Paper - The formation of the umbilical cord and the umbilical region of the anterior abdominal wall
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Wyburn GM. The formation of the umbilical cord and the umbilical region of the anterior abdominal wall. (1939) J Anat. 73(2): 289-310.9. PMID 17104757
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Formation of the Umbilical Cord and the Umbilical Region of the Anterior Abdominal Wall
| George McCreath Wyburn (1903-1985) |
|---|
| George McCreath Wyburn (1903-1985) was appointed regius professor of anatomy in the University of Glasgow (1948 - 1972) in succession to Prof. W. J. Hamilton, who has been appointed to the chair of anatomy at Charing Cross Hospital Medical School, London. Dr. Wyburn was a pupil of the late T. H. Bryce, and graduated at Glasgow in 1925 : after holding appointments in various hospitals, he returned to Glasgow as demonstrator in 1929. He became senior lecturer in anatomy in 1936, and was acting head of the Department during 1944-45. His researches include work on embryology, with special reference to bone formation, on the endocrinological aspects of reproduction, and on tissue-grafting (of skin, cartilage and cornea in particular). He was awarded the Struthers Gold Medal and Prize in 1939 for embryological research, and again in 1947 (with Dr. Paul Bacsich) for work done during the War on the repair of peripheral nerve injuries. (modified from Nature 162, 56-56 - 10 July 1948) |
By George M. Wyburn
Department of Anatomy, The University of Glasgow
Introduction
"It is a characteristic of many vertebrates that, associated with the provision of special arrangements for nourishing the young individual, the time of commencing an independent existence on its own account is greatly delayed. In such cases where a considerable proportion of the whole development takes place within the shelter of the egg shell or parental body we have to do with what is known as embryonic in contradistinction to larval development” (Graham Kerr).
The young larvae of the holoblastic Anamnia such as the frog quickly learn to fend for themselves and therefore have a very small store of nourishment easily housed withi.n the ventral wall of the gut.
In the meroblastic elasmobranchs there is a considerable store of nourishment to tide the developing young over the much longer period preceding the acquisition of independence. The larger yolk mass is for atime extra-embryonic. It is encircled by the endodermal cells, forming a primitive gut or enteron, and later enclosed by the extra-embryonic blastoderm. The yolk sac then becomes connected to the embryo by a constricted narrow part—the yolk duct; this in turn has its covering of mesoderm and ectoderm, and constitutes the somatic stalk. In the final stages the yolk sac is drawn into the body and the somatic stalk is incorporated in the body wall.
The umbilical cord of the Amniota constitutes the pathway for the transit of substances to and from the embryo. In birds and reptiles the extra-embryonic blastoderm (false amnion and endoderm) grows round and encloses the large yolk mass carrying with it an extension of the coelom between the somatopleure and splanchnopleure. In Amniota the allantois issues from the hindgut on the caudal and ventral aspect of the embryo. The true amnion, attached round the embryonic “rim”, forms the boundaries of the somatic stalk which thus includes the yolk stalk, a. portion of the exocoelom and more caudally the allantoic stalk.
The somatic stalk or umbilical cord of placental mammals is essentially of a similar nature to that described above, although the now increased functional importance of the allantoic element overshadows that of the yolk stalk.
This work consists of a study of the umbilical cord in the series of human embryos already utilized in previous publications (Wyburn, 1937-8), with the addition of an embryo of 8-5 mm., one of 4-2 mm., and one of 60 mm. Sections were also made of rabbit embryos in which the gut had returned into the abdominal cavity, but as these show no features of peculiar interest they are not described in detail.
The method of graphic reconstruction is adopted where possible, as it affords a ready appreciation of the relations in any given embryo, and obviates much tedious description of individual sections.
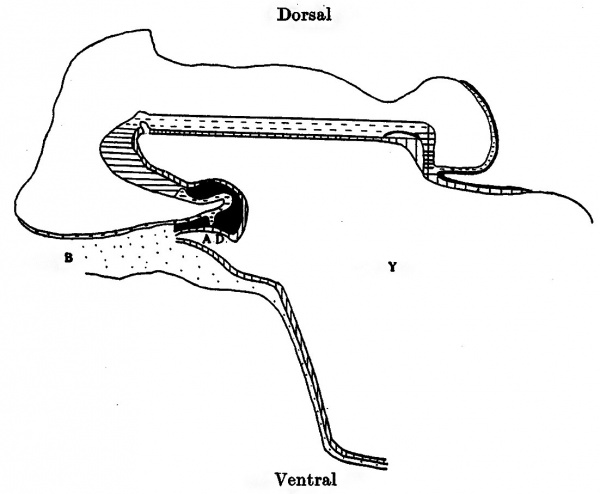
Text-fig. 1. Embryo 1-4 mm. (McIntyre 1). Reconstruction of a median sagittal section. x 50. Description in text. Horizontal lines = primitive streak. Interrupted horizontal lines = ectoderm. Vertical lines = endoderm, head process and prochordal area. Black = mesoderm. Y = yolk sac. A.D. = allantoic diverticulum. B = body stalk.
From the observations of the individual embryos one suggests the method of formation of the umbilical cord, completed in human embryos somewhere between the 5 and 7 mm. stage, of the formation and expansion of the umbilical cord coelom which attains its maximum size between the 30 and 40 mm. stage, and of the final obliteration of the cord coelom and closure of the um bilical ring subsequent to the return of the gut into the abdominal cavity. No great attention has been paid to the relations and fate of the contents of the umbilical cord such as the yolk duct and vitelline vessels, as these have been recounted with much thoroughness by Politzer & Sternberg in their comprehensive paper “Uber die Entwicklung der ventralen Korperwand und des Nabelstranges beim Menschen” to which frequent reference is made through out.
Description of Embryos
Embryo Mclntyre I (1.4 mm)
This embryo has already commenced to fold off from the yolk sac, and there is a small foregut and larger hindgut diverticulum. The ventral aspect consists mainly of the yolk sac which shows a slight The Formation of the Umbilical Cord 291 lateral furrowing indicating the site of later constriction. Cranially the anterior mesodermal field forms the anterior and lateral boundaries of the pericardial plate where the mesoderm exhibits cleavage. Elsewhere there is as yet no intraembryonic coelom.
VVith the formation of the tail fold the embryonic attachment of the body stalk comes to lie on the ventral aspect. This region has already been described (1937), and it was suggested that the stalk mesoderm was not only derived from primitive chorionic mesenchyme, but received accessions from the caudal margin of the embryonic disc.
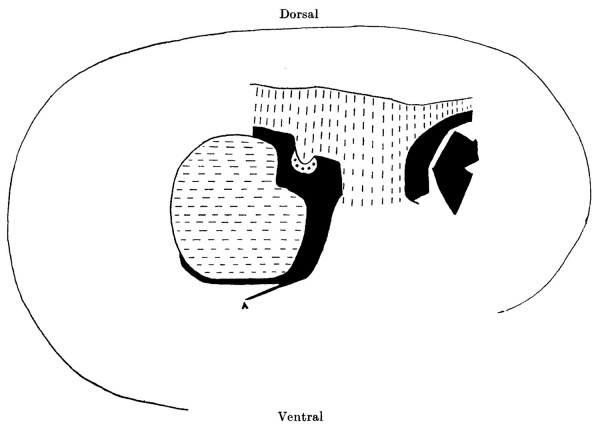
Text-fig. 2. Embryo 2.6 mm. Reconstruction of a median sagittal section. x 50. Description in text. The yolk sac is not shown. Interrupted horizontal lines = pcricardial cavity. Interrupted vertical lines = gut. Large dots = liver. Black zmesoderm. A = amnion.
Embryo McIntyre I differs from the four-somitc embryo of Sternberg in the absence of any extensive connexion of amnion and chorion, i.e. a “primary amnio-chorionic field”. There is no amnio-embryonic stalk as described by Florian in presomite embryos. In this respect it can be compared to Embryo Bi. XI (Florian) of ten somites to which it has also some external resemblance.
Embryo 2.6 mm
Following the pronounced involution of the embryonic body the ectodermal surface of the pericardium forms the cranial part of the ventral aspect. There is still a broad yolk-sac connexion which, however, in comparison to the 1.4 mm. embryo has a marked “waisting” at the junction with the midgut. Between the attachment of the amnion and the cranial aspect of the yolksac connexion is a broad band of mesoderm, the anterior mesodermal field, which is continuous dorsally with the splanchnopleure on the floor of the gut. A prolongation of the coelom—the posterior coelomic portal—separates the body stalk from the mesoderm on the caudal side of the yolk-sac connexion. As the tail fold develops this coelomic portal is taken still further into the embryonic body to form the pelvic cavity. The most caudal structure in the somatic stalk is the allantoic or body stalk. This has an oblique attachment, being directed to the left while the yolk sac is to the right. In consequence of this deviation of the body stalk the amnion clothes the greater portion of its left lateral aspect (Pl. I, fig. 2), and much of the right side is extra-amniotic.
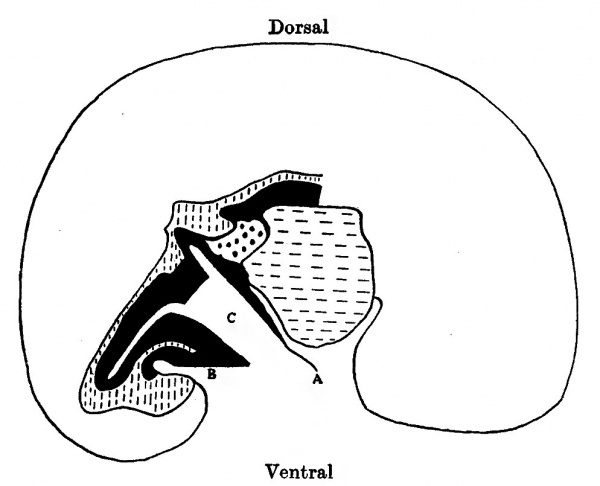
Text-fig. 3. Embryo 4.5 mm. Reconstruction of a median sagittal section. x 25. Description in text. Yolk sac is not shown. Interrupted horizontal lines = pericardium. Interrupted vertical lines = gut. Black = mesoderm. Large dots = liver. C = coelome. A = amnion. B = body stalk.
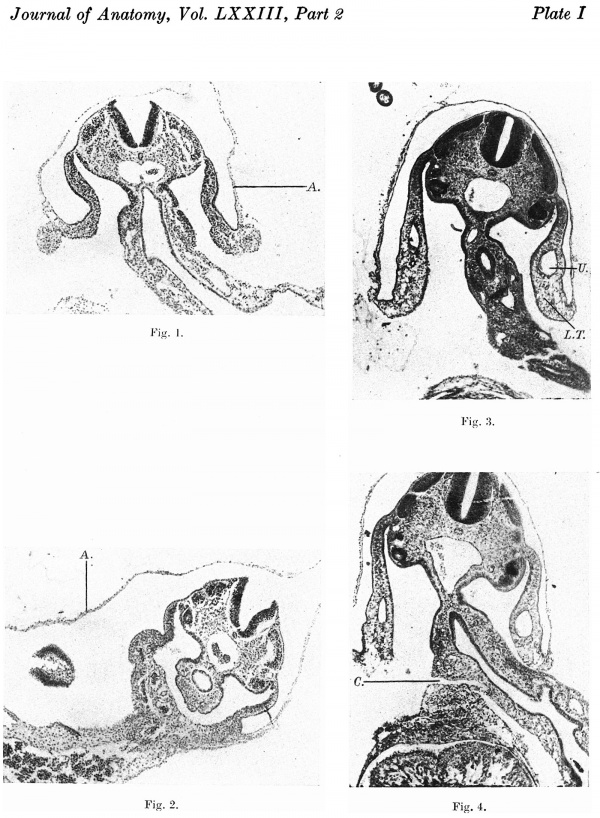
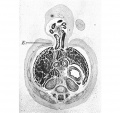
As has already been noted, the cranial aspect of the yolk-sac connexion is lying against the anterior mesodermal field. There is now a body cavity which has a relatively extensive communication with the extra-embryonic coelom on each side of the yolk-sac connexion, i.e. where the Ventral wall remains “open”. Each intercoelomic communication is bounded medially by the yolk-sac connexion and laterally by the body wall with the amnial reflexion. At the rim or point of amnial reflexion are the umbilical veins, the left larger than the right. Here the somatopleure of the lateral body wall becomes continuous with the mesoderm of the amnion. There is a marked increase of mesoderm at the site of amnial reflexion (Pl. I, fig. 1).
Embryo 4.5 mm
The further folding of the head and tail ends is responsible for the relative decrease in the extent of the “open” ventral aspect and the increased dorsi-ventral convexity for the greater depth of intra-embryonic coelom. Moreover, a part of the extra-embryonic coelom has been taken into the body anteriorly as well as posteriorly, and a coelomic prolongation now separates the proximal end of the yolk duct from the anterior mesodermal field and septum transversum (Pl. I, fig. 4). The connexion of the intestine to the yolk sac has become narrowed to a tube-like communication which at this stage could be called the vitelline duct.
The open ventral aspect is limited cranially by the amnion reflected on to the caudal Wall of the completely rotated pericardium, and caudally by the allantoic stalk‘ which in this embryo has a more or less median position.
The lateral boundaries are the somatopleure of the body wall and more ventrally the thickened mesoderm on the inner aspect of the amnion. This thickening of the amnial mesoderm is much more apparent than in the 2-6 mm. embryo. In its forward growth it carries with it the reflexion of the amnion on each side and forms lateral tissue plates” which extend from the “ sta1k” to the septum transversum (Pl. I, fig. 3). There is therefore in this embryo a part of the extra—embryonic coelom enclosed between the stalk, the cranial reflexion of the amnion and the lateral tissue plates, i.e. there is a short umbilical cord.
The earlier site of lateral amnial reflexion is indicated by the position of the umbilical veins, each of which runs in a ridge of tissue projecting from the internal aspect of the somatopleure. This is also the junction of the lateral body wall with the lateral tissue plates and of the exocoelom with the body cavity. The lateral tissue plates consist externally of amnial ectoderm, internally of endothelium from the amnial mesoderm and a thick middle coat of proliferated embryonic connective tissue. This young connective tissue may result from migration of somatopleure cells, but more probably is a derivative of the amnial mesoderm. The lateral tissue plates are deeper caudally and “taper” towards the septum transversum, so that in the wax reconstruction they have the appearance of cranial extensions or pillars of the “stalk”.
The smooth mesothelium of the cranial aspect of the stalk forms the caudal lining of the enclosed exocoelom. An umbilical loop of intestine is not yet formed.
Embryo 7 mm
This is the youngest embryo of the series to possess a formed umbilical cord. Coincident with this is the first appearance of a short supraumbilical abdominal wall, and thus the cranial attachment of the .cord is a little distance caudal to the pericardium.
Comparison of Text-figs. 3 and 4 gives some indication of the changes by which the “ open ” ventral wall of the embryo is now the site of attachment of the umbilical cord.
The lateral tissue plates whose formation had commenced in the 4-5 mm. embryo undergo further growth by proliferation of their connective tissue, and in their ventral extension carry with them the amnial reflexion. There is also proliferation of the mesoderm at the cranial reflexion of the amnion. Therefore the amnion at its embryonic attachment now encloses a circular band of embryonic connective tissue made up of the “stalk” caudally, the lateral tissue plates on each side, and cranially the proliferated amnial mesoderm—the umbilical cord.
- 1 From now on the allantoic stalk will be referred to as “stalk”.
- 2 The term “lateral tissue plate" shall be used to denote the amnion with its thickened mesoderm ventral to the umbilical veins.
The anterior mesodermal field, which in the 4-5 mm. embryo is caudal and dorsal to the pericardium, is here divided by the developing liver into the cranial portion forming the pericardio-peritoneal membrane and the ventrocaudal portion which in part forms the mesodermal basis of the supra-umbilical wall. This ventro-caudal mesoderm is continuous with the cranial mesoderm of the umbilical cord, the two together forming an intercoelomic septum which separates the umbilical cord coelom from the liver ventrally and from the body cavity more dorsally (Pl. II, fig. 5).
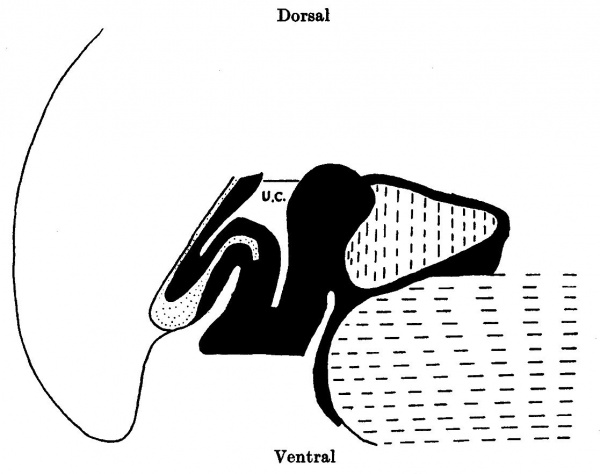
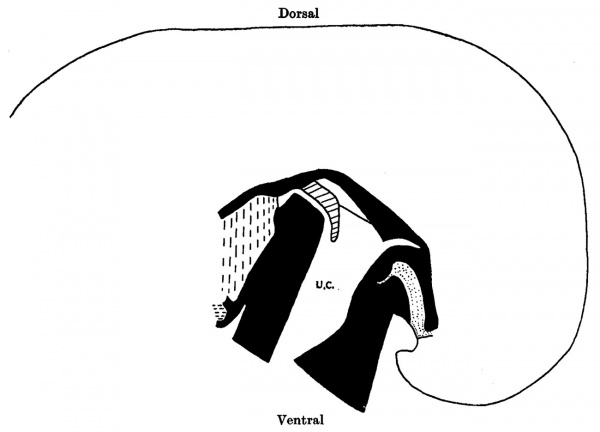
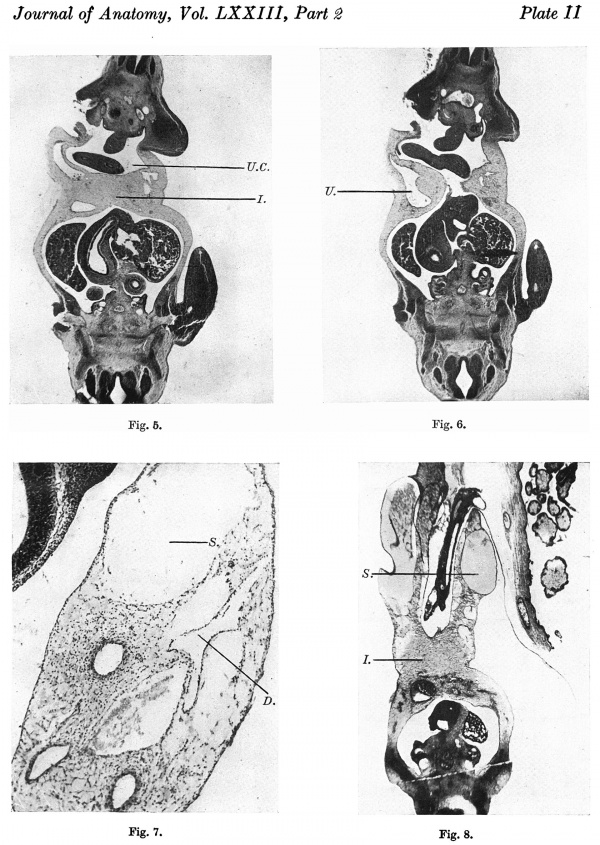
Text-fig. 4. Embryo 7 mm. Reconstruction of a median sagittal section. x 25. Description in text. Interrupted horizontal lines: pericardium. Interrupted vertical lines = liver. small dots = gut and allantoic diverticulum. U.C. = umbilical cord coelom. Black line indicates the plane of the umbilical veins.
The umbilical veins run in the dorsal part of the intercoelomic septum to reach the liver (P1. II, fig. 6).
The completed cord includes a part of the exocoelom—the umbilical cord coelom. In this embryo it consists of a wide proximal portion and a narrow slit-like prolongation (Pl. II, figs. 6, 7). The proximal portion has a caudal extension between the gut and the allantois, and dorsally a wide communication with the intra-embryonic coelom. It is bounded caudally by the “ stalk”, cranially by the intercoelomic septum, and on each side by the lateral tissue plates.
In a transverse section of the cord some distance distal to its attachment, there are a number of large spaces in the loose embryonic connective tissue. One of these spaces contains blood clot and has a distinct but not an endothelial lining (Pl. II, fig. 7). These large spaces result from confluence of a number of smaller ones. The umbilical cord coelom increases considerably in size in older embryos, very probably by the “ taking in” of these spaces and a proliferation and extension of the endothelial lining. Politzer & Sternberg remark on the normal occurrence of spaces in cord tissue and state “fiber ihre Bedeutung kénnen nur Vermutungen ausgesprochen werden, doch scheint es uns miiglich, dass ihre Auftreten, zumindest im proximalen Abschnitte des Nabelstranges, mit der Vergrosserung der Nabelstrangcoelomhohle in Zusammenhang steht”. V The midgut is differentiated and there is a definite loop of intestine in the umbilical cord coelom.
In Text-fig. 4- the position of the umbilical veins is indicated and this also represents the division between the intra- and extra-embryonic coeloms. It will be observed that the gut is lying in the ventral part of the body cavity so that in its growth it almost inevitably protrudes into the umbilical cord coelom.
The cranio-caudal width of the cord at its point of attachment to the embryo is 0-5 mm.‘ The length of the attachment cranial to the umbilical cord coelom is 0-24 mm., which is, in fact, the cranio-caudal measurement of the intercoelomic septum} while the wide umbilical cord coelom measures 0-4 mm.
Embryo 8.5 mm
This embryo has been recently acquired and therefore was not previously described.
The umbilical cord has an inclination towards the right, and there is a. small yolk sac also on the right side. The embryo is cut transversely, and the umbilical cord longitudinally. The cord extends almost to the chorion, but there is a small extra-amniotic stretch of “ stalk” distally and to the left.
The ventral aspect has straightened out somewhat compared to that of the 7 mm. embryo. This is due to the forward and caudal extension of the liver and the formation of a longer supra-abdominal wall. The cranial portion of the cord consists of a loose embryonic connective tissue which, dorsal to the cord attachment, fuses with the denser tissue on the ventral aspect of the liver to form the intercoelomic septum (Text-fig. 5). It should be noted that with the stretching of the ventral wall the intercoelomic septum is no longer in the dorsiVentral plane but makes an angle of about 45° with it and thus the cord is tending to become “ prised out” of the embryonic body. The length of the cord attachment is 1-2 mm., and of its cranial attachment 0-6 mm. This increase (cf. 7 mm.) is in fact the result of the more horizontal position of the intercoelomic septum. The widest part of the umbilical cord coelom is at its communication with the body cavity, the coelomic portal,3 and measures 0-6 mm.
- I Will be referred to from now on as “cord attachment”. ’ Will be referred to from now on as “cranial attachment” of cord. ’ The communication of extra» and intra-embryonic coeloms.
In a longitudinal section of the cranial half of the cord (Pl. II, fig. 8) can be observed the distal prolongation of the umbilical cord coelom containing the vitelline stalk. This part of the coelom is much larger than the corresponding extension in the 7 mm. embryo. On the left the amnion is refiected from the cord while still a short distance from the chorion, and at the point of reflexion the endothelium of the coelom becoines continuous with the mesothelium of the amnion. Proximal to this point the endothelium and amnial ectoderm are separated by a thickness of mesenchyme—the proliferated connective tissue of the lateral tissue plates.
Text-fig. 5. Embryo 8.5 mm. Reconstruction of a median sagittal section. x 25. Description in text. Interrupted horizontal lines = pericardium. Interrupted vertical lines = liver. Oblique lines = blood vessels. Black = mesoderm. Small dots = gut. U .C. = umbilical cord coelom. Black line indicates the plane of the umbilical veins.
A noticeable feature in this embryo as in the 7 mm. one is the fact that the umbilical cord actually penetrates the embryonic body for some distance, and accordingly there is a relatively shallow intra-embryonic coelom at this level. It is therefore not surprising that the growing intestine should find its way into the extra—embryonic coelom.
The liver still occupies but a small part of the abdominal cavity, and can have little or no influence on the position of the gut at this time.
Embryo 12.5 mm
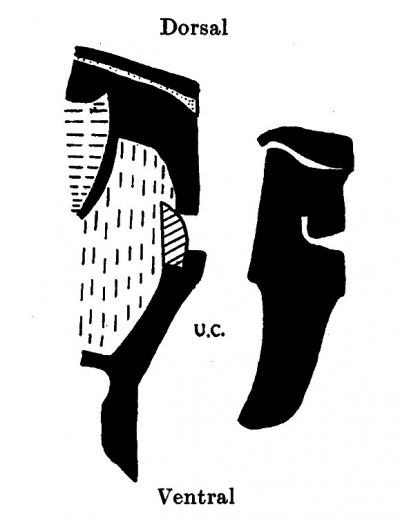
The cord attachment extends over 1-9 mm. and the The Formation of the Umbilical Cord 297 cranial attachment is over 1 mm. The increased length of the cranial attachment is simply due to the more horizontal position of the intercoelomic septum which in turn has been brought about by the caudal and ventral extensions of the now rapidly growing liver. As a consequence of both these factors the cord is still further “prised” out of the embryonic body, and the body cavity has a much increased dorsi-ventral depth. Above the cord is the supra-umbilical area, and caudal to its lower attachment is a small infra-umbilical area of abdominal wall.
|
|
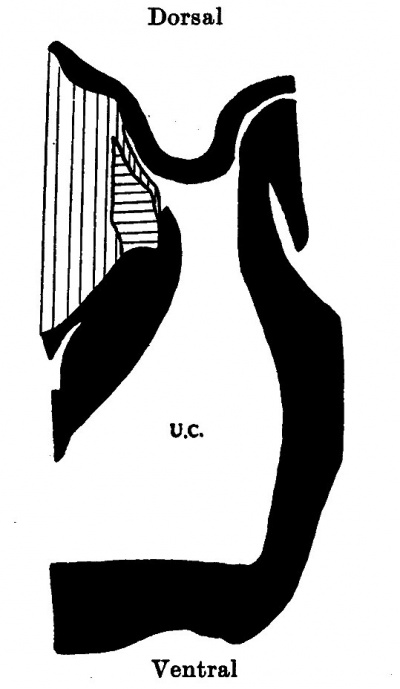
| Text-fig. 6. Embryo 12-5 mm. Reconstruction of a median sagittal section. x 13. Description in text. Interrupted horizontal lines = pericardium. Interrupted vertical lines = liver. Small dots = gut. Oblique line = blood vessels. Black = mesoderm. U.C. = umbilical cord coelom. | Text-fig. 7. Embryo 14 mm. Reconstruction of a median sagittal section. x 13. Description in text. Vertical lines = liver. Horizontal lines = blood vessel. Black = mesoderm. U.C. = umbilical cord coelom. |
The coelomic portal has a cranio-caudal width of 0.5 mm., but a short distance distal to this the umbilical cord coelom widens out to 1.1 mm. There is thus in this embryo a bottle-neck communication between the intra- and extraembryonic coeloms; a characteristic feature in all the older specimens.
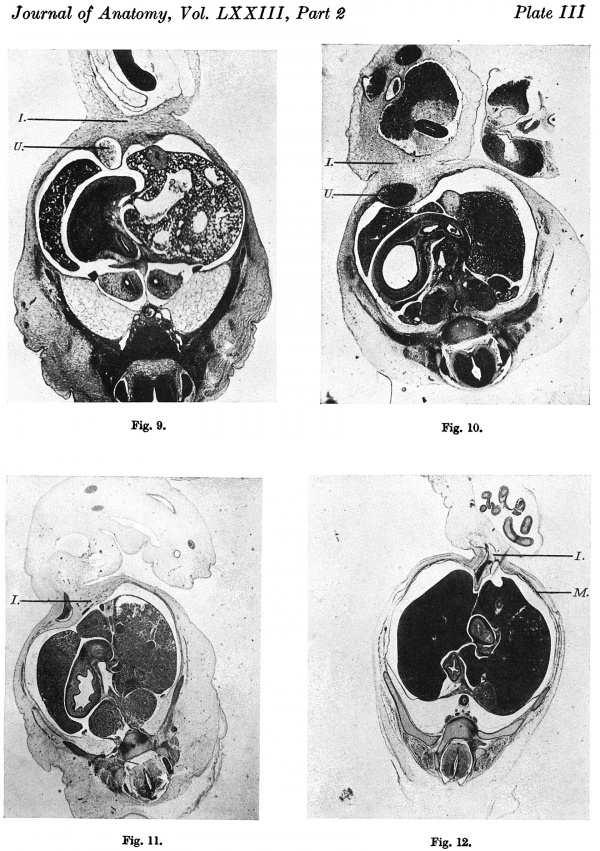
A section at the level of the cranial attachment of the cord (P1. III, fig. 9) shows the myotomic downgrowths fusing in the mid-line ventrally to form a denser connective tissue on the superficial aspect of the umbilical vein. At the level of the coelomic portal the downgrowths are not so definite and do not reach the sides of the cord. The stalk tissue with the vessels is on the right side of the cord.
The significance of the zone of mesodermal condensation on the caudal aspect of the cord has already been discussed (1937).
Embryo 14 mm
In this embryo the umbilical cord in the first part of its course turns towards the head and the umbilical vessels occupy the left aspect. The extent of cord attachment measures 1-6 mm.—an absolute and relative decrease from the corresponding figure in the preceding embryo. The cranial attachment is 0-8 mm., while the intercoelomic septum is now lying at an angle of about 45° to the horizontal.
A narrow coelomic portal (cranio-caudal width 0-6 mm.) is the entrance into a large umbilical cord coelom which but a short distance from the cord attachment has expanded to a cranio-caudal breadth of 2 mm., and is responsible for the increased circumference‘ of this part of the umbilical cord.
Although not so well marked as in the 12-5 mm. embryo, the myotomic downgrowths can be discerned in the upper half of the abdominal wall and traced into a mesodermal condensation on the anterior aspect of the umbilical vein (Pl. III, fig. 10). In the mid-abdominal region, however, i.e. at the level of the coelomic portal, the downgrowths fade away before reaching the ventral aspect.
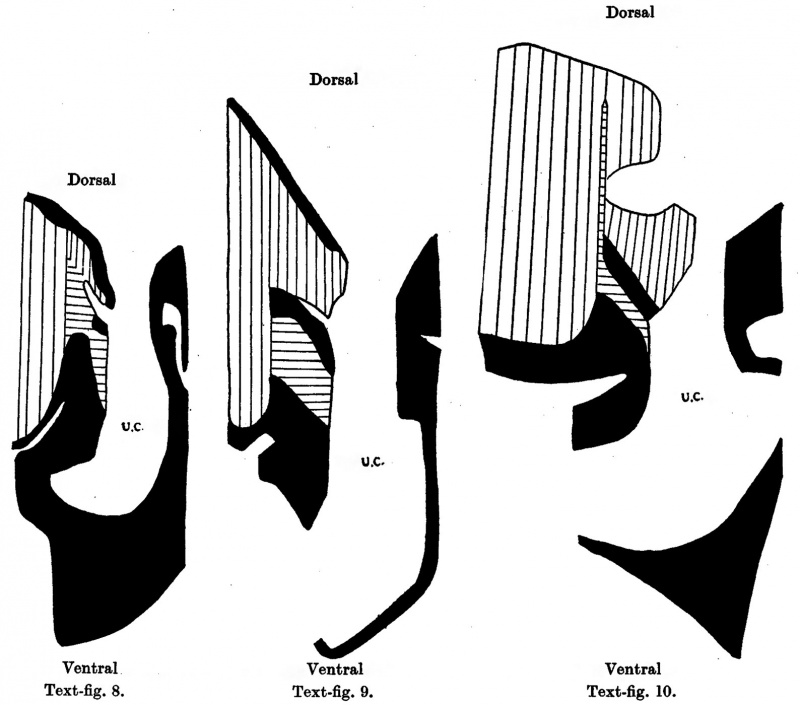
Embryo 16-1 mm
The area of cord attachment is 1-3 mm., the cranial attachment 0-5 mm., and the coelomic portal 0-7 mm.
Above the cord the mid-ventral wall is a narrow band of loose embryonic connective tissue. At the level of the cranial attachment of the cord the mesoderm ventral to the liver and the immediate cord tissue (Pl. III, fig. 11) is of a more compact texture, and this denser connective tissue can also be observed extending some little way into the pillars of the cord flanking the coelomic portal.
Embryo 23 mm
The extent of umbilical cord attachment is 2 mm., the cranial attachment is 0-7 mm., and the cranio-caudal width of the coelomic portal is 1 mm. In this embryo the intercoelomic septum is in the horizontal plane, and the liver in the mid-ventral line encroaches on the cranial half of the coelomic portal. A little distal to the embryo the cord coelom has a craniocaudal measurement of 1-6 mm. ' In the upper part of the abdominal wall the mesoderm of the mid-ventral line is of a loose texture, but at the level of the cord attachment this is replaced by a compact tissue which is continuous on the one hand with the myotomic downgrowth (Pl. III, fig. 12) and on the other extends into the proximal part of the intercoelomic septum; and more caudally into the pillars of the cord on each side of the coelomic portal. Along with the condensation of mesoderm on the caudal aspect of the cord there is thus a ring of compact tissue at the cord attachment which forms the circumference of the coelomic portal.
Embryo 40 mm
The extent of cord attachment extends over 2 mm. of the ventral body wall. There is a very small cranial attachment of 0-4 mm. and a coelomic portal of 1-3 mm. opening into a wide umbilical cord coelom which, however, on account of the obliquity of the section through the cord, could not be measured. From the embryo the umbilical cord takes a cranial direction, and its coelom, with the endothelial lining, can be followed as a narrow prolongation into the more distal sections.
| ||
| Text-fig. 8. Embryo 16.1 mm. Reconstruction of a median sagittal section. x 13. Description in text. Vertical lines = liver. Horizontal lines = blood vessels. Black = mesoderm. U .C. = umbilical cord coelom. | Text-fig. 9. Embryo 23 mm. Reconstruction of a median sagittal section. x 13. Description in text. Vertical lines = liver. Horizontal lines = blood vessels. Black = mesoderm. U .C. = umbilical cord coelom. | Text-fig. 10. Embryo 40 mm. Reconstruction of a median sagittal section. x 10. Description in text. Vertical lines = liver. Horizontal lines = blood vessels. Black = mesoderm. U .C. = umbilical cord coelom. |
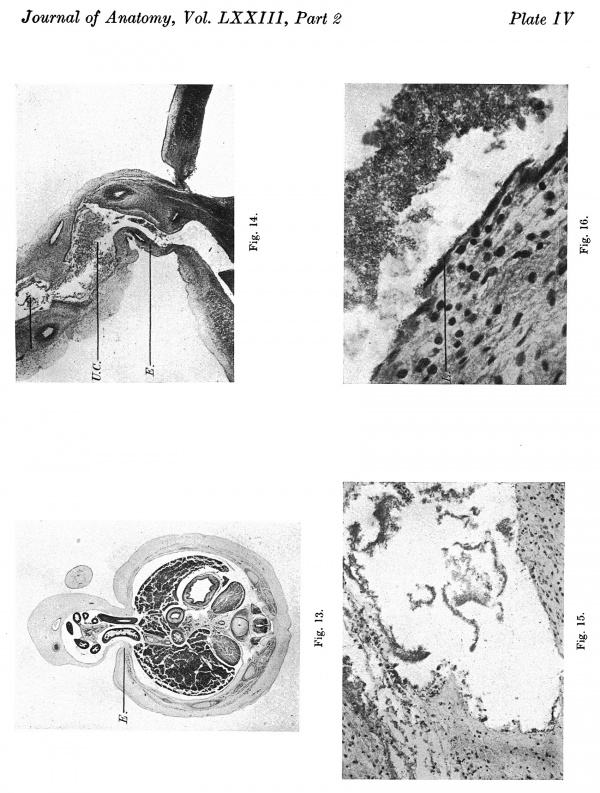
The liver occupies an even greater proportion of the abdominal cavity than in the 23 mm. embryo, and in the mid-line encroaches not only ventrally but also dorsally on the cranial half of the coelomic portal. At this level the intra- ' abdominal part of the intestine is lodged in a groove between the above ventral and dorsal portions of the liver. There is a broad linea alba in the supraumbilical abdominal wall formed by a fusion of the sheaths of the recti muscles which provides a fibrous condensation on the superficial aspect of the intraabdominal part of the umbilical vein. At the attachment of the cord the muscle sheaths fuse with the umbilical ring of compact tissue surrounding the coelomic portal (Pl. IV, fig. 13).
Embryo 42 mm
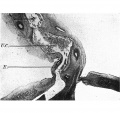
This embryo is included in the series as it demonstrates the presence of an empty umbilical cord coelom after the withdrawal of the gut. The cord coelom is much reduced in size from that in the 40 mm. embryo, and is occupied by a quantity of blood clot (Pl. IV, fig. 14-). In the more distal part there is an absence of the endothelial lining; the wall is irregular and formed simply of the connective tissue of the cord (Pl. IV, fig. 15).
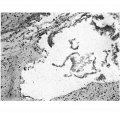
In the proximal cord coelom the endothelial cells are being separated off (P1. IV, fig. 16) preparatory to the ultimate degeneration and disappearance of the endothelium.
The anterior sheath of therecti muscles is continuous on all sides with the compact tissue encircling the now much narrowed coelomic portal (Pl. IV, fig. 14).
Embryo 60 mm
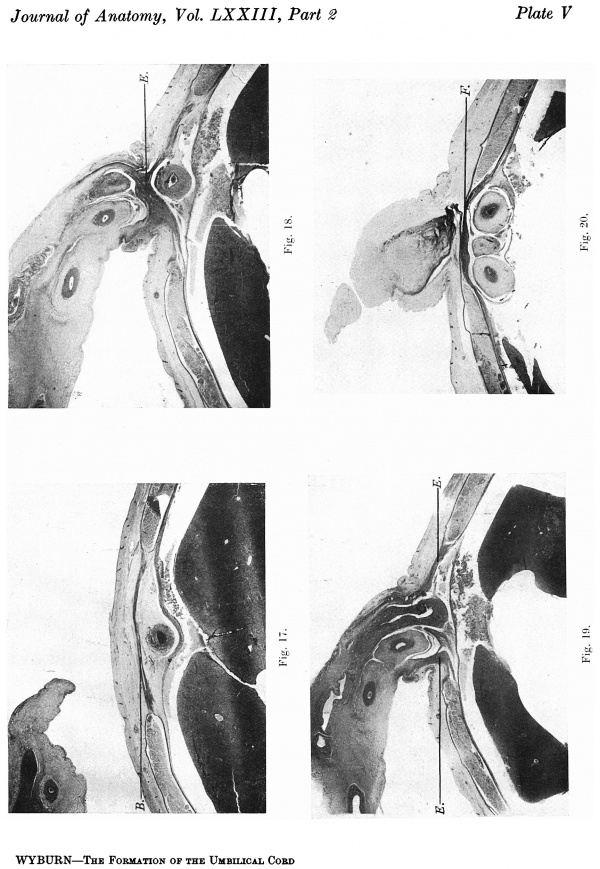
A description of the relevant sections will be given as these include only the anterior abdominal wall in the region of the cord attachment, and accurate graphic reconstruction was not possible. The length of the cord attachment is 32 mm. ‘ In the supra-umbilical region (21. V, fig. 17) the now well-formed broad linea .alba lies superficial to the umbilical vein whose coats are differentiated. Posteriorly between the vein and the peritoneum is a layer of fibrous tissue the umbilical fascia—-the variations and significance of which have been worked out by Gorelow. The character of the tissue of the umbilical cord shows considerable change from that in the 40 and 42 mm. embryos (Pl. V, fig. 18). There has been much proliferation of embryonic connective tissue which approximates to adult fibrous tissue. This proliferation is more apparent at the attachment of the cord in the vicinity of the blood vessels. The coats of the vessels themselves have increased in thickness, and the allantoic arteries have a layer of visceral muscle. In the distal sections of the cord there is a less dense and more open appearance. On each side the sheaths of the recti muscles are continued into the broad band of compact fibrous tissue which forms the cranial attachment of the cord, lies on the superficial aspect of the umbilical vein, and can in turn be followed into the supra-umbilical linea alba (Pl. V, fig. 18).
At the proximal end of the cord the umbilical vein takes the left and cranial side, while the allantoic structures are on the caudal and right aspects.
Pl. V, fig. 19, is a photograph of a section about midway through the cord attachment. The muscle sheaths run into the ‘cord tissue on each side. In this embryo there is no umbilical cord coelom and the body cavity is shut off from the cord by the peritoneum. At the caudal end of the cord attachment (Pl. V, fig. 20) there is a band of compact tissue superficial to the allantoic arteries and allantois. This merges on each side with the sheath of the rectus muscle and caudally is continued into the infra-umbilical division of the linea alba.
In this embryo the encircling band of compact tissue at the cord attachment is a particularly noticeable feature, and by its proliferation is responsible not only for the closure of the umbilical ring, but also a reinforcement of the immediate abdominal parietes.
Discussion And Conclusions
Formation of the umbilical cord
The umbilical cord is formed essentially by the closing in of the somatic stalk. In young embryos the somatic stalk is bounded by the attachment of the amnion round the broad ventral aspect. The approximation of the amnial reflexions is brought about by the ever increasing involution of the embryonic body. Following rotation of the pericardium the cranial reflexion of the amnion moves from the ventral to the caudal pericardial wall, and finally at the 7 mm. stage the amnion is reflected on to the newly formed supra-umbilical portion of the abdominal wall. The allantoic stalk covered posteriorly and laterally with amnion is rotated ventrally and while part of the stalk is thereby taken into the body of the embryo the relative distance between the caudal and cranial amnial reflexions, i.e. the breadth of somatic stalk, is decreased. In addition there is a relative narrowing in the transverse axis as a result of the formation of the vitelline duct coupled with the great dorsi-ventral convexity of the embryonic body. The lateral tissue plates make their first appearance in the 4.5 mm embryo. They arise as a proliferation of the embryonic connective tissue between the ectoderm of the amnion and its mesothelial or endothelial lining, and in their forward growth they carry with them the lateral amnial reflexions which will now lie some distance ventral to the umbilical veins and thus ventral to the embryonic rim. They connect the allantoic stalk to the septum transversum and represent the formation of the umbilical cord.
By a proliferation of the mesoderm the tissue plates continue to grow in length and thickness. There is also an increase of the corresponding mesoderm at the cranial reflexion of the amnion which might be said to form a third or anterior tissue plate. At a later stage—the 7 mm. embryo——a portion of the exocoelom has been enclosed by these thick endothelial lined tissue plates which encircle it laterally and in front. The caudal boundary is the cranial surface of the body stalk. This is the umbilical cord and it finally stretches from the embryo to the chorion.
Umbilical cord coelom
In any given embryo the level at which the umbilical vein enters the body wall indicates the boundary between intra- and extra-embryonic coeloms.
In the 2-6 mm. embryo there is, on the ventral aspect, a wide communication between the intra- and extra-embryonic coeloms. Posteriorly the coelom extends as a caudal recess some distance into the body of the embryo. This caudal recess is extra-embryonic coelom which has “rotated” into the embryonic body as the tail fold forms. Anteriorly the coelomic portal has the opening of the coelomic tubes on each side of the mid-line. There is as yet no median prolongation of the coelom and the cranial wall of the yolk—sac connexion lies against the septum transversum (Text-fig. 2). With the further involution of the embryo (e.g. embryo 4-5 mm.) the cranial wall of the now constricted yolksac connexion becomes separated from the septum transversum by an extension of the coelom, and a short length of gut intervenes between them.
The intra-embryonic coelom is therefore to some extent increased by the inclusion of part of the exocoelom.
The umbilical cord coelom in the first instance consists of a narrow slitlike distal part and a wide proximal part which receives the opening of the caudal recess and communicates with the body cavity. It enlarges, pari passu, with the growth of the umbilical loop of intestine until it attains a maximum size in embryos between 30 and 40 mm. In these older specimens the umbilical cord coelom occupies a proportionately greater extent of the cranio-caudal diameter of the cord, and therefore some of this increase in size is at the expense of cord tissue. The opinion has already been expressed that there is a confluence of spaces normally found in the cord mesenchyme, that these open into a cord coelom, and the endothelium extends into and forms a lining for them.
The coelomic portal is in the earlier stages the widest part of the cord coelom. It, however, soon becomes relatively narrow and constitutes a “ bottle-neck ” intercoelomic passage.
Eventration of the gut
In the 4.5 mm. embryo there is a short length of gut between the cranial margin of the yolk-sac connexion and the septum transversum. This is the first indication of a mid-gut loop and it is intra-embryonic. The proximal umbilical cord coelom, in the 7 mm. embryo, contains the umbilical loop of intestine, and there it remains and develops till about the 42 mm. stage.
Why should a portion of intestine grow for a period outside the body of the embryo‘? To state that it does so because of lack of space within the abdominal cavity leaves still unexplained this apparent temporary architectural miscalculation. There seems no adequate reason why the intestine should not grow as well if not better inside an abdominal cavity of the requisite size. “Es sieht daher tatsiichlich hier so aus, als ob die Natur unnotigerweise eine konstante embryonale Missbildung hervorriefe, die sie nachher die Miihe haben miisse, wieder zu reparieren” (Broman). There are two aspects of the problem of what has been described as the physiological hernia.
first, the method of production—in other words, how does the hernia occur? The various theories and suggestions appertaining thereto are the result of the study of the relevant factors in the developing human embryo. Secondly, why does the hernia occur? Observation of human ontogeny alone would leave this question still unsolved, and the final answer is only found in terms of comparative embryology.
The method of production
The most apparent factor in the production of the hernia is the connexion of the intestine with the yolk sac. In the early stages the broad yo1k—sac connexion maintains the gut in the ventral portion of the relatively shallow body cavity, so that when the mid-gut develops by constriction of the yolk sac it very quickly comes to lie in the proximal part of the umbilical cord coelom which at that time has a wide communication with the body cavity (Text-fig. 4). As the embryo becomes folded off and the body cavity enlarges there would be a tendency for the umbilical loop to re-enter the intra-embryonic coelom, but it is maintained in or actually drawn farther into the exocoelom by the yolk stalk. The vitelline duct itself becomes obliterated, according to Politzer & Sternberg, between the 7 and 9 mm. stage, and soon after severs its connexion with the intestine. There, however, still remains attached to the ileal mesentery the mesodermal yolk stalk containing the vitelline artery which does not disappear till about the 15 mm. stage (Broman), and this is an important factor in the maintenance of the extra-embryonic position of the gut. In the present series of embryos there is no trace of a yolk stalk in the 7 mm. embryo, but it is present very definitely in the older 8-5 mm. specimen. In a rabbit embryo in which the gut has returned to the abdominal cavity, there is still present a mesodermal stalk connecting the mesentery to the umbilical cord. Broman states that in the cat and the seal the yolk stalk persists for a much longer period than in the human.
It has been suggested (Mall) that the rapidly developing liver “squeezes” the gut into the exocoelom, but the umbilical loop of intestine can be observed in its extra-embryonic position at a stage, e.g. 7 mm., when the liver occupies only a small proportion of the abdominal cavity. The later great extension of liver tissue combined with the narrow intercoelomic communication will certainly help to maintain the extra-embryonic position of the gut, but hepatic growth is not responsible for its initial production.
Why should the hernia occur?
The primitive gut or enteron is formed by an extension of the endoderm round the yolk mass. In amphibians, such as the frog, the enteron with enclosed yolk is within the body of the quickly formed larva. Where the yolk furnishes nourishment over a more prolonged period, as in elasmobranchs, reptiles and birds, it would obviously be impracticable to harbour this inert mass within a growing embryo, and so the original plan is modified—the yolk sac remains for a time extra—embryonic and lies within an extra—embryonic extension of the coelom. The large and heavy yolk sac acts as a drag on the intra-embryonic intestine to which it is attached, and this portion will be brought without the embryo to form the umbilical loop in the exocoelom.
In reptiles and birds the yolk sac is taken into the body of the embryo at a late stage———the umbilical cord coelom is incorporated in the body cavity and its somatopleure with the body wall. ' In placental mammals the role of the yolk sac is of comparatively minor significance and confined to the early stages, but it still plays a part, however transient, in the physiology of the embryo, and its extra—embryonic modification serves as a basis for still more elaborate nutritional preparations. As a concomitant of the extra—embryonic position of the yolk sac there is a portion of the definitive intestine in the umbilical cord coelom. The part played by the yolk sac is soon over; its further development or inclusion within the embryo would serve no useful purpose. It therefore atrophies and severs its connexion with the intestine which it leaves within the exocoelom.
The physiological hernia does not therefore occur a priori but rather is incidental to the extra-embronic position of the yolk sac. It is a by—product of a plan which utilizes part of the primary enteron as a storehouse for nutriment. In mammals much of this scheme is still further modified, but, as the presence of the hernia indicates, it is not altogether discarded.
Later changes in the form of the umbilical cord
In the stages immediately succeeding the formation of the umbilical cord there is an increase absolute and relative in the cranio-caudal length of its embryonic attachment, for whereas at 7 mm. this area is one-fourteenth of the total embryonic length it accounts for one-seventh of the total length at 8-5 and 12-5 mm. After this the rate of growth lags behind that of the embryo as a whole, and at 42 and 60 mm. the proportion is only one to twenty. The umbilicus can therefore be regarded as a relatively fixed area.
At the level of its proximal attachment the umbilical cord consists of the embryonic connective tissue of the intercoelomic septum cranially and the stalk mesoderm caudally, which, together with the lateral amnial plates, form the respective boundaries of the umbilical cord coelom. ' The relative proportion of cord structure formed by intercoelomic septum and coelomic portal undergoes a change as development proceeds. The former, which at an early stage forms more than half, is, in the older embryos, reduced to less than half of the sagittal diameter of the cord at its proximal attachment, while the latter is increased accordingly. This increase, however, is by no means commensurate with the enlargement of the more distal-cord coelom, and the result is a narrow bottle-necked intercoelomic communication. The intercoelomic septum is situated in the first instance with its long axis dorsi-ventrally, and the cord is “sun ” for quite a considerable distance into the embryonic body. With the ventral and caudal extension of the liver the intercoelomic septum is rotated through 90° so that its long axis is now in the cranio-caudal plane and the cord is “eased out” of the body of the embryo.
Dense embryonic connective tissue encircles the attachment of the cord. It forms an umbilical ring of mesodermal condensation surrounding the coelomic portal, and is present in the 16-1 mm. embryo, but more emphatically so in the 23 mm. embryo. The compact tissue first appears in the stalk mesoderm, situated superficial to the allantoic vessels. Cranially it lies ventral to the umbilical vein and on each side extends into the tissue of the lateral pillars of the cord bounding the coelom. When the myotomic downgrowths reach the ventral aspect their anterior portions, i.e. the sheaths of the recti muscles, become continuous with the tissue of the umbilical ring.
Ventralization of the Gut
It is generally agreed that there is a comparatively rapid passage of the umbilical loop of intestine into the abdominal cavity in embryos of from 40 to 42 mm. I have been unable to find described any intermediate stage with a. partial reduction of the gut, but nevertheless it is obvious, as stated by Frazer, “ that there is no question of the entrance of the contents of the sac en masse into the abdomen ”, but that the coils of gut “ slip back in a continuous movement”.
In the chick the large yolk sac together with the sac containing the intestine is taken into the body of the embryo about the 19th day. The distal wall of the yolk sac, according to Lille, is fused with the allantois. This part of the allantois has a muscular wall, and by its contraction initiates the propulsion of the yolk sac towards the embryo. The movement is completed by the pressure of the inner wall of the amnion which possesses an interlacing system of muscle fibres. The umbilicus is closed by the act of inclusion (except for a small central aperture) as the inner wall of the amnion is attached on the one hand to the body wall and on the other to the distal pole of the yolk sac.
There are no muscular fibres in the mammalian amnion nor any other means of exerting pressure on the umbilical sac from without. Therefore it must be assumed that there is an intra-embryonic influence sufficiently effective to overcome the resistance of the narrow inlet. This could be (A) of the nature of a direct pull, or (B) a suction force by the establishment of a negative abdominal pressure (i.e. negative relative to that in the exocoelom).
Under A have been cited (1) a rotation, and increase in size, of the intraabdominal loop of intestine (Mall); (2) shortening of the vitelline vessels (Rathke) ; (3) a dorsal pull exerted on the gut by a strong caudal growth of the liver (Broman).
A negative intra-abdominal pressure would be caused by the expansion of the abdominal cavity and diminished growth rate of liver (Frazer). Certainly coincident with the entrance of the gut there is a decrease in the size of the liver, but this might be the effect rather than the cause, as the pressure of additional gut would cancel the negative pressure and allow the liver to “contract”.
The study of this series of embryos has failed to reveal any additional factor which would further aid in a solution of the problem.
As far as the question lends itself to elucidation in terms of physical forces the evidence favours the creation of a negative abdominal pressure and a “ suction” influence rather than a direct pull.
To be reckoned along with any mechanical explanation, however plausible, of embryological phenomena, is the biological urge towards the completion of a specific form which finds its modern expression in the terms “organiser”, “growth centre”, etc., and which so far" has defied all effort to place it within any known hierarchy of physical laws.
Obliteration of the umbilical cord coelom and closure of the umbilical ring
The passage of the umbilical loop of gut into the abdominal cavity leaves a still open coelom in the umbilical cord, as has been observed in the 42 mm. embryo. Apart from this direct evidence of the presence of the empty exocoelom, any question of its incorporation in the embryonic body would be negatived by the following observations: (a) the extent of the cord coelom in the older embryos (23 and 40 mm.), (b) its prolongation into the distal cord, and (c) the bottle-necked coelomic portal.
The first step towards obliteration is probably a certain amount of collapse of the wide proximal cord coelom, then a degeneration of the endothelial lining (Pl. IV, fig. 16) would permit of the filling up of the space by the proliferation of contiguous fibrous tissue. Such an increase of connective tissue is strikingly apparent in the 60 mm. embryo, where there is not only a greater density of tissue, but it is of a more mature type. It is particularly concentrated in the region of the proximal attachment of the cord, and could be regarded as the result of increased activity of the ring of fibrous tissue already described. This proliferating zone is responsible for the closure of the umbilical ring and forms a collar encircling the base of the cord and extending into the abdominal wall. Above and below it plays a part in the formation of the supra- and infraumbilical portions of the linea alba, and is continued into and reinforces the rectus sheath on each side.
It is perhaps of some significance that in the human and probably other placental mammals the umbilical cord as in some lower vertebrates makes its contribution to the body wall, and that this contribution traced to its ultimate source is a derivative of the primary mesoderm.
Congenital herniae due to maldevelopment of the umbilical region Recorded in the literature are many and varied forms of congenital deficiencies of the anterior abdominal wall with a correspondingly formidable array of names and a somewhat confusing choice of classification. The Formation of the Umbilical Cord 307 Ectopia vesicae and absence of an anterior abdominal wall (eventration) have already been discussed (1937, 1938), and here remarks will be confined to herniation which results from maldevelopment of the umbilical cord and neighbouring parietes.
A. Arrested formation of the umbilical cord
In the Hunterian Pathological Collection of Glasgow University is a specimen (no. 50-113) of a 6 months foetus labelled “Hernia into the umbilical cord”. The greater portion of the abdominal wall is occupied by a large thinwalled sac about the size of a hen’s egg which contains most of the abdominal viscera. A stalk or cord arises from the apex of the sac. There is no neck to the sac, but a wide circular aperture opens into the body cavity proper: this is the Exomphalos of Fraser’s classification. In the specimen there is a supra- and infra-umbilical portion of the abdominal wall and some attempt at closure of the somatic stalk to form an umbilical cord (cf. cases of eventration), but the bandyof mesodermal condensation normally present at the embryonic attachment of the cord is absent, and in consequence there is no proper umbilical ring and the coelomic portal has expanded pari pass-u with the exocoelom.
B. Persistence of the physiological hernia
Specimen no. 50-112 of the Hunterian Collection is also labelled “Hernia into the umbilical cord”, but is an entirely different condition from the preceding example. The large sac, which has been opened, contains the major portion of the gut including the caecum and appendix, and communicates with the intra-embryonic coelom by a narrow constricted neck. The intestinal wall is firmly adherent to the upper rim of this umbilical ring. Thunig thinks that adhesion of the intestine to a patent umbilical ring is a primary aetiological factor in persistent physiological hernia. It is equally possible that a premature narrowing of the coelomic portal by a precocious proliferation of the fibrous tissue of the umbilical ring might defeat attempts at reduction. With the growth of the contained gut and a further relative constriction of the neck a condition akin to incarceration would exist, and secondary inflammatory adhesions develop.
It should be noted that as the caecum and appendix are normally the last parts of the gut to enter the abdominal cavity they will perforce be included in the hernial sac in all cases of the above condition.
C. Non-closure of the umbilical ring
If the umbilical ring remains patent there may be a persistent umbilical cord coelom, or a new peritoneal sac may be formed. In either case the entrance into the sac of a loop of bowel will result in a hernia into the umbilical cord which, of course, occurs after the reduction of the physiological hernia. Such a hernia may be symmetrical or asymmetrical, depending on the position of the allantoic stalk in the cord. Clinically this is the commonest type of umbilical hernia and is the “ simple umbilicalhernia” of Schwalbe, the “ small umbilical hernia” of Politzer 85 Sternberg, the postnatal umbilical hernia of Fraser, and includes “congenital hernia” into the cord and infantile hernia (R. Maingot).
It has been shown that the closure of the umbilical ring is effected by a proliferation of the encircling band of compact mesoderm. Partial failure or absence of this proliferation would leave a patent umbilical ring—a potential hernial site.
Defects of the parietes in the umbilical region
From observations on the older embryos of the series, one concluded that there is a contribution of mesoderm from the tissue of the umbilical cord to the abdominal parietes.
The linea alba immediately above and below the cord attachment and the sheath of the rectus muscle on each side are formed by a fusion of the anterior portion of the myotomic downgrowths with connective tissue from the compact condensation round the base of the umbilical cord. It is suggested by Schwalbe that partial deficiencies of the abdominal wall are due to a mesodermal failure of one or two particular somites related to a scoliotic deformity of the vertebral column. Difficult to reconcile with this view is the fact, also stated by Schwalbe, that the abdominal muscles are normal and the recti form the lateral boundaries of the parietal deficiency.
A defective proliferation or insufficiency at one or other point of the umbilical ring of compact mesoderm would result in a weakness of the corresponding part of the umbilical region of the abdominal wall. This might affect the linea alba above or below the umbilicus, or the immediate lateral parietes—all of them sites of potential hernia. Such a condition has been variously designated as amniotic umbilicus (Tennant), amniotic linea alba (Thunig), or, where a hernia has developed, amniotic hernia (Freshman). An aggravation of the deficiency in the subumbilical region is exemplified in ectopia vesicae, while its counterpart above would cause a condition approaching partial eventration. Perhaps the most common result of such a parietal defect is a small asymmetrical hernia above or to one or other side of an apparently normal umbilical cord.
In each and all of the above malformations leading to herniae in this region of the abdominal wall, one is of the opinion that the initial error or fault can be traced to that mesoderm which by its proliferation forms the amnial tissue plates of the umbilical cord, closes the umbilical ring, and contributes to the umbilical part of the abdominal parietes. The earlier the flaw the more serious the consequences, as in severe forms of exomphalos, while its later occurrence is the aetiological factor in the minor defects of “ small umbilical ” or “ amniotic ” herniae. Is it of more than passing interest that in deficiencies of the anterior abdominal wall the offending tissue is the mesenchyme of the early embryonic rim—the site of fusion of primary and secondary mesoderm?
Summary
- The umbilical cord is formed by the growth of lateral tissue plates which extend on each side from the allantoic stalk to the septum transversum.
- The umbilical cord coelom enlarges by the extension of the endothelial lining into the large spaces in the cord tissue formed by the cofluence of smaller spaces.
- The temporary physiological herniation of a part of the gut is a concomitant of the extra-embryonic position of the yolk sac which itself is an expression of a modification to meet nutritional requirements.
- The observations support the suggestion already offered by Frazer that the return of the gut into the abdominal cavity is dependent on a “negative” intra-abdominal pressure.
- The obliteration of the umbilical cord coelom is effected by a proliferation of the denser fibrous tissue which forms an encircling collar at the embryonic attachment of the cord.
- This circular zone of mesodermal condensation contributes to the linea alba above and below, and to the rectus sheaths on each side of the umbilicus.
- Some fault of the junctional mesoderm, i.e. the tissue at the embryonic rim where there is union of primary and secondary mesoderm, is responsible for congenital herniation which results either from maldevelopment of the cord itself or from deficiency of the neighbouring parietes.
I have again to record indebtedness to my Chief, Prof. Blair, for his continued guidance and help. Dr P. Bacsich was consulted on several questions of histological interpretation.
The 42 mm. embryo was kindly given on loan by Prof. T. Nicol and Dr Gladstone, of King’s College, London.
References
BROMAN, I. (1932). “Das Ratsel des Physiologischen Nabelbruches.” Verh. amt. Gee. Jena, Heft Lxxv.
FLORIAN, J. (1930). “The formation of the connecting stalk and the extension of the amniotic cavity towards the tissue of the connecting stalk in young human embryos.” J. Anat., Lo'nd., vol. LXIV.
FRASER, J. (1926). The Surgery of Childhood. London: Arnold.
FRAZER, J. E. (1931). Manual of Embryology. London: Bailliere, Tindall and Cox.
FRESHMAN, E. (1933). “Congenital umbilical hernia.” Lancet, 23 September.
GORELOW, M. A. (1934). “Zur Anatomic des Nabelkanals.” Arch. klin. Chir. No. cLxxxI.
KERR, J. G. (1919). Text Book of Embryology, vol. II. London: Macmillan and Co.
LILLE (1908). Development of the Chick. London: George Bell and Sons.
MAINGOT, R. (1936). Post-Graduate Surgery. Medical Publications Ltd.
MALL, F. P. (1897). Haudbuch d. Entwgesch. des Menacheu. Bd. II.
POLITZER, C. & STERNBERG, H. (1930). “ Uber die Entwicklung der ventralen Korperwand und des Nabelstranges beim Menschen.” Z. gee. Anat. 1. Z. Amt. EntwGeach. Bd. xcn, Heft 4. (1931). “Beitriige zur Pathologischen Anatomie und zur Allgemeinen Pathologie.” Beitr. path. Amt. Bd. Lxxxvm.
SCHWALBE, E. (1909). Die Morphologie der Missbildumgen, Bd. 111, Heft 1.
STERNBI-zleo, H. (1927). “Beschreibung eines menschlichen Embryos mit vier Ursegmentpaaren.” Z. gee. Anat. 1. Z. Anat. EntwGesch. Bd. Lxxxn.
TENNANT, R. (1921). “Hernia into the umbilical cord.” Brit. med. J. 19 February.
TI-IUNIG, A. (1936). “Hernia into the umbilical cord, and related anomalies.” Arch. Surg., Chicago, vol. Xxxm.
WYBURN, G. M. (1937). “The development of the infra-umbilical portion of the abdominal wall, with remarks on the aetiology of ectopia vesicae.” J. Arum, Lo'nd., vol. LXXI.
—— (1938). “The development of the supra.-umbilical portion of the anterior abdominal wall.” J. Arum, L¢md., vol. Lxxn.
Explanation of Plates
Plate I
Figs. 1, 2. Photomicrographs of sections of embryo 2.6 mm. x circa 60. Figs. 3, 4. Photomicrographs of sections of embryo 4.5 mm. x circa 60.
A. = amnion. L.T. = lateral tissue plate. U. = umbilical vein. C. = coelom. Description in text.
Plate II
Figs. 5, 6. Photomicrographs of section of embryo 7 mm. x circa 18. Fig. 7. Photomicrograph of section of distal part of umbilical cord of embryo 7 mm. x circa 85. Fig. 8. Photomicrograph of section of embryo 8-5 mm. x circa 18.
U .0. =umbilical cord coelom. I . =intercoelomic septum. D. = distal slit-like portion of umbilical cord coelom. S. = space in umbilical cord. U. = umbilical vein. Description in text.
Plate III
Fig. 9. Photomicrograph of section of embryo 125 mm. x circa 12.
Fig. 10. Photomicrograph of section of embryo 14 mm. x circa 12.
Fig. 11. Photomicrograph of section of embryo 16-1 mm. x circa 11.
Fig. 12. Photomicrograph of section of embryo 23 mm. x circa 11.
U . = umbilical vein. I .= intercoelomic septum with denser mesoderm extending into cord. M. = myotomic downgrowth.
Description in text.
Plate IV
Fig. 13. Photomicrograph of section of embryo 40 mm. x circa 6.
Fig. 14. Photomicrograph of section of embryo 42 mm. x circa 7.
Fig. 15. Photomicrograph of section of umbilical cord coelom of embryo 42 mm. Site indicated by arrow in Fig. 14. x circa 75.
Fig. 16. Photomicrograph of endothelial lining of umbilical cord coelom of embryo 42 mm. x circa 225.
E. = denser mesoderm extending into cord “pillars” and continuous with rectus sheath. U.C. = umbilical cord coelom. L. = degenerating endothelial cells. Description in text.
Plate V
Figs. l7—20. Photomicrographs of sections of the ventral body wall of embryo 60 mm. x circa 6. B. = linea alba formed from rectus sheath. E. = denser tissue of midventral line extending into cord. F. = denser tissue on superficial aspect of allantoic vessels. Description in text.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The formation of the umbilical cord and the umbilical region of the anterior abdominal wall. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_formation_of_the_umbilical_cord_and_the_umbilical_region_of_the_anterior_abdominal_wall
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G