Paper - The early embryology of the auditory ossicles in man
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hanson JR. Anson BJ. and Bast TH. The early embryology of the auditory ossicles in man. (1959) Q Bull Northwest Univ Med Sch. 33: 358-379. PMID: 14399619
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Early Embryology of the Auditory Ossicles in Man
Hanson JR., Anson BJ. and Bast TH.
Antecedent phases of all otological investigation, carried out at Northwestern University and the University of Wisconsin dealt with developmental stage in which the otic capsule, the auditory ossicles and the related brachial arches were well formed in cartilage.
As a recent supplement to these more general discussions, three articles have offered detailed information on the morphogenesis of the otic capsule, the stapes and the incus (with atlas arrangement of figures). A fourth article, comparably concerned with the malleus, is in preparation.
- The illustrations were prepared from the following series in the G. L. Streeter Collection. Carnegie Laboratory. Bnltimore. Figs. I and 2. Series 617; Figs. 3 and 4. 6517; Fig. :'..).,9; Fil(. (j. 6.'j2~ ; Figs. 8 and n, H aO; und froll1 the T. 1-1. BURt Collection. Clli\"cl" it.\' of \\"is("onsin. Fig. 7. seliC's 180; Fi"•. lOund 11. 10; Fig. 12, 17~; Figs. I~. 1·1. I .; u nd 16. I ·; Fig. 17 . 168; F ig. 18. 29 ; Fi l(. 19. B 12~; !'ig. 20u. 1:3; Fi". 20b. 29.\ ; Fig. 20c. (;2 : Fig. 20d . 9S.
It now remains to report upon the developmental steps, taken before the stage of 28 mm, in which localized portions of the mesenchyma in the branchial region suddenly become distinguishable from the tissues of which they are primordially a part and, at the same time. assume an appearance and a position predictive of their adult form and topography.
In order to determine accurately the origin of the ossicles it is necessary to examine them prior to the aforementioned stage, beginning with specimens in which the future ossicles and the related branchial arches have not reached pre-cartilaginous constituency. In the past, investigators attempted to explain the origin of the ossicles without benefit of early embryos. In such studies. limited as they were to the use of embryos in which the branchial arches and their derivatives were already composed of cartilage, conjecture inescapably took the place of fact in the attempt to account for the genesis of the auditory ossicles in man.“
Microscopic sections used alone are of limited value in following the rapidly changing architecture of the branchial arches, but reconstructions provide the needed aid in determining what contribution the mesenchymal condensations give the developing structures.7 The following discussion will be based chiefly upon the use of such “models.”
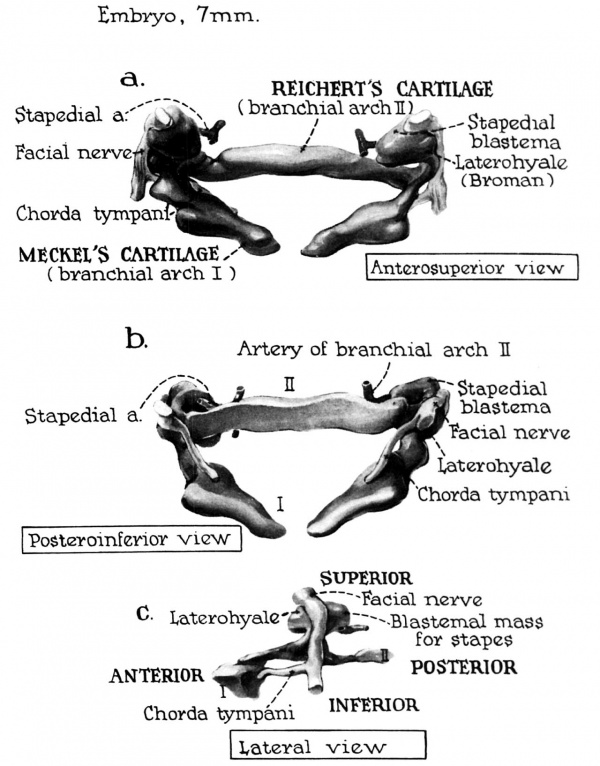
In the fifth week of fetal life the stapes is not yet a definitive portion of a blastemal lobe which is situated at the proximal extremity of the hyoid (Reichert’s) bar (fig. I). This lobe is grooved by the facial nerve. which serves to divide it into a stapedial primordium and the laterohyale, both of which temporarily retain hyoid continuity (fig. 2). In the Sl.t'i-mm. stage the division is more definitely marked. The portion which connects the above-mentioned structures is called the interhyale (fig. 3) ; it will give rise to the stapedial tendon.
In the earliest stages the two visceral bars are broadly connected by an inter- branchial bridge of mesenchyme (figs. l :m(l 3). the greater part of which is in the territory of the second arch as indicated by its relationship to the pharyngeal g1'oo\'e (figs. 3 and -I). This blastemal mass is located between, and continuous with. the middle segment of the hyoid bar and the proximal portion of the mandibular (.\Ieckel’s) bar. This circumstance indicates that the man- ubrium of the malleus and long crus of the incus are mainly of second arch origin.
In the 8-mm. specimen. which is more advanced in ossicular development than the 9.1 mm. stage. the interbranehial bridge is separating from the hyoid bar along a sulcns which transmits the chorda tympani (fig. .3). Separation is complete in the 11.7-mm. stage. at which time a secondary connection between the arches is formetl as the incus. now of distinguishable outline. comes to approximate the stapes (fig. Iii).
During the fifth week the stapedial artery attains a central position in the blastemal lobe. thereby seeming to aid in the conversion of the rounded mass into a ring-like structure with an obturator foramen (figs. 5 to 9). Occasionally a ring fails to form. and an anomalous stapes is the result: the l1ol'sesl1(ie-sllapetl stapes in a l-l-mm. embryo is an example of this defect (fig. 7).
The malleus and incus in the l.3..") mm. embryo. although still precartilaginous. become demarcated as the mannbrium and long crus begin to assume char- acteristic form and as a gl'o0\'t‘ appears between the head of the malleus and body of the incus (fig. 8). At this stage the ring-shaped stapes impinges upon the otic capsule. The laterohyale and short crus of the incus end in loose ad\'entitia (fig. 9). In the 17-min. embryo. both the incus and malleus are clearly iden- tifiable as chondrified structures. The malleus temporarily remains in contin- uity with the mandibular (.\leckel’s) bar (fig. 10).
The interhyale passes from the junction of the stapes and incus to the hyoid bar (fig. 11) where the latter makes an acute angle to become the laterohyale (in contact with capsular tissue). The primordium of the stapedial muscle is identifiable in the 21-mm. stage (fig. 12) as an outgrowth from the interhyale. located posterior to the angle of Reichert 's cartilage and medial to the facial nerve (figs. 13 and H). The tendon of the stapedial muscle is derived from the interhyale in the portion between the developing stapes and Reichert’s bar (figs. 15 and 1(3). The stapedial ring now fuses with the otic capsule. the conjoined tissue thus becoming the lamina stapedialis. The periphery of the lamina. through (litferentiation of its tissue. will become the annular ligament. The zone of change is the site of the future vestibular fenestra (fig. 15>.
continuity between the interhyale (seemingly the primordium of the stapedial muscle as well as the tendom and the laterohyale is lost in the ll)-mm fetus: at this stage the constituent cells begin to assume the appearance of muscle (fig. 17).
As the muscle develops and the incudo-stapedial joint is formed. the tendon usually gains an attachment to the neck stapes. However, the frequent partial insertion of the stapedial tendon into the incus (figs. 18 and 19) supports the belief that the long crus of the incus. as well as the stapes, is a derivative of the second arch.
Both of the cartilaginous arches contribute not only to the s-ubstance of the atulitory ossicles, but also to that of related structures in the head and neck.‘
While the inalleus is undergoing ossification, the zone of continuity with Meckel’s cartilage narrows rapidly (fig. 20a). This alteration in form is due t.o a retrograde change in the cartilage which precedes differentiation of the tissue into that of the future anterior ligament of the mallcus. As soon as bone is formed in the head and neok of the mallcns the anterior process (an osseous rod before bone-formation begins in the ossicle itself) fuses with the malleus (fig. 20b). Distally the cartilage will be
ll)S()l'l)(’(l in the inaudible. Reichert’s cartilage passes through an even more dramatic series of changes in attaining adulthood. At its proximal e.\;tremit_v. that part of the cartilage which is (lcrived from the laterohyale of the
early embryo forms :1 transito1'_v lateral boundary for the developing facial canal (fig. 200). By the 215-mm. stage, how- ever, membrane bone is developing internal to the -artilage-—thus interven- ing between cartilage and nerve (and stapedial muscle, derived from the interhyale). Concurrently, the otic capsule is ossifying. In early infancy, Reichert’s cartilage is merely a remnant within new bone, and the facial canal has acquired new and permanent walls (fig. 20d). I)istally the tissue of the original arch is converted into the styloid process of the temporal bone, the stylohyoid ligament and the lesser cornu of the hyoid bone.
The 2 illustrations appear on the succeeding pages.
Fig. 1. In the embryo of 4.5 weeks (7 mm) the precursor tissue for the auditory ossicles is a common blastemal mass which is distinguishable from the surrounding mesenchyma only because of the greater concentration of its constituent cells (compare fig. 2).
A lobe-like portion of this mass is localed at the cmnial end of the second visceral bar (precursor of Reichert's cartilage), where it is grooved by the facial nerve. Part of this mass is the primordium of the stapes. However, it is still separate from the aggregation of which will become the otic capsule (the latter not included in the reconstruction). The stapedial artery extends from the source-vessel to encroach upon the interiomedial aspect of the blastema (fig. la); the chorda tympani branches from the facial nerve to pass medial to thefirst visceral bar, the forerunner of Meckel's cartilage (fig. 1b).
A mesenchymal bridge of cells between the first and second visceral bar extends from the proximal end of the mandibular bar (at I in fig. 1c) to a point on the hyoid bar (at II) just distal to the blastemal lobe of the stapes (fig. 1c). The primordia of the malleus and incus are not yet distinct structures within this condensation of cells which will soon give rise to them.
File:HansonAnsonBast1959 fig03.jpg
Fig. 3. In the embryo of 5 1/3 weeks (0.6 mm.) the branchial apetures have increased greatly in
size through hyperplasia of the mesenchymal cells amt the outlines of the ossieutar prinmwlia hare
become more clearly defined.
The second visceral bar projects farther tou'ar(t the otie labyrinth than /he first anrl has at its pro.ri- mal extremity the blasternal lobe with the ])I"tIlt()l‘(llltIIt of the stapes. The _,"aeial nerre has groom'Il the lobe more deeply, the su,lcns serving to mark ofl more ctistinetly the stapedial from the laterohyale portion. The primorzti um of the stapes is still separated from the otie capsule (not shown) by a zone of cells which is less dense than the /nesenehyme of either the sttlpes or the capsule. The general location of the stapes (lateral to the otie capsule and medial to the faeial nerve) is one I('h.ieh persists throughout fetal life and into adulthood.
Although a stapedial artery is not yet centrally l0('(Il(‘(l within the blas/ema, an artery is fonml (not shown in the reconstruction) at the periphery of the lobe as it iras in the 1‘-nun. stage.
The malleus and incus are not yet tlifferentiatett from the interhran.ehiat bridge of mesenehynu- which now broadly connects the two visceral bars aeross the first pharyngeal groore (jig. Jr at arrmr). Since the groove is the boundary externally between the arehes (I am! I I ), rlmlble origin of the two ossicles is thereby indicated.
The chorda tympani, branching from the facial nerre, passes from the hyoltl arch (I I ). in_t'eri¢.-r
to the blastemal mass, into the mandibular arch ( I L It appears as if the nerve were playing an important role in the separation of the blasterna from the serond I'isceral hm‘.
File:HansonAnsonBast1959 fig04.jpg
Fig. 4. The first pharyngeal groove makes a deep impression on the lateral aspect of the blastemal mass zrhieh is established as the primary connection between the first and second visceral arches. The first visceral bar is located closer to the groove than is the second bar, meaning that the major portion. of the cellular mass belongs to the second arch.
Although no longitudinal grooves or diflerences in cellular density exist to distinguish the primordium of the malleus from that of the incus, the relationships to adjacent structures and the course of the chorda tympani (about to branch from the facial nerve) are indications that this mesenchymal mass is the source-tissue of both of these ossicles.
.-is seen in neighboring sections, the chorda tympani, after branching from the facial nerve, passes medial to the pharyngeal groove and into the first visceral arch, where it joins the mandibular branch of the trigeminal nerve. The pathway of this nerve from the lateral to the medial side of the blastema serves to separate the primordium for the malleus and ineus from the second visceral bar. A large portion of this mass is located on the second arch side of the pharyngeal groove, suggesting that most of the primordial tissue which will develop into the manubriusm of the malleus and long crus of the incus comes from mesenehymal cells which are of second arch origin. This contradicts the usual belief that the malleus and incus are derived entirely from the first arch,
Fig. 5. In the 8-mm. (5-week) embryo branchzfal devclagtnzent zs athvtrtcert over that of the -.'I.t2'-mm. stage in respect to the followirztg: deeper entry of the stapcdzal artery veto the .s't(Iped'tal aulage; _t:_1rth‘¢-r separation of the laterohyale from the stapes by the faeml nerve; (l[IIIO.s't complete rupture of contuuuty of the intterbmnchial bridge adjacent to the second ms-coral bar (througlathe lIt.fl'lt(?7lC€ 0} the chnrda tympani). Ifhether the 8-mm. specilnen is adranccrl, 07‘ thc .’t.f>‘-nun. spcczlncrr ‘cs retarded zn I[¢'t‘('[0p- ment, cannot, of course, be deternn'm-rt.
Fig. 7'. In. the embryo of 14 mm. (6%; -weeks), although general advancement in development is
evidenced, the stapes has not attained the form of a ring; it has been grooved by the artery, but has
assumed the form of a horseshoe. This observation would indicate a congenital origin for the excessirely thinned and malformed ossir'les frequently seen (luring emlaural surgery.
The interbranchial bridge remains on the right side, but the continuity on the left side is broken: only a remnant of this connection remains (fig. 7c). The interhyale is a rather prominent link between the stapes and the hyoid bar (fig. 7a). A groove has begun to separate the malleus from the incus, but the latter is not yet in contact with the stapes.
Fig. .9. The precartilage stage -is reached in the embryo of 15.5 mm. (7 weeks). However, only the distal portion of the first and second visceral bars (n'e so adeanced; the pro.cirnal portions of both bars and the prirnordia of the ossicles are still com posed of condensed mesench_I/me (more compact than in the previous stage). The stapes is now definitely annular in form, with a distinct obturator foramen whose margin is separated from the contained stapedial arterg by a less dense zone of mesenchyme.
The crura of the stapes are round in cross—section. The stapes itself is annular. The medial part seems to be imbedded in the precursor tissue (precartilage) of the developing otic capsule. It is as if this annular collection of cells, by pressing inward against the otie capsule, were causing capsular cells to condense in the territory of the future lamina stapedialis ( which will contribute to the base of the stapes and the annular ligament).
The facial nerve passes between the laterohyale and the stapedial ring just above the level of the interhyale. The laterohgale and the short crus of the ineus (that is, its Iuesenchymal forerunner) end freely in loose mesenchymal tissue; they are not get in contact with the otic capsule. In the period between this and the preceding stage the i nterh gale has undergone further decrease -in size.
thy. I I). I1’ _I/ the 1 7-mm. ()"-week) stage, the mandibular and hyoid bars have developed into cartilage
through the q/‘eater fraction. of their length, and hence may be properly termed Meckel’s and Reichert’s
carttlagcs. The prozrimal e.rtreImt_I/ of each. bar and the ossteles are still composed of precartilage;
/IOII‘(‘l’(‘,I', the malleus and turns are more rtistinctty outlined ~— and less a part of the mesenchyma
from urhich they were deriI'ed.
The taterohg/ale and the short crus of the incus approximate and fuse to the under surface of the rleI'etop't'n_q capsule between. the 15.5 and 17-mm. stages (fig. 10a, at reader's left). The secondary nature of this c0ntin.I1,it_I/ with the (tevelomng capsule means that the capsule makes no contribution to these prz'numI2'a.
Fig. 11. Although the interhyale has become considerably narrower in the embryo of 1?’ mm. (7 weeks), it still serves as a definitive connection between the visceral bar (Reichert's cartilage) and the stapes. The second visceral bar may be regarded as bifurcating at its proximal extremity, the diver- gent parts continuing as the laterohyale and the interhyale, with the facial nerve passing over and between. them. The laterohyale is now continuous with the capsule and the interhyale is (as it was from the beginning) continuous with the stapes. The size, shape and relationships of the interhyale have changed markedly since its difierentiatioen from the blastemal lobe of the 7-mm. embryo; its mes- cnchymal composition has not yet been altered, despite the fact that it will soon assume the ‘role of tendon for the stapedial muscle.
The otic capsule, continuing its increase in size, shows transition from mesenehyme to preeartilage to cartilage. The stapedial impression into the capsular wall is thus increased by diflerential growth, but the division between the annulus stapedialis and lamina stapedialis remains apparent. The cells of the stapes, however, blend with those of the incus, there being no articulation as yet at the im-mlo- stapedial junction.
A small stapedial artery (arrow) is seen medial to the stapedial ring; it can be traced through the obturator foramen to its source lateral to the ossicle.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The early embryology of the auditory ossicles in man. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_early_embryology_of_the_auditory_ossicles_in_man
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G