Paper - Factors Involved In The Formation Of The Filum Terminale
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. Factors involved in the formation of the filum terminale. (1919) Amer. J Anat. 22(1): 1-11.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Factors Involved In The Formation Of The Filum Terminale
From The Department Of Embryology, Carnegie Institution of Washington, Baltimore, Maryland.
Three Text Figures
Introduction
In a study recently published by the writer[1] on the development of the cartilaginous capsule of the ear in human embryos it was pointed out that the changes in size and form which the capsule undergoes during its development are accomplished not only by a progressive differentiation, but also in part by a retrogressive differentiation of its constituent tissues. The margins of the cartilaginous cavities are in a continual state of change; they exhibit an unstable equilibrium between two opposing tendencies: on one hand, toward the deposit of new cartilage, and on the other, toward the excavation of the old. The margins thereby are always advancing; or receding, and as a result of this there is provided a suitable suite of chambers for the contained membranous labyrinth in all stages of its development.
It is the feature of retrogressive differentiation or dedifferentiation that I wish particularly to recall here. The fact that certain areas of cartilaginous tissue'revert to an earlier embryonic type and are subsequently redifferentiated into a tissue of a Widely different histological character, is very clearly shown in the case of the otic capsule, and is a factor of great embryological significance. Such a process of retrogressive change, combined with redifferentiation of the same tissue, greatly increases the facilities for and the range of certain structural adjustments that occur in many regions in the development of the human embryo.
Another instance of dedifferentiation has recently been pointed out by Kunitomo.[2] This writer has published the results of a careful study of the tail region in alarge number of human embryos, representing the period of greatest development of the caudal appendage, and also the later period of its gradual reduction. He shows that in very young specimens the spinal cord reaches the extreme tip of the tail and throughout its length is quite uniform in structure. Somewhat later (11 to 15 mm stage) it can be divided at about the level of the thirty-second vertebra into two parts—a cranial or main part, having a Wide central canal and thick walls in which can be recognized well-developed mantle and marginal zones, and a caudal slender part, having a narrow canal with walls consisting only of an ependymal zone. Kunitomo shows that it is this caudal atrophic portion that eventually forms the filum terminale. The main part lying cranial to the thirty-second vertebra undergoes uninterrupted and progressive differentiation, whereas the portion caudal to this undergoes regressive changes and, with the exception of the extreme tip, finally becomes converted into a fibrous strand, the tip forming the coccygeal medullary vestige. This, therefore, is another instance in which an absorptive adjustment is brought about by the reversion of the tissue to an earlier embryonic type with a certain amount of subsequent redifferentiation.
Kunitomo further calls attention to the fact that in the formation of the filum terminale, in addition to the dedifferentiation
of the caudal end of the medullary tube, there is also the
mechanical disproportion between the growth of the medullary
tube and that of the vertebral column. How much of one and
how much of the other of these two factors is responsible for the
further development of the filum terminale Was not determined
by him. It has occurred to the writer that this question could
be answered by the determination of‘the elongation of the nerve roots. ln the younger stages the spinal cord and the vertebral
column lie alongside of each other in a metameric manner, corresponding in position segment for segment. Owing to their
disproportion in growth, there occurs a relative displacement of
their segment levels, so that, for instance, the thirtieth segment
of the cord comes to lie opposite the twentieth segment of the
vertebral column. The segment levels of the vertebral column
are, of course, evident; in the spinal cord they are just as plainly
marked byithe attachment of the nerve roots, for these become
attached to the cord before the displacement begins, and thus
permanently mark the various segmental levels. In the case of
each segment of the spinal cord there are two fixed topographical
points: the spinal ganglion, Which is held in the intervertebral
foramen and registers the original position of the segment relative
to the vertebral column, and the place at Which the dorsal root
is attached to the cord and Which moves as the cord moves. By
locating those points for the different stages one can determine
the exact elongation of the nerve roots, and this in turn is the
index of the relative displacement of the spinal cord as regards
the vertebral column. Conversely, it Will be seen that the
alteration not explained by mechanical displacement must be
attributed to the retrogressive changes referred to above. The
determination of the amount of displacement Was made by
comparison of selected stages by means of profile reconstructions
of the smaller specimens and actual dissection of the older ones.
I Was assisted in this by Mr. James F. Didusch, of the Carnegie
Embryological Laboratory, Who made careful dissections of these
structures in several older fetuses, two of which Will be used for
illustration. The results of this determination are given in the
following note as a matter of interest to those Who have read
the paper by Kunitomo, and also because it offers an opportunity
to emphasize the significance of dedifferentiation of tissues in
the processes of development in the human embryo.
The part played by dedifferentiation in the caudal region of the spinal cord is more apparent in the younger stages of development, as pointed out by Kunitomo. The so-called ‘absorption’ of the tail is completed before the embryo reaches a length of 30 mm. It is also well known that the remodeling which takes
place in the gill region completes the obliteration of the gill bars
before the embryo is 20 mm. long. One well might expect these
processes of dedifferentiation and redifferentiation to be more
active in the earlier stages. They are not confined, however, to
this period, for in the case of the ear capsule they were found to
be very active throughout fetal life. In the case of the spinal
cord dedifferentiation is well demonstrated in the period represented by embryos between 11 and 30 mm. long. A comparison
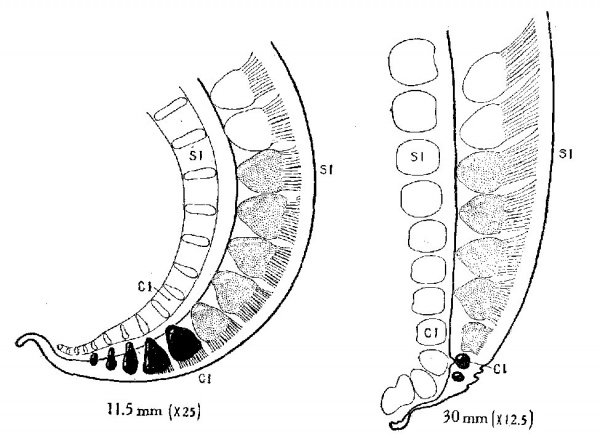
of these two stages can be made in figure 1. It will be noted in
the first place that the spinal ganglia show a regression varying
from arrest in development to complete disappearance. All but
two of the coccygeal ganglia have disappeared in the 30-mm.
specimen, and the remaining two are of about the same size as
the same two ganglia in the 11.5-mm. specimen.[3]
As for the cord itself, the changes are equally marked. In the
younger stage (embryo 11.5 mm. long) the extreme caudal end
of the spinal cord, the part belonging to the non—vertebrated tail,
shows little differentiation, consisting only of indifferent cells
resembling embryonic ependyma. In the coccygeal region, however, the development is more advanced. Opposite the five
coccygeal ganglia the wall of the cord is differentiated into distinct ependymal, mantle, and marginal zones, with well—developed
rootlets entering into it from the first two ganglia. Sections
through it show nothing to indicate that this region is not going
on to complete its differentiation into the adult condition. When,
for comparison, one examines the very same region in the older
specimens (fig. 1, embryo 30 mm. long) it is found that its condition, relative to the remainder of the cord, has undergone a
marked change. While the precoccygeal cord has continued to
increase in the thickness of its walls and in the elaboration of the
mantle and marginal zones, the coccygeal region is less advanced in its development than it Was in the younger stage. Whereas in
the 11—mm. embryo there existed, a distinct elaboration into
ependymal, mantle and marginal zones, the mantle zone is completely missing in the 30—mm. embryo, and We find thin Walls
consisting only of ependymal cells covered by a thin marginal
zone. The coccygeal spinal cord in the 30—mm. embryo is in an
earlier embryonic state than that of the 11.5—mm. embryo; that is, it has undergone dedifferentiation. In later stages the proc-
ess goes still farther and, as has been pointed out by Kunitomo,
this ependymal tube eventually becomes converted or redifferentiated into a fibrous strand.
Fig. 1 Profile reconstructions showing the spinal ganglia and their dorsal roots in the tail region of the human embryo. The last two lumbar ganglia are shown in White, the saéral ganglia are stippled, and the coecygeal ganglia are solid black. It will be noted that in the period included between these two stages marked regressive changes have aflected the entire coccygeal region of the spinal cord, with complete disappearance of the last three eoccygeal ganglia, in sharp contrast to the sacral region of the cord, which undergoes uninterrupted development. The reconstructions are taken from embryos No. 544, 11.5 mm, long, and No. 75, 30 mm. long, belonging to the Carnegie Collection.
How much of the spinal cord is involved in this retrograde process can be seen by comparing the two stages shown in figure 1. In the region of the attachment of the fifth sacral nerve the Wall of the cord remains thick and develops a Well-differentiated mantle zone. About opposite the first coccygeal nerve in the 30-mm. embryo the mantle zone abruptly disappears, and there is a corresponding enlargement of the lumen of the cord, thereby producing the thin—Walled ventriculus terminalis. There is some variation in different embryos as to the segmental level caudal to which the mantle zone has dedifferentiated and also in the manner of transition from the well-developed sacral cord into the atrophic coccygeal cord, including sometimes the doubling or partial obliteration of the central canal. The transition is quite abrupt, involving only one segment. In the 30-mm. embryo in figure 1 the cord at the level of the first coccygeal nerve shows some decrease in the size of its mantle zone area. Opposite the second coccygeal nerve the mantle zone is entirely gone. The second coccygeal ganglion present in this case Would probably soon have disappeared.
The ventriculus terminalis at this stage tapers caudally and
may be said to extend to the third coccygeal segment. Caudal to
this the differentiation of the cord is more complete and results
in the gradual obliteration of the lumen and the replacement of
the ependymal substance by a fibrous strand, embedded in
which can be found isolated groups of persistent ependymal
cells. At its extreme tip there is often found a more or less
detached group of such cells which undergoes cystic enlargement
and constitutes the coccygeal medullary vestige. The interval
of cord lying between this and the ventriculus terminalis constitutes what is later known as the filum terminale. Thus far
itsformation is based upon the process of dedifferentiation; its
subsequent growth and elongation is accomplished by an interstitial increase of its constituent fibres, and not by the further
invasion of the process of dedifferentiation into the sacral region
of the cord. This Will become evident on examination of
figure 2.
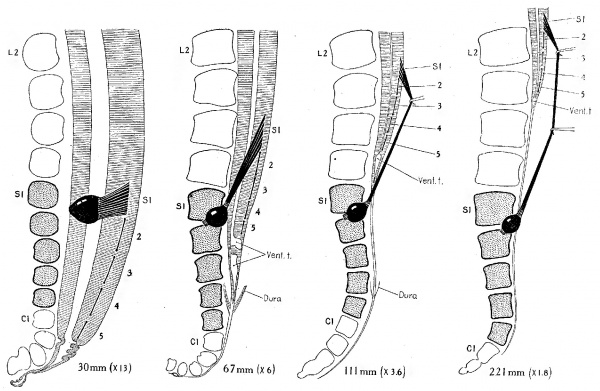
Fig. 2 Topographical relations of the C2|.ll(l.‘1.l end of the spinal cord in the hum:1.n fetus from the eight-h to the twenty-fifth week. Comparison of these stages shows the rate and extent of caudal displacement of the vertebral column, in relation to the telzninal ventricle and the attachment of the sacral nerve roots, which constitute definite and fixed points on the spinal cord. In each case the dorsal root of the first sacral nerve is drawn in, and the attachment of the dorsal roots of the other sacral nerves is shown by heavy lines. The twenty-fifth to twenty-ninth vertebrae were regarded as sacral vertebrae, irrespective of form, as shown in stipple. The fetuses are listed in the Carnegie Collection follows: No. 75, 30 mm.; No. 1656, 67 mm.; No. Template:CE1673, 111 mm.; N0. Template:CE1345, 221 mm. The first two are from profile reconstructions, the last two from dissections.
It has been pointed out that in embryos 30 mm. long a ventriculus terminalis is formed opposite the second and third coccygeal vertebrae, owing to a retrogressive thinning out of the walls of the spinal cord, with a consequent irregular enlargement of the central canal. In fetuses with a crown-rump length of 100 to 200 mm. the Ventriculus terminalis can be recognized in gross dissection with the naked eye, as a piriform, translucent area at the tip of the conus medullaris. Apparently in the natural condition it presents smooth outlines, but in prepared sections its thin walls are thrown into what are evidently shrinkage folds. This ventricle bears a permanent relation to the rootlets of the fifth sacral nerve at their entrance into the cord, as is shown in figure 2. In the four stages there represented it lies just caudal to the entrance of the fifth sacral nerve. The rootlets of the first coccygeal nerve in these specimens were so delicate that they could not be traced with certainty and were therefore omitted. The roots of the five sacral nerves, however, could be very accurately followed, and are all indicated in the figures. Their entrance into the substance of the cord constitutes in each case a firm attachment and remains a fixed topographical point. By comparing the four stages from 30 mm. to 221 mm. it will be seen that the ventriculus terminalis and the points of attachment of the sacral roots maintain the same relative positions, there being no further encroachment of the former into the territory of the more cephalic lying spinal cord. In other words, there is no further dedifferentiation of the sacral region of the cord after the embryo has attained a length of 30 mm. The cephalic migration that is subsequently experienced by the ventriculus terminalis and points of attachment of the sacral nerve roots, relative to the bodies of the vertebrae, is clearly a result of the fact that the vertebral column gradually extends farther caudalward than the spinal cord, and since the nerve roots and the filum terminale are attached at both ends they are correspondingly elongated. The latter process is not a simple stretching, for, as these structuréslelngthen they actually become thicker. In other words, there is a compensatory inter- stitial growth. Thisiincrease in thickness is not apparent in figure 2, as the older stages are ‘shown at a progressively decreasing scale of enlargement.
The rapidity and extent of the caudal thrust of the vertebral
column—that is, its caudal displacement in relation to the
terminal ventricle—can be seen in figure 2. This covers a little over the first half of fetal life (twenty-five weeks). In the
adult the corresponding points fall at the interval between the
bodies. of the first and second lumbar vertebrae. Thus in the
first twenty—five weeks there is an ascent of the ventriculus
terminalis from the level of the second coccygeal to the third
lumbar vertebra, or a distance of nine segments, and there
remain but two segments before the adult position is reached.
One may say that the principal part of the migration is accomplished during the first half of fetal life.
The dura mater and its relations can be plainly recognized in
the 67-mm. fetus, Where it can be seen to reach and adhere to
the filum terminale at the lower border of the fourth sacral
vertebra, thereby sealing off the lower end of the subdural
space. It is of interest to note that it undergoes very little
change from its position here and that which it occupies in the
adult. In the 11l—mm. fetus it extends to about the same level
and ends in the same manner. In the adult it terminates about
two segments higher up. Thus the dural sac conforms more to
its bony environment than does the spinal cord and shows very
little of the migratory adjustment of position that is noted in
the latter. VVe therefore find the ventriculus terminalis
gradually receding cranialward from the caudal end of the sac.
In figure 2 the specimens are enlarged upon a decreasing
scale of magnification according to age, so that the segments of
the different stages are brought to about the same size. This
has been done in order to facilitate the comparison of segment
levels. The actual elongation of the spinal root of a given nerve
is greater, therefore, than would appear from the figure.
Measurements of the dorsal root of the first sacral nerve from
the margin of the ganglion to the point of entrance into the cord yield the following figures: 30-mm. fetus, 0.65 mm. long; 67-mm. fetus, 4.75 mm. long; 111-mm. fetus, 12.25 mm. long; 221-mm. fetus, 32 mm. long.
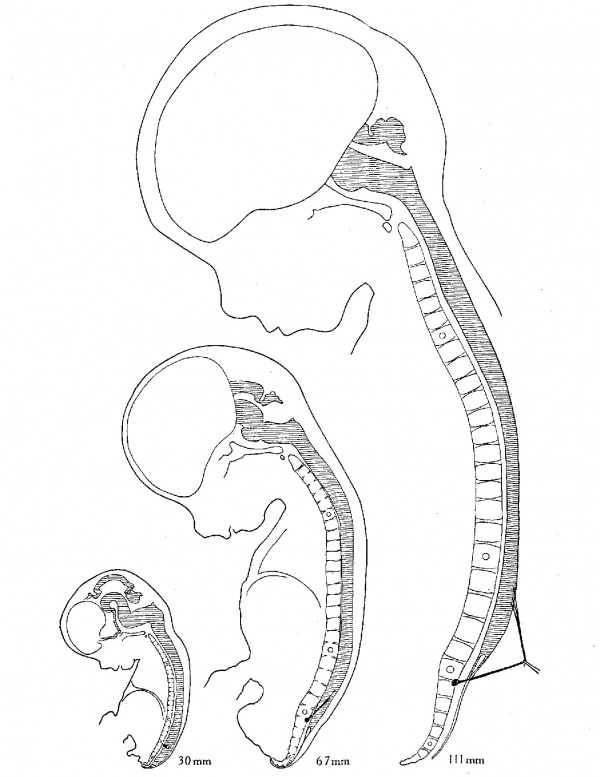
The actual elongation of the first sacral root is indicated for
the first three of these stages in figure 3, in which the topography
of the spinal cord and the vertebral column is drawn on the same
scale of enlargement. The dorsal root of the first sacral nerve is indicated by a heavy black line; the first thoracic, first
lumbar, first sacral, and first coccygeal vertebrae are marked
by small circles. Comparison of the stages, as shown in this
figure, gives perhaps a better representation of the actual topo-
graphical changes that occur in this apparent ascent than does
figure 2.
Fig. 3 Topography of the spinal cord and the dorsal root of the first sacral nerve in three fetal stages. These are taken from three of the same specimens shown in figure 2, but here they are shown on one scale of enlargement in order to indicate the actual changes in size. The 30-mm. specimen is shown both in figure 2 and figure 1.
From these results we may conclude that in the human
embryo the greater part of the coccygeal and post-coccygeal
cord that is, the part caudal to the thirtieth segment undergoes dedifferentiation, the more cephalic part of it persisting as
the ventriculus terminalis and the more caudal part redif-
ferentiating into a fibrous strand—the filum terminale, with the
coccygeal medullary vestige at the tip. The first twenty-nine
segments of the spinal cord are not affected by this process of
dedifferentiation, but continue in a progressive development.
When the embryo reaches 30 mm. in length there begins a disproportion in the rate of growth as between the vertebral column
and the spinal cord, the former elongating more rapidly than
the latter. This results in a relative displacement of the two,
the ventriculus terminalis in the 221-mm. fetus (twenty-five
weeks) lying niner segments higher than it did originally, and by
the time the adult form is attained two more segments have been
added" to this disproportion. We may say, therefore, that the
filum terminale represents that portion of the spinal cord caudal
to the second coccygeal segment (thirty-first segment), which
has undergone dedifierentiation and has finally become converted into a fibrousstrand. This strand, like the sacral nerve
roots, elongates by interstitial growth in adaptation to the
ascending displacement of the spinal cord. The caudal tip of
the dural sac maintains its relation to the vertebrae rather than to the spinal cord and remains attached to the filum terminale in the sacral region at a more or less fixed point.
References
- ↑ Streeter GL. The development of the scala tympani, scala vestibuli and perioticular cistern in the human embryo. (1917) Amer. J Anat. 21: 300-320.
- ↑ Kunitomo, K., 1918. The development and reduction of the tail and of the caudal end of the spinal cord in the human embryo. Contributions to Embryology, vol. 8, Publication No. 271, Carnegie Inst. of Wash.
- ↑ Throughout this paper the twenty-fifth to the twenty-ninth segments have been uniformly regarded as sacral. The slight variation which is known to exist in this respect is too small to be taken into account in our general conclusions, and for convenience the regional terms, lumbar, sacral, and coccygeal, will be used, upon the assumption that the specimen concerned has the usual regional distribution of its segments.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - Factors Involved In The Formation Of The Filum Terminale. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Factors_Involved_In_The_Formation_Of_The_Filum_Terminale
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G