2015 Group Project 2
| 2015 Student Projects | ||||
|---|---|---|---|---|
| 2015 Projects: Three Person Embryos | Ovarian Hyper-stimulation Syndrome | Polycystic Ovarian Syndrome | Male Infertility | Oncofertility | Preimplantation Genetic Diagnosis | Students | ||||
| 2015 Group Project Topic - Assisted Reproductive Technology | ||||
| This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Ovarian Hyper-stimulation Syndrome (OHSS)
Ovarian Hyper stimulation Syndrome (OHSS) is an iatrogenic complication of Assisted Reproduction Technology (ART), in which women take medications to stimulate oocyte growth. It is generally identified by cystic enlargements of the ovaries and fluid accumulation in the peritoneal cavity due to the increased capillary permeability and ovarian neoangiogenesis [1]. It is an occurrence which is dependent on the controlled stimulation of the ovaries in preparation for IVF i.e. administration of human Chorionic Gonadotropin (hCG).
OHSS was first described in 1943 as “syndrome d’hyperluteinisation massive des ovaries”. It was during this time that gonadotropins were prepared from animals such as sheep to bring on ovulation in women. The first recorded death as a result of OHSS occurred in 1951 and was due to renal failure as a result of oligouria, a complication of the syndrome [2] [3].
This wikipage aims to provide clear information on various areas surrounding OHSS such as diagnosis, prevention and complications and will look into the genetics behind the disorder and the various animals models used to research it.
<html5media height="350" width="450">https://www.youtube.com/watch?v=aXmjJw234a4</html5media>
Overview of Ovarian Hyperstimulation Syndrome [4]
Epidemiology
Ovarian Hyper-Stimulation Syndrome (OHSS) rarely occurs sporadically, and if it does, it is usually the result of an underlying genetic problem. The majority of OHSS is due to the ovaries being stimulated to mature and release an abundance of oocytes, in response to hormones Human Chorionic Gonadotropin (hCG) and Follicle Stimulating hormone (FSH), during IVF. Rarely, Clomifene Citrate therapy can cause OHSS.[5]
OHSS only affects 0.5-5% of women undergoing Ovarian Hyper-stimulation, but despite its small prevalence, it is a potentially fatal outcome of a non-vital procedure and remains a prevalent problem for fertility specialists [6] [3]. Age has been cited as a factor affecting those with OHSS, with younger women more at risk. Additionally, women with allergies were seen to have a higher incidence of OHSS. It is important to note that at this point in time, there is no positive correlation between gonadotropin dose and OHSS.
Of the 0.5-5% of women affected with OHSS mentioned above, 2% of those women will require hospitalisation. As of 2011, it has been reported that the incidence of OHSS was increasing, resulting in approximately 3 deaths per 100,000 women undergoing ovarian stimulation per year [7]. This is particularly worrying as in 2008, in the United States alone, there were around 150,000 IVF cycles undertaken.
|
With the field of Assisted Reproductive Technologies expanding and more women partaking in IVF treatments, the threat of women developing OHSS is an ongoing and increasing one [7]. |
| Characteristics of women with OHSS by complication group [8] | |||
|---|---|---|---|
| Characteristic | No Complications
(N = 212,041) |
Moderate OHSS
(N = 1,523) |
Severe OHSS
(N = 655) |
| Maternal Age (Mean ± SD) | 35.6 ± 4.6 | 33.0 ± 4.3 | 33.1 ± 4.4 |
| Maternal Age (%) | |||
| <30 years | 10 % | 21.1 % | 20.6 % |
| 30-34 years | 30.4 % | 43.2 % | 40.5 % |
| 35-39 years | 37.8 % | 27.2 % | 32.5 % |
| >40 years | 21.8 % | 8.4 % | 6.4 % |
| Nulligravida (%) | 45.8 % | 51.5 % | 51.8 % |
| Infertility Diagnosis (%) | |||
| Male factor | 37.7 % | 41.0 % | 33.4 % |
| Endometriosis | 13.5 % | 13.5 % | 10.7 % |
| Ovulation Disorders | 14.0 % | 29.5 % | 22.4 % |
| Diminished ovarian reserve | 16.9 % | 3.2 % | 2.4 % |
| Tubal factors | 20.4 % | 22.6 % | 22.3 % |
| Uterine factors | 5.1 % | 4.3 % | 3.1 % |
| Other factors | 13.9 % | 13.1 % | 10.4 % |
| Unexplained factor | 12.7 % | 11.8 % | 18.6 % |
Causative Agents
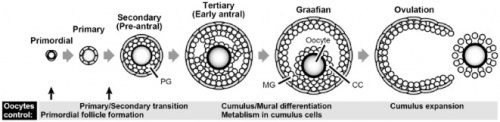
Normally a woman produces one egg per month from the ovaries, which travel down the Fallopian tube to be fertilized or be released from the body. In cases where women have difficulty falling pregnant, they are given medication which help them to produce and release eggs (as shown in the diagram to the right), further increasing their chances of fertilization and pregnancy [10]. Ovarian Hyper stimulation Syndrome generally occurs during infertility treatments when the ovaries are overstimulated by the fertility medication which causes the ovaries to swell and leak fluids into the belly and abdomen. The prevalence of OHSS onset is linked with excessive number of follicle development in response to the administration of injectable Follicle Stimulating Hormones (FSH) followed by the Human Chorionic Gonadotrophins (hCG) which triggers the release of the oocytes [11].
Follicle Stimulating Hormones (FSH) [12] are glycoprotein hormones that are produced and secreted from the Anterior Pituitary gland into the bloodstream, which regulate the developmental, growth, maturation and the reproductive processes within the body. In females, the Follicle Stimulating Hormones initiates the stages of growth and development of immature ovarian follicles within the ovaries before the release of an egg from a follicle at ovulation. When FSH is administered to a patient who is suffering from infertility, the increase FSH levels affect the rate of development and production, in turn increasing the amount of follicles which are ready to be released during ovulation.
Symptoms
OHSS is classified based on a criteria from mild, moderate and severe [5]:
Mild Symptoms- abdominal bloating, minimal weight gain, nausea, diarrhoea and a feeling of fullness.
Moderate Symptoms- Substantial weight gain (on average 2 or more pounds a day), increased abdominal girth, darkend urine and excessive thirst in addition to the mild symptoms.
Severe Symptoms- In addition to the symptoms associated with Mild and Moderate OHSS, in severe OHSS, you see shortness of breath, calf and chest pains and pleural effusion.
Classifications of Ovarian Hyper-stimulation Syndrome: [1]
| Classification | Symptoms |
|---|---|
| Mild | Grade 1 - Abdominal distention and discomfort
Grade 2 - Abdominal distention, discomfort with nausea, vomiting and/or diarrhea and ovarian enlargement from 5~12cm |
| Moderate | Grade 3 - All features of Mild OHSS with ultrasonographic evidence of ascites |
| Severe | Grade 4 - All features of Moderate OHSS with the addition of clinical evidence of ascites and breathing difficulties present
Grade 5 - All of the above symptoms along with a change in the blood volume, increased blood viscosity due to coagulation and diminished renal function |
Diagnosis
Whilst symptoms of OHSS can occur as soon as 24 hours post hCG administration, they are usually seen in women 7-10 days post administration. Initially women with OHSS will present with abdominal bloating, as a result of fluid in the peritoneal cavity and an increase in ovary size. When women present with severe OHSS they are often dehydrated, due to increased vascular permeability, and have hemoconcentration. The above results in a decrease in intravascular volume, leading to oligouria [13].
History
Initially a thorough history of the patient is taken during which the clinician looks for evidence of ovarian stimulation, followed by ovulation. During this history taking, the clinician will inquire into any weight gain noticed, urine output, and the woman's ability to maintain oral hydration. A key diagnostic tool for clinicians regarding women who are taking gonadotropins is to identify if they are at an increased risk of developing OHSS. Some risk factors include woman aged less than 30, women who have polycystic ovaries, woman with a previous history of OHSS and women who have had greater than 20 oocytes retrieved [13].
Physical Exam
After a history of the patient is taken, the next step is to perform a physical exam on the patient. Women who present with abdominal bloating will produce a shifting dullness upon abdominal percussion. Additionally the clinician will test the woman's vital signs, measure her abdominal girth, weight and will look for evidence of ascites or increase in calf size (usually unilateral)[13].
Investigations
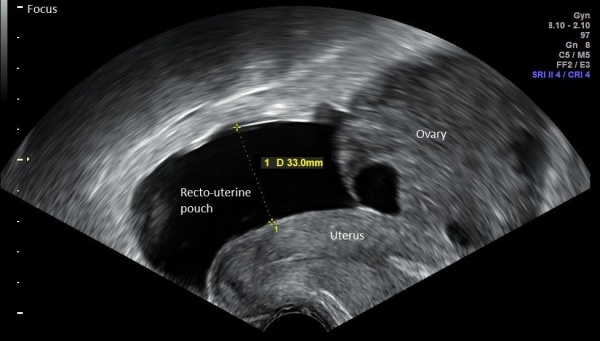
If the clinician further suspects a women of having OHSS, various investigations can be done such as an ultrasound.The intraperitoneal fluid is best imaged via vaginal ultrasound due to the enlarged ovaries making it difficult to image the pelvis using transabdominal ultrasound. The clinician can also order laboratory testing to look for urine specific gravity and and complete blood count to look for hemoconcentration with a hematocrit. Additionally, liver function tests can be ordered to look for elevated function. The clinician will also look for evidence of elevated D-dimers and fibrinogen and decreased levels of anti-thrombin 3 [15].
Ultrasound
Typical appearances of the ultrasound include [16]:
- Bilaterally and symmetrically enlarged ovaries (>12cm)
- "Spoke-Wheel appearance" - presence of multiple cysts of varying size
- May also see ascites (fluid)
Severity
Once a clinician has deduced a women is suffering from OHSS, the severity of their condition needs to be established as either mild, moderate or severe. This is done by referring to the criteria under the sub-heading Symptoms.The subsequent course of treatment for the woman will be based upon this evaluation.
|
Further investigations can be done including a Chest X-ray to check for pleural effusions and oedema and USS with Doppler's to check for ascites or possible ovary torsion. [13] |
Differential Diagnosis
Conditions presenting with similar symptoms or ultrasound images include [16]:
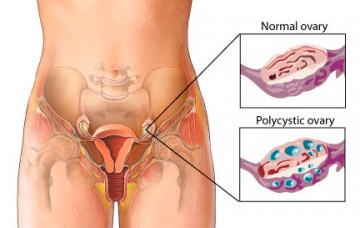
- Polycystic Ovaries (PO)
- The difference is that with PO is that the cycts are typically smaller than OHSS cysts and there is no evidence of ascited or pleural effusion
- Mucinous Ovarian Malignancy
- A type of Ovarian Epithelial tumour
- Ectopic Pregnancy [17]
- A pregnancy in which the foetus develops outside of the uterus e.g. in the fallopian tube
Pathophysiology
The definition of Ovarian Stimulation is enlarged ovaries with many luteinized cysts, that can present with secondary complications. What distinguishes Ovarian Stimulation from OHSS is the presence of vascular hyper-permeability that results in fluids being redirected elsewhere in the body.
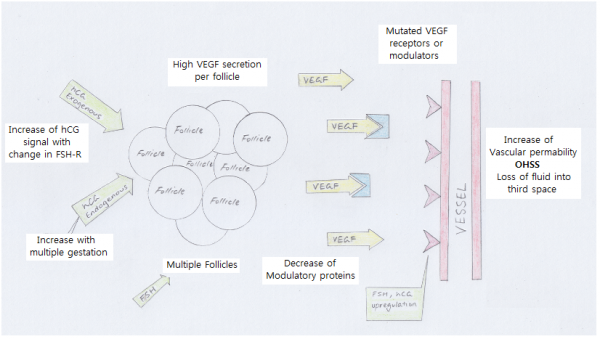
The key process to OHSS appears to be caused by Vascular Endothelial Growth Factor (VEGF), that is released along with other cytokines, estrogen and progesterone, due to the ovary undergoing luteinization as a result of stimulation by hCG. VEGF increases vascular permeability and as a result the capillaries become more "leaky" to the fluids in them. These fluids can then escape the capillaries and accumulate in the pleural and abdominal cavities as ascites. The woman then becomes hypovolemic and is at an increased risk of circulatory, renal and respiratory issues such as arterial thromboembolism due to the thickening of the blood. Note, the blood is thickened as fluid is leaving the capillaries, leaving behind red blood cells and other cellular components of the blood [5].
The increase in expression of VEGF and VEGF Receptor 2 (VEGFR2) is attributed to the greatly increased amount of their mRNA present in the body after stimulation with hCG [18]. VEGF interacts with VEGF2 and VEGF Receptor 1 (VEGFR1) to create a strong angiogenic effect. Both VEGFR2 and VEGFR1 belong to a family of receptors called tyrosine kinases. VEGF2 is involved in the regulation of angiogenesis and vascular permeability whilst VEGF1 has a slightly contradictory role in that is is involved in the maintenance of the tight junctions between endothelial cells in blood vessels. A study by Gómez et. al. [18] showed that women experiencing OHSS had substantially higher plasma levels of VEGF and lower levels of VEGFR1.
Complications
1-2% of women who undergo ovarian stimulation develop a severe form of OHSS . Complications from severe OHSS include:
- Fluid collection in the abdomen
- Electrolyte disturbances (sodium and potassium)
- Kidney failure
- Ovary twisting
- Rupture of a cyst in an ovary
- Breathing problems
- Blood clots in large vessels (most commonly the legs)
- Pregnancy loss from miscarriage or termination
- Rarely, death
A study by Nouri et. al. [20] found that women with Polycystic Ovarian Syndrome (PCOS) and those that were induced with hCG experienced longer recovery times from severe OHSS than women who were not pregnant. They looked at a cohort of women hospitalised for the first time with severe OHSS and subject them to the same treatments. Based on a defined criteria, they established the recovery time of these women and compared the times between those that were pregnant to those that were not pregnant. They also found that whether a woman had PCOS or ovulation induction did not affect her risk of developing OHSS if she was not pregnant. It is only a risk factor for pregnant women.
Thromboembolic events
A frequent and deadly complication of OHSS are thromboembolic events due to increased blood clotting. This increase in blood clotting has been attributed to a variety of complications such as hemoconcentration (which thickens the blood), hypovolemia and an increase in the permeability of blood vessels as a result of increased vasoactive substances in the body of ovarian origin. Thromboembolic events include venous thromboses often in the upper extremities and arterial thromboses such as those in the cerebrovascular region. These events can lead to amputations of extremities, brain damage, miscarriage and death. To avoid this, anti-coagulants are given to pateitns with OHSS and they are fitted with compression stockings (see Treatment) [21].
|
The risk of a woman developing OHSS is higher if she is having a twin pregnancy, as she is exposed to higher concentration of hCG. [22] |
Treatment
General treatment
- Acetaminophen with or without a narcotic agent- used to treat abdominal discomfort
- Women are encouraged to drink 2-3 litres of water a day to prevent hemoconcentration
- Women are advised to avoid sexual intercourse and vigorous exercise at the risk of torsion or rupture of the ovaries
- NSAID's with anti-platelet properties should NOT be used as they may effect renal function in a women with OHSS
- Patients with significantly painful ascites or breathing problems may undergo Paracentesis (drainage of the ascites)
- Culdocentesis (extraction of fluid from recto-uterine pouch) can be done to decrease the likelihood of a woman with moderate OHSS progressing to severe OHSS.[13]
Mild to Moderate OHSS
Mild OHSS can often resolve on its own, however, moderate OHSS may include treatments such as[5]:
- Anti-nausea medication and prescription painkillers
- Regular physical examinations and ultrasounds
- Daily weigh-ins and waist measurements
- Measuring the amount of urine produced each day
- Frequent blood tests for monitoring dehydration and electrolyte imbalance
- Maintaining a high balance of fluids
- Drainage of excess abdominal fluid by inserting a needle in the abdominal cavity
- Wearing support stockings which help prevent blood clots/thrombosis
Severe OHSS
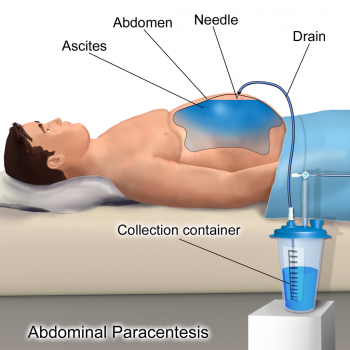
Severe OHSS requires hospital care in order for constant monitoring and treatment such as [5]:
- Intravenous fluids with a crystalloid solution (100-150mL/hr)
- Intravenous Albumin is administered is IV fluids are insufficient (15-20mL/hr of 25% albumin for 4 hrs)
- In addition to Acetaminophen, Opioid analgesics can be administered for pain relief
- Antiemetics can be used to subside nauseas and/or vomiting
- Woman administered to hospital of OHSS are considered at high risk of thromboembolic complications and are given low molecular weight heparin, an anti-coagulant
- Cabergoline- lessens OHSS symptoms
- GnRH agonist- suppresses ovarian activity
- If at risk of OHSS, alternates include using a GnRH agonist instead of hCG however its effects on pregnancy rates are questionable. Using a GnRH agonist to replace the use of hCG for final oocyte stimulation will see a 6% decrease in delivery rate.
- Daily monitoring of creatine, urea, creatine clearance C-reactive protein (to rule out infection) are all required in combination with weekly tests such as liver and renal function tests and chest x-rays (to check for pleural effusion) [3].
If there are serious complications then additional treatments are required:
- Surgery for a ruptured ovarian cyst
- Intensive care for the liver or lung complications
Paracentesis
For women with severe/grade 3 OHSS, paracentesis, the removal of fluid from the body using an aspiration needle, can be undertaken. It is a diagnostic and a therapeutic method that can be done trans-abdominally and trans-vaginally. It is used to relieve symptoms, improve haemodynamics e.g. Urinary output, and shorten the women's hospital stay. Complications of paracentesis include bleeding, infection and organ injury, however are note common [23].
|
The most important aspect of treatment for any women with OHSS is close and constant monitoring with her healthcare professional. Counselling may also be provided to women and their partners or families in order to provide them with the best possible knowledge base to manage and treat their OHSS. It is important to note that there is no one cure or treatment for OHSS. Treatment involves managing and eliminating the symptoms until such time as the syndrome resolves itself [5]. |
Prevention
There are three key avenues through which the incidence of OHSS may be prevented. These include identifying risk factors to predict OHSS development, modifying treatment regimes on the basis of the identified risk factors and intervention to prevent progression to OHSS once the patient has undergone Controlled Ovarian Stimulation (COS).
Risk factors
Primary risk factors
Pre-existing factors likely to exacerbate the ovarian stimulation response. Primary risk factors include [24]:
- Young age
- Low body weight
- History of elevated response to gonadotropins
- Polycystic Ovary Syndrome (PCOS)
- Isolated PCOS characteristic
- Previous history of OHSS.
- High pretreatment basal Anti-Mullerian Hormone (AMH) concentration
- Large Antral Follicle Count (AFC)
Secondary risk factors
Secondary risk factors involve the monitoring of ovarian response parameters once COS has been initiated. These parameters are monitored for [25]:
- rapidly rising E2 levels
- E2 concentration larger than 2500 pg/mL
- large number of developing follicles (10-14mm) on the day hCG is administered
- large number of oocytes retrieved
These factors in combination, act as a predictive tool to assess the likelihood of severe OHSS development, with a 83% sensitivity and 84% specificity. [25]
Primary Prevention
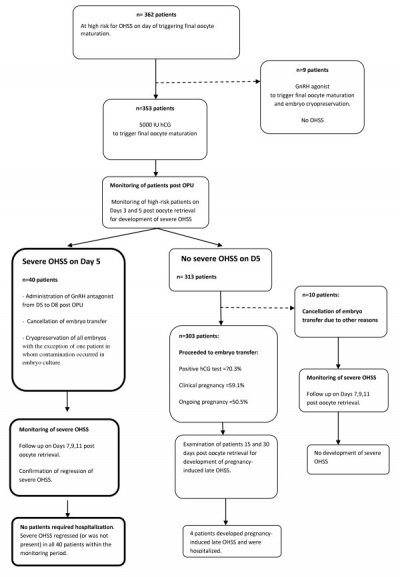
Primary Prevention involves the modification of treatment regimens on the basis of OHSS risk classifications, in order to prevent OHSS occurrence.
Ovulation Induction
Unifollicular Ovulation Induction through Ovulation Induction (OI) is a primary means of avoiding OHSS in women with Polycystic Ovarian Syndrome, who are at an increased risk. In order to promote unifollicular development, the ovaries are stimulated with a low starting dose of FSH (75 IU).[19] Studies suggests that a minimum gonadotropin dose lowers OHSS risk and thus a step-up regimen is utilised to achieve an ovarian response, whereby the FSH dosage is increased every 7 days until follicular development of greater than 10mm is noted.[24] Alternatively, a step-down regimen may be followed, whereby a high initial FSH dosage is lowered according to the ovarian response. Another method to increase FSH levels during OI, is through Aromatase Inhibitors which promote folliculogenesis by increasing pituitary secretion of FSH, and downregulate oestrogen production through a negative feedback loop. However, Aromatase Inhibitors have not been shown to reduce OHSS incidence in comparison to other methods of OI.[24] During OI, a key objective is to prevent early cycle cancellation due to premature luteinisation from pituitary secretion of LH. In order to downregulate LH secretion, Gonadotropin-releasing hormone agonists (GnRHa) are administered in addition to gonadotropins. Alternatively, GnRH antagonists, which have a rapid onset of action, may be administered to downregulate endogenous LH secretion, with relative success as depicted in the diagram to the right.[19] Comparative studies have shown that the GnRH antagonist protocol has a greater effect in lowering the incidence of mild to severe OHSS. However due to the rare nature of OHSS, and insufficient sample sizes, the difference was not found to be significant. [27]. Generally, the duration of exposure to gonadotropins and subsequent risk of OHSS may be minimised through mild stimulation protocols which administer FSH only in the mid to late follicular phase.[19]
Adjuvant Metformin Therapy
Adjuvant Metformin Therapy has been found to lower the risk of OHSS by 63%.[28] It involves the administration of metformin at a daily dosage of 1000 to 2000mg, 2 months prior to Controlled Ovarian Stimulation [24]. Metformin lowers the elevated insulin levels in PCOS which consequently reduce intraovarian androgen levels. This leads to a reduced sensitivity and expression of granulosa cell-follicle stimulating hormone receptors which result in a less exaggerated response to gonadotropins. Furthermore, it is suggested that Metformin prevents OHSS by controlling vascular permeability through the inhibition of various vasoactive molecules, including VEGF.
Avoiding hCG during Luteal Phase Support
Following Controlled Ovarian Stimulation, the steroid levels of E2 and P4 are reduced during the luteal phase due to the negative feedback on the pituitary. This leads to low endogenous LH levels, which consequently reduce endometrial receptivity as well as the luteal phase duration itself. As a result, implantation and pregnancy rates are reduced and early pregnancy loss rates are increased.[19] Luteal Phase Support (LPS) serves to combat these adverse events with the use of hCG. However, hCG has been found to increase the risk of OHSS. Alternatively, the use of progesterone has been found to not only halve the risk of OHSS, but also induce similar improvements in pregnancy and miscarriage rates as hCG.[29]
Secondary Prevention
Secondary Prevention aims to prevent progression to OHSS once COS has been initiated and the patient has been found to mount an exaggerated response. [19]
Coasting
Coasting is a first-line secondary preventative strategy. It consists of the withdrawal of gonadotrophins when a critical number of follicles and/or E2 concentration is reached. hCG is administered once the E2 concentration reduces to a safe level, before the process of oocyte retrieval commences. This preventative strategy is conducted for a period of less than 3 days in order to avoid compromising IVF outcomes.[24]
Cryopreservation
Cryopreservation of embryos after oocyte retrieval is another avenue which may avert OHSS progression. The cryopreserved embryos are only reimplanted once the patient's hormone serum levels have normalised. Using oocyte vitrification, crystal formation within embryo tissue is avoided and so cryopreserved embryos have been found to produce better pregnancy rates with a 32% increase, than fresh embryo transfer.[24] Recent studies suggest cryopreservation itself does not reduce OHSS rates, but must be followed by a GnRHa trigger to avert OHSS.[31]
Cycle cancellation
Cycle cancellation is a guaranteed method to prevent early OHSS whereby hCG is withheld. This is a last resort strategy as it carries the risk of significant psychological distress and financial loss for the patient. [19]
Effect on the Newborn
Women who develop OHSS while undergoing Assisted Reproductive Technologies (ART) treatment are not only more likely to achieve a pregnancy, but have a live birth as the pregnancy outcome. This live birth is also more likely to be a multiple birth of two, three or more children. [8] It has been postulated that a multi-fetal pregnancy leads to a more rapid increase in hCG levels, resulting in an increased risk of OHSS. However, the causal nature between multi-fetal pregnancy and OHSS development is disputed and further research is required to distinguish correlation from causation. [32]
OHSS development is also associated with an increased risk of adverse outcomes including stillbirth, premature birth and low birth weight.[8] Although the cause for the increased risk in adverse pregnancy outcomes is unknown, it is suggested that as the incidence of OHSS is reduced through various prevention strategies, the risk of such outcomes may be also be reduced.
The following link is to a podcast of a woman describing her pregnancy in which she underwent IVF, had OHSS and gave birth to healthy twins[33]
Genetics
Despite OHSS typically being of iatrogenic origin as a result of ovarian stimulation with gonadotropins, research has been conducted into the potential genetics behind OHSS as many sporadic and familial cases have been observed. Mutations in the receptor for hormones such as Follicle Simulating Hormone (FSH), Lutenizing Hormone (LH) and hCG have all been targets of OHSS genetics research. Interestingly, they too also arise from a common ancestral gene.
Follicle Stimulating Hormome
Dr. Botros Rizk, of the University of South Alabama College of Medicine [34], has written extensively regarding the genetics behind OHSS. In particular, he talks about FSH receptors (FSHR) and their role in the syndrome. He hypothesises that mutations in these receptors could be activating or inactivating, leading to an increased risk of developing OHSS or sterility respectively. Currently, 744 single nucleotide polymorphisms (a type of mutation) have been found in the gene encoding the FSHR, 8 of which are located in its exons (coding regions of the gene). How the ovary responds is resultant on the FSHR genotype. For example, Ser680Asn, a polymorphism in the FSHR gene has been shown to aid in predicting the severity of a woman's OHSS. Ordinarily FSH stimulates the growth of ovarian follicles, however, when mutated, it is stimulated by the hCG resulting in excessive follicle development.
Spontaneous OHSS, OHSS that arises and cannot be attributed to any form of ovarian stimulation or Assisted Reproductive Technology, has been linked to activating mutations in the FSHR [35]. This familial disorder is an autosomal dominant one. In most cases the FSHR can be stimulated by the presence of Thyroid Stimulating Hormone (TSH) or hCG, in the absence of FSH (the ligand for FSHR).
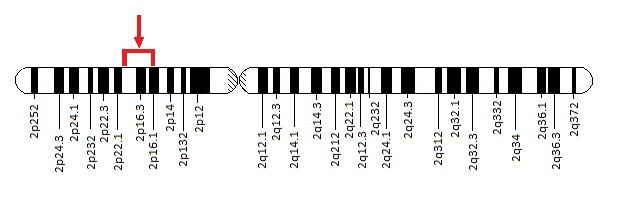
Lutenizing Hormome
The Lutenizing Hormone Receptor (LHR) gene in humans is comprised of 11 exons. Animal studies have shown that many primates exhibit a similar gene which is comprised of only 10 exons, however a gene lacking the 10th exon has been identified and termed the type 2 LHR. Expression of the type 2 LHR has been seen in humans. The type 2 LHR, compared to the wild-type LHR appears to be repaired with regard to its function. Defects in the type 2 LHR include a decrease in efficiency of transport to the plasma membrane and irregularities in signal transduction. Inactivating mutations in the LHR have been seen to cause infertility in women as well as amenorrhoea. Activating LHR gene mutations are asymptomatic and are not associated with OHSS in women.
Bone Morphogenic Protein
An imporant growth factor, derived from oocytes called BMP-15 (Bone Morphogenic Protein 15) is vital for female fertility. It belongs to the family of growth factors, Transforming Growth Factor β (TGF-β) and is heavily involved in folliculogenesis. It has been found that mutations in the BMP-15 gene caused infertility in female sheep. Conversely, it has also been indicated in enhanced fertility when BMP-15 is present is high amounts in follicular fluid [34].
A case study done by Hanevik et. al. in 2013 [37] found that a Single Nuclear Polymorphism (SNP) in the BMP-15 gene is responsible for a high response to ovarian hyper-stimulation. The aim of the study was to test the effect the SNP had on BMP-15 with regard to high and low responders buy taking blood from 53 high responders, 38 low responders and 100 non-responders (controls) and analysing 5 noted SNP's. Results from their study showed a correlation between a high response to ovarian hyper-stimulation and the BMP-15 9G allele. The article does state however, that further research is required into the molecular effects of SNP's on the function of BMP-15.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor (VEGF) has been indicated as one of the key agents causing vascular permeability and subsequent ascites in OHSS. The gene, which is located on chromosome 12, is made up of 8 exons (coding regions), of which exons 6 and 7 do not always appear due to the exons being spliced out.The splicing of this gene allows for various isoforms of the gene to exist of which VEGF 121 and 165 appear to play a role in angiogenesis. There are two VEGF Receptors, VEGFR-1 and VEGFR-2 that belong to the tyrosine kinase family of receptors (for more information on VEGF see Pathophysiology). Because of its distinct role in OHSS, it has been targeted as an area for research for potential treatments. The idea is that if the genetic expression of VEGF and its receptors can be controlled, OHSS can be avoided or treated.[34]
A different study by Hanevik et. al. , [38] done in 2012 involved analysing blood samples from 53 women with OHSS and 100 women without it (controls) and analysing 6 SNP's in the VEGFR2 gene to find any genetic variations. They found a correlation between women with the VEGF +405cc genotype and the development of OHSS indicating women that undergo controlled ovarian hyper-stimulation and posses his genotype, are at an increased risk of developing OHSS.
Current Research on Animal Models
| Inhibition of Cyclooxygenase-2 (COX-2) by Meloxican decreasing the incidence of OHSS in Rat Model [39]
Reasearch was carried out to investigate the effects of selective inhibition of the enzyme COX-2 on the Ovarian Hyperstimulation Syndrome (OHSS) using Female Wistar rates as the subjects. The research aimed to find results by measuring the number of antral and luteinized follicles, ovarian weight, vascualr endothelial growth factors and COX-2 immunohistochemistry. The rats being tested were all 22 days old and were divided into four equal groups; Group 1 (Control group) was subject to a 0.1 ml of Intraperitoneal Saline from days 22 ~ 26 Group 2 (Mildly-stimulated group) subject to 10IU of pregnant mare serum gonadotrophin (PMSG) on day 24 and then 10IU of Human Chorionic Gonadotrophin (hCG) on day 26 Group 3 (OHSS positive group) was subject to 10IU of PMSG from days 22 ~ 26 and then administered 30IU of hCG on day 26 to induce OHSS Group 4 (OHSS positive variant group) received 15mg/ml of Meloxicam 2 hours prior to administration of 10IU PMSG from days 22 ~ 26 Results showed there was no difference in ovarian weight in samples from Group 1 and Group 2, however Group 3 showed signs of significant ovarian weight increase which in group 4 was suppressed by the introduction of Meloxicam. No differences were observed in the number of antral follicles amongst the four test groups. Results from Group 2 and Group 3 showed that the granulosa cells of preovulatory follicles and the stromal cells were highly VEGF immunoreactive, however the Meloxicam treated Group 4 showed less immunoreactivity than Group 2 and Group 3 which indicated the correlation between Meloxicam and the diminished VEGF expression. Group 3 presented an increased COS-2 immunoreactivity which was highly diminished than in Group 4. The research concluded that in a rat model, the enzyme Meloxicam has a beneficial effect on OHSS by reducing the increase of ovarian weight and the expression of VEGF associated with OHSS, the effects of which may be mediated by the inhibitory capacity of COX-2 on Meloxicam |
| Inhibition of Ovarian VEGF secretion by activation of Dopamine Receptor 2 [40]
This research was carried out to investigate a possibility in whether a Dopamine Receptor 2 agonist (D2-ag) can assist in the prevention of Ovarian Hyperstimulation Syndrome, using rat models, by decreasing the ovarian vascular endothelial growth factor (VEGF) production. Using Immature Wistar rats (22 days old) as their animal model, the rats were initially stimulated with Gonadotrophins to mimic the onset and effects of OHSS and then subjected to treatment with a D2-agonist and/or a D2-antagonist (D2-ant). The vascular permeability was measured at the endpoint after day 26 by measuring the peritoneal extravasion of a previously injected dye and ovaries from all subjects were collected to assess the effects of D2-ag and D2-ant on the production of Ovarian VEGF. The expression of VEGF mRNA was measured by quantitative real time PCR and the levels of VEGF proteins were measured by Western Blots. Results showed that the D2-ag caused a large reduction in the vascular permeability which was in turn associated with the great decreased in VEGF protein production in the OHSS rat ovaries, whereas the introduction of D2-ant showed opposite results with a increase in the vascular permeability leading to the increase of VEGF protein production in the ovaries. Ovarian VEGF mRNA levels were found to be unaffected by the introduction of these drugs in OHSS rat subjects. Conclusions were drawn on the fact that Dopamine Receptor 2 agonists prevent the increase of vascular permeability in subjects with OHSS by decreasing the ovarian production of VEGF and also that due to the dose-dependent inhibitory effect of the D2-ag on ovarian VEGF, current OHSS therapies used in humans can benefit by increasing the intraovarian concentration of D2-ag. |
Glossary
Aromatase Inhibitor - A drug that inhibits enzyme aromatase, which in turn suppresses estrogen synthesis.
Ascites - An accumulation of fluid in the peritoneal cavity with resultant abdominal swelling.
Cabergoline - A dopamine receptor agonist used to treat hormone imbalance.
Controlled Ovarian Stimulation - female infertility treatment using medications to stimulate the ovaries to develop follicles.
Cryopreservation - Cooling and storing biological material at extremely low temperatures.
E2 - Estradiol - Potent estrogen sex hormone.
FSH - Follicle Stimulating Hormone
Gonadotropin - Any hormone that has a stimulating effect on the gonads.
GnRH agonist - Gonadotropin Releasing Hormone - A class of compounds that mimic the effect of the natural Gonadotropin Releasing Hormone.
GnRH antagonist - Gonadotropin Releasing Hormone - A class of compounds that are similar in terms of the structure of natural Gonadotropin Releasing Hormone but has a antagonistic effect.
hCG- Human Chorionic Gonadotropin
Hemoconcentration - An increase in the concentration of circulating red blood cells in response to a decrease in blood plasma volume.
Hypovolemic - A decrease in circulating blood volume.
Iatrogenic - Illness caused as a result of a medical examination or treatment.
Intravenous fluids - Is the infusion of liquid substances directly into a vein.
IVF - In-vitro Fertilization
LH - Luteinizing Hormone
Metformin - A biguanide, antidiabetic drug used as an adjuvant to modify the effect of other agents.
NSAID - Non-steroidal Anti-Inflammatory Drug e.g. Ibuprofen
Oocyte vitrification - technique for cryopreservation of oocytes.
OHSS - Ovarian Hyper-stimulation Syndrome
Oliguria- Decreased urine output/small amounts of urine produced.
P4 - Progesterone - endogenous progestogen sex hormone.
PCOS - Polycystic Ovarian Syndrome. An endrocrine system disorder whereby the ovaries are enlarged with small collections of fluid.
References
- ↑ 1.0 1.1 <pubmed>22065820</pubmed>
- ↑ Marie M. Budev, DO, MPH; Alejandro C. Arroliga, MD; Tommaso Falcone, MD, [ http://utilis.net/Morning%20Topics/REI/Ovarian%20Hyperstimulation.pdf ], 'Ovarian Hyperstimulation Syndrome'
- ↑ 3.0 3.1 3.2 <pubmed>12638783</pubmed>
- ↑ Howcast (2013, August 27) Ovarian Hyperstimulation Syndrome | Infertility. Retrieved from https://www.youtube.com/watch?v=aXmjJw234a4
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Wikipedia, [ https://en.wikipedia.org/wiki/Ovarian_hyperstimulation_syndrome ], 'Ovarian Hyperstimulation Syndrome'
- ↑ <pubmed>12498425</pubmed>
- ↑ 7.0 7.1 <pubmed>21828116</pubmed>
- ↑ 8.0 8.1 8.2 <pubmed>19591989</pubmed>
- ↑ <pubmed>24717179</pubmed>
- ↑ <pubmed>19573285</pubmed>
- ↑ <pubmed>26190539</pubmed>
- ↑ <pubmed>9020850</pubmed>
- ↑ 13.0 13.1 13.2 13.3 13.4 <pubmed>22416285</pubmed>
- ↑ 14.0 14.1 Häggström, Mikael. "Medical gallery of Mikael Häggström 2014". Wikiversity Journal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 20018762 Cite error: Invalid
<ref>tag; name 'Wikiversity Journal of Medicine' defined multiple times with different content - ↑ Government of South Australia Health [ http://www.sahealth.sa.gov.au/wps/wcm/connect/9b61ed004ee5348da663afd150ce4f37/Ovarian-hyperstimulation-syndrome-WCHN-PPG-17072012.pdf?MOD=AJPERES&CACHEID=9b61ed004ee5348da663afd150ce4f37 ], 'South Australian Paediatric Clinical Guidelines OHSS'
- ↑ 16.0 16.1 Radiopaedia [ http://radiopaedia.org/articles/ovarian-hyperstimulation-syndrome-1 ], 'Ovarian Hyperstimulation Syndrome'
- ↑ Australian Medical Student Journal [ http://www.amsj.org/wpcontent/uploads/files/articles/amsj_v2_i1/AMSJ_v2_i1_pg58-60.pdf ], 'Ovarian hyperstimulation syndrome'
- ↑ 18.0 18.1 <pubmed>21082502</pubmed>
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 <pubmed>20416867</pubmed>
- ↑ <pubmed>24996451</pubmed>
- ↑ <pubmed>23378404</pubmed>
- ↑ <pubmed>9756273</pubmed>
- ↑ S. Monica Soni, HMS 3, Gillian Lieberman, MD [ http://eradiology.bidmc.harvard.edu/LearningLab/genito/Soni.pdf ], 'Ovarian Hyperstimulation Syndrome'
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 <pubmed>26074966</pubmed>
- ↑ 25.0 25.1 <pubmed>19007627</pubmed>
- ↑ <pubmed>22938051</pubmed>
- ↑ <pubmed>21082508</pubmed>
- ↑ <pubmed>25406011</pubmed>
- ↑ <pubmed>26148507</pubmed>
- ↑ <pubmed>26309801</pubmed>
- ↑ <pubmed>24753847>
- ↑ <pubmed>19573292</pubmed>
- ↑ Pregnancy Perfect[ http://pregnancyperfect.com/emilyley/ ], 'TWINS, IVF, OHSS AND C-SECTION WITH EMILY LEY'
- ↑ 34.0 34.1 34.2 <pubmed>19573286</pubmed>
- ↑ <pubmed>23499866</pubmed>
- ↑ Genetics Home Reference [http://ghr.nlm.nih.gov/gene/FSHR'
- ↑ <pubmed>21565556</pubmed>
- ↑ <pubmed>22587628</pubmed>
- ↑ <pubmed>18166186</pubmed>
- ↑ <pubmed>25217874</pubmed>
- 2015 Course: Week 2 Lecture 1 Lecture 2 Lab 1 | Week 3 Lecture 3 Lecture 4 Lab 2 | Week 4 Lecture 5 Lecture 6 Lab 3 | Week 5 Lecture 7 Lecture 8 Lab 4 | Week 6 Lecture 9 Lecture 10 Lab 5 | Week 7 Lecture 11 Lecture 12 Lab 6 | Week 8 Lecture 13 Lecture 14 Lab 7 | Week 9 Lecture 15 Lecture 16 Lab 8 | Week 10 Lecture 17 Lecture 18 Lab 9 | Week 11 Lecture 19 Lecture 20 Lab 10 | Week 12 Lecture 21 Lecture 22 Lab 11 | Week 13 Lecture 23 Lecture 24 Lab 12 | 2015 Projects: Three Person Embryos | Ovarian Hyper-stimulation Syndrome | Polycystic Ovarian Syndrome | Male Infertility | Oncofertility | Preimplantation Genetic Diagnosis | Students | Student Designed Quiz Questions | Moodle page
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 28) Embryology 2015 Group Project 2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/2015_Group_Project_2
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G