User:Z3418989
Welcome to the 2014 Embryology Course!
- Links: Timetable | How to work online | One page Wiki Reference Card | Moodle
- Each week the individual assessment questions will be displayed in the practical class pages and also added here.
- Copy the assessment items to your own page and provide your answer.
- Note - Some guest assessments may require completion of a worksheet that will be handed in in class with your student name and ID.
| Individual Lab Assessment |
|---|
|
| Lab 12 - Stem Cell Presentation Assessment | More Info | |
|---|---|---|
| Group | Comment | Mark (10) |
| 1/8 |
|
7 |
| 2 |
|
7.5 |
| 3 |
|
7.5 |
| 4 |
|
8.5 |
| 5 |
|
8.5 |
| 6 |
|
8.5 |
| 7 |
|
7.5 |
Lab Attendance
Lab 1 --Mark Hill (talk) 12:50, 6 August 2014 (EST)
reference 1
<pubmed>25084016</pubmed>
reference 2
<pubmed>25101180</pubmed>
reference 3
<pubmed>25100708</pubmed>
Lab 2 --Z3418989 (talk) 11:22, 13 August 2014 (EST)
Lab 3 --Z3418989 (talk) 11:40, 20 August 2014 (EST)
Lab 4--Z3418989 (talk) 11:51, 27 August 2014 (EST)
Lab 5----Z3418989 (talk) 11:47, 3 September 2014 (EST)
Lab 6--Z3418989 (talk) 12:03, 10 September 2014 (EST)
Lab 7--Z3418989 (talk) 11:23, 17 September 2014 (EST)
Lab 8--Z3418989 (talk) 11:36, 24 September 2014 (EST)
===Lab 9--Z3418989 (talk) 12:18, 8 October 2014 (EST)
Lab Assessment 1
Research article 1
Summary
IVF children have been noticed to have cardiovascular problems and remodeling. However not much is known of how IVF treatment could cause these cardiovascular problems and this is the main concern of this article. The scientists’ previous studies have led them to discover that ART like IVF may cause differentially expressed proteins (DEPs) in the IVF placenta.
The umbilical veins and cord blood from 45 IVD and 48 naturally conceived (CV) newborns was collected and tissue samples were collected and then put to undergo in vitro fertilization under various conditions. E_2 cord blood levels was also examined. Using a randomizing program, 3 IVF and 3 NC umbilical veins were selected for proteomic analysis by the iTRAQ, a proteomic analysis technology. First the 6 umbilical vein sample proteins were extracted, separated with chromatography and identified using different methods like a mass spectrometer and the MASCOT search engine (Gao et al, 2014).
Also the E_2 in human umbilical vein endothelial cells (HUVECs) and cord blood was measured. For further validation of proteomic results PCR and Western blotting analysis was conducted on 11 and 4 umbilical veins respectively (Gao et al, 2014).
Results showed 47 DEPs (20 up-regulated; 27 down-regulated) were found in IVF newborns in comparison to NC newborns. Q-PCR and Western blotting done showed validated results as proteins lumican, nestin and PTGDS were up-regulated and vimentin was down regulated as observed with proteomic results. The bioinformatics analysis conducted showed that umbilical vein DEPs had a connection with development of many systems including cardiovascular system development and carbon metabolism (Gao et al, 2014). This study indicates there is different in expression of proteins in IVF-newborns compared to NC newborns and that DEPs might correlate with IVF-related cardiovascular issues (Gao et al, 2014).
Research article 2
Summary
This experiment aims to investigates, in rams, the effects of fish oil on level of reactive oxygen species (ROS), spermatozoa death incidence and in vitro fertilization (IVF) (Behzad, 2014).
9 Rams were randomly selected and split into control (5) and fish oil (4) groups. A diet was administered of essentially 0% fish oil to the control groups and 2.5% to the fish oil group and other things such as equal amounts of vitamin E. After 21 days semen from both groups was collated via an artificial vagina. Semen continued to be collected weekly following this. This continued on for 70 days during breeding season. (Behzad, 2014). Every week after the initial 21 days ROS level and spermatozoa death incidence was measured via flow cytometry. However during only the first (day 21) and last (day 70) weeks of sperm collection sperm was analyzed using a sperm analyzer program called CASA and swimming up technique was used to prepare sperm for IVF. (Behzad, 2014).
The results showed a greater volume, concentration and sperm motility in fish oil groups. They found higher fertilization rates in fish oil groups; 56% compared to 49%. In third week of samples O_2 and spermatozoa death incidence was lower in fish oil groups (Behzad, 2014).
Therefore this articulate argues that dietary omega-3 which is found in fish oil could be used to increase fertilization rates in vitro fertilization.
--Mark Hill These are both good articles and summaries (5/5)
Lab Assessment 2
Promising System for Selecting Healthy In Vitro Fertilized Embryos in Cattle[1]
--Mark Hill The file and associated reference and copyright information is correct. My only comment would be to reformat the image to a smaller size, this is a large image file (1 Mb) . (5/5)
Lab Assessment 3
Anatomy and variations of palmaris longus in fetuses.[1]
Development of the rectus abdominis and its sheath in the human fetus.[2]
Sonic hedgehog acts cell-autonomously on muscle precursor cells to generate limb muscle diversity.[3]
The normal growth of the biceps brachii muscle in human fetuses.[4]
--Mark Hill These are useful references, you have included the reference title as a single sentence, you should have included why you had selected these references for your section. (4/5)
Lab Assessment 4
cord stem cell therapy
Induction of Highly Functional Hepatocytes from Human Umbilical Cord Mesenchymal Stem Cells by HNF4a Transduction
In this article they describe how human umbilical mesenchymal stem cells were turned into hepatocyte/liver like cells. Using plasmid transfection of these cells Hepatocyte nuclear factor 4 alpha (HNFα) was overexpressed. HNFα is known to be crucial in liver development and hepatic differentiation. Then the expression of different proteins and genes were observed by Western blotting and RT-PCR methods. What they found was that the liver like cells with overexpressed HNFα caused hepatic specific proteins, genes, liver enriched transcription facors and the Wnt/β-Catenin pathway (Hang et al, 2014); all of which play a role in hepatic development. Hepatic specific proteins like ALB and AFB were observed to be expressed. Liver enriched transcription factors control expression genes involved in liver function. After HNFα transfection they found that HNF6, CEBP/ α and HNF3 β were overexpressed. Hang et al (2014) postulated that HNFα might enhance hepatic differentiation via liver enriched factors. And as expected during hepatic differentiation Hang et al observed the Wnt/β-Catenin pathway to be inactivated by HNFα administration. The results show that human umbilical mesenchymal stem cells can be used to form hepatic like cells and HNFα further to activate important genes for hepatic differentiation. These findings provide the experimental basis for clinical procedures like liver generation after a hepatectomy and liver transplantation.
Induction of Highly Functional Hepatocytes from Human Umbilical Cord Mesenchymal Stem Cells by HNF4α Transduction.[1]
Human umbilical cord mesenchymal stromal cells suppress MHC class II expression on rat vascular endothelium and prolong survival time of cardiac allograft
Umbilical cord mesechymal stem cells (UC-MSCs) are known to have immunomodulatory effects and this is the basis of this investigation. UC-MSCs were taken from human umbilical cords. MHC class II transactivator gene construct (CIITA) was introduced to form a transgenic rat line. After the addition of MHC class II to the vascular endothelium, they were analyzed by immunological staining. UC-MSCs were introduced to one group of transgenic rats. Survival time of the cardiac allograft of the transgenic groups with the UC-MSCs were compared with the group without the UC-MSCs. What they found was that with repeated infusion of UC-MSCs that the cardiac allograft survival time increased. Ying et al (2014) go onto describe that basically UC-MSCs reduced MHC class II expression on vascular endothelium of transplanted hearts and increased regulatory anti-inflammatory cytokines like IL10, transforming growth factor (TGF)-β1 and suppressed proinflammatory cytokines like IL2, IFN-γ. This had the combined effect of increasing the survival time of the rat cardiac allograft. Why this is therapeutically relevant is that it shows great potential of use of umbilical cord mesenchymal stem cells for organ transplantation potential (Ying et al, 2014).
Human umbilical cord mesenchymal stromal cells suppress MHC class II expression on rat vascular endothelium and prolong survival time of cardiac allograft.[1]
Vascular "shunts" in embryo
There are three major shunts; foramen ovale, ductus arteriosus and ductus venosus; two shunts direct pulmonary blood to systemic circulation and the third connects the vena cava and umbilical vein. These shunts close following birth and third shunt becomes non-functional once the umbilical cord is cut. Foramen Ovale is an interatrial septum opening. After birth with the closing of this shunt the fossa ovalis remains in its remnant place. Foramen Ovale is between the right and left atrium and allows between them and has a valve to prevent backflow (during fetal period). Ductus arteriosus helps in supporting the fetal lung and is a small muscular vessel joining the pulmonary trunk and aorta which allows blood from pulmonary trunk to go to the aorta. Pressure drop in lungs after first birth causes smooth muscle in ductus arteriosus to constrict and eventually degenerate. What is left is connective tissue remnant known as ligamentum arteriosum. Ductus venosus transports oxygenated blood from the placenta to the fetus’ heart. The degenerated remnant of ductus venosus is known as ligamentum venosum.
Lab Assessment 5
Meckel's Diverticulum
Meckel’s diverticulum Meckel’s diverticulum is caused by the vitelline duct not being destroyed, resulting in a blind pouch of the intestine [1]. This pouch is in the lower part of the small intestine, the ileum, and presents itself at birth. [2] The vitelline duct transports nutrients from the yolk sac to the fetus. Normally around week 5 to 7 of embryonic development the vitelline duct begins to narrow and eventually gets obliterated. When the vitelline duct doesn’t get obliterated a number of conditions may arise, however 97% of times it is a Meckel’s diverticulum [3] and it occurs in approximately 2% of the population [4]. In fact Meckel’s diverticulum is the most common abnormality of gastrointestinal tract development. Meckel’s diverticulum contains all layers of the small intestine and is on the anti-mesenteric border of the ileum; the side of the small intestine where the vitelline sac/yolk used to be attached and the border opposite to where the blood and nerve supply is provided [5]. Meckel’s diverticulum has its own unique blood supply from the vitelline artery.
Meckel’s diverticulum is normally asymptomatic but if there are symptoms most of the time it presents itself as the presence of stomach or pancreas tissue at the edge of the diverticulum. Meckel’s diverticulum can cause bleeding, inflammation, rupture or blockage. It can even cause intussusception which is the movement of the upstream intestine in the downstream intestine.[6] This may clinically present itself as intestinal bleeding which results in bloody stools which is present more in children younger than 5. Intestinal blockage may occur in early months of life. Inflammation which can occur presents itself similarly to appendicitis and is treated by surgical removal. [7]
References
- ↑ <pubmed>15729078</pubmed>
- ↑ http://www.nlm.nih.gov/medlineplus/ency/article/000234.htm
- ↑ http://emedicine.medscape.com/article/931229-overview#showall
- ↑ <pubmed>4755212</pubmed>
- ↑ <pubmed>25006469</pubmed>
- ↑ http://www.eapsa.org/Meckel_s_Diverticulum/4294.htm
- ↑ http://www.eapsa.org/Meckel_s_Diverticulum/4294.htm
Lab Assessment 7
Research Article
Tbx1 is a gene that is important in pharyngeal apparatus development and is required for derivatives such as the thyroid. Tbx1 acts on mesoderm which lies next to the thyroid and this in turn controls thyroid size and early primordium. Tbx1 also known to regulate Fgf8 in the mesoderm. The article thus describes the investigation of the possibility that Fgf8 is a mediator of Tbx1 mediated reactions between mesoderm and the thyroid and that the Tbx1-Fgf8 pathway is important in early thyroid development [1]. Lania et al (2009) describe the function of Tbx1 to be affecting how many cells in the primordium and affects thyroid at primordium formation or prior to it [1]. They found the primordium of Tbx1 mutants to grow but never reach the normal size. Lania et al (2009) test their hypothesis of the Tbx1-Fgf8 pathway by stopping expression of Fgf8 in Tbx1 mutant mice, and they found that there was thyroid hypoplasia. They also found this with Tbx1 deficient mice. Lania et al (2009) describe that Fgf8 cDNA when expressed in Tbx1 domain in Tbx1 mutant mice had a therapeutic effect on a thyroidal primordiam size deficiencies [1]. There association between Tbx1 and Fgf8 is confirmed in this experiment and they hypothesize that the Tbx1-Fgf8 controls primordium growth by controlling proliferation of endodermal thyroid progenitor cells [1].
layers and tissue involved with Tooth development
Teeth in the embryo are derived from the ectoderm and the mesoderm. Specifically the ectoderm of the first pharyngeal arch, mesoderm and the neural crest ectomesenchyme. The dental lamina is a thin ectodermal layer which proliferates to form two horse shoe shaped structure. Extoderm forms the ameloblasts which participates in enamel formation[1]. The enamel organs which are cellular aggregation forms swellings on the dental lamina which eventually is where the tooth will form and will affect the size and structure of the crown of the tooth as well as the adjacent mesoderm structures known as the dermal papillae [2]. The dermal papillae gets enclosed by some of the enamel organ whilst some is left unenclosed, which eventually forms a sac known as the follicular sac. Each of these structures have different fates; the enamel organ differentiates to form the enamel cap; the dental papillae form the dentine and pulp chamber; follicular sac forms the periodontal membrane [3]. These structures form specialized teeth cells like the odontoblasts, ameloblasts and cementoblasts. The neural crest ectomesenchyme form the odontoblasts. Odontoblasts contribute to the outer dental pulp and makes dentin which is calcified tissue which surfaces structures like the enamel and pulp[1].
- ↑ 1.0 1.1 <pubmed>12640730</pubmed>
- ↑ http://www.britannica.com/EBchecked/topic/1512077/tooth-germ
- ↑ http://www.britannica.com/EBchecked/topic/1512077/tooth-germ
Lab Assessment 8
For the testis to develop the XY chromosome must be present. Primordial Germ Cells (PGCs) in early gastrulation migrate through primitive streak. These migrated PGCs then congregate in-between the hindgut and yolk sac. They then migrate to the germinal ridge which now begins the stage of development of the testis. The gonadal ridge is formed by the proliferation of epithelium and mesenchyme of mesothelium on its medial side. The PGCs are originally found with endodermal cells of umbilical vesicle. The dorsal part which gets involved with the embryo and the PGCs migrate into the underlying mesenchyme. In Week 4-5 the intermediate mesoderm forms the pronephros near the pharyngeal arches. The pronephros disintegrates and forms the mesonephros or intermediate kidney. The mesonephros has two mesonephric ducts which open up into the cloacal cavity. The mesonephros extends caudally towards the hind gut. The anterior portion of the hind gut separates itself to form the urogenital sinus into which the mesonephric ducts initially open up into. Superior part of the primitive urogenital sinus forms the bladder. The inferior part of the primitive urogential sinus is where either male or female gonad structures develop from.
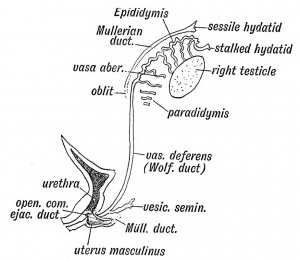
SRY gene on Y chromosome expresses testis determining factor (TDF). In week 8 TDF differentiates sertoli cells which express Mullerian duct inhibitory factor (MDIF) which degenerates the paramesonephric duct. In week 9 by the action of TDF, leydig cells for them to express testosterone which differentiates mesonephric ducts. The mesonephric ducts associated with medullary sex chords forms the rete testis by condensing and anastomising in the indifferent gonad. The mesonephric duct associated with gonad forms the ductus deferens and also forms the vas deferens. Tunica albuginea also develops which forms the connective tissue over the testis, which forms when the seminiferous cords connection with the epithelium is lost. Medullary sex cords form seminiferous tubules. The developing testis suspends itself by the mesorchium.
Hill, M.A. (2014) Embryology ANAT2341 Lab 8 - Sex Determination
Remnant of the Wolffian Body in the Male