BGDA Practical 3 - Implantation
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
Trophoblast cells at the site of adplantation proliferate and form an additional layer the syncitiotrophoblast layer. This layer of cells rapidly divide, secrete enzymes that degrade the endometrial extracellular matrix and secrete human Chorionic Gonadotropin (hCG). Trophoblast cells also disperse through the maternal decidua and are associated with the open maternal blood vessels and the anchoring villi attachment (trophoblast column).
Implantation Dynamics
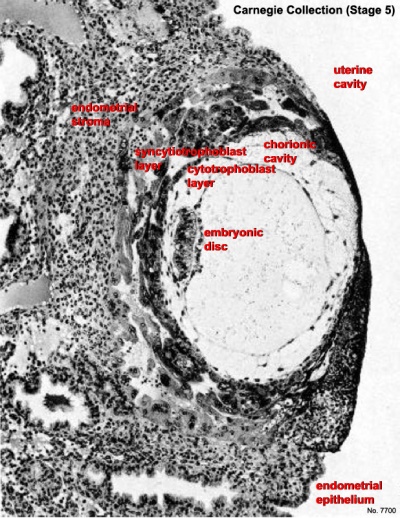
The uterine epithelium (white cells) are invaded by the trophoblast cells (green, syncitiotrophoblasts) with the inner cell mass now having 2 layers: an epiblast (blue) and hypoblast (yellow). The blastoceol is covered in cytotrophoblast cells (green).
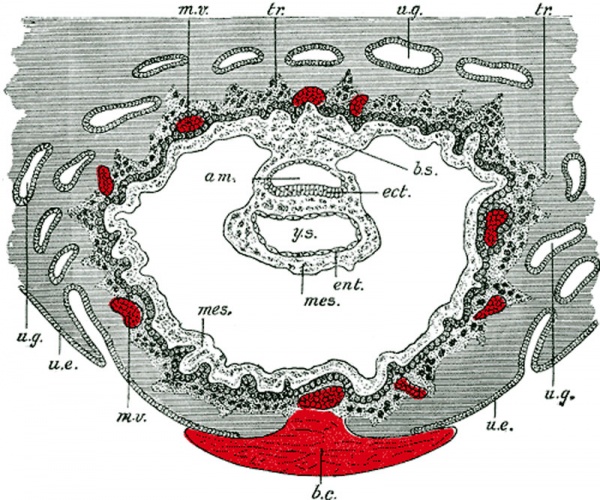
Later in the movie the amniotic cavity forms adjacent to the epiblast layer(blue) and spaces in the syncitiotrophoblast layer are filled with maternal blood, lacunae.
| <html5media height="580" width="500">File:Week2_001.mp4</html5media> |
This animation shows the process of implantation, occurring during week 2 (GA week 4) of development in humans. The beginning of the animation shows adplantation to the the uterus lining (endometrium epithelium). The hatched blastocyst with a flat outer layer of trophoblast cells (green), the inner cell mass which has formed into the bilaminar embryo (epiblast and hypoblast) and the large fluid-filled space (blastocoel).
|
Identify the embryoblast and trophoblast layers of the conceptus.
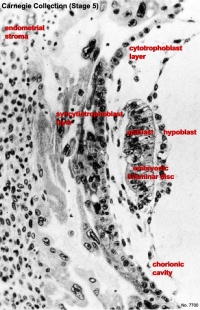
Carnegie Stage 4 represents the beginning of implantation. The blastocyst initially attached to the uterine endometrium (adplantation), syncitiotrophoblasts then secrete enzymes that digest extracellular matrix, allowing the blastocyst to sink into the uterine wall, eventually being completely enclosed within the uterine wall. Note the majority of growth occurs in the trophoblastic shell. The inner cell mass divides initially into 2 layers; epiblast and hypoblast (bilaminar embryo). Hypoblast cells migrate around the original blastoceol cavity forming the primary yolk sac. A second cavity (amniotic) forms between the inner cell mass and the cytotrophoblast shell; this cavity is lined by epiblast cells.
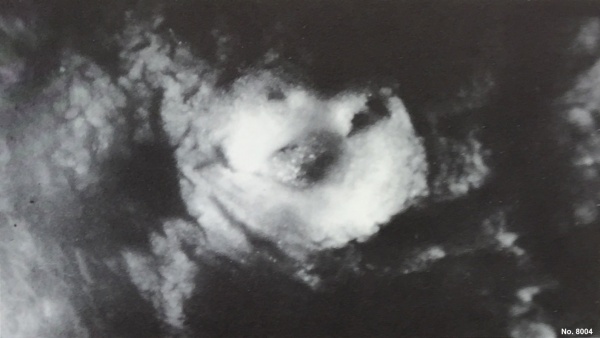
This is a uterine surface view showing the site of implantation (pale region in centre of image). The conceptus can be identified located at the dark central region within the pale region.
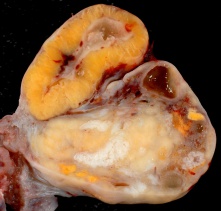
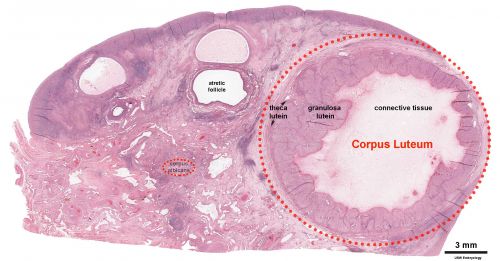
Corpus Luteum
| Ovary Overview | Corpus Luteum Layers |
|---|---|
|

|
|
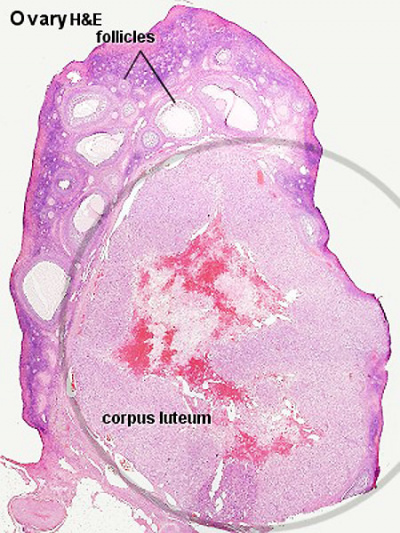
An endocrine signal (human Chorionic Gonadotropin, hCG) secreted from the implanting conceptus syncytiotrophoblast cells maintains the ovarian corpus luteum, which in turn provides hormonal support to the uterine functional lining, preventing menstruation. The corpus luteum is formed during the luteal phase (secretory phase) of the menstrual cycle by proliferation of both follicular granulosa cells (granulosa lutein cells) and thecal cells (theca lutein cells), which produce progesterone and oestrogen (USA spelling, estrogen).
Following ovulation
If implantation does not begin until very late in the current menstrual cycle, or not at all, then that cycle will continue with loss of both the functional layer and the conceptus. Many human fertilization events never form an embryo or develop as a pregnancy. In the maternal endometrium, decidualization occurs in response to elevated levels of the ovarian steroid hormones, oestrogen (E2) and progesterone. |
| Pregnancy | |
|---|---|
| Time (GA) weeks | Levels (mIU/mL) |
| 0 - 1 weeks | 0 - 50 |
| 1 - 2 weeks | 40 - 300 |
| 3 - 4 weeks | 500 - 6,000 |
| 1 - 2 months | 5,000 - 200,000 |
| 2 - 3 months | 10,000-100,000 |
| second trimester | 3,000 - 50,000 |
| third trimester | 1,000 - 50,000 |
| Non-pregnant | < 5.0 |
| Post-menopausal | < 9.5 |
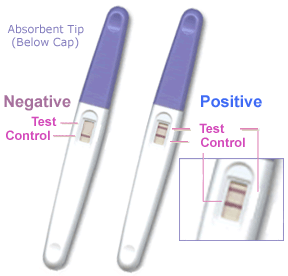
| Pregnancy test - greater than 20 mIU/mL (milli-international units per milliliter) is a positive result. Levels peak at 8 to 10 weeks of pregnancy, then decline and are lower for rest of pregnancy. | |
Histology
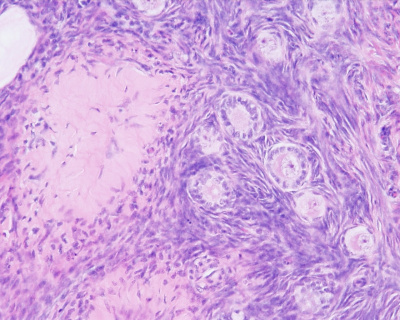
Corpus Albicans
The degenerating remnant of the ovulating follicle.
Interactive Component
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Additional Information
| Additional Information - Content shown under this heading is not part of the material covered in this class. It is provided for those students who would like to know about some concepts or current research in topics related to the current class page. |
Complications in Early Pregnancy
Pontius E & Vieth JT. (2019). Complications in Early Pregnancy. Emerg. Med. Clin. North Am. , 37, 219-237. PMID: 30940368 DOI.
Early in pregnancy women frequently experience nausea, vomiting, and vaginal bleeding. Nausea and vomiting can be mild, managed by dietary modifications and medications, or severe, requiring intravenous fluids and medications. Care should be used when selecting medications for nausea to avoid additional side effects or potential harm to the developing fetus. When evaluating vaginal bleeding in early pregnancy, ectopic pregnancy must be ruled out. If an intrauterine pregnancy is seen, threatened miscarriage should be considered and the patient appropriately counseled. If neither intrauterine pregnancy nor ectopic pregnancy can be established, a management algorithm for pregnancy of unknown location is presented.
Pelvic Inflammatory Disease and Ectopic Pregnancy
Rates of pelvic inflammatory disease and ectopic pregnancy in Australia, 2009-2014[1]
- Calculated yearly PID and EP diagnosis rates in three states (Victoria, New South Wales, Queensland) for women aged 15-44 years using hospital admissions and emergency department (ED) attendance data, with population and live birth denominators. * Ectopic Pregnancy - in 2014 the rate of all admissions was 17.4 (95% CI 16.9 to 17.9) per 1000 live births and of all ED presentations was 15.6 (95% CI 15.1 to 16.1).
- Comparing 2014 with 2009, the rates of all EP admissions (aIRR 1.06, 95% CI 1.04 to 1.08) and rates in EDs (aIRR 1.24, 95% CI 1.18 to 1.31) were higher.
- Pelvic Inflammatory Disease and EP remain important causes of hospital admissions for female STI-associated complications. Hospital EDs care for more PID cases than inpatient departments, particularly for young women." Australian statistics | bacterial infection
Birth Control
There are a number of different chemical and mechanical methods of birth control. The most comon is the "birth control pill" taken daily and made up of two hormones, estrogen and progestogen and these stop a woman's ovaries from releasing an egg each month (ovulation), which means that a pregnancy cannot begin. Recently the drug RU486, which is an abortive rather contraceptive drug, has been the centre of political and medical discussions in Australia.
Chemical
- Estrogen - the hormone estrogen in birth control pills act on the pituitary gland (suppress FSH and LH) which then blocks ovulation.
- progesterone - the hormone progesterone in birth control pills act on the uterus to both alter the lining to prevent implantation and forms a cervical mucus plug that mechanically blocks acceess of sperm. There is also an inhibition of sperm capacitation.
- Injectable Control - there are commercial (Lunelle, USA) injectable estrogen/progestin contraceptives administered on a monthly basis.
- mifepristone (RU486) - is a progesterone receptor antagonist (antiprogesterone) which can prevent between 92-100 % of pregnancies on oral intake of a 10-600 mg dose within 72 h of unprotected intercourse. (alternative commercial name: Mifegyne)
Links: Clinical Methods - Birth Control
Maternal Immune
How does the implanting conceptus avoid immune rejection by the maternal immune system? There are a number of maternal and embryonic mechanisms that are thought to act to prevent immune rejection of the implanting conceptus, a few mechanisms are shown below.
Chemokine Gene Silencing - Remove the attraction of maternal immune cells.
A mouse study[2] has shown that the normal immune response to inflammation, accumulation of effector T cells in response to chemokine secretion does not occur during implantation. This is prevented locally by epigenetic silencing of chemokine expression in the decidual stromal cells.
Corticotropin-Releasing Hormone - Kill the maternal immune cells.
Both maternal and implanting conceptus release CRH at the embryo implantation site. This hormone then binds to receptors on the surface of trophoblast (extravillous trophoblast) cells leading to expression of a protein (Fas ligand, FasL) that activates the extrinsic cell death pathway on any local maternal immune cells ( T and B lymphocytes, natural killer cells, monocytes and macrophages).[3] This cannot be the only mechanism, as mice with dysfunctional FasL proteins are still fertile.
Links: implantation
Terms
- bilaminar - having 2 layers
- blastocyst - the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the conceptus to adplant and then implant into the uterine wall.
- corpus albicans - (Latin, corpus = body, albicans = whitish) The histological structure formed by luteolysis of the corpus luteum in the ovary. If implantation does not occur and the hormone hCG is not released the corpus luteum degenerates and the structure is white, not yellow, because of the absence of steroid hormone synthesis/accumulation.
- corpus luteum - (Latin, corpus = body, luteum = yellow) The remains of ovarian follicle formed after ovulation that acts as an endocrine organ (produce progesterone and oestrogen) supporting pregnancy and preventing menstruation (loss of the endometrial lining). Formed during the luteal phase (secretory phase) of the menstrual cycle by proliferation of both follicular granulosa cells (granulosa lutein cells) and thecal cells (theca lutein cells), which produce progesterone and oestrogen. If fertilization and pregnancy does not occur, the corpus luteum degenerates to form the corpus albicans. Regnier de Graaf (1641 – 1673) first observed it in the ovary of a cow as a yellow structure, the yellow colour is caused by accumulation of steroidal hormones.
- inner cell mass- the clump of cells found inside the blastocyst. These cells will go in to form the embryo, these are the "stem cells" (we here about in the media) that are totipotential, they can form any tissue in the embryo. Mature oocyte-the female germ cell released at ovulation from the ovary.
- trilaminar embryonic disc- the 3 layered embryo stage.
- Trophoblasts- (Gr. trophe = nutrition) outer layer of cells on blastocyst that will generate the embryonic part of the placenta.
References
- ↑ Goller JL, De Livera AM, Guy RJ, Low N, Donovan B, Law M, Kaldor JM, Fairley CK & Hocking JS. (2018). Rates of pelvic inflammatory disease and ectopic pregnancy in Australia, 2009-2014: ecological analysis of hospital data. Sex Transm Infect , , . PMID: 29720385 DOI.
- ↑ Nancy P, Tagliani E, Tay CS, Asp P, Levy DE & Erlebacher A. (2012). Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science , 336, 1317-21. PMID: 22679098 DOI.
- ↑ Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A & Chrousos GP. (2001). Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat. Immunol. , 2, 1018-24. PMID: 11590404 DOI.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
BGDA: Lecture 1 | Lecture 2 | Practical 3 | Practical 6 | Practical 12 | Lecture Neural | Practical 14 | Histology Support - Female | Male | Tutorial
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology BGDA Practical 3 - Implantation. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/BGDA_Practical_3_-_Implantation
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G