ANAT2241 The Virtual Microscope
| ANAT2241 This practical support page content is not part of the virtual science practical class and provides additional information for student self-directed learning purposes. All practical class pages are located on Moodle - ANAT2241 |
General Objective
An introduction to the use of the virtual microscope.
Specific Objective and Learning Activities
To learn the correct use of virtual microscopy using computers and to employ various virtual histological databases for enhancing learning as well as for revision purposes.
In Histology, you are expected to study the features of histological preparations as virtual images, which were scanned from real stained sections, which were mounted on glass slides and listed in the Learning Activities. In these classes you may encounter structures and terminology not defined in lectures. You will need to read about these structures in your textbooks.
Learning Activity
Read sections in your course manual on General Hints on Staining Procedures and Routine Staining Protocols.
Introduction
Histological sections, which are slices of tissue usually from 5 - 8µm thick (see Dimensions below), can be examined efficiently as follows:
- Use the Slide Catalogue in your course handbook to determine:
- The animal from which the section is taken.
- The stains used (thereby defining the colours of the major tissue components). Note that stains are not examinable.
- Low power sketches or notes made may help you to remember the main histological features of a section, e.g., which major tissue components are present.
- Note the 2-D shapes in the section and the major tissue components present and try to determine the approximate 3-D shape of the whole organ from which the section was taken. Is the section cut randomly through the organ? Is there an obvious lumen in the section?
Abbreviations
- XS - cross section
- TS - transverse section
- LS - longitudinal section
- LM - light microscope or light micrograph
- EM - electron microscope, or electron micrograph
- 2-D = 2-dimensional
- 3-D = 3-dimensional
Dimensions
1mm = 103 micrometres (µm) = 106 nanometres (nm)
A micrometre is often called a "micron" (µm); 1µm = 10-6m
Resolving Powers
- Unaided eye - approx. 0.1 mm = 100µm
- Light microscope - approx. 0.1 µm = 100nm
- Electron microscope - approx. 1 nm
What are Virtual Slides?
High magnification scanned digital images of tissue sections on glass slides acquired using a x40 microscope lens.
Stored in a multi-resolution file format and viewed in a web browser window with software capacity to “click and drag” and “zoom in” on the image.
Simulates the examination of glass slides with a real microscope, but with the added benefits of always having optimal focus and contrast, with orientation maintained and avoiding section-to-section variability.
Advantages
- Common tissue - allows all students to examine the same scanned tissue section.
- Annotation - can add your own labelled structures to help with revision.
- These annotations can be shared with others.
- Can see demonstrator annotations (if available).
- Access - can access images from any internet connected computer.
Microscopy Methods
This Lab is an introduction to cell biology methods using microscopy. It includes a brief historic background and relevant modern technological advances. The focus is more on the application of these techniques in cell biology, rather than a comprehensive understanding of the physics and technology underlying the techniques.
- "Diffraction inevitably limits the resolution of microscopy to around half the wavelength of light" Ernst Abbe (1873, German physicist)
This rule has recently been bent, not broken.....
Also take the time to look at the Textbook References and some of the Cell Biology Images on this Wiki. Later in the course we will be visiting the Confocal Microscope Facility, so spend some time reading about this technique.
MBoC Figure 9-8. Four types of light microscopy
Microscopy Timeline
- 1665 - Robert Hooke publishes Micrographia, a collection of biological micrographs.
- 1674 - Anton van Leeuwenhoek improved simple microscope for biological specimens.
- 1833 - Brown published a microscopic observation of orchids, describing the cell nucleus.
- 1898 - Golgi first saw and described the Golgi apparatus by staining cells with silver nitrate.
- 1931 - Ernst Ruska first transmission electron microscope, TEM).
- 1934 - Frits Zernike describes phase contrast microscopy.
- 1957 - Marvin Minsky patents principle of confocal imaging.
- 1953 - Frits Zernike receives the Nobel Prize in Physics for invention of the phase contrast microscope.
- 1955 - George Nomarski theoretical basis of Differential interference contrast microscopy.
- 1981 - Gerd Binnig and Heinrich Rohrer develop the Scanning Tunneling Microscope (STM).
- 1981 - Daniel Axelrod develop Total Internal Reflection Fluorescence Microscope (TIRFM).
- 1981 - Allen and Inoué perfected video-enhanced-contrast light microscopy.
- 1986 - Ernst Ruska, Gerd Binnig and Heinrich Rohrer receive the Nobel Prize in Physics for invention of the electron microscope (ER) and scanning tunneling microscope (GB and HR).
- 2000 - Hell and collaborators develop Stimulated Emission Depletion Microscopy (STED)
- 2008 - Freudiger and Wei Min develop Stimulated Raman Scattering Microscopy (SRS)
Fixation
Techniques for tissue and cell fixation.
- Fresh Frozen - Cryostat Sectioning Movie
- Precipitation
- Aldehyde Cross-linked
Histology Stains
This table includes many of the common histology stains. Note that depending upon the stain used the same tissue can appear quite different. Check the Slide information panel to see the original histological stain.
Haematoxylin and Eosin
One of the most common staining techniques in pathology and histology. Acronym "H and E" stain. (H&E, HE)
| Haematoxylin | Eosin |
|---|---|
|
|
| Histology Stains - Common Stains and Their Reactions | |||||
|---|---|---|---|---|---|
| Haematoxylin | mucins - light blue | ||||
| Eosin | colloid - pinkmuscle - red | ||||
| Iron Haematoxylin | |||||
| Van Gieson | muscle: yellow/browncartilage - pink | ||||
| Verhoeff's Elastin | elastic fibres - black | ||||
| Tartrazine | |||||
| Silver Impregnation | reticular fibres - black | ||||
| Methyl Green | |||||
| Nuclear Fast Red | |||||
| Gomori's Trichrome | keratin - redmuscle - purple/red | ||||
| Heidenhain's Azan | muscle - red | ||||
| Osmium Tetroxide | myelin, lipids - black | ||||
| Alcian Blue | mucins, - blue | ||||
| Periodic acid-Schiff (PAS) | mucins, glycogen, glycocalyx - magenta | ||||
| Phosphotungstic Acid-Hematoxylin (PTAH) | muscle bands - blue | ||||
| Masson's Trichrome | cartilage, mucins - blue or green; muscle - red | ||||
| Luxol Fast Blue | myelin - blue | ||||
| Aldehyde Fuchsin | elastic fibres, mast cells - deep purple | ||||
| Light Green | |||||
| Gallocyanin | nucleic acids, Nissl granules - dark blue | ||||
| Romanowsky (e.g. Leishman's) | acidophils - red, basophils - blue, azurophilic - purple | ||||
| Aldehyde Pararosanilin | elastic fibres - purple | ||||
Artefacts
- Chatter artefact - fine parallel lines (Virtual slide - tendon)
- Tissue cut - often a neat straight edge to tissue (where cut has been made) to allow the tissue to sit flat.
- Tissue fold - darker well demarcated line with apparent disruption of the tissue structure (Virtual slide - artery)
- Tissue tearing - irregular "tear" with apparent disruption of the tissue structure. Can be due to something intrinsic to the tissue that is hard.
- Shrinkage - clear white spaces due to fixatives dehydrating tissues.
- Uneven staining - Tissue has an "mottled" appearance that does not coincide with any tissue structure.
See also - Chatterjee S. (2014). Artefacts in histopathology. J Oral Maxillofac Pathol , 18, S111-6. PMID: 25364159 DOI.
Support Tutorial
2018 - Please note the Slide software has been updated and some of the original software descriptions below no longer apply.
UNSWTV - Tutorial Using the Virtual Microscope
This 15 minute video tutorial will take you through the organization and basic controls of the Virtual Microscope.
| Transcript - Virtual Slide Tutorial |
|---|
|
The following text is an approximate transcript for the UNSWTV video tutorial "Using the Virtual Microscope" |
| Hi There
Welcome to the support tutorial for UNSW courses using "The Virtual Microscope". In particular ANAT2241 Histology - Practical 1 An introduction to the virtual microscope. This current tutorial does not go through practical class content but will help you both use and understand "The Virtual Microscope" controls.
Have your practical manual ready with pens and pencils to make both notes and drawings as you work through the class materials. You may also choose to keep notes on your own computer as you work through the class.
From the Practicals table select practical 1 "The Virtual Microscope" If you are on your own computer or outside UNSW a log in message will appear "To access the Virtual Slides from outside UNSW, please log in." Click OK The log in screen will appear. Enter your student number and zpass. The first time you log in, then the the default Virtual Slides welcome will open. You can then go through your course portal to each practical class.
This time you should go directly to your practical page. This current tutorial ail not go through practical class content but will introduce the "The Virtual Microscope" controls. In practical classes, ensure you
You will need to allocate sufficient time to looking through all these slides during the practical class, unless the demonstrator advises otherwise. You will also see from the whole slide thumbnail:
Note that the slide sequence will usually match that in your practical manual and may also be the order that they will be demonstrated. Other histological resources and materials outside virtual slides may also be used in your classes.
Note - This tutorial will not discuss this tissue, only the controls used to change its viewing. Click a slide thumbnail. Please read note about Computer Requirements A new browser page will open with the slide tissue visible in 2 windows. All Virtual slides will have this same initial layout, but tissues may open at different initial magnifications.
Always observe the whole section before making any changes to the magnification. For your own reference purposes, it may be useful to either take a screenshot or drawing of this initial view and any useful future view. Screenshots can be imported into a number of programs that allow you to add your own annotations (labels, notes, arrows or boxes).
Under the thumbnail image are a number of image controls (contrast and magnification) initially visible, and a location control not visible. ContrastThe black and white box relates to image contrast and the slider located beside it. Dragging the black triangle to the left, decreases contrast. Making the image darker. Dragging the black triangle to the right, increases contrast. Making the image lighter. You can change the contrast on any virtual slide, the demonstrator will often suggest if this is required for optimal viewing.
MagnificationUnder the contrast controls are 2 magnifying glass icons and a drop-down window with a number shown.
When you click the "+" (green plus) icon:
When you click on this window the menu opens and a range of magnification values are now available to be selected. The overall magnification range may vary for different slides but the maximum available for any slide 40x, some only reaching 20x. When a magnification is selected it will automatically zoom centred on the current view.
LocationThe location of your view can be changed in both the main and thumbnail windows. This control is not immediately visible and requires you to "click and drag" over the image. Main window - when you left click the mouse, a hand will appear and you can now drag the slide image to a different location. Note the righthand thumbnail yellow and black box will also change to match the viewed location. Thumbnail window - when you left click the mouse, a arrows appear over the yellow and black box and you can now drag the slide image to a different location.
Important noteThe demonstrator will be using the same virtual slides displayed on the class projectors and monitors. Follow the demonstrators descriptions closely (they know the material).
--Mark Hill 15:24, 21 February 2013 (EST) |
Computer RequirementsFlash Player will not function on any Apple iPad (iPad or mini-iPad). It can function on their laptop and desktop computers. Virtual slides requires a Flash Player (Adobe) plug-in to be installed in the browser application and will not function without this player. All practical class computers have this player installed. If you are using your own desktop or laptop computer this plug-in may need to be installed before virtual slides will display. Ensure that you are downloading and installing the latest player and only from the official Adobe website. |
Virtual Slides Computer Requirements
- Virtual slides requires a Flash Player (Adobe) plug-in to be installed in the browser application and will not function without this player.
- Flash Player will not function on any Apple iPad (iPad or mini-iPad). It can function on their laptop and desktop computers.
- Note - all online practical support pages will display on any computer, tablet (including iPad) and mobile phone platform capable of running a web browser.
- All practical class computers have this player installed.
- If you are using your own desktop or laptop computer this plug-in may need to be installed before virtual slides will display.
- Ensure that you are downloading and installing the latest player version and only from the official Adobe website (http://get.adobe.com/flashplayer).
- Note - you should also be using the latest available browser software.
- If you install this plug-in yourself you may need to shutdown and restart the browser application before it will function.
Electron Microscopy
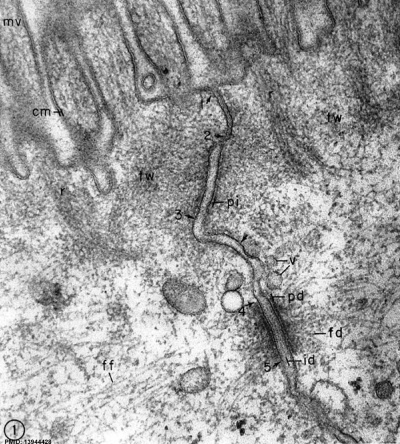
EM appearance and structure of: nucleus, nucleolus, nuclear envelope, nuclear pore, mitochondria, endoplasmic reticulum (smooth & rough), ribosomes, Golgi apparatus, lysosomes, microtubules, microfilaments, plasma (cell) membrane, microvilli, stereocilia, cilia, and junctional complexes.
(Stain - Osmium) used as the main staining technique for electron microscopy.
Cilia
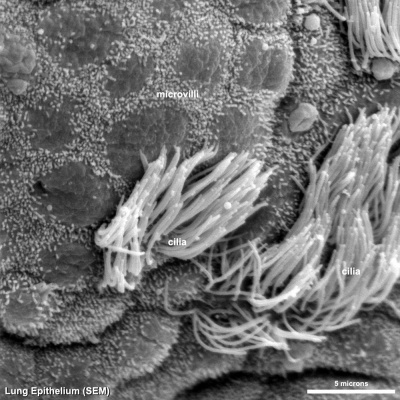
|
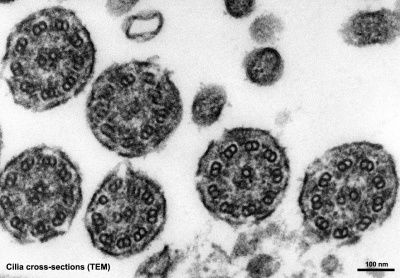
|
| Cilia and microvilli on respiratory epithelium. (scanning EM) | Cilia cross-sections showing microtubules. (EM) |
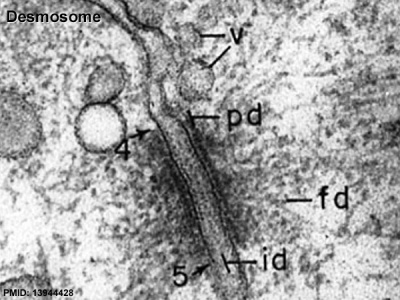
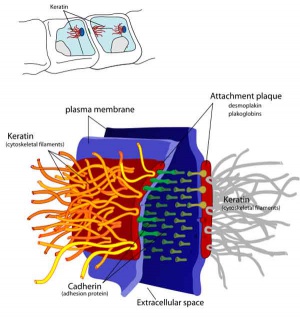
Junctional Complexes
Desmosome
Course Links
- Histology Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | ANAT2241 Support | Histology | Histology Stains | Embryology Glossary
| Common Histology Stains | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Practical Support
- Pages can be accessed from any internet connected computer.
ANAT2241 Support Links: The Virtual Microscope | Covering and Lining Epithelia | Glandular Epithelia | CT Components | CT Types | Bone, Bone Formation and Joints | Muscle | Nervous | Blood | Eye | Cardiovascular | Respiratory | Integumentary | Gastrointestinal | Gastrointestinal Organs | Lymphatic and Immune | Endocrine | Urinary | Female Reproductive | Male Reproductive | Histology Stains | Histology Drawings | Practicals Health and Safety 2013 | Moodle - 2019
ANAT2241 This practical support page content is not part of the science practical class and provides only background information for student self-directed learning purposes.
Cite this page: Hill, M.A. (2024, April 27) Embryology ANAT2241 The Virtual Microscope. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/ANAT2241_The_Virtual_Microscope
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G