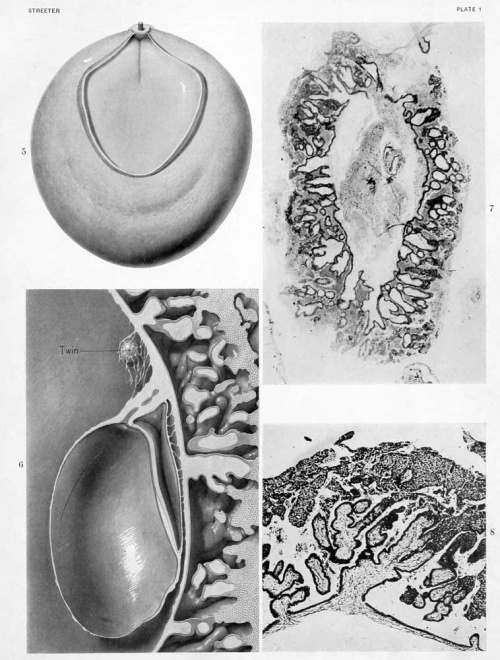
Where the villi project from the surface of the chorionic membrane the same general structure is maintained. The villi present a rather uniform calibre and some indication of their shape and manner of branching may be obtained by an examination of text-figures 3 and 4, and figures 6 and 8, plate 1. In general their tips merge directly into the incrusting trophoderm, and where this occurs it is no longer possible to differentiate sharply between the Langhans layer and the syncytial layer. One gains the impression that the former merges into the latter tissue, where the tips of the villi come in contact with the trophoderm, the syncytial layer continuing along its margins.
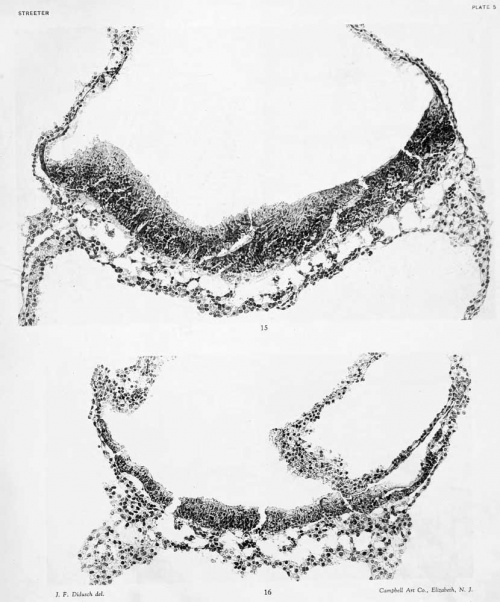
Fig. 3. Profile reconstruction showing the Mateer ovum in the median sagittal plane. Enlarged 15 diameters.
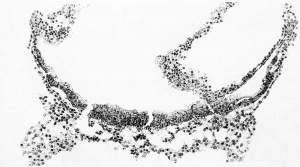
The trophoderm completely incrusts the ovum as a trabeculated shell. Although it seems to consist of a uniform tissue, there is considerable variation in its detailed structure in certain areas, these areas merging gradually into each other. This variation applies to the size of the cells, the distinctness of their outline, and the compactness of their cytoplasm. As has been noted in some areas, the trophoderm seems to merge directly with the Langhans layer of the villi and histologically to be a continuation of it. In many places along the periphery it merges into decidua, and much of the trophoderm along the margins gives the appearance of rapid transition into syncjtial tissue like that found on the villi. In some cases this transition occurs directly within the substance of the trophodermic mass, and we therefore find small syncytial masses completely imbedded in trophoderm. One also finds more or less detached masses of syncytium scattered everywhere through the irregular spaces of the trophoderm and in the intervillous spaces. These masses present the greatest variety of size and form; some of them seem to be entirely detached, others project into the spaces and are attached only at one end. In some cases they form an enveloping coat for the adjacent trophoderm. They frequently show vacuolization and present a wide variety in the form, number, and character of their nuclei. As a rule the margins of the trophoblastic spaces show a tendency toward the formation of a cell-border which merges into the adjoining trophoderm. In some places this marginal arrangement resembles a thickened endothelium. Along other margins one finds all varieties of transition into syncytial masses. In many respects the conversion of the trophoblastic margins into syncytial ohinips resembles a process of excavation, and in tliis sense the syncytial clumps would have to be regarded as degenerate trophoblast. In text-figure 4 several varieties of syncytial masses (marked /) can be seen in different degrees of formation and in the process of detachment from the adjoining trophoderm. The picture presented by these masses is very suggestive of their retrograde character. The vascularization of the chorion will be described in connection with that of the cmbrvo and of the yolk-sac.
Fig. 4. Detailed structure of chorionic membrane and villi, showing the transition into trophoblast and decidua. Syncytia masses in different stages of formation are marked "f." Section 168, enlarged 125 diameters.
Embryo
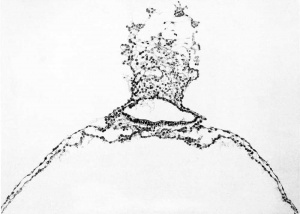
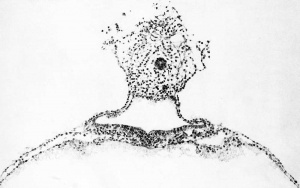
The form and relation of the embryo and adnexa are shown in plate 1, figures 5 and 0, and tyi)ical sections through this region are reproduced in figures 9 to IG, plates 2, 3 and 4, which will be repeatedly referred to. For convenience of description these structures will be taken uj) separately as the embryonic plate, amnion, yolk-sac, and body-stalk.
Embryonic Plate
This is somewhat oval in outline. Caudally, it narrows rapidly and is somewhat pointed where it terminates at the body-stalk. In its widest diameter it measures 0.75 mm.; it is 1 mm. long in its median axis. The sections cut it transversely only in the caudal portion; for the greater part of the plate they meet it tangentially or in a very oblique direction. For its form we nuist depend principally upon the reconstruction. From this it can be seen that there is a distinct primitive groove in its caudal fourth, measuring 0.25 mm.; otherwise the plate is smooth, with a slight tendency toward the formation of bilateral, low marginal ridges. There is, however, no evidence of a medullary groove. In the more transverse sections, both at the caudal and rostral ends, the plate consists of stratified ectoderm about four cells thick and measuring 0.015 mm. midway between the median line and the lateral margin of the plate. Apparently this thickness is fairly uniform throughout the plate, although in many of the sections, owing to their obliquity, it would seem to be much thicker.
Laterally, along the margins of the plate, the ectoderm folds backward to become continuous with the amnion. At this point there is a transitional area consisting of ectoderm a single cell thick, which resembles the ectoderm of the embryonic plate more than that of the amnion. Since we have no knowledge as to exactly how much of the embryonic plate enters into the formation of the medullary plate, we can not as yet be sure of the destiny of this transitional area — whether to allot it to the amnion or to the integument of the body of the embryo. It would seem probable that it might be considered as an area of very active growth of the amnion. This would mean that the area of amnion formation is most active around its margins.
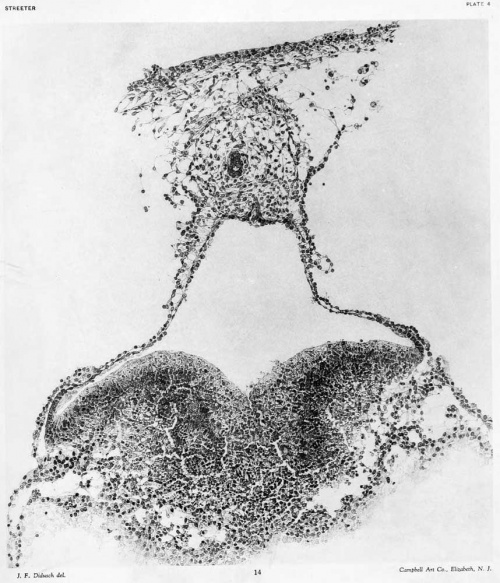
The primitive groove can be seen very clearly in the sections (figs. 12, 13, and 14, plates 2 and 3), and can be traced into the most caudal sections. Where one would expect to find the primitive node the sections become very oblique, so that it can not be outhned with great certainty. It is probably represented, however, in figure 14, plate 3. In this region the ectoderm fuses more or less c; mpletely with the endoderm, and lateral to the point of fusion can be seen a flattened area of mesoderm (fig. 13, plate 3). This mesodermal tissue is in the form of a reticula syncytium, which in most regions is closely attached to the ventral surface of the embryonic plate. It is somewhat more loosely attached to the endoderm by irregular trabeculae. In its more lateral areas it is slightly more condensed into a tissue from which are derived the somites and the more laterally situated unsegmented mesoderm. As to the presence of a head process there seems to be no evidence, although the oblique direction of the sections makes it impossible to rule it out with certainty. The most careful scrutiny, however, fails to reveal any sign of a head-process canal.
Amnion
The amniotic cavity is much flattened and consists of scarcely more than a cleft. In this respect figures 15 and 16, plate 4, are very misleading, owing to the tangential direction of the sections. As has been seen, the amniotic ectoderm is directly continuous with the margins of the embryonic plate through a transitional zone. The amniotic ectoderm consists of a single layer of flattened cells which extends to the margin of the body-stalk, fitting tightly against its ventral border and conforming to its shape. This membranous layer of ectoderm is supported by a thin layer of parietal mesoblast, which is also somewhat membranous in character. On reaching the body-stalk the parietal mesoblast passes laterally, so as to partially inclose it. Made up of these two layers, the amnion forms a vexy thin membrane which lies in the narrow space between the chorionic membrane and the embryonic plate. Over the greater extent of its dorsal surface many loose strands of mesoderm partly attach it to the chorionic membrane. In figure 5 the cut edges of the amnion can be seen along the margins of the embryonic plate.
Careful search was made for an amniotic duct and it was found that the amniotic ectoderm, where it lies in contact with the body-stalk, shows at one point an active proliferafion and the formation of a wedge which partially penetrates the body-stalk (fig. 14, plate 3). This may, perhaps, represent a tendency toward the formation of an amniotic duct.
Body Stalk
Loose strands of mesoderm arc scattered at irregular intervals throughout the space between the amnion and chorionic membrane, whereas the center of the exocoelom is quite free from them. Within this area of looser mesenchyme is situated the more compactly arranged body-stalk, the form and structure of which can be seen by comparing figure 0, plate 1, and figures 13 and 14, plate 3. Lying at its center is the allantoic stalk, aside from which it consists entirely of a meshwork of mesoderm in which the process of angiogenesis can be seen to be under way. At the margins of the body-stalk the mesoderm is flattened into the mesothelium, which partially separates it from the exoccelom. The amniotic ectoderm bears a very intimate relation to the body-stalk, conforming closely to the shape of its anterior and ventral surface. It is at this point, as has been mentioned before, that the proliferating wedge of ectoderm penetrates into the substance of the bodystalk, representing the amniotic duct.
Yolk-Sac
The yolk-sac is intact and forms a thin-walled, flattened vesicle, rather evenly distended, and measuring L5 by L4 by 0.9 mm. in its greatest diameters. It contains a moderate amount of finely granular coagulum, which is somewhat more abundant in the dorsal portion and around the margins. Over its dorsal pole the wall of the yolk-sac consists of two distinct layers — endoderm and visceral mesoblast — separated by a narrow cleft, which is bridged here and there by irregular trabeculae. In its ventral two-thirds the visceral mesoblast is intimately adherent to the endoderm, so that in many places it is difficult to make out more than one thin layer. The endoderm is quite uniformly made uj) of a thin, stretched out, single layer of membrane-like cells. The appearance of the uniformly thin wall of the yolk-sac is interrupted at intervals l)y small masses constituting the foci of blood-vessel formation. Sketches of these margins are shown on plate 5, and this process will presently be discussed.
Except at the line along which it fuses with the ectoderm, the endoderm shows very little difference from that in other regions. At the extreme caudal end of the embryonic plate, however, it consists of an area of taller and more cuboidal cells. This area evaginates at the center to penetrate into the substance of the abdominal stalk (fig. 9, plate 2) for a distance of 0.37 mm. as the allantoic duct. This is everywhere within the more compact body-stalk. About midway along its course the endoderm of the stalk seems to have broken off and retracted; in the interval, in some of the sections, the empty space previously occupied by it seems to be still present. The allantoic stalk, in some places along its course, shows a distinct lumen, while in other places this can not be recognized.
Angiogenesis
Evidences of blood-vessel formation can be recognized in all parts of the chorion and the body-stalk and in certain areas of the yolk-sac. The mesoderm of the latter is very different in character from that of the body-stalk and chorion. To a lesser degree the mesoderm of the body-stalk and chorionic membrane differs from the mesodermic stroma of the villi; thus the picture of developing blood vessels varies according to the region examined. We will therefore consider first the conditions existing in the chorion and body-stalk, which are closely allied, and then proceed to the vessel-formation of the yolk-sac, which has its own peculiar type of development.
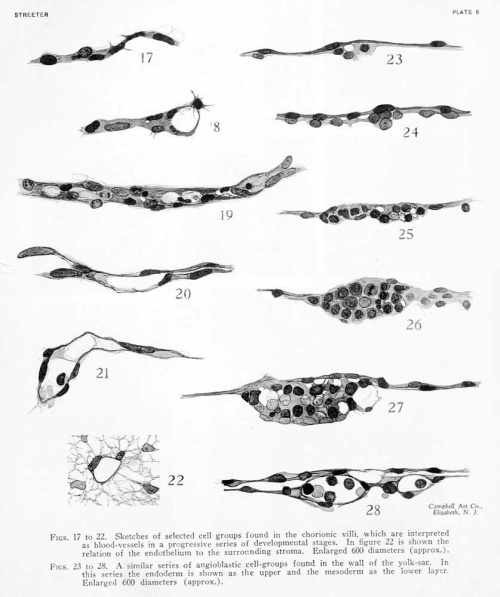
As regards the chorionic membrane and villi, a careful survey shows very little difference in the number or degree of development of the blood-ves.sels in the different regions. In all parts of the chorion one can find the earliest types of vessels, consisting of simple protoplasmic, multinucleated strands, and the intervening stages between this and completed endotheUal tubes. Selected stages of this process were sketched and are shown on plate 5, figures 17 to 22, which are arranged according to their apparent degree of differentiation. One gains the impression that the vessels are more numerous in the villi than in the chorionic membrane. This may, however, be due to the fact that the chorionic membrane is compressed into a compact sheet, consisting of 3 to 5 layers of flattened nuclei and their intermediate process-like strands. Owing to their arrangement these nuclei resemble endothelium, which makes the identification of endothelial formation uncertain. At the points where the mesoderm of the chorionic membrane evaginates to form the stroma of the villi, the trabeculae of the tissue are somewhat more loosely arranged and this makes it possible to identify the younger vessels with more certainty. The villi themselves offer a most favorable place for the study of angiogenesis. The arrangements here are extremely simple and the topography of the villi is such that one can select either cross or longitudinal sections at will. We have to take into consideration only a rather uniform stroma of the villus and its double-layered covering of epithelium. In the meshes of the stroma can be found the condensed strands which represent blood-vessels in their various stages of formation.
In the specimen we are describing, a great many of the villi do not show any sign as yet of blood-vessels. The viUi devoid of blood-vessels are, as a rule, the smaller ones, and these are distributed evenly over all parts of the chorion. In some cases one can find a section showing a large villus with a mature main vessel, from which less mature strands can be seen extending into the terminal branches of the villus. These strands may be in the center of the villus or may extend obliquely so as to terminate close against the epithelium. The process by which blood-vessels are formed in the mesodermal tissue of the villi is initiated by the condensation of slender, cytoplasmic strands, along which are scattered irregularly placed and actively proliferating nuclei. We may speak of these as angioblastic strands which can be only incompletely resolved into separate cells. The next stage in the process consists in the differentiation of some of the component parts of the angioblastic strand into ondotliolial cells, which can bo distinguishod by the shape of their nuclei and by their tendency to so arrange themselves as to form the contour of the strand. The cytoplasm of the other components of the strand undergoes Uquefaction, either in the formation of large vacuoles or by resolution into a very fine mesh, which also disappears. The appearances in this respect probably represent the vacuolization phenomena demonstrated in the living chick by Professor Sabin, whose preparations I have had the privilege of examining and whose observations are reported elsewhere in this volume. In some cases we find a few round nuclei persisting, either adherent to the endothelial wall or suspended between the two endothelial walls by slender threads. These are doubtless to be regarded as future blood-cells. Whereas the spaces in the vacuolated strands are first seen to be incompletely closed off from the spaces of the surrounding stroma, further differentiation of the endothelium gradually completes their boundaries, thus forming completely closed tubes as established vessels. In other words, we are dealing with the formation of multinucleated strands, some of the elements of which become differentiated into endothelium, while the remainder is either completely liquefied or persists as blood-cells within the peripherally formed endothelial elements.
Figure 17 represents an angioblastic strand with its longitudinally arranged, elongated nuclei. In some places there is a slight indication of cleavage of the cytoplasm of such a strand, but this is always incomplete. Around its margins it is more or less continuous with the delicate trabecular of the surrounding stroma. In figure 18 a similar strand is shown extending as a lateral process from the margin of a vessel that is farther advanced. Figure 19 illustrates the transition of a solid strand into an endothelial tube. Here the nuclei are in active proliferation. It can be plainly seen that the cells along the margin are elongating into typical endothelium, as regards both the nuclei and the adjacent cytoplasm. The cytoplasm of the central part of the strand shows enlarging vacuoles. At other points, such as the
right-hand end of the figure, instead of showing a simple, large space, the cytoplasm becomes converted into a very degenerate mesh. As a result of these two processes there is a general liquefaction, or conversion into plasma, of the central portion of the angioblastic strand. Most of the nuclei seen in the strand in figure 19 have either divided or are about to divide. However, it is evident that some of them, together with their surrounding cytoplasm, must undergo degeneration.
Figure 20 shows a strand in which there is left only the differentiated endothelium. The incompletely closed lumen of this strand, as far as one can judge from the sections, still seems to communicate with the spaces of the surrounding stroma. In figure 21 we meet with a condition in which the endothelium forms a completely closed tube. A transverse section of a similar vessel is shown in figure 22. In figure 21 a few trabecular still traverse the lumen connecting the opposite endothelial walls. There can also be .seen within the lumen an occasional large, round, nucleated cell, showing a scant amount of cytoplasm , which apparent ly represent s an embryonic blood-cell. This is the most mature type of blood-vessel encountered in the specimen. Up to this time there is apparently not a very active formation of blood-cells.
Owing to the uniform distribution of these different types of vessels throughout the chorion, there is strong evidence of the general differentiation of blood-vessels in loco rather than from a single focus restricted to any particular area.
In the region of the body-stalk one can recognize two areas of mesoderm: a more condensed area immexliatcly surrountling the allantoic stalk, who.se lateral margins are definitely inclosed by the formation of mesothelium and a much le.ss compact area intervening between the former and the chorionic membrane. The more compact area is doubtless to be regarded as the forerunner of the permanent umbilical cord, whereas the looser area eventually is taken up by the exocoelom. In both of these regions blood-vessel formation can be seen taking place, and is in about the same degree of development as that noted in the chorionic membrane and vilH. In some places throughout the looser areas of the body-stalk are small endothelial vesicles, which are completely detached from the adjoining exocoelom.
Blood-vessel formation can be detected over the greater part of the parietal mesoblast covering the amnion. In the body-stalk, as in the chorion, young bloodvessels are for the most part empty.
Angiogenesis in the yolk-sac presents a somewhat different picture from that seen in other parts of the ovum. In the first place it is circumscribed, being limited to the caudo-ventral half of the yolk-sac and is most marked at the extreme caudoventral pole; in the second place, the angiogenetic picture is quite different from that described as typical for the chorionic villi.
The wall of the yolk-sac consists of a thin, stretched-out, endodermic membrane, which is shown in figure 23, plate 5. This is covered in by the visceral mesoblast, shown below in the figure. In the more dorsal part of the yolk-sac the mesoblast is much thicker and more membranous and is separated from the endoderm by a distinct, narrow cleft. The cleavage between these two extends twothirds of the distance from the dorsal pole to the equator and entirely encircles the embryonic area. Ventral to the cleavage rim a thin coating of visceral mesoblast fuses tightly with the endoderm, in some places being so thin that one can scarcely be sure that there is anything more than endoderm present. The simplicity of these regions of the wall of the yolk-sac constitutes very favorable conditions for the study of blood-vessel formation.
Sketches were made of selected areas of the wall, showing blood-vessels in their different stages. These are arranged in figures 23 to 28, plate 5, in their approximate order of development. All of the figures are so arranged that the endoderm is above and the mesoderm below. In figure 23 is shown a small, isolated clump of proliferating mesoblast cells in an area where the endoderm is only scantily covered. From such an angioblastic node we can find all stages of transition up to completed endothelial tubes. In figure 2-i is another clump, sUghtly larger, but otherwise of much the same character. From their appearance one could not be sure that such clumps were destined to form blood-vessels. Since, however, there is at this time no other process taking place, we may assume that these are earlier stages of the
condition met with in figure 25. In this figure there is shown a characteristic, compact, multinuclear plate, in which the cellular boundaries can be only partially made out. In such plates one can very early recognize that certain cells at the periphery are becoming elongated and flattened into endothelial cells. These shape themselves so as to compactly inclose the more centrally placed cells. It is also characteristic of these angioblastic plates that the cytoplasm of the more centrally placed cells undergoes liciuefaction similar to that in the long strands in the chorionic villi. The hquef action of such cells seems to vary, since it is found in some of the smaller angioblastic plates and is absent in some of the larger ones.
In figure 26 is shown a larger angioblastic mass with less vacuohzation and liquefaction of the cells than in the preceding figure. However, endothelial cells differentiating around the contour of the mass can be distinctly recognized. The component cells of the mass show evidences of very active proUferation ; they are either in mitosis or in pairs of small, recently divided nuclei. In figure 27 the condition is more advanced and a considerable amount of liquefaction may be seen among the more centrally placed cells. In addition to large vacuoles, one finds in such a mass that many of the nuclei are becoming very large and pale, apparently preliminary to their complete disappearance. Although the endothelium can be apparently recognized, it has not yet completely closed off the area from the exocoelom, and even less so from the endoderm. In a few cases completely formed endothelial tubes are found on the yolk-sac, as shown in figure 28. These may contain one or more cells with large, round nuclei, but never so many as are present in the angioblastic masses seen in figure 27. We must conclude, therefore, that there is in these cases a considerable conversion of the cellular mass into clear plasma, leaving relatively few complete cells, none which as yet show any evidence of the presence of hemoglobin.
Comparison of the Mateer Embryo and other Young Human Embryos
As an aid in placing our specimen in its proper relative position in the series of embryos that have been described in the literature, the more important of these will be briefly reviewed. They will be taken up in the order of their apparent degree of development, which will be determined by the consideration of the morphology as well as by the actual size of the embryo and the chorion. The manner of handling and the amount of shrinkage and folding affect the size greatly, particularly as regards the dimensions of the chorion. The disproportion between size and development is even greater where pathological elements have entered. On the other hand, in young stages up to the time of the appearance of the primitive groove, the size of the chorion, owing to its rapid growth as compared with that of the embryo, appears to be a consistent index of the development of the ovum. In older specimens it is necessary to take into account also the morphology of embryo and chorion.
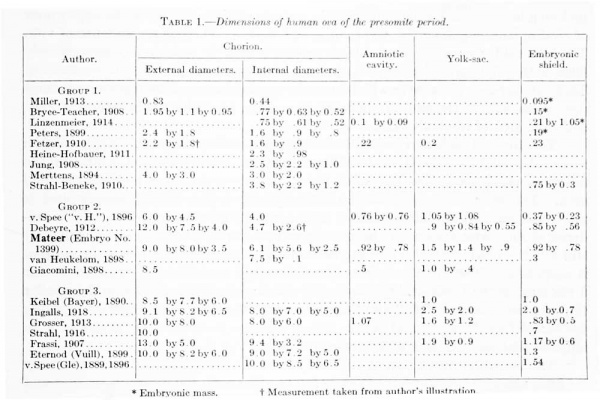
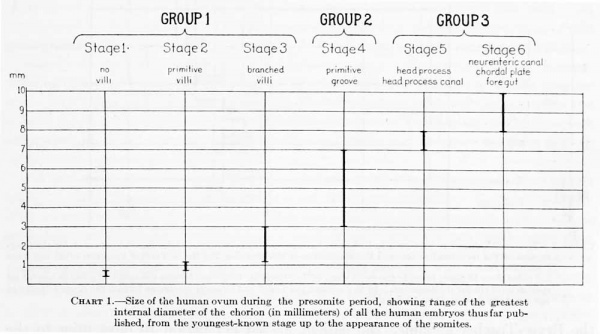
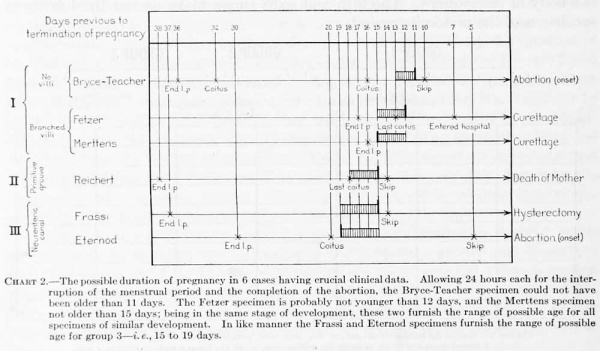
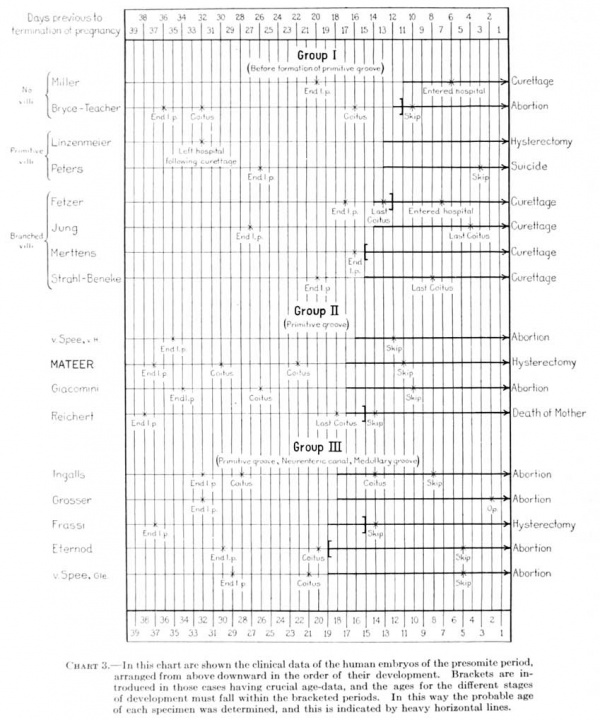
For the most part the literature dealing with young embryos relates to the histological character of the implantation area and to the interaction between the trophoblastic shell and the uterine mucosa; the structure and form of the embryo, with which we are especially concerned, are given with much less detail. This is due in part to the inadecjuacy of the material, the chorion and trophoblast being usually in a better state of preservation than the embryo. There will be considered here only presumably normal embryos in which the somites have not yet made their appearance. These will be taken up in three groups: (1) Those in which the primitive groove is not yet formed; (2) those in which the primitive groove is present; and (3) those having, in addition to the primitive groove, a neurenteric canal and medullary folds. A list of these, with their measurements, is given in table 1.
Table 1. Dimensions of human ova of the presomite period
Group 1. Embryos before the Formation of a Primitive Groove
The youngest stage in the development of the human embryo that has thus far been observed is represented by a blastocyst already embedded in the uterine mucosa, but devoid as yet of villi. Two such specimens have been described, one by Miller (1913) , the other by Bryce and Teacher (1908) . The one described by Miller is smaller and in it the embryonic rudiment consists of a sohd mass of cells, whereas in the Bryce-Teacher specimen an amnio-embryonic vesicle can be recognized. Certainly the Miller specimen must be regarded as normal, and although the BryceTeacher specimen shows some evidence of degeneration it also should be provisionally regarded as normal. When more specimens of about the same age are available for comparison it is quite possible that the evidence may prove that the latter is not normal.
The specimen described by Miller was found in sections of material obtained at curettage. It was not a complete series, only five sections showing the ovum. Fortunately, however, three of these passed through the embryo, which is represented by a solid cell mass 0.095 by 0.072 mm., undergoing cleft formation preliminary to the development of the amniotic cavity. The embryo is surrounded by a trophoblastic shell with an external diameter of 0.83 mm. and an internal diameter of 0.44 mm. The trophoblast is partially differentiated into cytotrophoblast (Langhans layer) and plasmoditrophoblast (syncytium), the two partially merging into one another. Peripherally, the plasmoditrophoblast forms irregular loops inclosing large blood lacuna;, about 12 in a single section, and gives the appearance of eroding and engulfing the surrounding capillaries. There is no evidence of viHi. The interval between the trophoblastic ectoderm and the embryo is almost entirely filled with a fine, granular deposit, through which pass simple fibroblastic strands. These are arranged in a layer which lines the trophoblast, and are especially numerous on one side of the embryo, between it and the trophoblastic ectoderm, apparently the area of the body-stalk.
The Bryce-Teacher ovum, both from its size and in its development, is to be regarded as slightly older than the Miller specimen. It differs from the latter in the following particulars: The trophoblast shell is surrounded by a necrotic decidual area. The distinction between the cytotrophoblast and plasmoditrophoblast is more definite, and the latter forms a more complicated network. There is no evidence of any arrangement of the mesoderm into parietal and visceral lamellae still fills the blastocyst as a delicate tissue in the fine meshes of which the embryo is suspended. The latter consists of two detached vesicles; the larger (diameter 0.168 mm.) is the amnio-embryonic vesicle with a wall of cubical cells; in the smaller the yolk-sac (diameter 0.042 mm.) the cells are flattened. That these two vesicles are completely detached and not in proper relation to each other would tend to indicate that the specimen is not entirely normal.
The beginning formation of villi can be seen in the ova described respectively by Linzenmeier (1914) and by Peters (1899). Of these, the former is smaller and probably younger; it is, however, distinctly older than the Bryce-Teacher specimen, in respect to both the chorion and the embryo. The mesoblast is divided into parietal and visceral layers, with a well-defined exocoelomic cavity. The parietal mesoblast forms a continuous layer within the trophoblastic ectoderm, completing the formation of the chorionic membrane. Short processes from its mesoblastic layer i)roject outward into the trophoblastic shell, producing the first vilU; seven or eight of these may be seen in a single section. The mesoblastic layer of the chorionic membrane is continuous with the visceral layer of mesoblast surrounding the embryo, at the seat of the future; body-stalk. The embryo consists of two closely opposed vesicles; the larger (greatest diameter 0.105 mm.) is clearly differentiated into amniotic ectoderm and a thickened ectodermal embryonic plate; the smaller constitutes the yolk-sac and is about half as large as the amniotic vesicle. It is partially separated from the former by a layer of mesoderm. Whether an allantois is as yet present could not be definitely determined from the author's description. From the illustration the wall of the yolk-vesicle seems to consist of a very thin membrane.
In the well-known Peters ovum, which is much more completely described, the conditions are closely similar to those in the ovum just referred to. In it the villi are more numerous and distinct and the chorionic vesicle is larger. In form and position this embryo is almost identical with the Linzenmeier specimen, the amnion lying in contact with the mesoblastic layer of the chorionic membrane. Both possess closure-caps (Gewebspilz) consisting of partially organized fibrin and blood, into which the trophoblast sends branches.
As will be seen, the embryos of the first group, before the formation of the primitive groove, fall naturally into three substages: (1) thase without vilU, (2) those possessing primitive vilH, and (3) those in which the villi have already begun to branch. The first two have just been considered, and we may now take up those with branched villi. There are three ova, described respectively by Fetzer (1910), Heine-Hofbauer (1911), and Jung (1908), which are a little larger and a little further differentiated than that of Peters. The three are very much alike in form and about the same size. They have numerous and well-defined chorionic vilH, which show a beginning tendency to branch. In all of the specimens the yolk-sac is smaller than the amniotic cavity and its wall consists of a single layer of flat endodermal cells. The embryonic plate presents an oval surface and in section consists of two or three layers of high cyUndrical cells, whereas the amniotic ectoderm, in sharp contrast, consists of only a single layer of flat cells.
The Herzog (1909) ovum is about the same size as those just mentioned. Its chorionic wall, however, is much folded, so that originally it was probably considerably larger. It contains a tubular structure in the body -stalk, obviously torn from the embryo in the process of preservation and thus is completely detached. This epithelial tube was at first interpreted as an allantois, but, as subsequently determined by Professor F. T. Lewis (1917), it is clearly an amniotic duct, no allantois being present in this embryo. Apparently the yolk-sac is larger than the amniotic cavity (largest diameter of yolk-sac 0.3 mm., amniotic vesicle 0.16 mm.).
The Strahl-Beneke (1910) ovum, which the authors have published in a splendid monograph, would fall between our first and second groups. The histological ai)pearance of its chorion differs only sUghtly from those just referred to. In form it is more elongated, presenting a spindle shape that is not repeated in any other ovum and probably should not be regarded as typical. The embryo shows certain new features as evidence of a more advanced stage of development. In the body-stalk there is a solid epitheUal strand connecting the tip of the amniotic sac with the chorionic ectoderm and apparently representing an amniotic duct. On the ventral side of the yolk-sac are thickened, solid mesodermal strands which are possibly predecessors of blood-vessels. These are not to be confused with the ringlike arrangement of mesodermal cells which simulates vessels and may be seen in all mesodermal parts of the ovum. In figure 32 (Strahl-Beneke) there is seen a clear area in the ectodermal plate which suggests the transition of the cells into a canalis neurentericus, although a distinct canal is not present. The authors also speak of a head-process, including under that term the free mesodermal cells which, in the middle line, beneath the embryonal area, Ue between the ectoderm and endoderm, as seen in their figures 41 and 42. More certain than either of the last two phenomena is the beginning appearance of the primitive streak. In their figures 25 to 34 it can be seen that the ectoderm of the caudal end of the embryonic plate fuses with the mesoderm, producing the appearance of a primitive streak, although there is no distinct groove. In this ovum, as in the Herzog specimen, the yolkvesicle is distinctly larger than the amniotic vesicle, differing in this respect from all those previously mentioned. The Herzog and Strahl-Beneke specimens are on the border-land between groups 1 and 2, and, e.xcept for the absence of a primitive groove, could be grouped with the "v. H" ovum of Graf v. Spee.
Together with the Strahl-Beneke specimen should be mentioned the Merttens (1894) ovum, which is about the same size. Unfortunately, the sections are incomplete, being fragments from curettage, so that it is not possible to determine much regarding the structure of the embryo. The chorion, however, is well preserved and acquires great importance because of the valuable clinical history that accompanies the specimen. Another of about the same age as the Strahl-Beneke ovum is No. 763, of the Carnegie Collection, which has been mentioned by Mall (1915, p. 22), and a photograph of which is shown in our figure 7, plate 1. The specimen was found in curettage material and the series is therefore incomplete. The tissue, however, is in an excellent state of preservation and the structure of the chorionic membrane and vilU is very well shown, as can be seen in the photograph. The internal diameters of the chorion in the largest section are 2.5 by 1.2 mm., and the average length of the villi is 0.5 mm.
Group 2. Embryos in which the Primitive Groove is Present
The embryos belonging in tliis group have a primitive groove but no medullary groove or neurenteric canal. Five such specimens are referred to in the literature, and in this group the Mateer embryo must be placed. They are all much alike in size and form and evidently there is very little difference in their degree of development. As a group they are of a size intermediate between groups 1 and 3.
The well-known "v. H" ovum of Graf v. Spee (1896) is perhaps a httle less developed than the others. In the relatively small size and spherical form of its
amniotic vesicle it resembles some of the older specimens of group 1 and its embryonic shield is quite like that of the Jung specimen except for the presence of a primitive groove. It has, however, a larger chorion (average internal diameter 4 mm.) with freely brandling villi. The yolk-sac is much larger than the amniotic cavity,
and there is a well-developed allantoic duct extending from it into the body-stalk.
On its ventral pole are found numerous blood-islands. In all these respects it conforms to the other specimens of group 2.
Lewis (1912) has pictured and briefly described the Minot embryo (Harvard series, No. 825), and I am informed that a more complete description of it is now in course of preparation. This embryo resembles very closely the "v. H" embryo of v. Spee, both in size and in form. It differs in that the amniotic vesicle is more flattened and the embryonic shield correspondingly larger. Whether tlie spherical form of the amniotic vesicle normally precedes the flattened form usually met with in slightly larger specimens, remains to be determined by a comparison of more material than is available at present. It is quite probable that the form of the vesicle is dependent to a great extent upon such factors as the handling and preservation of the tissues and the technique of embedding. In the Minot specimen there is a distinct primitive knot where the ectoderm and endoderm are definitely blended. Numerous blood-vessels are present in the wall of the yolk-sac and also in the bodystalk and the chorion.
In addition to the two just mentioned, there are three other specimens closely resembUng our own. These have been described respectively by Debeyre (1912), van Heukelom (1898), and Giacomini (1898). Of these, the Debeyre ovum is in the best state of preservation. In it the embryonic shield is in the form of an elhptical and dorsally convex plate, consisting (according to the author's description) of a single layer of cylindrical epithelial cells showing numerous karyokinotic figures. In consisting of a single layer of cells it differs from the embryonic shield in the other specimens mentioned, in all of which it was described as stratified. Debeyre explains the appearance of stratification as due to the distribution of nuclei at different levels. There is a well-marked primitive streak, along which exists a close union between the ectoderm and endoderm, and a primitive groove 0.54 mm. long. At the caudal end of this there is a cloacal membrane where the ectoderm and endoderm again join. No trace of a neural groove or neurenteric canal could be found. The amnion incloses a flattened space conforming to the shape of the embryo. There is no amniotic canal present. An allantois 0.4 mm. long is present and has a distinct lumen, but no terminal dilatation. The yolk-sac is lined with endoderm, which has the appearance of a protoplasmic syncitium sown with nuclei; the contours of the cells, for the most part, can not be made out. There are no epithelial buds or glandular diverticula. The supporting mesoderm is extremely variable and at places is entirely lacking. At the ventral pole of the yolk-sac, apparentlj' arising from the mesoderm, are numerous blood-islands in the following forms: (1) Full and consisting of many layers of cells arranged concentrically; (2) an opaque mass of amorphous substance sown irregularly with nuclei of uniform size; (3) cellular elements arranged around a central cavity; (4) uniform cells surrounding a cavity which may or may not have partitions and which contain differentiated elements, some having large nuclei with little protoplasm; (5) irregular strands of closely packed mitotic cells. Blood-islands are also present in the bodystalk, but none are to be seen in the region of the embryonic plate.
In the van Heukelom specimen the chorion is torn and collapsed and the tissues are in a rather poor state of preservation. From its general form and size, however, one can see that it closely resembles the other specimens of this group. In the brief anatomical description mention is made of the presence of the primitive groove, allantois, and blood-islands in the walls of the yolk-sac, and what were possibly blood-vessels in the chorionic membrane.
The Giacomini specimen is in form very much like the "v. H" ovum of v. Spee, but from the figures and description it is apparent that it had undergone marked maceration. Owing to mechanical injury the yolk-sac is partially detached from the amniotic vesicle. The primitive groove and allantois are present. In the wall of the yolk-sac are cell groups that seem to be blood-islands, but no definite vessels were found in the body-stalk or chorionic membrane.
The two ova described respectively by Reichert (1873) and Rossi Doria(1905), both of whicli are frequently referred to and which are about the same size as those in this group, can not, however, be used for this comparison, as no description of the
embryo is given by either of the authors.
A summary of the features occurring in all of the embryos of this group would include the presence of a primitive groove and an allantois, with evidences of bloodvessel formation in the wall of the yolk-sac and (in most of them) also in the bodystalk and chorionic membrane. Furthermore, in all of them the yolk-sac is considerably larger than the amniotic cavity. The chorion is covered with freely branching villi and its ectoderm is clearly differentiated into two layers. The mesoderm Uning the chorion forms a well-defined sup{)orting membrane, from which processes extend as the cores of the villi. The internal diameter of the chorion of such an ovum is from 4 to 6 mm.
All of these characteristics are present in the Mateer specimen. In addition, the latter, as we have seen, also shows evidences of blood-vessel formation in the viUi, which was not reported in the others. It may be that the Mateer ovum is more advanced in development and is thus on the border-land between groups 2 and 3. On the other hand, it is apparently in a better state of preservation than the others of this group, a factor of great importance in the recognition of the early stages of vasculogenesis. In the Other specimens the process may have been obscured by the poor preservation of the tissues.
Group 3. Embryos having Medullary Folds and a Neurenteric Canal
The first three specimens to be mentioned in this group are those described respectively by Grosser (1913), Strahl (1916), and Ingalls (1918). These resemble each other very closely, both in form and in size, and all are in a state of good preservation. In each the greatest external diameter of the chorion is about 10 mm., the greatest internal diameter about 8 mm., and the yolk-sac nearly 2 mm. in its greatest diameter. The only marked difference in size is found in the embryonic shield, which in the Grosser and Strahl specimens is slightly less than 1 mm. long, while in the Ingalls specimen it is 2 mm. long. In all of these embryos there is a distinct head-process with its contained canal,' together with a completion-plate. One can also now speak of medullary folds, and at the caudal end of the primitive groove the ectoderm and endoderm unite in the formation of a well-defined cloacal membrane. Thus, both in the differentiation of these particular features and in their size, these ova represent a stage definitely in advance of that seen in the specimens of group 2.
A complete desoription of this canal is Rivpn by Ingalls, who designates it as the archrnlcrk canal, holding, contrary to Hubrccht and Kcibel that in the formation of the head-process all the essentials of gastrulation are rcprrsentetl, and that its huncn is in reality aii arehentcrir ranal. Furthermore, aerordinR to him, the frequently used
term chrrrdal, or iwlochimial canal is inadequate, sinee only a part of the wall enters into the formation of the chorda.
Similarly, an objection might be raised to the use of the term ninrcnkric canal as a designation for the canal in its
earlier form, as in the strict sen.se this can refer only to the caudal portion of the canal of the head-process — the short, sharply defined canal coiinecting the caudal end of the floor of the medullary groove with the (;ut, iis is best se«'ll in the older si>ccimon8 of this group.
In the Grosser specimen an amniotic duct is described as connecting the amniotic ectoderm with the ectoderm of the chorionic membrane. A similar structure has been seen in the much younger Strahl-Beneke (1910) embryo. In the Strahl (1916) specimen there exists in the body-stalk a small remnant of what seems to be an amniotic duct in process of disappearing. In the Ingalls specimen, however, neither the presence of the amniotic duct nor evidence of its recent disappearance could be made out. There is a short amniotic diverticulum extending from
the amniotic cavity towards the allantois, which is regarded by Ingalls (p. 21) as comparable to the canalis amnioallantoideus connecting these cavities in certain reptiles.
The Bayer ovum described by Keibel (1890), judging from its size and general form, would represent about the same stage of development as those just mentioned. Unfortunately, the preservation is not good, and much of the detailed structure of the specimen is lost. The embryonic shield shows a well-developed primitive streak, but whether a head-process and a neurenteric canal are present
could not be verified.
The Frassi (1907) specimen, which was studied in Professor Keibel's laboratory, is a little larger than those thus far mentioned in this group. It possesses a medullary groove bordered by evidences of medullary folds. A canalis neurentericus connects the caudal end of the floor of the groove with the amniotic cavity. A definite cloacal membrane is present. The amniotic and vitelUne cavities are distended and thin-walled ; in structure they are essentially the same as those found in all of the specimens of groups 2 and 3. The same is true of the allantois, the chorion and,. the chorionic villi. These features do not offer definite criteria for determining the relative degree of development of the specimens of these two groups, nor can the observations on blood-vessel formation be safely used for this purpose, for the reason that the reported presence or absence of blood-islands and blood-vessels in the various parts depends largely upon the state of preservation of the specinxen, and also upon the observer's familiarity with the early form of these structures. The Frassi specimen showed no evidence of an amniotic duct.
The last two specimens that should be included in this group are the frequently referred to Vuillet specimen of Eternod (1899) and the Glaevecke specimen of v. Spee (1899). One should, perhaps, add to these the Triepel (1916) specimen, which seems to be in about the same stage of development, although in not quite as good condition. The v. Spee ovum is slightly more advanced, both in form and size, than that described by Eternod; otherwise there is a very close resemblance between the two. Each has a distinct medullary groove, bordered by broad medullary folds corresponding to the head-region. A well-defined chordal plate hes along the ventral surface of the medullary groove and terminates caudally at a large, distinct neurenteric canal, where its cells are continuous with those of the ectodermal cells of the medullary plate. Caudal to the neurenteric canal, in the region of the primitive streak, the axis of the embryo bends sharply ventralward to terminate in the body-stalk. Just before this point of termination the ectoderm and endoderm unite in the formation of a cloacal membrane. The dorsal wall of the yolk-cavity shows a beginning constriction, which marks off the gut-area. In this respect the V. Spee is shghtly more advanced than the Eternod specimen ; in it a distinct pouch, corresponding to the fore-gut, is marked off. As regards blood-vessels, it also shows an advance over any of the embryos thus far referred to.
Throughout groups 2 and 3 we find evidences of blood-vessel formation in the wall of the yolk-sac, in the body-stalk, chorionic membrane and chorionic villi; here, however, for the first time, developing blood-vessels are found in the body of the embryo. Distinct strands, which are to enter into the formation of cardiac endotheUum in the v. Spee specimen, are pictured by Evans (1912). Eternod describes in his own specimen a complete blood-circulation. It includes a heart with three or four aortic arches and paired aorta extending back to the body-stalk, where they break up into branches that are distributed to the villi. The blood is returned by two large veins that unite to form a vena umbilicaUs impar; this in turn bifurcates into two chorio-placental veins which extend along the junction of the embryo and yolk-sac, to unite in front at the venous end of the heart. From Eternod's description, these vessels are only partially permeable. For the greater part they are in a mesodermal condition which (he says) renders them difficult to trace. It is therefore quite possible that the actual differentiation of the bloodvessels is not so far advanced nor the circulation so complete as one would be led to beUeve from the author's description. Evans (p. 592) regards it as certain that the structures described by Eternod as aortic arches are not such, but only the components of a vascular plexus. We can, however, safely assume that angiogenesis is to be recognized in the body of the embryo at this time.
In a summary of the embryos of this group, as compared with those of group 2, there is to be included the formation of the medullary groove and medullary folds, the formation of the head-process and its contained canal, the differentiation of the neurenteric canal and the chordal plate, the formation of the cloacal membrane, the beginning constriction of the gut-portion from the remainder of the yolk-sac, and finally the evidences of angiogenesis within the body of the embryo.
Probable Age of the Mateer Embryo
The sequence of morphological events that mark the developmental period from the earliest-known form up to the appearance of the first somites is now fairly well established and, as can be seen from the preceding review, the embryos therein referred to may be arranged in a consecutive series of clearly defined stages. These may be briefly summarized as follows:
In the first stage the chorionic villi have not yet appeared and the embryonic rudiment consists of two simple vesicles or of a .solid mass of cells in the process of forming these vesicles. In the second stage the villi have made their appearance but are still of a primitive character. In the embryo a mesoblast can be recognized, and is separated into parietal and visceral layers farming an exocoelomic cavity, and the ectoderm can be resolved into the amniotic ectoderm and that forming the embryonic plate. In the third stage the villi are branched and the size of the chorion has increased relative to that of the embryo. The first three stages a.s a group include all those specimens in which the primitive groove has not yet formed. The fourth stage is marked by the presence of a primitive groove; there is also a well-developed allantoic duct, the yolk-sac has become larger than the amniotic vesicle, and the first steps in the formation of blood-vessels can be recognized in the wall of the yolk-sac and in the chorion. In the fifth stage, in addition to a primitive groove, there is a distinct head-process with its contained canal, and also a completion-plate. Medullary folds can be recognized, and at the caudal end of the primitive groove the ectoderm and endoderm unite in the formation of a cloacal membrane. In the sixth stage there is further differentiation of the neurenteric canal, formation of the chordal plate, beginning constriction of the gut-endoderm from the remainder of the yolk-sac, and finally evidences of angiogenesis within the body of the embryo. The fifth and sixth stages make up our third group of specimens of the presomite period.
Chart 1. Size of the human ovum during the presomite period, shoning range of the greatest internal diameter of the chorion (in millimeters) of all the human embryos thus far puh)lished, from the youngest-known stage up to the appearance of the somites.
In addition to these morphological changes there is a corresponding change in the size of the different ova. This is shown in chart 1, in which the size of the ovum is represented by its most easily determined dimension — the largest diameter of the chorionic cavity. The range of this dimension, as can be seen in table 1 and in chart 1, falls within limits that correspond consistent I3' to the different stages of development. In the only specimen in which there is a departure from this dimensional curve (Strahl-Beneke ovum) the discrepancy is due to an extreme elongation of the chorionic sac.
From such morphological and dimensional criteria it is possible to determine the relative development of any two specimens with a considerable degree of accuracy, which is of the greatest importance in arriviiig at the true age of an embryo. Whereas the clinical data for any individual specimen may be incomplete, when used conjointly with data from similar specimens it may prove of decisive value. In the case of two specimens of the same developmental period, if we know, for instance, that one can not be younf>;er than 15 days and the other not older than 18 days, we have then established the probable age-limits for all the specimens of that group. It may be explained here that by the term age is meant the fei'tilization age; that is, the time elapsing from the fertilization of the ovum to the cessation of development. This term was hitroduced by Mall (1918), who distinguishes it from the copulation age, which he shows to be approximately 24 hours longer.
Decisive age-data of the character referred to are available in connection with the sbc specimens listed in chart 2, and we are thus enabled to place limits within which the age of the specimens belonging to our three main groups must fall. In the Bryce-Teacher specimen the patient missed her period 10 days prior to the onset of the abortion. If we allow an additional day as the minimum time necessary for the interruption of the expected period, the total minimum age would be 11 days. In the Fetzer specimen the last coitus, according to information given by the patient, was at least G days before entering the hosipital, and I'.i days before the removal of the specimen by curettage. If 24 hours are allowed for fertilization to take place the result is a minimum age of 12 days. In the Merttens specimen the \a&t menstrual period began 21 days prior to the curettement and lasted 5 days. Allowing one day for fertilization, there are left 15 days as the maximum age. Since the Merttens and Fetzer sjK'cimens are of about the same stage of development we are justified in concluding that they can not be older than 15 days or ycnuiger than 12 days, as indicated in chart 2. The Bryce-Teacher specimen, being in a distinctly younger stage of develoi:)ment than either of these, must be considered as correspondingly younger, though it can not be younger than 11 days.
Chart 2. The possible duration of pregnancy in 6 cases giving crucial clinical data. Allowing 24 hours each for the interruption of the menstrual period and the completion of the abortion, the Bryce-Teacher specimen could not have been older than 11 days. The Fetzer specimen is probably not younger than 12 days, and the Merttens spicimen not older than 15 days; being in the same stage of development, these two furnish the range of possible age for all specimens of similar development. In like manner the Frassi and Eternod specimens furnish the range of possible age for group 3— i.e., 15 to 19 days.
The morphological description of the Reichert (1873) specimen is in many respects inadequate. From its dimensions, however, it must fall in our second group, the embryos of which have a primitive groove but no head-process canal. The patient in this case died of suicidal poisoning 14 days subsequent to the omission of her menstrual period. If an additional day is allowed for the interruption of her period it makes a minimum age of 15 days for this group. There are no clinical data from the other specimens of this group which would make po.ssible a conclusion as to the maximum age for this stage of development. It must he somewhere between the maximum age of the preceding and that of the succeeding group — that is, between 15 and 19 days.
In the tliird group data as to maximum and minimum age are furnished by the Frassi (1907) and Etcrnod (1898) ova. In the Frassi case the patient reported that her last menstrual period began two weeks before the hysterectomy, but upon subsequent careful questioning it was learned that she had missed her period 14 day's prior to the operation. Allowing an additional day for the interruption of the period, this leaves a minimum age of 15 days. In the Eternod specimen the abortion of a well-preserved, normal ovum followed 21 days after a single coitus. Assuming one day for the process of fertiUzation, there are left 19 days as the maximum age. From the foregoing it is apparent that the period of development from the time of the formation of the ectodermic vesicle (Bry ce and Teacher) up to the time just preceding the appearance of somites (Eternod) does not cover more than 8 daj's (eleventh to nineteenth day).
While the data in each of these selected cases tend to substantiate that of the others, some of them nevertheless are not entirely above question. In the case of the Bryce-Teacher specimen one might raise the point that, although at least 11 days elapsed from fertilization to the beginning of the abortion, we can not be sure that the embryo continued in its development up to that time. It may have died a few days previous, thus making a minimum age of less than 11 days. In the Fetzer case we are allowing 24 hours for fertilization following the last coitus, which is probably adequate, although we know that spermatozoa may retain their vitality much longer than this. In the Merttens specimen there can be no doubt as to the accuracy of 15 days as the maximum age. The same is true of the Reichert and Frassi specimens, in which 15 days is certainly their minimum age. In the case of the Eternod specimen we must depend on the reUabiUt}' of the patient's statements and at the same time assume that 24 hours is sufficient for fertilzation. That we are safe, however, in accepting the confirmatory trend of these data is supported by the recent observations of Huber on the rat, in which it was possible to obtain timed specimens.
In addition to the above 6 specimens in which decisive data as to maximum and minimum age are present, there are 11 other specimens of the presomite period, including our own, which have available chnical records. These are arranged in the order of their development in chart 3, and the probable duration of pregnancy in the individual specimens is arrived at by their adjustment to tlie maximum- and minimum-age data, as indicated by the heavy brackets. The ages fall witliin these hmits and thus form a curve winch would correspond to the gradual increase in size and the differentiation of the individual specimens. The essential clinical facts a.s far a.s reported are indicated in the chart and need not l)e detailed here. It should be said, however, that in the cases terminating in abortion 24 hours arc allowed as an average duration of that process; that is, development is regarded as arrested 24 hours before the completion of the abortion.
Chart 3. In this chart are shown the clinical data of the human embryos of the presomite period, arranged from above downward in the order of their development. Brackets are introduced in those cases having crucial aKC-<lata, and the anon for the dilTerent stages of development must fall within the bracketed periods. In this way the probable ago of (■.■u-h specimen was determined, and this is indicated by hcavj- horiiontal lines.
The Mateer specimen is probably not younger than the Reichert specimen, which in turn can not be younger than 15 days. On the other hand, it is distinctly younger than the Eternod specimen, which is not older than 19 days. If the Ingalls and Grosser specimens, which are also younger than the Eternod specimen, are placed at 18 days, then the Reichert, Giacomini, and Mateer specimens, being still younger, would fall back on the 17th day, which is probably very close to their actual age.
On examining chart 3 it will be found that, if the age-deductions are correct, pregnancy in 16 of the cases hsted began respectively 1, 1, 3, 4, 5, 9, 10, 11, 13, 13, 14, 14, 17, 18, 19, and 20 days following the end of the last menstrual period.' In no case did it begin during the period or in the 4 days inunediately preceding it. It would appear that conception takes place most frequently (25 per cent of the above cases) at about the 13th or 14th day following the cessation of the menstrual period. In nearly one-half of the cases it occurred at the end of the second and during the third week, which has previously been regarded as a comparatively sterile period.
Twin Embryo
Before concluding I wish to call particular attention to the two small vesicles which are to be found in the region of the body-stalk, mention of which was made under the serial description of sections (sees. 68 to 79). The opinion was there expressed that these structures constitute respectively the amniotic vesicle and yolk vesicle of another embryo. Inasmuch as an opportunity is thus afforded to observe twin-formation at an extremely early stage, this specimen has an important bearing upon the problem of that process.
As we have seen, intervening between the amnion and the chorionic membrane there is an area which is partially filled with parietal mesoblast. Caudally, this area surrounds the body-stalk proper, which can be distinguished by its more compact structure. Around the base of the body-stalk the parietal mesoblast walls itself in from the exoccelomic cavity by a membranous arrangement of its superficial cells in the form of a mesotheUum. The latter flares outward and becomes indistinct as it approaches the chorionic membrane, so that in tliis region the parietal mesoblast is more irregularly marked off from the exocoelom, constituting an area of scattered mesoblastic tissue in which the exoccelomic development is not yet complete.
A section through this region is shown in figure 37, plate 6, in which will be recognized the chorionic membrane above and a portion of the yolk-sac of the main embryo and its abdominal stalk below. In the loose tissue lying between these are the two detached vesicles, which together constitute the twin. The smaller, with a lumen 0.03 mm. in its largest diameter, is interpreted as the yolk-sac. It is shown
under greater enlargement in figure 36; here it can be seen that its wall is made up of a thick sheet of protoplasm interspersed with a single layer of nuclei. This
endodermic layer is inclosed by an irregular membranous layer of mesoderm.
- In the Linzenraeier specimen, owing to the excessive and irregular character of the menstruation, the data could not be safely used.
A series of sections through the larger structure, which is interpreted as the amniotic vesicle, is shown in figures 29 to 35. This vesicle, the inside diameters of which are 0.1 by 0.1 by O.OG mm., consists of an ectodermic layer clearly subdivided into embryonic ectoderm and amniotic ectoderm. The embryonic ectoderm forms a sharply marked-off plate on the side towards the body-stalk of the larger embryo. Like the yolk-sac, it is surrounded by a membranous sheet of mesoderm in which here and there are vesicular arrangements of its cells, in some instances closely resembling empty young blood-vessels.
In accepting tliis structure as a monozygotic twin, the discrepancy in size between it and the principal embryo suggests the possibility of its being stunted. Whether it is essentially normal in form and simply retarded in its development can only be determined by comparison with a much larger group of specimens than is to be found in the literature up to the present time. The amniotic vesicle seems to be well preserved and shows relatively normal differentiation. It corresponds in many respects to those seen in the ova described by Peters, Fetzer, Jung, and Strahl and Beneke. Our specimen differs from these, however, in the complete detachment of the yolk-sac. This condition might justify us in considering it as abnormal. It will be recalled, however, that the amniotic vesicle and the yolk-sac were as widely separated in the Bryce-Teacher ovum which has hitherto been regarded as normal. Inasmuch as the twin in the Mateer specimen is at least stunted and possibly abnormal, it would cast some doubt upon the probability of a detached yolk-sac ever being a normal condition.
If the pregnancy had not been terminated in this case it is probable that the larger embryo would have gone on to maturity, and that the smaller one would have remained stationary in the form of two small epithelial vesicles, and so would have been entirely overlooked. Careful search at the placental attachment of the umbilical cord might frequently reveal the presence of similar minute epithelial vesicles, the remains of stunted twins, and thus we might find that the tendency toward twinning in man is even greater than is now supposed.
Bibliography
Bryce TH. and Teacher JH. Contributions To The Study Of The Early Development And Imbedding Of The Human Ovum 1. An Early Ovum Imbedded In The Decidua. (1908) James Maclehose and Sons. Glasgow.
Debeyre, a., 1912. Description d'un embryon bumain de O^i^.g Joui.de I'Anat. etde la Physiol., vol. 48, p. 448-515.
Eternod, a. C. F., 1894. Communication sur un ocuf humain avec embryon exce-ssivcmcnt jeune. C. R. du Xl" Cong, inteinat. m(?dical. Rome; Arch. ital. de Biol, 1895, vol. 22. , 1899. Premiers stades de la circulation sanguine dans I'reuf et I'embrvon hiunains. Anat. Anz., vol. 15, p. 181-189.
Evans HM. The development of the vascular system. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp570-708.
Fetzer, 1910. Ueber ein durch Operation gcwonnenes mensrhliches Ei, dass in seiner Entwickeluns etwa dem Petersschen Ei entspricht. Anat. Anz.. Bd. 37, Ergiinzhft., p. 116.
Frassi, L., 1907. Ueber ein junges menschliches Ei in . situ. Arch. f. niikr. Anat. u. Entwick., Bd. 70, p. 492-505.
GiACOMiNi, C, 1898. Un ceuf humain de 11 joure. Arch. ital. d. Biol., vol. 29, p. 1-22.
Grosser, O., 1913. Ein mensehhcher Embryo mit chonlakanal. Anat. Hefte, 1 Abth., Bd. 47, p. 649-686.
Heixe 0. HoFBAUER, 1911. Beitrag zur friihesten Eientwicklung. Zeitschr. f. Geburtsh. u. Gyn., Bd. 68, p. 665-688.
Herzog, M., 1909. -V contribution to our knowledge of the earliest known stages of placentation and embryonic development in man. Amer. Jour. Anat., vol. 9, p. 361^00.
Van Heukelom, S., 1898. Ueber die menscbhche Placentation. Arch. f. Anat. u. Physiol., Anat. Abth., p. 1-35.
Huber, G. C, 1915. The development of the albino rat, Mus norvegicus albinus. Memoirs Wistar Inst., No. 5.
Ingalls NW. A human embryo before the appearance of the myotomes. (1918) Contrib. Embryol., Carnegie Inst. Wash. No.23 Publ. 227, 7:111-134.
Juno, P., 1908. Beitrage zur friihesten Ei-einbettung beim menschlichen Weibe. Beilm.
Keibel F. Ein sehr junges menschliches Ei. (1890) Arch. f. Anat. u. Physiol., Anat. Abth., p. 250.
Template:Ref-GrosserLewisMcmurrich1912 p. 300.
Lewis FT. A comparison of the Herzog and Strahl-Beneke embryos. (1917) Anat. Rec, 11: 386.
LiNZE.XMEiER, G., 1914. Ein junges menschliches Ei in situ. Arch. f. Gj-n., Bd. 102, p. 1-17.
Mall FP. On the fate of the human embryo in tubal pregnancy. (1915) Contrib. Embryol., Carnegie Inst. Wash. Publ. 221, 1: 1-104.
Mall FP. On the age of human embryos. (1918) Amer. J Anat. 23: 397-422.
Mall, F. P., 1915. On the fate of the human embryo in tubal pregnancy. Contributions to Embrj-ology, vol. I, Carnegie Inst. Wash. Pub. Xo. 221. , 1918. On the age of human embryos. Amer. Jour. Anat., vol. 23, p. 397-422.
Merttens, J., 1894. Beitrage zur normalen und pathologischen Anatomie der menschliihen Placenta. Zeitschr. f. Geburtsh. u. Gyn., Bd. 30, p. 1-97.
Miller, J. W., 1913. Corpus luteum u. Schwangerschaft. Das jungste opeu.tiv erhaltene menschliche Ei. Berlin klin. Wochenschr. Bd. 50, p. 865.
Newman, H. H., 1917. The biology of twins. University of Chicago Press.
Peters, H., 1899. Ueber die Einbettung des menschlichen Eies. Leipzig u. Wien.
Reichert, Karl B., 1873. Beschieibung einer fruhzeitigen monschhchen Frucht im blilschenformigen Bildungszustand, nebst vergleichenden Unteusuchungon iiber die blaschenfoimiger Friichte der Saugethiere und des Menschen. Phys. u. med. Abhandl. d. kgl. Akad. der W'iss. zu Berlin.
Rossi, Doria, T., 1905. Ueber die Einbettung des menschlichen Eies, studirt an einen kleinen Ei der zweiten Woche. Arch. f. Gynak., Bd 76 p. 433.
V. Spee, F. Graf, 1896. Xeue Beobachtungen uber sehr friihe Entwickelungs-stufen des menschlichen Eies. Arch. f. Anat. u. Physiol., Anat Abth.
Strahl, H., 1916. Ueber einen jungen menschlichen Embryo nebst Bemerkungen zu C. Rabl's Gastrulationstheor'e. Anat. Hefte, Bd. 54, p 115 146.
Strahl, H., and R. Beneke, 1910. Ein junger menschlichcr Embryo. Wiesbaden.
Triepel, H., 1916. Ein mensehhcher Embrj-o mit Canalis ncurentericus. Chordulation. Anat Hefte, Bd. 54, p. 151-183.
Plates
- Paper Links: Fig 1 | Fig 2 | Fig 3 | Fig 4 | Fig 5 | Fig 6 | Fig 7 | Fig 8 | Fig 9 | Fig 10 | Fig 11 | Fig 12 | Fig 13 | Fig 15 | Fig 16 | Table 1 | Chart 1 | Chart 2 | Chart 3 | Plate 1 | Plate 2 | Plate 3 | Plate 4 | Plate 5 | Plate 6 | Plate 7 | Paper | Carnegie Institution of Washington - Contributions to Embryology
Plate 1
Fig. 5. Frontal view of wax-plate reconstruction of Mateer embryo, showing yolk-sac and embryonic plate outlined by the cut edge of the amnion, which is shown as removed. The stump of the body-stalk can be seen with the allantois at its center. Extending forward from the caudal end of the embryonic plate is a well-defined primitive groove. Enlarged 60 diameters.
Fig. 6. Median view of wax-plate reconstruction of Mateer embryo showing the form of the amniotic cavity and its relation to the chorionic membrane and the yolk-sac. The allantois projects through the bo<ly-stalk and is interrupted near its center. In the loose tissue caudal to the body-stalk is an epithelial mass constituting the amniotic vesicle of the twin embryo. Enlarged &) diameters.
Fig. 7. Photograph of section through embryo No. 763, Carnegie Collection. This specimen would belong among those in our group 1 having branched villi. The embryo, consisting of two vesicles, can be seen in the center. The series being incomplete, the definite form of the embryo can not be made out. This specimen is distinctly younger than the Mateer specimen and its viUi are to be compared with those in figure 8. Enlarged 30 diameters.
Fig. 8. Photograph of a typical larger villus of the Mateer specimen, showing transition into trophoblast. For a detail of these structures see text-figiu'e 4. Enlarged 50 diameters.
Plate 2
Plate 3
Figs 9 to 16, shown on plates 2, 3, 4 and S, represent detailed drawings of typical sections through the region of the embryonic plate indicating the arrangement of the three germ-layers The section number, are as follows: Fig. 9. Section Ko. 78; Fig. 10, Section No. 75; Fig. 11. Sec ion No. /O, Fig. 12. Section No. 67; Fig. 13, Section No. 64; Fig. 14, Section No. 59; Fig. 15, Section No. 26; Fig. 16. Section No. 50. .\11 of the figures are enlarged about 180 diameters.
Plate 4
Figs 17 to 22 Sketches of selected cell groups found in the chorionic villi, which are interpreted as blood-vessels in a progressive series of developmental stages. In figure 22 is shown the relation of the endothelium to the surrounding stroma. Enlarged 600 diameters (approx.).
Plate 5
Figs 23 to 28. A similar series of angioblastic cell-groups found in the wall of the yolk-sac In this series the endoderm is shown as the upper and the mesoderm as the lower layer. Enlarged 600 diameters (approx.).
Plate 6
Figs 29 to 35 Photographs of serial sections through the amniotic vesicle of the twin embryo, showing the ectodermic character of its wall and the investing layer of mesoderni. In figures 30 to 33 the region of the amniotic plate is clearly marked off. Enlarged 300 diameters.
Plate 7
Fig. 36. Photograoh of section through the yolk-vesicle. Enlarged 300 diameters.
Fig 37 Section (No. 73) through region of abdominal stalk, showing the relation of the two vesicles (constituting the twin), to the larger embryo and the chorionic membrane. Enlarged 11 diameters.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Contributions to Embryology Carnegie Institution No.43. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.43
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G