Paper - A Human Embryo of Thirteen Somites
| Embryology - 6 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Wallin IE. A human embryo of thirteen somites. (1913) Amer. J Anat. 15(3): 319-331.
| Online Editor |
|---|
| This 13 somite embryo would correspond to Carnegie stage 11. Listed as Carnegie Collection Embryo no. 1201a.
The embryo is also later described in relation to neural development and shown in Bartelmez (1923) Fig. 5. Bartelmez GW. The subdivisions of the neural folds in man. (1923) J. Comp. Neural., 35: 231-247.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Embryo Of Thirteen Somites
Ivan E. Wallin
Anatomical Laboratory, Bellevue Hospital Medical College, New York City
Seven Figures
The embryo which forms the basis of this work was given to me by Dr. Rudolph Boencke in the spring of 1911. It has been placed in the collection of the Department of Anatomy at the
University and Bellevue Hospital Medical College and is called embryo no. 4.
The embryo was aborted two weeks after the last menstrual period. There was no record of coitus. After fixation and with the amnion intact the embryo measured 2.3 mm. in length. It was cut into transverse sections 5 u in thickness, and stained with iron haematoxylin. The embryo yielded 287 sections.
Wax plate reconstructions were made of the complete embryo, the heart, the foregut, also of the caudal part of the medullary tube with the hind-gut and the belly stalk vessels. A graphic reconstruction was made representing the embryo cut in the mid-sagittal plane. All the reconstructions were made at a magnification of 200.
The embryo appears to be normal in every respect and the following points of structure have been determined.
External Form
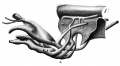
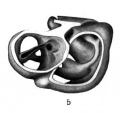
In its general configuration this embryo is very similar to Pfannenstiel III described by Low (’08). The body has a regular dorso-ventral curve and has a slight twist so that the head is situated to the right of the mid-sagittal plane. The yolk sac communicates with the primitive gut by means of an extensive yolk stalk. The latter has its greatest diameter in the cephalo~caudal direction and its lateral width is greatest at the cephalic end. Caudal and to the right of the yolk stalk the belly stalk leaves the embryo passing ventrally and curving to the right and caudad. Lateral to the yolk stalk the embryonic coelom has an extensive communication with the extra-embryonic coelom.
The heart produces a prominent bulging of the right side of the body immediately caudad to the head. The most prominent part of the bulging marks the flexure in the heart tube between the bulbus cordis and the ventricle. ‘The neck flexure has not advanced to any prominent degree. There are two prominences on the dorsal surface of the head region, one at the cephalic end of the mid—brain and the other at the cephalic end of the hindbrain. Caudally the body curves gradually in a ventral direction. There is no distinct caudal flexure.
The medullary tube is open to the exterior at both ends. The cephalic neuropore exhibits an unusual appearance for an embryo of this age. It is Very wide and gives a great breadth to the head when viewed from the ventral aspect. The lateral lips of this neuropore curve dorsally and form the ventral boundary of a deep groove which is directed cephalo—caudally. The caudal end of this groove runs into the stomodeum. This part of the nervous system which represents the forebrain has not kept apace with the development of the remainder of the tube. It apparently is a persistence of the condition which is present in an earlier stage of development. Eternod’s (’95) embryo of eight somit'es and the embryo of seven somites described by Dandy (’10) exhibit cephalic neuropores which appear to be in about the_same stage of development.
There are no indications of otic invaginations. Two pairs of entodermal pouches are in contact with the ectoderm. The points of contact are indicated on the surface by shallow depressions. In figure 2 their positions have been indicated on the surface by broken lines.
The amnion lies close on to the body of the embryo. The head fold crosses the ventral aspect of the heart at about its middle. The lateral folds follow the lateral lips of the coelom. The tail fold is situated on the dorsal aspect of the belly stalk.
Nervous System
The nervous system has not proceeded Very far in its differentiation. The brain flexures do not agree With the His models of this stage, but correspond more to the older embryos described by Thompson (’07) and van den Broek (’11). The most distal portion representing the forebrain is still open and is bent almost at right angles to the mid-brain. The long axis of the forebrain lies in a cephalo-caudal plane and almost parallel with the long axis of the hind-brain. The most cephalic point of the nervous system_ is thus represented by the junction of the forebrain and the mid-brain. Near the caudal extremity of the forebrain there is a thickening together with an evagination of the brain ectoderm. This evagination is almost in contact with the ectoderm of the stomodeum and undoubtedly represents the infundibulum. Cephalad to the infundibulum and about in the middle of the lateral expansions of the cephalic neuropore there is a slight depression of the ectoderm on each side which represents the beginning of the optic Vesicles.
The mid-brain is quite extensive as is apparent from an examination of figure 3. Its floor is smooth and exhibits a thickening at the cephalic end. Caudally there is a flexure of the floor between the mid-brain and the hind-brain. The floor of the mesencephalon is thickened at its cephalic end. The trigeminal ganglion is present as a distinct mass of cells. Its position is represented in figure 3 by a broken circle. The hind-brain passes gradually into the spinal cord. A distinct neck flexure is not present.
The medullary tube has its greatest diameter at the cephalic
extremity. It diminishes gradually in size caudally. At the
caudal neuropore it exhibits a slight enlargement.
Digestive System
The stomodeum is a broad and deep invagination of the ectoderm between the heart bulging and the head. It touches the entoderm of the pharynx and forms with it the beginning of an oral plate. There is no indication of an hypophysis. The ectoderm lining the stomodeum is thickened especially in the roof.
The cephalic extremity of the pharynx projects beyond the oral
plate and nearly reaches the floor of the forebrain, a small
amount of mesoderm intervening.
The median thyreoid anlage is a very prominent evagination
of the entoderm of the floor of the pharynx. It projects between
the layers of splanchnic mesoderm at the arterial end of the
heart immediately caudad to the endothelial aorta and the first
aortic arches. The cephalic wall of the evagination is considerably thicker than the caudal. Cephalad to the thyreoid anlage
the first branchial pouches are evaginated from the lateral wall
of the pharynx and immediately caudad to the thyreoid the
second pair of pouches are present. The first pair of pouches
are the larger. Their long axes are directed laterally, cephalad.
and slightly dorsal. Opposite the venous opening of the heart the liver anlage is present as a thickening of the gut entoderm. Lung buds have not developed in this stage.
A cross section of the foregut has a crescentic outline with the concavity directed dorsally. The tube is widest at the point where the first pair of branchial pouches is developed. The cephalic part of the foregut is flattened dorso-ventrally. Caudally the dorso-ventral diameter increases gradually to the end of the foregut where it becomes greater than the lateral diamter.
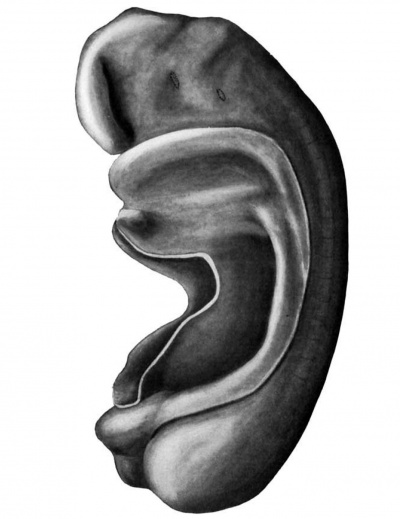
Fig. 1. Wax plate reconstruction of complete embryo seen from left side. The broken lines indicate the points where the entodermal pouches touch the ectoderm. X100.
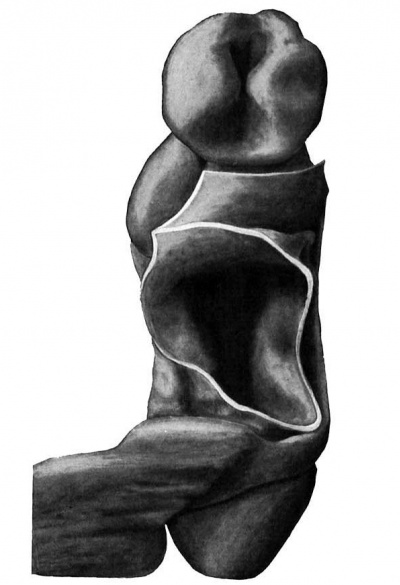
Fig. 2. Wax plate reconstruction of coiiiplete embryo seen from the ventral aspect. x 100.
The gut entoderm extending out into the yolk stalk retains its thickness only a short distance (fig. 3).
The hind-gut is shorter than the foregut. Its dorso-ventral
diameter is comparatively large while its lateral diameter is small.
The allantois is evaginated from the ventral wall. The lumen
of the diverticulum is very small at its proximal end, but throughout the rest of its extent it is distinct. At first the allantois
lies between the allantoic arteries. At its distal end it comes
to lie between the venous and arterial trunks or sinuses of the
belly stalk. The end of the allantois is not recurved as found
by Lewis (’12) but ends as a straight tube. The hind-gut exhibits a dorso-ventral constriction immediately cephalad to the
allantoic diverticulum. Caudal to the allantois the hind-gut widens out to form the cloaca. The entoderm of the ventral wall of the cloaca is fused with the body ectoderm and forms a thick cloacal membrane. At the most caudal part of the cloaca there is a; thickening of the entoderm together with a slight evagination which is suggestive of a post anal gut.
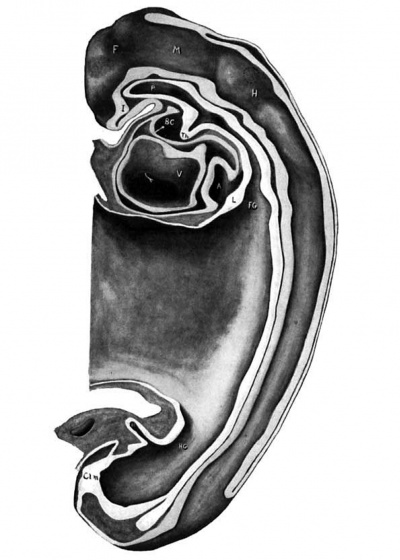
Fig. 3 Graphic reconstruction representing the embryo cut in the mid-sagittal plane. The broken circle above the letter H represents the position of the trigeminal ganglion. x100.
A, Atrium C'l.M, Cloacal membrane L, Liver anlage Al., Allantois F’, Forebrain M, Mid-brain Al.A, Allantoic artery FG, Foregut P, Pharynx Al.V, Allantoic vein H, Hind-brain Th, Thyreoid BC, Bulbus cordis HG, Hind-gut V, Ventricle I, Infundibulum
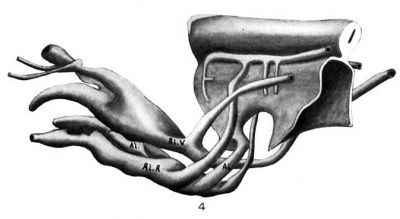
Fig. 4 Wax plate reconstruction of caudal end of the medullary tube and hind-gut with the belly stalk vessels viewed from the side. X 100.
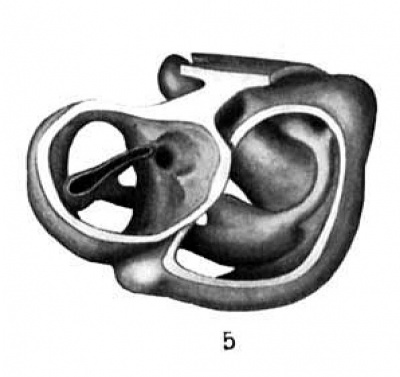
Fig. 5 Wax plate reconstruction of a section of the heart with the endothelial tube in position viewed from the cephalic aspect. X 100.
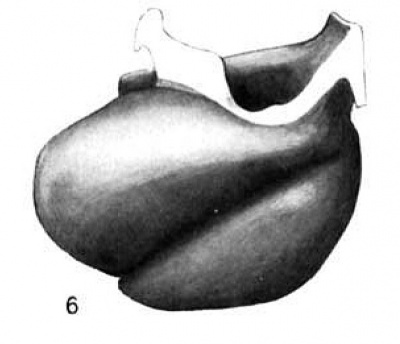
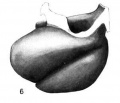
Fig. 6 Wax plate reconstruction of the heart viewed from the cephalic aspect. X 100.
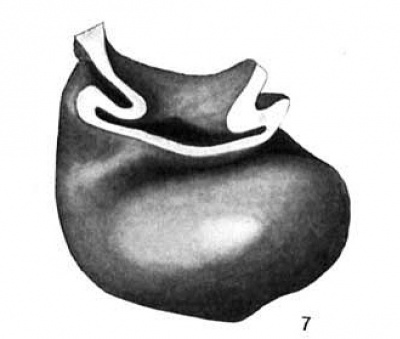
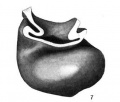
Fig. 7 Wax plate reconstruction of the heart viewed from the caudal aspect. X 100.
Notochord
The notochord is about in the same stage of development as the one described in a 2.5 mm. embryo by Kollmann (’90). The notochord is intimately connected with the gut entoderm throughout its length with the exception of the caudal end. The caudal end, or tail bud, is cut off from the entodertn and lies imbedded in the mesoderm between the neural ectoderm and the gut tube. There is no distinct notochordal canal as described by Mall (’91), Eternod (’99) and Grosser (’13). In places the cells of the notochord are vacuolated and apparently in a stage of developing a canal. The relationship of the notochord to the gut entoderm is a very intimate one. In the .region of the mid-gut the notochord is composed of but a single layer of cells which appear to be a modified part of the gut entoderm. Where the notochord is composed of more than a single layer of cells the basal layer is directly continuous with the single layer of cells forming the gut entoderm. It is impossible to give any other interpretation than that the Trotochord is developed from the gut entoderm. In places the cells of the notochord are arranged in two lateral masses giving the appearance of bilateral symmetry. This condition is undoubtedly accounted for by the arched nature of the original notochordal plate. In the subsequent proliferation of cells they would grow laterally and unless there were an especially active growth of cells in the central part a gap would naturally intervene between the two lateral groups of cells. At the cephalic end the notochord has more the appearance of a rod and is almost pinched off from the entoderm. On account of the plane of the sections it is not possible to determine with certainty the cephalic limit of the notochord.
Mesodermal Structure
There are thirteen pairs of mesodermal somites. These are hardly discernible on the surface. The first pair is situated at a level of about midway between the neck flexure and the hind-brain flexure. The last pair is opposite the point where the allantois leaves the hind—gut. A myocoele may be observed in most of the somites. The cells of the somite are arranged in a radial manner.
The pleuro-peritoneal coelom communicates with the extra-embryonic coelom on the two sides of the yolk stalk. In its
cephalic portion it communicates with the pericardial coelom.
The lateral lips bounding the open part of the pleuro-peritoneal
coelom have a thickened edge produced by the allantoic Veins
which_run cephalad in this position.
The septum transversum is present as a single layer composed
of the cephalic wall of the yolk stalk fused with the caudal part
of the pericardium.
The excretory system is represented by pronephric tubules. The morphological details of these, as far as I have studied them, agree with the description given by Felix (’12).
Vascular System
The heart tube is composed of three parts, bulbus cordis, ventricle and atrium. The atrium is situated in the mid~line of the body immediately cephalad to the septum transversum. Its greatest diameter is transverse. From the left extremity of the atrium the atrial canal runs to the left, ventral and cephalad to the ventricle. The ventricle pursues a course from the left to right, ventrally and somewhat caudad. At the right extremity of the ventricle the heart tube makes a sharp bend so that its continuation, the bulbus cordis, comes to lie parallel with the ventricle. The bulbus cordis has a fairly constant size up to its cephalic end where it diminishes slightly. It ends in the mid—line of the body.
The ventral wall of the cephalic end of the bulbus cordis is continuous with the pericardium as is also the case with the caudal wall of the venous end of the heart (figs. 3, 6, 7). The
dorsal wall of the bulbus cordis has a distinct mesentery connecting it with the dorsal pericardium. Near the point where the atrial canal joins the ventricle the ventricle has a mesentery which joins the pericardium at the place Where the mesentery of the bulbus cordis joins it. Caudal to the junction of these two mesenteries there is a small space dorsal to the atrium which is free from mesentery and represents the future transverse sinus of the pericardium.
From the dorsal wall of the bulbus cordis a tube-like diverticulum is present (fig. 5). I have been unable to find any ref-
erences in literature to anything similar to this. The tube runs
in the mesentery of the bulbus cordis and at its distal end it
comes into close proximity to the ventricle. It is probable that
this tube represents a vestige of the space between the two
laminae in the closing up of the heart tube and the formation of
the mesocardium. Two other tubular spaces of a similar appearance may be seen in the mesentery. They have no communication with the cavity of the myo—epicardium. I observed a
similar diverticulum from the bulbus cordis in a 4.06 mm. embryo
belonging to the collection of the Department of Anatomy of
Syracuse University. It may be noted that this bulbus cordis
diverticulum does not contain any endothelium. The endothe-
lial fibrillae, however, appear to extend into it.
The endothelium in no place approximates the walls of the
myo—epicardium. The caliber of the endothelial tube varies in
thepdiflerent chambers of the heart, being quite constant in the
bulbus cordis, enlarged in the ventricle, and greatly reduced in
the atrial canal. In the atrium it widens out into the right and
left lateral expansions of the atrium. At its cephalic end the
endocardium is continued by the ventral aorta which immediately divides to form the first pair of aortic arches. At the
venous end of the heart the most distal part of the endocardium
represents the sinus venosus. There is no constriction between
the sinus venosus and the atrial part of the endocardium. The
endothelial fibrillae which have been observed by various authors and to which Mall (’12) ascribes the source of the intima may
be seen in connection with the endocardium in its entire length.
The blood vessels are collapsed in places so that it is not possible to trace them'in their entire extent. The communication
between the first pair of aortic arches and the dorsal aortae
could not be seen. The dorsal aortae are distinct throughout
their course lying dorsal to the gut tube. There is no indication
of a second pair of aortic arches. The first pair come off at a
point cephalad to the first mesodermal somites. Vitelline vessels containing blood are easily discernible in the wall of the
yolk sac and yolk stalk. Vitelline veins run dorsally in the
cephalic part of the yolk stalk to gain the caudo—ventral aspect
of the sinus venosus opposite the fourth pair of somites. The
allantoic veins (fig. 4) begin in the belly stalk as a single trunk
or sinus. As the sinus approaches the body of the embryo it
bifurcates to form the two allantoic veins which diverge and
run laterally and cephalad to gain the lateral lips of the coelom.
In this position they run in a cephalad direction to the septum
transversum Where they enter the caudo—dorsal part of the sinus
venosus. The allantoic arteries leave the dorsal aortae at a point
opposite the place where the allantois is evaginated from the
hind—gut and caudal to the last pair of somites. The arteries
run ventrally on either side of the allantois in the belly stalk.
At a point more distal than the bifurcation of the allantoic ve-
nous trunk the allantoic arteries anastomose to form a single
trunk. I have been unable to find any trace of the anterior and
posterior cardinal Veins. At the cephalo-dorsal aspect of the
sinus venosus on the left side there is a-short bud-like diverticulum Which may represent the future ductus Cuvieri.
I Wish to take this opportunity to thank Dr. Boencke for this valuable embryo and Profs. H. D. Senior and F. W. Thyng for assistance and advice in connection with this piece of work.
Bibliography
VAN DER BROEK, A. J . P. 1911 Zur Kasuistik J unger Menschlichen Embryonen. Anatomische Hefte, 44.2, 275-302.
Dandy WE. A human embryo with seven pairs of somites measuring about 2 mm in length. (1910) Amer. J Anat. 10: 85-109.
ETERNOD, A. C. F. 1895 Communication sur un oeuf humain avec embryon excessivement jcune. Arch. Ital. de Biologie, 22
1899 II y a un canal notochordal dans l’embryon humain. Anat. Anz., 16, 131-143.
Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979.
GROSSER, O. 1913 Ein Menschlicher Embryo mit Chordakanal. Anat. Hefte. 7, 653-686.
HIS, W. 1880 Anatomie Menschliche Embryonen, Leipzig.
KOLLMANN, J . 1890 Die Entwickelung der Chorda dorsalis bei dem Menschen. Anat. Anz., 5, 308-321.
Lewis FT. The early development of the entodermal tract and the formation of its subdivisions. (1912) chapter 17, vol. 2, in Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
Low A. Description of a human embryo of 13-14 mesodermic somites. (1908) J Anat Physiol. 42(3): 237-51. PMID 17232769 | PMC1289161
Mall FP. A human embryo twenty-six days old. (1891) J Morphol. 5: 459-480.
Thompson P. Description of a human embryo of twenty-three paired somites. (1907) J Anat Physiol, 41(3):159-71. PMID 17232726
Cite this page: Hill, M.A. (2026, March 6) Embryology Paper - A Human Embryo of Thirteen Somites. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_Human_Embryo_of_Thirteen_Somites
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G