Prenatal Diagnosis
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
This current page is a general starting point for the topic of prenatal diagnosis, the links below are to resources that give more specific information about some diagnostic techniques available at different stages of pregnancy. The two major classes of techniques are invasive and non-invasive testing and the results of these tests most commonly show normal development. When abnormal development is identified this can be due to genetic, environmental, unknown causes or a combination of these effects. (More? Abnormal Development)
A trend in developed countries of an increasing maternal age, long associated with increased genetic abnormalities, has emphasised the need for good diagnostic low risk tests that allow informed decisions early in a pregnancy. (More? Genetic) In contrast, in developing countries environmental effects such as infections and nutrition can impact upon embryonic and fetal development (More? Environmental | Nutrition)
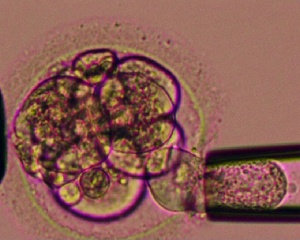
Recently with the growth in Assisted Reproductive Technologies (ART) or commonly known as In Vitro Fertilization (IVF), there is a new "form" of prenatal diagnosis that involves genetic testing of the blastocyst before implantation. (More? Assisted Reproductive Technology)
A Combined First Trimester Screening test (cFTS) involves a maternal ultrasound scan and a blood test at 11-13+6 weeks pregnancy.
Non-Invasive Prenatal Testing (NIPT) include new techniques that analyzes cell-free fetal DNA circulating in maternal blood or from fetal cells in the cervical canal.
There are other pages that refer to postnatal diagnostic testing. (More? Neonatal Diagnosis)
- This Embryology site is a developmental educational resource, it does not provide specific clinical details, you should always refer to a health professional.
Assisted Reproductive Technology | In Vitro Fertilization | Journal - Prenatal diagnosis
|
|
|
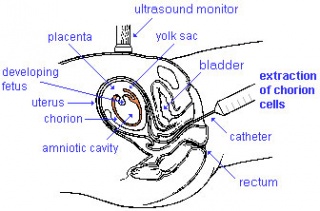
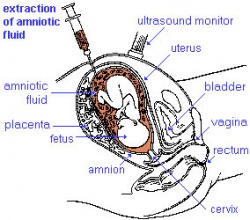
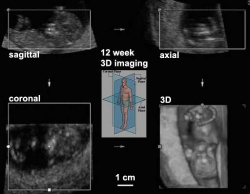
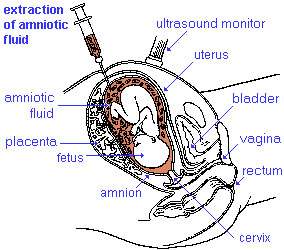
| chorionic villus sampling | amniocentesis | ultrasound |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Prenatal Diagnosis | Non-Invasive Prenatal Testing |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Maternal Blood Test
Maternal blood tests are usually offered in the first trimester.
- full blood count
- blood group and antibodies
- free b-hCG and PAPP-A
- glucose challenge - for diabetes
- random blood glucose
- sickle cell and Thalassaemia (Haemoglobinopathy) - for at risk women (Ethnic groups with high risk include: Mediterranean, Middle Eastern, African, Asian, Pacific Islander, South American, New Zealand Maori)
- Infections
- syphilis
- rubella
- hepatitis B
- hepatitis C
- HIV antibodies
Other maternal tests:
- ultrasound - check dates, number of fetuses and development
- midstream urine - infections
- chlamydia screening
Amniocentesis
|
Amniocentesis is a prenatal diagnostic test carried out mainly between 14th to 18th week of pregnancy. Amniotic fluid is taken from the uterus, sent to a diagnostic laboratory and embryonic cells isolated from the amniotic fluid. No anaesthetic is required, and a result is usually obtained in about three to four weeks. When the test is carried out by an obstetrician experienced in the technique, the risk of a miscarriage related to the test is about 1 %.
|
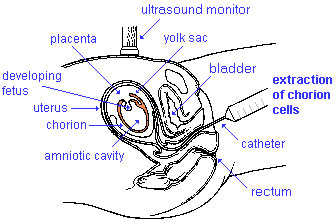
Chorionic Villus Sampling
| Chorionic Villus Sampling (CVS) test is done in the 10th to 12th week after the first day of the mother's last menstrual period. The test is done by looking at cells taken from the chorionic membrane or placenta. No anaesthetic is required, and a test result is usually available in two to three weeks.
|
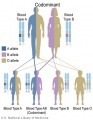
Cordocentesis
Percutaneous umbilical blood sampling (PUBS, fetal blood sampling, umbilical vein sampling)
This chromosome analysis test is done at in the 18th week or later of high-risk pregnancies. The technique may be used when either alternative tests (amniocentesis, CVS, ultrasound) are either inconclusive or not achievable (severe oligohydramnios).
The risk of a miscarriage related to the test is about 3 per cent (occurring in 3 in 100 pregnancies).
Coelocentesis
This is a technique of sampling of extracoelomic fluid usually for an early prenatal diagnostic technique.
Fetal Fibronectin
As a prenatal diagnostic test, a positive fetal fibronectin test result can indicate a higher risk of preterm delivery, but may also has false positive results. The negative result is more reliable as an indicator of reduced risk of preterm birth.
(fFN) is an extracellular matrix glycoprotein produced by fetal cells. Fetal fibronectin appears to act as an adhesive between the interface of the chorion and the decidua (fetal membrane and uterine lining).
Non-Invasive Prenatal Testing
A published survey of clinicians from 28 countries worldwide[14] reported that NIPT is available in their country (n=43) and that they offer NIPT in their current practice (n=38). Eighteen respondents from 14 countries reported that there are plans to introduce NIPT into routine antenatal care in their country. Test prices varied widely, ranging from $US 350 - $US 2900, and several respondents observed that high test prices limited or restricted widespread use of NIPT.
See also recent Non-Invasive Prenatal Testing (NIPT) articles in Australian Family Physician[15] and JAMA (USA).
Cervical Canal Trophoblasts
Beginning in 2009, a diagnostic trial indicated that fetal cytotrophoblast cells could be collected by transcervical sampling for genetic analysis.[16] A recent 2016 study[8] has identified fetal genome profiling as early as gestation weeK 5 GA using this technique.
- Links: Trophoblast
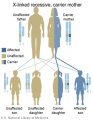
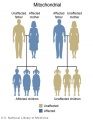
Genetic Testing
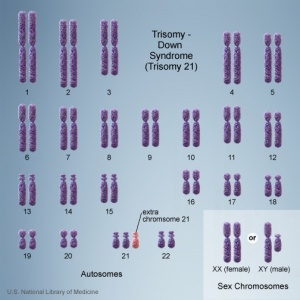
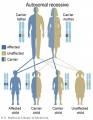
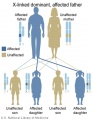
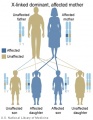
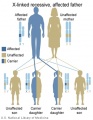
There are clinically more and more tests becoming available as we learn more about the genetic basis of some diseases. The most common diagnostic test relates to the current trend in an increasing maternal age, which has long been associated with an increase in genetic abnormalities, the most frequent of these is trisomy 21 or Down syndrome.
Inheritance Genetics
- Links: Genetic risk maternal age | Trisomy 21
Australia
Trisomy 21
- Department of Health 9.3 Screening tests in the first trimester combined test (nuchal translucency thickness, free beta-human chorionic gonadotrophin, pregnancy-associated plasma protein-A)
- NSW Health (2007) Prenatal Testing/Screening for Down Syndrome & Other Chromosomal Abnormalities PDF.
The Royal Hospital for Women (2014) Trisomy 21 Screening, Including Non-Invasive Prenatal Testing (Nipt) PDF
- Better Health Victoria trisomy disorders
A 2010 publication from NHMRC Medical Genetic Testing: information for health professionals covers background information on all types of genetic tests, not just those associated with prenatal diagnosis.
Types of genetic tests
- Somatic cell genetic testing involves testing tissue (usually cancer) for non-heritable mutations. This may be for diagnostic purposes, or to assist in selecting treatment for a known cancer.
- Diagnostic testing for heritable mutations involves testing an affected person to identify the underlying mutation(s) responsible for the disease. This typically involves testing one or more genes for a heritable mutation.
- Predictive testing for heritable mutations involves testing an unaffected person for a germline mutation identified in genetic relatives. The risk of disease will vary according to the gene, the mutation and the family history.
- Carrier testing for heritable mutations involves testing for the presence of a mutation that does not place the person at increased risk of developing the disease, but does increase the risk of having an affected child developing the disease.
- Pharmacogenetic testing for a genetic variant that alters the way a drug is metabolised. These variants can involve somatic cells or germline changes. Even if these variants are heritable (that is germline changes), the tests are usually of relevance to genetic relatives only if they are being treated with the same type of medication.
USA
A new site developed by NIH "GeneTests" provides medical genetics information resources available at no cost to all interested persons. It contains educational information, a directory of genetic testing laboratories and links to other databases such as OMIM.
- Links: GeneTests | Medline Plus - Genetic Testing
Comparative Genomic Hybridization
This new test under development is based upon microarray-based comparative genomic hybridization (array CGH).
All fetal cells should have complete copies of maternal and paternal genomes. The test compares regions of fetal DNA that deviate from this "pattern" due to either too much or too little DNA, alterations reflect regions of the genome that are either copied or deleted. These genetic changes may therefore cause disease.
Ethics of Testing
Major developmental abnormalities detected early enough can be resolved far more easily than those discovered late in a pregnancy.
What are the ethical questions that are raised by prenatal testing? Future individual rights or parents rights? But what about diseases, like Huntington's, where a diagnostic test can be made but there are no current treatments for the postnatal (95% of cases adult onset) disease?
Huntington's disease
Guidelines for the molecular genetics predictive test
- Recommendation 2.1 "the test is available only to individuals who have reached the age of majority."
- Recommendation 7.2 "the couple requesting antenatal testing must be clearly informed that if they intend to complete the pregnancy if the fetus is a carrier of the gene defect, there is no valid reason for performing the test."
References
- ↑ Milachich T. (2014). New advances of preimplantation and prenatal genetic screening and noninvasive testing as a potential predictor of health status of babies. Biomed Res Int , 2014, 306505. PMID: 24783200 DOI.
- ↑ Van De Looij A, Singh R, Hatt L, Ravn K, Jeppesen LD, Nicolaisen BH, Kølvraa M, Vogel I, Schelde P & Uldbjerg N. (2020). Do fetal extravillous trophoblasts circulate in maternal blood postpartum?. Acta Obstet Gynecol Scand , , . PMID: 32323316 DOI.
- ↑ Xue Y, Zhao G, Li H, Zhang Q, Lu J, Yu B & Wang T. (2019). Non-invasive prenatal testing to detect chromosome aneuploidies in 57,204 pregnancies. Mol Cytogenet , 12, 29. PMID: 31249627 DOI.
- ↑ Becker DA, Tang Y, Jacobs AP, Biggio JR, Edwards RK & Subramaniam A. (2019). Sensitivity of prenatal ultrasound for detection of trisomy 18. J. Matern. Fetal. Neonatal. Med. , 32, 3716-3722. PMID: 29712489 DOI.
- ↑ Alanen J, Korpimaki T, Kouru H, Sairanen M, Leskinen M, Gissler M, Ryynanen M & Nevalainen J. (2019). First trimester combined screening biochemistry in detection of congenital heart defects. J. Matern. Fetal. Neonatal. Med. , 32, 3272-3277. PMID: 29683008 DOI.
- ↑ Ngo TTM, Moufarrej MN, Rasmussen MH, Camunas-Soler J, Pan W, Okamoto J, Neff NF, Liu K, Wong RJ, Downes K, Tibshirani R, Shaw GM, Skotte L, Stevenson DK, Biggio JR, Elovitz MA, Melbye M & Quake SR. (2018). Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science , 360, 1133-1136. PMID: 29880692 DOI.
- ↑ Badeau M, Lindsay C, Blais J, Nshimyumukiza L, Takwoingi Y, Langlois S, Légaré F, Giguère Y, Turgeon AF, Witteman W & Rousseau F. (2017). Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database Syst Rev , 11, CD011767. PMID: 29125628 DOI.
- ↑ 8.0 8.1 Jain CV, Kadam L, van Dijk M, Kohan-Ghadr HR, Kilburn BA, Hartman C, Mazzorana V, Visser A, Hertz M, Bolnick AD, Fritz R, Armant DR & Drewlo S. (2016). Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Sci Transl Med , 8, 363re4. PMID: 27807286 DOI.
- ↑ Benn P, Curnow KJ, Chapman S, Michalopoulos SN, Hornberger J & Rabinowitz M. (2015). An Economic Analysis of Cell-Free DNA Non-Invasive Prenatal Testing in the US General Pregnancy Population. PLoS ONE , 10, e0132313. PMID: 26158465 DOI.
- ↑ Feichtinger M, Stopp T, Göbl C, Feichtinger E, Vaccari E, Mädel U, et al. (2015) Increasing Live Birth Rate by Preimplantation Genetic Screening of Pooled Polar Bodies Using Array Comparative Genomic Hybridization. PLoS ONE 10(5): e0128317.
- ↑ Norwitz ER & Levy B. (2013). Noninvasive prenatal testing: the future is now. Rev Obstet Gynecol , 6, 48-62. PMID: 24466384
- ↑ Kitzman JO, Snyder MW, Ventura M, Lewis AP, Qiu R, Simmons LE, Gammill HS, Rubens CE, Santillan DA, Murray JC, Tabor HK, Bamshad MJ, Eichler EE & Shendure J. (2012). Noninvasive whole-genome sequencing of a human fetus. Sci Transl Med , 4, 137ra76. PMID: 22674554 DOI.
- ↑ Guo X, Bayliss P, Damewood M, Varney J, Ma E, Vallecillo B & Dhallan R. (2012). A noninvasive test to determine paternity in pregnancy. N. Engl. J. Med. , 366, 1743-5. PMID: 22551147 DOI.
- ↑ Minear MA, Lewis C, Pradhan S & Chandrasekharan S. (2015). Global perspectives on clinical adoption of NIPT. Prenat. Diagn. , 35, 959-67. PMID: 26085345 DOI.
- ↑ [Noninvasive prenatal testing Noninvasive prenatal testing] Volume 43, No.7, July 2014 Pages 432-434.
- ↑ Imudia AN, Suzuki Y, Kilburn BA, Yelian FD, Diamond MP, Romero R & Armant DR. (2009). Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: a pilot study. Hum. Reprod. , 24, 2086-92. PMID: 19497946 DOI.
Journals
Prenatal Diagnosis communicates the results of clinical and basic research in prenatal and preimplantation diagnosis in humans, and animal and in vitro models,
http://www.ncbi.nlm.nih.gov/pubmed?term=%22Prenat+Diagn%22[jour]
Articles
Liston R, Sawchuck D & Young D. (2007). Fetal health surveillance: antepartum and intrapartum consensus guideline. J Obstet Gynaecol Can , 29, S3-56. PMID: 17845745
Search Pubmed
November 2010 "Prenatal Diagnosis" All (63715) Review (8631) Free Full Text (6349)
Search Pubmed: Prenatal Diagnosis
- ART - Assisted Reproductive Technology a general term to describe all the clinical techniques used to aid fertility.
- blastomere biopsy - An ART preimplantation genetic diagnosis technique carried out at cleavage stage (day 3), excluding poor quality embryos, detects chromosomal abnormalities of both maternal and paternal origin. May not detect cellular mosaicism in the embryo.
- blastocyst biopsy - An ART preimplantation genetic diagnosis technique carried out at blastocyst stage (day 4-5), removes several trophoblast (trophoderm) cells, detects chromosomal abnormalities of both maternal and paternal origin and may detect cellular mosaicism.
- cell-free fetal deoxyribonucleic acid - (cfDNA) refers to fetal DNA circulating and isolated from the plasma portion of maternal blood. Can be performed from GA 10 weeks as a first-tier test or as a second-tier test, with women with increased probability on combined first trimester screening offered cfDNA or diagnostic testing.
- false negative rate - The proportion of pregnancies that will test negative given that the congenital anomaly is present.
- false positive rate - The proportion of pregnancies that will test positive given that the congenital anomaly is absent.
- free β human chorionic gonadotrophin - beta-hCG subunit of hCG used as a diagnostic marker for: early detection of pregnancy, Trisomy 21, spontaneous abortion, ectopic pregnancy, hydatidiform mole or choriocarcinoma.
- multiples of the median - (MoM) A multiple of the median is a measure of how far an individual test result deviates from the median and is used to report the results of medical screening tests, particularly where the results of the individual tests are highly variable.
- negative predictive value - The probability that a congenital anomaly is absent given that the prenatal screening test is negative.
- Non-Invasive Prenatal Testing - (NIPT) could refer to ultrasound or other imaging techniques, but more frequently used to describe analysis of cell-free fetal DNA circulating in maternal blood.
- polar body biopsy - (PB biopsy) An ART preimplantation genetic diagnosis technique that removes either the first or second polar body from the zygote. As these are generated by oocyte meiosis they detects chromosomal abnormalities only on the female genetics.
- positive predictive value - The probability that a congenital anomaly is present given that the prenatal screening test is positive.
- pre-implantation genetic diagnosis - (PGD, pre-implantation genetic screening) a diagnostic procedure for embryos produced through Assisted Reproductive Technology (ART, in vitro fertilisation, IVF) for genetic diseases that would generate developmental abnormalities or serious postnatal diseases.
- prenatal screening sensitivity - (detection rate) The probability of testing positive on a prenatal screening test if the congenital anomaly is present.
- prenatal screening specificity - The probability of testing negative on a prenatal screening test if the congenital anomaly is absent.
- quadruple test (maternal serum testing of a-fetoprotein Template:AFP, free B-hCG or total hCG, unconjugated estriol, and inhibin A) is a fetal chromosomal anomaly test usually carried out later in pregnancy (GA 14 to 20 weeks).
- single nucleotide polymorphisms - (SNPs) the variation in a single DNA nucleotide that occurs at a specific position in the genome.
- triple test - (maternal serum testing of a-fetoprotein Template:AFP, free B-hCG or total hCG, and unconjugated estriol) is a fetal chromosomal anomaly test usually carried out later in pregnancy (GA 14 to 20 weeks).
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Australian and New Zealand - College of Obstetricians and Gynaecologists Intrapartum Fetal Surveillance Clinical Guidelines - Second Edition (June 2011)
- Canada - Society of Obstetricians and Gynaecologists of Canada
- Mayo Clinic [www.mayoclinic.org/tests-procedures/noninvasive-prenatal-testing/basics/definition/prc-20012964 Noninvasive Prenatal Testing]
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Prenatal Diagnosis. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Prenatal_Diagnosis
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G