Paper - Development of the Wolffian body in Sus Scrofa Domesticus
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Angle EJ. Development of the Wolffian Body in Sus Scrofa Domesticus.(1918) Trans. Amer. Micro. Soc. 37(4): 215-238.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Development of the Wolffian body in Sus Scrofa Domesticus
Edward J. Angle, A.M.M.D.
The results embodied in this paper are from studies undertaken some years ago. At that time it was realized that the investigation was far from complete and publication was delayed with the hope of further study — a hope never realized — of the source of origin of the several portions of the urinary tubules. In the light of recent research the publication of the paper at this late date is largely that the illustrations may prove of some permanent value.
Historical
The Wolffian bodies (Corps de Wolff, Urniere, mesonephros, Primitive Kidney) were discovered by Casper Fr. Wolff in the year 1759, who regarded them as representing the embryonic period of the true kidney (Metanephros). They received their present name from H. Rathke in 1825; this only applied to the Wolffian bodies of birds, as Rathke termed the same organs in mammals Okensche Kérper. In 1824 Jacobson introduced the name of primordialniere and discovered that in birds these bodies secreted uric acid, which was conducted away by the allantois. The first mention of the mesonephros in man was made by J. Frey Meckel (1809) in his work on comparative anatomy. Meckel describes in fairly accurate language the mesonephros of an embryo 1 mm. long; but evidently was in doubt as to what the organ was, as he later asks the question — “ are these structures the common source from which lungs, liver, kidneys, adrenals and sexual organs later have their origin?”
The older writers (Wrisberg, Dzondi, Oken, Emmert and Hochstetter) had many fanciful theories regarding the role of the Wolffian body, regarding it either as the beginning of the kidney or as the horn of the utereus.
Rathke (25) led the way to a true conception by discovering the origin of the true kidney in an embryo chick of six days and showed that the Wolffian body was related to the kidneys as the gills are to the lungs.
The glomerulus of the mesonephros was discovered by J. Muller (30). The honor is due Miiller for having first accurately and correctly followed the developmental changes of this organ in a large series of embryos.
The smallest of Muller’s embryos hada length of 20 mm. In this embryo he describes the adrenals, which are quite large and covered by the true kidney, the kidney and ureter, the Wolffian bodies with their conducting sexual portions. The Wolffian body is described as a long flat organ which is in relation on its lateral surface with the sexual duct. Miiller emphasizes the fact of the early disappearance of the Wolffian body in man; for in his next embryo which was 3 cm. long he found between excretory duct and sexual gland (does not mention whether testes or ovary) a long spur which is the remaining trace of the Wolffian body. The chief merit of Miiller’s work consists in his having discovered the réle which the Wolffian body plays in the development of the sexual ducts.
Among the noted early investigators was Valentin (35) whose work principally relates to mammals. In the Wolffian body, Valentin distinguished two portions; an outer half which consists only of canals and an inner portion made up principally of coiled vessles (Malpighian bodies). Valentin adds parenthetically that in spite of great pains it is frequently difficult to determine the direct connection of the Wolffian tubules with the Wolffian duct.
When one considers the imperfection of optical instruments in use at the period when Miiller, Rathke and Valentin lived, one can only wonder at the accuracy of their observations. In mammals, von Baer (37) says, that the primordialnieren arise and disappear as in birds and that their structure clearly points to the general characters of secretory glands.
Bischoff (42) remarks that the Wolffian bodies are only to be found in very young human embryos and that in the second month only faint traces of the glands are to be found. This author agrees with J. Miiller, Rathke, Jacobson, E. von Baer that the Wolffian body is an excretory organ. Koebelt (47) observed the atrophy of the Wolffian body in man and higher mammals and from his work concludes that the epididymis of the male is a homologue of the epoophoron in the female.
Waldeyer (70) devoted his attention to the early developmental changes of the Wolffian body in the chick, mammals and man and found the phases of development in the two latter in no way different from the former. From the fact that the Wolffian canals in their several portions are lined with different forms of epithelium, Waldeyer came to the conclusion that there were two types of canals in the gland and from this fact differentiated them into a sexual and a urinary portion; from the former arises the epoophoron or epididymis and from the latter the paroophoron or paradidymis. This opinion was concurred in by Rathke, Dursy and J. Muller.
All investigators prior to 1870 regarded the Wolffian tubules as arising either as evaginated blind sacks from the Wolffian duct or from differentiation of the mesoblastic tissue of the middle plate.
In the year 1874 a new theory was promulgated by the independent investigations of three men, Semper (75), Balfour (74) and Schultz (75) who came to the conclusion that in Selechians the segmental Wolffian canals are in relation with the coelom by means of nephrostomes. According to Semper these segmental canals arise from hollow invaginations of the pleuro-peritoneal epithelium. Balfour regarded the canals as arising from solid buds from the intermediate cell mass, the buds later acquiring a lumen.
Gotte (75), working independently of the preceding authors, found in amphibia that the Wolffian tubules arise as hollow outgrowths from the urogenital fold of the peritoneum. This observation was confirmed by Spengel (76) and Fiirbringer (78). Spengel and F. Meyer, working independently discovered in 1875 that the amphibian Wolffian body possesses peritoneal funnels and the former regards the Wolffian body of amphibians as possessing a segmental formation and holds that its peritoneal funnels deserve as in Selachians, the name of segmental nephrostomes. Fiirbringer (78) has shown in Petromyzon that the first anlage of the Wolffian body originates from segmentally arranged cell cords which arise from the peritoneal epithelium.
In 1875 K6lliker (75) investigated the origin of the Wolffian tubules of amniota which agrees with the anamnia in its essential features. In reptiles according to Braun (77) the Wolffian tubules arise from buds which had previously been evaginated from the coelom epithelium; these buds are segmental as in Selachians and solid as in mammals, and become segmental vesicles. The connection with the peritoneum soon vanishes. Thus we see in the seventies a strange concord of opinion; in all vertebrates which had been investigated the theory of Semper and Balfour regarding Selachians was confirmed.
Sedgwick (80) advanced the view later that the Wolffian tubules in the chick do not arise as peritoneal evaginations as announced by Semper and Balfour but arise through a differentiation of the Wolffian Mesoblast. Soon after this Weldon (83) showed for Lacerta that the Wolffian tubules do not originate, as held by Braun, from peritoneal evagination but according to the theory of Sedgwick. The theory advanced by Sedgwick for birds and Weldon for reptiles was opposed by Janosik (85) who had investigated the subject in the chick. Mihalkovics (85) agrees with Sedgwick and Weldon that in Sauropsida the Wolffian canals do not arise as a growth from the coelomic epithelium but by differentiation of the Wolffian mesoblast.
Hoffmann (89) investigated the Wolffian canals of Lacerta and found that they arise similar to those in Selachians, with the difference that the constriction of the nephrostome from the lateral plates occurs at an earlier period.
According to Martin (88) in rabbits the anlage of the canals is differentiated from the middle plate and later loses connection with the mesoblastic somites.
Kollman (92) is authority for the statement that the middle plates in amniota are segmental. This conclusion was arrived at by surface observation and confirmed by sections. In man the same conditions could not be directly shown but can be assumed as the Wolffian canals are arranged segmentally and the Wolffian vesicles show segmental characters.
In the same year Field (91) made a study of amphibians. He came to no conclusions in Amblystoma although he considers it probable that the tubules arise from a proliferation of the peritoneal epithelium but not from a true invagination.
Description
The Wolffian body is the chief occupant of the embryonic Wolffian ridge; in Anamnia it is the chief renal organ throughout life; in Amniota on the contrary it disappears during embryonic life, being entirely replaced by the true kidney (metanephros), with the exception of a small portion of the cephalic end which is retained and becomes a constituent of the developing sexual gland.
In its primitive form the Wolffian body consists of a series of transverse tubules emptying into the Wolffian duct. As Sempter (75) has shown for Plagiostomes there is one tubule for each body somite.
A mesonephros in the simple form in which it is first produced developmentally is retained permanently, as Gegenbaur has shown, only in Bdellostoma, a species of Cyclostomes. Here the organ consists according to J. Miiller of short transverse tubules whose proximal closed ends are invaginated by glomeruli and which open after a short course into the Wolffian duct. In all remaining vertebrates the mesonephros is metamorphosed into a more voluminous and complicated organ and shown manifold changes over the simple form. Here we find a distal strongly convoluted tube opening into the Wolffian duct by means of a collecting tube; the proximal distended portion of the canal becoming a Bowman’s capsule and lastly a peritoneal opening leading up to the glomerulus. This latter however is not found in the amniota as maintained by Hertwig (92) who claims that it is present in the three higher classes of vertebrates. I have searched diligently for traces of this in the chick, rabbit, cat and pig, and have found no evidence of the presence of such a canal. The Wolffian body develops in the intermediate cell mass which is formed when the mesoblastic somites are constricted off from the lateral plates; it arises through a union of the median portions of the latter and is best known under the name of middle plate. It has been amply shown that the coelomic epithelium of the middle plate represents without exception the anlage of the sexual glands and I shall attempt to show that the middle plate itself represents the anlage of the excretory apparatus and that the latter contains no traces of coelomic epithelium; thus is shown the descent of the entire anlage from mesoblast.
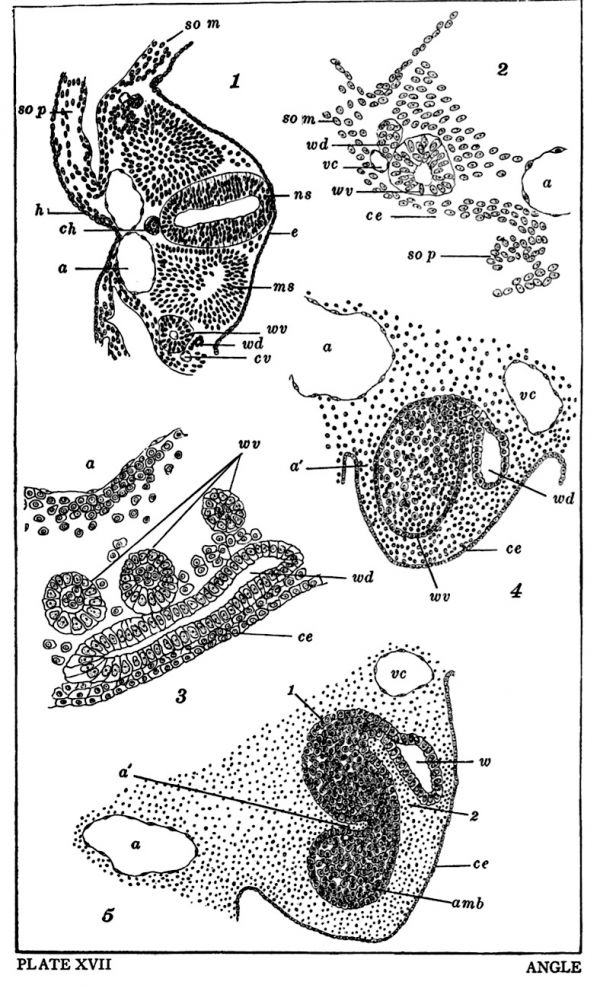
Preceding the appearance of the anlage of the Wolffian tubules there appears an accumulation of mesoblastic cells on the mesial and ventral side of the Wolffian duct. These cells assume a radial appearance and become hollowed out to form small vesicles. These vesicles were termed mesonephric vesicles by Remak (50) and segmental vesicles by M. Braun (77). Braun found in lizards the number of vesicles to correspond with the number of segments but in birds Mihalkovics (85) has found the vesicles more numerous than the mesoblastic somites. In Sus I have found from two to three vesicles for each somite and consequently the term segmental vesicle of Braun is inappropriate for birds and mammals. The Wolffian vesicles are either oval or circular in outline when viewed in sections and are lined with columnar epithelium. These epithelial cells have large clear and well defined nuclei and each cell possesses a deeply staining nucleolus. In Figures 1, 2, and 3, the relations of the Wolffian vesicles to surrounding parts are clearly shown; Figures 1 and 2 are transverse sections from the proximal portion of the Wolffian body of an embryo 2.5 mm. long. The Wolffian vesicle (w. v.) is seen in the above figures to be situated ventral and medianwards from the Wolffian duct (w. d.) and is in close relation ventrally with the coelom epithelium of the middle plate. Dorsally the vesicles are in relation with the mesoblastic somites (m. s.) and medianwards with the aorta (a). The Wolffian duct in these figures has not as yet acquired a lumen. Just posterior and a trifle lateral to the Wolffian duct a small blood vessel is visible, this is the vena cardinalis (v. c.) which is closely related to the growth of the Wolffian body. As the Wolffian body grows and enlarges the cardinal vein is forced to assume a position dorsal to the Wolffian ridge. Its position is readily seen in figures 7, 21, 9 and 10. The first two of these sections are from embryos three mm. long and the two latter from embryos four mm. long. Its shape varies greatly as will be seen in comparing the figures 9 and 10. In embryos a little older (S mm.) it will be seen in figures 12, 18 and 19 that the cardinal vein (v. c.) is situated near the dorso-median angle of the Wolffian ridge, and is in close: relation with the Malpighian bodies (m. b.) which are fully developed in embryos of 5 mm. length.
The Wolffian vesicles (w. v.) are shown in an oblique section in figure 3, which is from the distal end of the Wolffian body of a three mm. embryo. The vesicles here are in relation laterally with the Wolffian duct (w. d.) and medianwards with the aorta (a). The small amount of mesoblastic tissue surrounding the vesicles is particularly noticeable.
The origin of the anlage of the Wolffian canals is a subject which has engaged the serious attention of embryologists for the past score of years and has given rise to a voluminous literature. Among the amniota, birds have received the attention of a majority of investigator, reptiles still less and mammals least of all, which is not at all commensurate with their position and importance in the animal scale. The names of Kdlliker, Renson, Kollmannm, Egli, His, H. Meyer, Nagel and Mihalkovics are in the foreground of investigators on the development of urogenital organs of mammals.
Three views have been advanced for the origin of the Wolffian tubles:
(a) The Wolffian Tubules Arise Similarly to the Tubules of Other Glands That is as Hollow Evaginations from the Wolffian Duct. This theory was advanced by Remak in 1850 and was accepted by Waldeyer (70) with the distinction that the tubule only is an outgrowth from the duct, the Malpighian body arising from the mesoblast independently and later joins the tubule. It is only necessary to examine sections of young embryos in which the Wolffian vesicles are yet separate from the Wolffian duct to show the incorrectness of this view (w. v., Figures 1, 2 and 3).
(b) A More Modern Theory is that the Tubules take their Origin from an Evagination of Cell Cords or Buds from the Coelomic Epithelium of the Middle Plate. This was first advanced by Balfour (75) and Semper (75) for Selachinas. Among workers on amniota the adherents of this theory are Braun (77), Weldon (83), Kélliker (79), Kollmann (82), Siemerling (82), Sedgwick (81) and Rensen (83). Sedgwick held this theory only for that portion of the Wolffian body which develops anterior to the sixteenth mesoblastic somite. In reptiles according to Braun the tubules arise from funnel shaped invaginations of the coelom epithelium which are then constricted off from the latter and become the segmental vesicles, and which are present in numbers corresponding to the body segments. These vesicles secondarily unite with the Wolffian duct. The vesicle proper becomes the future Malpighian body while the tubule arises from a short canal which connects the vesicle with the Wolffian duct. Weldon holds the same theory as Braun but merely makes the statement without any evidence.
According to Kolliker in embryo chicks of the sehond day, there are to be seen, on the median side of the urogenital ridge club-shaped buds of epithelial cells which are growing in towards the connective tissue of the Wolffian blastem. KGlliker observed fine fissures in these cell cords, which he regarded as portions of the coelomic cavity constricted off with the cells. The connection with the coelomic cavity is lost only after the tubules have made their union with the Wolffian duct. Kolliker observed the same in the rabbit with the exception that no fissures were present.
Kollmann examined embryos of mouse and rabbit and confirmed in toto the view of Kélliker. Renson (in chick, rabbit and rat) divides the Wolffian body into two portions, a proximal extending from the seventh to the eleventh somite and a distal, extending from the eleventh somite to the pelvis. In the first named region the tubules arise from isolated buds of the pleuro-peritoneal epithelium while in the latter the tubules are differentiated from the intermediate cell mass which had previously arisen from an ingrowth of the pleuro-peritoneal epithelium in the form of a longitudinal plate. The cells which become anlagen of canals are arranged around small lacunae. The lacunae are the remains of small fissures when the longitudinal plate was constricted off from the coelom epithelium. The remainder of the lacunae form the cavities of the Malpighian bodies. Renson regards the pronephros and mesonephros as being homologous organs; a view which is untenable at the present time.
Hertwig (92) in his text book of embryology says: “The collective evidence of investigators shows that the Wolffian canals arise from the pleuro-peritoneal epithelium of the middle plate from which solid cell cords are formed and pass in towards the side of the Wolffian duct. In the higher vertebrates the development of the primitive kidney is to a certain extent abbreviated, in so far as the separate cords of cells which arise at the constricting off of the primitive segments lie very close together and constitute an apparently undifferentiated cell mass out of which the mesonephric tubules subsequently appear to have been differentiated. The source of its material (mesonephros) is either directly or indirectly the epithelium of the body cavity as it has been possible to prove in many cases in Selachians, amphibia and amniota.”
(c) The third view is that the Wolffian tubules are derived independently of previous existing epithelium through differentiation of the Wolffian mesoblastic tissue. This view was first advanced by Remak (50) and accepted by His (80), Bornhaupt (67), Egli (76), Sernoff (76), Mihalkovics (85) and H. Meyer (90). Balfour (79), Sedgwick (80) and Fiirbringer (78) hold this view for that portion of the Wolffian body developing distal from the sixteenth somite. Mihalkovics (85) has made a very thorough and exhaustive study of the development of the Wolffian body in the lizard and chick and finds no evidence whatever to substantiate the views of Braun, Kolliker and Rensen. Mihalkovics has shown that the tubules of chick and lizard which correspond to the seventh to the eleventh somite inclusive arise from the coelom epithelium and that each tubule is connected with the coelom cavity by means of a funnel shaped nephrostome. At the median side of each nephrostome and projecting out from the root of the mesentery is a free glomerulus. It is admitted by all modern investigators that the above constitutes the head-kidney or pronephros which is in no way connected or homologous with the Wolffian body. The error of Balfour, Sedgwick and others arose no doubt from the fact that they regarded the pronephros as the anterior portion of the Wolffian body. I have verified the work of Mihalkovics in the chick and find no nephrostomes or free glomeruli farther distal than the body somite. Sedgwick makes the sixteenth somite the point of differentiation. His (80) in description of embryo “a” says that the thickness of the walls of the Wolffian duct at an early period is double the size of that structure later on. This fact would cause him to conclude that the tubules arise from the duct by a fold and a consequent thinning out at this point if the collective evidence of vertebrates did not point to their formation from the Wolffian mesoblast. Nagel (89) in his description of two human embryos rejects the coelom theory in toto and while admitting that his embryos were much too old to give information on this point says if it were not for the opinion of His (see above) he would be inclined to believe that the tubules arise as outgrowths from the Wolffian duct. In order to prove unequivocally that the Wolffian tubules arise from the mesoblastic tissue of the middle plate one must have embryos of such ages which will show the complete cycle of changes from undifferentiated mesoblastic tissue to fully formed Wolffian vesicles. From this point of view I am unfortunate in the selection of my subject material as in my youngest embryos (2.5 mm.) the segmental vesicles are already well formed and differentiated from the surrounding tissue (see w. v. figures 1 and 2). If the vesicles arose from the coelom epithelium one would expect to find some indication of this occurrence at the point to where they were constricted off from the latter, opposite to the vesicles, but by observing the vesicles (w. v.) in figures 1 and 2, it will be seen that no fissures, thinning out of the epithelium, or depression of the latter are to be found.
The anlage of the Wolffian canals develop in a distally extending direction and in the embryo from which figures 1 and 2 are taken the vesicles are well formed at the proximal end. At the distal end of this embryo the cells of the mesoblastic tissue are just arranging themselves around a common center and no lumen is present. Another point which adds considerable confirmatory evidence is the fact that the immature vesicles at the distal end are no nearer the coelom epithelium than the more fully developed vesicles of the proximal end; which should be the case if the vesicles arose from the coelom epithelium. In the embryo (3 mm. long) from which figures 7 and 21 are taken one finds separating the coelom epithelium from the underlying blastem, first a compact layer of connective tissue (c. t.) and second an intercellular space (i. s.) each of which amounts to more than the thickness of a tubule. With the exception of the point at which the tubules adjoin the Wolffian duct the coelom epithelium is separated from the underlying structures im this embryo. As previously stated, I admit that my evidence is not complete but all the facts which I found point strongly to the origin of the Wolffian canals from the mesoblastic cells of the middle plate. I hope in the near future to obtain younger embryos which will unequivocally settle this point. While the theories regarding the origin of the anlage of the Wolffian canals are numerous there is a corresponding scarcity of accounts describing the changes by which the primary vesicles are metamorphosed into a fully developed canal, ending distally in a Malpighian body and proximally opening into the Wolffian duct. Sedgwick (80) gives the following account which is decidedly indefinite, “from the inner and dorsal wall of the vesicle a glomerulus is ultimately developed. The whole structure grows enormously and gives rise to the Malpighian body and complicated coils of the later Wolffian tubule. The question as to whether or no there are outgrowths from the Wolffian duct to meet the independently developed Wolffian tubules is not easy to answer. I am not now in a position to give a definite answer and will merely state that there are appearances in my sections which incline me to the opinion that there are outgrowths from the Wolffian duct which in the case of the primary Wolffian tubules are solid but hollow in the case of the secondary and tertiary tubules.”
Waldeyer (65) regarded the tubule proper as an outgrowth from the Wolffian ducts while the Malpighian body develops separately in the intermediate cell mass and later joins the tubule. Braun (77) holds in reptiles that there is a short connecting canal given off from the Wolffian duct which joins the segmental vesicle and that by the lengthening out of this canal the tubule proper is developed; while the Malpighian body is formed from the vesicle itself. The most painstaking and the only complete account which I can find is by Mihalkovics (85)and he gives in detail, illustrated by a number of figures, the various changes assumed by the vesicle in its conversion into a Wolffian tubule. He gives an account of this process in both the lizard and the chick and as they agree in all essential points it will serve our purpose to relate briefly a summary of this change occurring in the chick. The Wolffian vesicles are situated at the median side of the Wolffian duct and their contiguous surfaces are in close contact and at the point of union, there is a melting away of the cells and a communication is formed connecting the lumen of both vesicle and duct. At the same time that the above is occurring the round form of the vesicle becomes flattened by the sinking in of its dorsal wall and as a result we see in cross section, a half moon shaped body the lateral wall of which is joined to the median side of the Wolffian duct, and its convex wall is ventral and at the median side of the urogenital ridge, close to the coelom epithelium and its median point directed towards the aorta. In the concavity of the half moon is an aggregation of connective tissue which is the anlage of the future glomerulus. The short canal which connects the vesicle with the Wolffian duct is the anlage from which, when fully developed, a tortuous tubule arises; while the Malpighian body alone arises from the half moon shaped Wolffian vesicle.
This account of Mihalkovics for the cliick is entirely different from what I have found in Sus. In the pig the Wolffian vesicle assumes an oval form with its long diameter directed dorso-ventralwards, the walls of the Wolffian vesicle and duct being in close contact. Shortly after this the two are connected by a short canal, which is given off from the dorso-median wall of the Wolffian duct and uniting at the dorso-lateral border of the vesicle. In figure 4 the vesicle (w. v.) is seen united to the Wolffian duct (w. d.) by a short curved canal as above described. By comparing figures 1 and 2 with figure 4 it will be seen at this stage that the middle plate has increased considerably in size and now projects into the body cavity and from this period on will be designated as the Wolffian ridge. The vesicle having become oval has receded back from the coelom epithelium (c. e.) and its long diameter is vertical to the body axis. Otherwise the relations of the vesicle to surrounding tissues and organs are not changed from what was described in figures 1 and 2. A lumen in the canal connecting vesicle and Wolffian duct is not present at this early period (4). As to the origin of this canal whether derived from the vesicle or from the Wolffian duct I can not positively state, but it would seem that it is derived from the latter, from the fact that its cells like those of the Wolffian duct have taken the stain with great avidity while the cells lining the vesicle have pale nuclei. By comparing figures 4 and 5 it will be seen that the next stage of development is brought about by the sinking in of the median wall of the vesicle at point ‘a’ and causes the latter to assume somewhat of an ‘S’ shape (figure 5) whereby the anlage of the three portions of each tubule and Malpighian body can be differentiated. The proximal portion of the tubule (5) is quite narrow and it now has a distinct lumen and curves dorsally and passes under the ventral border of the cardinal vein (c. v.) and shortly afterwards unites with the second portion of the tubule at point 1 (figure 1). The second portion of the tubule extends from 1 to 2 and is spindle shaped(figure 5). This second portion curves ventralwards with a slight lateral deviation and then becomes constricted at point 2, then makes a sharp curve medianwards and passes over into the third portion of the tubule. This third portion extends from point 2 to anlage of the Malpighian body and like the first portion is quite narrow. The third portion is directed medianwards and is parallel with the ventral surface of the Wolffian ridge. The anlage of the Malpighian body is the expanded distal end of the third portion of the tubule (5) and its median surface is in close relation with the aorta (A). In figure 6, a trifle older stage is shown and the several portions of the tubule are more clearly defined than in figure 5. From the preceding account it will be seen that the two distal portions of each tubule and the Malpighian capsule are derived from the Wolffian vesicle. By comparing figures 4, 5 and 6, it will be seen that the lumen of the two distal portions of the tubule and the Malpighian capsule are filled with darkly stained formative cells while in figure 6 no such cells are present in the proximal (first) portion of tubule. This fact is additional evidence that the first portion of the tubule arises as an outgrowth from the Wolffian duct. In figure 8 the first portion of the tubule and the Wolffian duct are also seen to enclose these building cells but I think it purely accidental here and believe they have migrated from the other portions of the tubule, after union with the Wolffian duct; for in figure 3, from a section showing Wolffian vesicles (w.v.) and Wolffian duct, the former are seen to enclose these formative cells while the latter has a clear lumen.
Mihalkovics (85) represents the glomerulus as developing pari passu with the tubule. In Sus this does not seem to be the case. In an embryo of three mm. from sections of which figures 21 and 22 are taken the canals in the proximal three-fourths of the gland have assumed their typical curves, but the expanded distal end of tubule which is the anlage of the Malpighian body (a. m. b.), shows no evidence of invagination. In figure 20, the anlage of Malpighian body shown in figure 22 is seen more highly magnified; it is to be noticed that no evidences of invagination are to be seen. In embryos from 3 to 3.5 mm. the changes relative to the invagination of the Malpighian capsule and the formation of the glomerulus are first to be seen. The origin of the Malpighian tuft of vessels (glomerulus) has, so far as I have been able to ascertain, received very little attention from workers in this field of embryology. The only detailed account I have found is by Mihalkovics (85) who accepts the theory advanced by Gétte (74) and Fiirbringer (78) for amphibia and Braun (77) for reptiles. Mihalkovics found in the chick that the invagination of the Malpighian capsule went on pari passu with the development of the tubule and that first a collection of mesoblastic cells are noticed around the dorsal wall of the capsule and these later are invaginated into the capsule and become the anlage of the glomerulus. At this period no branches are seen approaching the Malpighian body from the aorta. Soon after invagination has occurred, groups of darkly stained cells are to be seen among the connective tissue of the glomerulus anlage. According to Mihalkovics these darker stained cells are first transformed into colorless and then colored blood corpuscles; surrounding connective connective tissue becoming the coiled vessels. Mihalkovics quotes Romiti and Schafer as giving this origin for the blood corpuscles and their enclosing vessel walls, for other organs. I do not doubt the perfect physiological propriety of this view but as a matter of fact it does not occur here. In figure 7, from an embryo 3 mm. long, the changes preparatory to formation of the glomeruli are to be seen. It will be noticed in this figure that the aorta (a) is relatively of large size and that opposite the median point of the Malpighian capsule, there is an evagination of the aorta and at this point a diverticulum is given off from the latter, which passes outwards into the connective tissue of the Wolffian ridge and comes in close relation with the dorsal wall of the Malpighian capsule. The wall of the aorta is continuous with the wall of the diverticulum and the latter is seen to be filled with numerous blood vessels enclosing blood corpuscles. In some cases I find no diverticulum from the aorta, but a number of small blood vessels instead which ramify on the dorsal surface of the capsule; preparatory to invagination of the latter In figure 17, from an embryo of 4 mm. in length the glomerulus is commencing to invaginate while in figure 16 a fully developed Malpighian body, from a 5 mm. embryo, is shown; the glomerulus being entirely invaginated and surrounded by a Malpighian capsule. The cells seen in the glomeruli of figures 16 and 17 are the nuclei of the endothelial cells of the coil vessels, and the wavy outline of the latter is seen in figure 16. In figure 16 in the cells lining the Malpighian capsule the transition from cylindrical to cubical and later to connective tissue is clearly shown. Figure 13 also represents a mature Malpighian body but owing to greater pressure there is less space between glomerulus and capsul thane is seen in figure 16. In figures 18 and 19, from an embryo 5 mm. long the Malpighian bodies are fully developed and the large branches given off to the glomeruli from the aorta are seen.
Each fully developed Wolffian canal consists of three typical portions, a dorsal (first), ventral (third) and middle (second) which are connected by two sharp curves. The dorsal portion cylindrical in form affords the connection with the Wolffian duct and then curves dorsalwards along the lateral edge of the Wolffian ridge and then passes medianwards along the ventral edge of the cardinal vein and approaches close to the aorta, on the inner side of the ridge, where it makes a sharp curve and passes over into the spindle shaped middle portion of tubule. The middle portion passes ventralwards and then curves under the first portion and here makes a sharp curve and passes into the anterior portion of the tubule which is directed medianwards and passes close to and almost parallel with the ventral surface of the Wolffian ridge and then expands into the capsule of the Malpighian body, at the median ventral angle of the ridge. The above described relations are readily seen in figures 8 and 12, the first or posterior portion of the tubule extends from the Wolffian duct (w. d.) to point designated (1) where there is a sharp curve. The middle or second spindle shaped portion extends from point (1) to (2) where we find the second sharp curve. The anterior or third portion of tubule extends from point (2) to the Malpighian capsule. In figures 9 and 10 (left section) the proximal two-thirds of first portion of tubule (t. w.) isseen. In figure 12 a complete tubule with its Malpighian body is shown. In figure 8 the tubule is seen arising from the ventral side of the Wolffian duct, an occurrence which I have only found two times in examining several thousand sections of this region. In figure 12 at point (s) (in first portion of the tubule) there is seen a sharp secondary curve. In figure 8 from a somewhat younger embryo this secondary curve is present but less sharply defined. I do not find a description of this secondary curve in the writings of any author who has investigated the Wolffian body. While each Wolffian canal shows three typical positions it is impossible to find any two canals which are identical throughout their entire course. In embryos of 5 mm. from sections of which figures 18 and 19 are taken, it is no longer possible to recognize the entire course of a tubule. As the Wolffian body develops the tubules lengthen out and new curves arise, giving the canals a highly tortuous and convoluted course.
With the formation of the primary tubules and their glomeruli the growth of the Wolffian body is by no means complete. Two factors contribute to the further growth of this organ; first the lengthening out of the several portions of each tubule, the intensification of the primary curves and by the addition of new ones; second by the formation of secondary, tertiary and quaternary canals. I shall designate as secondary canals all tubules developing subsequent to the primary set. As the origin of the primary mesonephric tubules gave rise to several theories, we have likewise a number of different views regarding the origin of the secondary.
(a) The first view—The secondary tubules and their glomeruli arise either by fission or buds from the primary set. Either of these processes may have as a starting point the wall of the Malpighian capsule or the tubule itself. Braun (77) found in reptiles and Spengel (76) in amphibia that the primary glomeruli are first divided by fissures which continue along the corse of the tubule until the Wolffian duct is reached. In Selachians according to the statements of Sedgwick (80) and Balfour (74) the glomeruli is the starting point of proliferation; cell buds grow out from the latter and towards the Wolffian tubules lying in front of them with which their blind ends fuse. After this union has been effected they detach their other end from the parent tissue. Renson (83) held the same view for birds but gives no adequate proof.
In discussing the origin of the secondary canals in the human embryo Nagel (89) says one finds numerous accumulations of epithelial cells in the middle of the sections and which might lead one to think the further growth of the tubules occurs through differentiation of the Wolffian tissues. But the examination of whole series of sections shows most clearly that these epithelial collections stand in direct relation with the previous formed canals and that they represent the solid ends of the same. Nowhere is there to be seen the transition of the cells of the Wolffian tissue to the epithelial cells which would be the case of the latter arose from the former. The solid end pieces of the canals are sharply defined from the surrounding tissues as the canals themselves. From this analysis Nagel concludes that the later development of the Wolffian canals in man occurs through a process of buds or outgrowths of the previously formed canals. Sedgwick (80) in describing this process in the chick does not seem to arrive at a definite conclusion but thinks that the secondary arise from the dorsal walls of the primary set of tubules.
(b) Second view—The secondary canals arise like the primary from invaginations of the coelom epithelium. Fiirbringer (78) is an advocate of this theory and says that the secondary canals arise from the coelom epithelium on the median side of the primary canals and passes into the Wolffian tissue in the form of cell cords which later lose their primary connection.
(c) Third view—The secondary canals and glomeruli arise independently of the primary through a process of differentiation of the Wolffian mesoblast. This view was first advanced by Bornhaupt and later confirmed by Balfour (79) and Mihalkovics (85). My own investigations are in perfect accord with this later view and I will attempt to show that in Sus the secondary canals arise independent of the coelom epithelium and primary tubules, through a differentiation of the mesoblastic cells of the Wolffian ridge. Mihalkovics (85) in reptiles and birds finds no evidence that the secondary canals arise from the primary through fission or buds. According to Sedgwick (80) the secondary canals of the chick arise dorsal from the primary and the tertiary dorsal from the secondary; but Mihalkovics has shown that the secondary canals may arise either ventral, dorsal or intermediate from the primary. Investigations of the origin of the secondary canals in Sus is difficult from the fact that the secondary canals do not appear until the primary are quite fully formed. In figures 8, 21 and 22 the several portions of each tubule are readily seen, no secondary canals have as yet appeared. Like the primary, the secondary canals develop in a proximal-distalward extending direction. In figure 21 from the proximal region of the Wolffian body of an embryo 3 mm. long, I find the first changes which lead up to the formation of the secondary canals; midway between the spindle shaped second portion of the primary canal and the aorta there are to be seen several collections of mesoblastic cells which are closely packed together. These cells take the stain with great intensity and contrast strongly with the surrounding connective tissue. I find no branches from the aorta approaching these groups of cells nor any thickening or invaginations extending in from the overlying coelom epithelium. These cells are at quite a distance from the latter and even though epithelial cords were present it would be difficult to conceive their passage through connective tissue, intercellular spaces and primary tubules and finally reach the designated point in figure 21. In figure 11 from an embryo 4 mm. long we see the next stage in the development of a secondary canal (t. w.); here a basement membrane is present and the darkly stained mesoblastic cells are assuming a radial arrangement and lumen i just appearing in the vesicle. The shape of this secondary vesicle is oblong, its width being about onehalf of its length; while cross sections of primary vesicles are nearly round (figures 1, 2 and 3). The next period of development is also seen in figure 11 where the anlage of a secondary canal is just ventral to the above described vesicle and has assumed somewhat of a ladle shaped form. By comparing the anlage of these two tubules (figure 11) it will be seen that the median portion of the vesicle becomes the anlage of the Malpighian body while the lateral portion becomes the tubule proper. This occurs in much the same way as described in the primary vesicles (figures 4, 5 and 6), although the process of differentiation of the vesicle into a tubule is somewhat abbreviated in the case of the secondary canals. As to the division of a Malpighian body by fissure or buds growing out from it—I have carefully examined the sections of a dozen embryos ranging in size from 3 to 5 mm. and nowhere find evidence of such occurences. In regard to Nagel’s view (89) that the secondary canals arise as outgrowths from the primary I can feel sure in saying that it does not occur. One can find numerous sections similar to figure 14 which appear like the outgrowth of a secondary tubule from a primary, but such is not the case for by following this outgrowth in consecutive sections it will be found to continue into a secondary tubule and the apparent blind sack to be caused by a sharp curve which the tubule made before joining the collective portion of the primary canal. According to Mihalkovics (85) secondary canals in the chick are formed either dorsal, ventral or medianwards from the primary. By comparing figures 9, 10 and 21, it will be seen that the first portion of the primary tubule passes very close to the lateral surface of the Wolffian ridge and then curves backward to the cardinal vein and lies directly in front of the ventral surface of the latter. From this it will be seen that there is but little space for secondary canals to develop dorsal from the primary and I have only found one instance of this occurrence which is shown in figure 15. The secondary canals do not arise ventralwards from the primary for a like want of space (figure 9) but are found to develop medianwards from the primary (figure 11). The secondary glomeruli are situated lateral and dorsal from the primary; the latter occupying a position near the inner portion of the gland just dorsal to the germinal epithelium (g. e.). The relations of primary and secondary Malpighian bodies are shown in figure 10.
In the chick of five or six days Mihalkovics finds from 12 to 18 Wolffian tubules opening into the Wolffian duct in each body somite and that it is no uncommon occurrence to find three tubules emptying into the duct in the same section and besides the tubules which open direct into the Wolffian duct he finds from 20 to 40 indirect tubules in each somite. These indirect tubules empty into the collective (first) portion of a direct canal. This would make a total of from thirty to sixty direct and indirect tubules for each body somite. I find in Sus from 2 to 3 tubules emptying into the Wolffian duct in each body somite. In embryos of four, five, eight and fifteen mm. respectively the number of direct canals remains practically the same, that is two to three to each somite. In embryos of four to five mm. length (figures 10, 11-18, 19) from two to three Malpighian bodies are to be seen in each section in the middle two-thirds of the Wolffian body. In embryos ranging in size from 8 mm. to 1-5 10 cm. one frequently finds from six to eight Malpighian bodies in a single section. From this one naturally comes to the conclusion that all or nearly all of the secondary canals in Sus are indirect; emptying into the collective portion of a primary canal. The examination of a number of sections demonstrates the correctness of this as can be seen in figures 14 and 19. In figure 11 the proximal end of the anlage of a secondary canal is in contact with the median wall of a primary tubule and later will open into it. I have only found one instance in which two tubules open into the Wolffian duct in the same section. This is shown in figure 15, the outer of the two tubules being a secondary while the inner is a primary one. Thus it appears that an occasional secondary tubule opens directly into the Wolffian duct, but is quite a rare occurrence.
Lincoln, Nebraska.
Literature Consulted
Batrovr, A. 1874. A preliminary account of the development of the Elasmobranch Fishes.
Q.J.MSS. 1874. 1879a. Head-Kidney in the Chick. Q.J.M.S. 1879. 1879b. Text book of comparative Embryology. 1879.
BAER, E. von 1837. Ueber Entwickelungsgeschichte der Thiere. Koenigsberg. 1837.
Biscuorr, TH. L. W. 1842. Ent. der Sdugethiere und des Menschen. Leipsig. 1842.
BoRNHAUPT 1867. Untersuchungen ueber die Ent. des Urogenitalsystems beim Hiinche. Riga. 1867. Bravn, M. 1877. Urogenital system, reptiles. Arb. Zool. Zoot. Inst. Wuerzburg. IV, 113-228.
Ecu, Tu. 1876. Sexual Organs. Zurich. 1876.
Frep, H. H. 1891. The development of the pronephros and segmental duct in amphibia. Bull. Museum Comp. Anatomy of Harvard Univ. Vol. XXI, No. 5, 1891.
FUERBRINGER, 1878. 1878. Zur Verleichenden Anatomie und Ent. der Excretionsorganie der Vertebraten. Gegenbaur’s Morph. Jahrbuch. Vol. IV, 1878.
GASSER, E. 1877. Die Entstehung des Wolff’schen Ganges bei embryonen Hiihnern U. Gisen. Archiv. f. M. Anat. Bd. XIV. 1877.
GoeTTE A. 1875. Die Entwickelungsgeschichte der Unke. Leipzig. 1875.
HeErtwic, O. 1892. Text-book of Embryology of Man and Mammals, translated from the second German edition by Dr. Mark. Macmillan & Co. 1892.
His, W. 1868. Untersuch. uber die Erste Anlage des Wirbelthierleibes. Leipzig. 1868. 1880. Anatomie Menschlicher Embryonen. Heft. I-II. Leipzig. 1880.
Horrmany, C. K. 1889. Zur Entwickelungsgeschichte der Urogenitalorgane bei den Reptilien. Z. f. W. Zool. Bd. XXXXVIII. 1889.
JACOBSON. 1824. Det. Kongl. danske Videnskabernes Selskab etc. Kjgbenhavn.
JANOSIK. 1885. Histologisch-embryologische Untersuchungen uber das Urogenitalsystem. Sitzungsber. des Kais. Akod. d. W. zu Wien. Bd. LXXXXTI. 1885. 1887. zwei junge Mensch. Embryonen. A.f.M.A. Bd. XXX. 1887.
Kose r. 1847, Der Nebeneierstock des Weibes. Heidelberg. 1847.
KOLLMANN. 1892. Die Rumpfsegmente Mensch. Embryonen von 13-35 Urwirbeln. Archiv. f. Anat. u. Ent. 1892.
K6uurgcer, A. 1875. Uber die erste Ent. des Siugethierembryos. Verh. d. Phys. -Med. Ges. zu Wiirzburg. 1875. 1879. Ent. des Menschen und der hoheren Thiere. Zweite Auflage. 1879.
MARTIN. 1888. Uber die Anlage der Urniere beim Kaninchen. Archiv. f. Anat. u. Ent. 1888. Mever, H.
1890. Die Ent. der Urniere beim Mensch. A.g.M.A. Bd. XXXVI. 1890.
MECKEL, J. FR. 1809. Beitrage zur Vergleichenden Anatomie. Bd. I. 1809.
MIBALKOVICS. 1885. Untersuchunger tiber die Ent. des Harn u. Geschlechtsapparates der Amnioten. Int. Monat. f. Anat. Bd. II. 1885.
Mrn0cr, C. 1892. Text-book of a human Embryology. William Wood & Co. 1892.
MEYER, FR. 1875. Beitrag zur Anatomie des Urogenitalsystems der Selach. u. Amphibien. Sitzungsber. der Naturf. Ges. zu Leipzig. 1875.
MULLER, J. 1830. Bildungsgeschichte der Genitalien aus Anatomischer Untersuchungen Embryonen des Menschen u. der Thiere. Duseldorf. 1830.
NAGEL, W. 1889. Ent. des Urogenitalsystems des Menschen. A. f. M. A. Bd. XXXIV. 1889.
RATHKE, H. 1825. Beobachtungen u. Betrachtungen uber die Ent. der Geschlechtswerkzeuge etc. Neue Schriften d. Gesellsch. in Danzig. Bd. I.
REMAK. 1850. Untersuchungen tiber die Entwickelung der Wiebelthiere. Berlin. 1885,
RENSEN, G. 1883. Development of head Kidney & Mesonephros in Birds and Mammals. A.f.M.A. XXII. 1883.
Ricxert, J. 1892. Entwickelung der Excretionsorgane. Ergebnisse der Anatomie und Entwickelungsgeschichte. Bd. I. Wiesbaden. 1892.
SEDGWICK, A. 1880. The development of the kidney in its relation to the Wolffian body in the chick. Q.J.M.S. Vol. XX. 1880. 1881. Early development of Anterior portion of the Wolffian duct and body in the chick. Q.J.M.S. Vol. XXI. 1881. Semon, R. 1891. Urogenitalsystem. Jena Zeit. Naturw. Bd. XXVI. 1891.
ScHAFER, E. G. 1890. Quains Anstomy. Tenth edition. Vol. I. Part 1. 1890. ScHuttz, A. 1875. Zur Ent. des Selachieries. A. f. M.A. Bd. XI. 1875.
SEMPER. 1875. Des Urogenitalsystem der Plagiostomen und seine Bedeutung fur das der tibrigen Wirbelthiere. Arb. Zool.-Zoot. Inst. Warzburg. 1875.
SIEMERLING. 1882. Beitrige zur Embryologie der Excretionsorgane des Vogels. Marburg. 1882.
SERNOFF. 1876. Beitrige zur Anatomie und Ent. der Geschlechtsorgane. Inaug. Diss. Zurich. 1876.
SPENGEL. 1876. Des Urogenitalsystem der Amphibien. Arb. aus d. Zool.-Zoot. Inst. Wirzburg. Bd. III. 1876.
VALENTEN, G. 1835. Handbuch der Entwickelungsgeschichte des Menschen. U.S. W. Berlin, 1835.
WALDEYER, W. 1865. Anatomische Untersuchung eines Menschlichen Embryo von 28-30 Tagen. Leipzig. 1865. 1870. Eierstuck und Ei. Leipzig. 1870.
WELDON. 1883. Note on the early development of Lacerta Muralis. Q. J. M.S. Vol. XXXII. 1883.
WIEDERSHEMM, R. 1890. Urogenitalsystem, Reptiles. A. f. M. A. Bd. XXXIII. 1890.
Wyse, J. W. von. 1889. Excretory organs Selachians. A. f. M.A. Bd. XXXIII. 1889.
Worrr, C. Fr. 1759. Theoria Generationis. Halae. 1759.
List of Reference Letters
- a - aorta
- amb - anlage Malpighian body
- c - coelom or body cavity
- ca - capsule
- ce - coelom epithelium
- ch - notochord
- ct - connective tissue
- e - epiblast
- g - glomerulus of the Malpighian body
- ge - genital epithelium
- gr - genital ridge
- is - intercellular space
- h - hypoblast
- mb - Malpighian body
- mc - medullary canal
- mes - mesentery
- mp - middle plate
- ms - mesoblastic somite
- m - mesoblast
- ns - spinal cord
- so m - somatopleuric layer of mesoblast
- sp m - splanchnopleuric layer of mesoblast
- ti - intestine
- tw - Wolffian tubule
- tw1 - primary Wolffian tubule
- tw2 - secondary Wolffian tubule
- vc - Cardinal vein
- vs - spermatic vein
- wd - Wolffian duct
- wv - Wolffian vesicle
- wr - Wolffian ridge
Explanation of Plates
Plate XVII
Fig. 1. Cross section form the proximal end of the Wolffian body of an embryo 2.5 mm.long. III—4 xX 100.
Fig. 2. Left side of figure 1 more highly magnified I—5 x 190.
Fig. 3. An oblique section passing through the distal end of a 3 mm. embryo. The Wolffian duct and three Wolffian vesicles areshown. III—5 X 280.
Figs. 4 and 5. Cross sections from the distal end of a 4 mm. embryo. I—5 X 190.
Plate XVIII
Fig. 6. Cross section from the distal end of a4 mm.embryo. I—5 X 190.
Fig. 7. Cross section from the middle third of the Wolffian body of a 3 mm. embryo. III—4 x 100.
Fig. 8. Cross section through the middle third of the Wolffian body of a 3 and 5-10 mm.embryo. III—3 x 140.
Plate XIX
Fig. 9. Cross section through the proximal end of Wolffian body of a 4 mm. embryo. I—5 X 190.
Fig. 10. Cross section through Wolffian bodies of middle third of a 4 mm. embryo 1—3 X 66.
Plate XX
Fig. 11. Cross section through the middle third of the Wolffian body of a 4 mm. embryo. IV—3 xX 125.
Fig. 12. Cross section through the distal end ofa 5 mm. embryo. IV—3 X 125.
Fig. 13. Shows the Malpighian body seen in fig. 12 more highly magnified. II— 5 X 280.
Fig. 14. Cross section through the middle third of Wolffian body of a 4 mm. embryo, showing Wolffian duct and proximal portions of Wolffian tubules. I—S5 X 190.
Plate XXI
Fig. 15. Cross section through the middle third of Wolffian body of a 4 mm. embryo, showing Wolffian duct and proximal portions of Wolffian tubules. I—5 X 190.
Fig. 16. Cross section through a fully developed Malpighian body of a 5 mm. embryo. I—5 X 190.
Fig. 17. Cross section through a Malpighian body in which the glomerulus is undergoing invagination froma 4mm.embryo. I—7 xX 300.
Plate XXII
Fig. 18. Cross section through middle third of the Wolffian body of a 5 mm. embryo. X 160.
Fig. 19. Cross section through the distal end of a Wolffian body of a 5 mm. embryo. I—4 x 39.
Plate XXIII
Fig. 20. Anlage of Malpighian body before invagination of capsule has occurred. From an embryo of 3mm. III—5 xX 280.
Fig. 21. Cross section through the proximal end of Wolffian body of a 3 mm. embryo. IV—3 X 125.
Fig. 22. Cross section through the middle third of the Wolffian body. From the ame embryo as Fig. 21. III—4 x 125.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - Development of the Wolffian body in Sus Scrofa Domesticus. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Development_of_the_Wolffian_body_in_Sus_Scrofa_Domesticus
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G