Book - Manual of Human Embryology 17-1
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Grosser O. Lewis FT. and McMurrich JP. The Development of the Digestive Tract and of the Organs of Respiration. (1912) chapter 17, vol. 2, in Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Lewis FT. The early development of the entodermal tract and the formation of its subdivisions. (1912) chapter 17, vol. 2, in Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
The Early Development of the Entodermal Tract and the Formation of its Subdivisions
Peters's Embryo - Yolk-sac
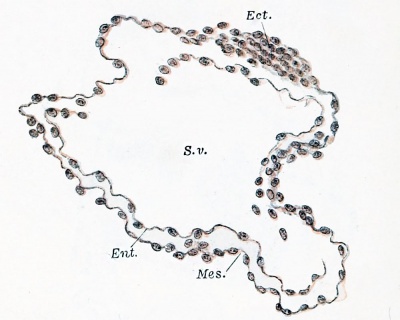
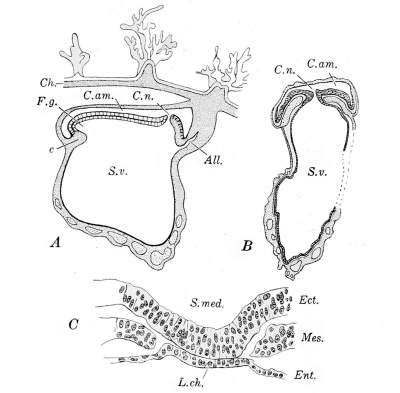
It has been inferred from comparative studies that the human entodermal tract arises as a solid mass of cells, and diagrams of this hypothetical stage have been published by Keibel, Schlater, and others. In the youngest embryos which have been observed, however, the entodermal cells surround a cavity. This is the condition in an embryo obtained by Peters (1899) at the autopsy of a suicide who had taken caustic potash one month after her last catamenia. Bryce and Teacher (1908) estimate the age of this embryo as 13 % to 14% days. It is generally conceded to be the youngest properly preserved human embryo yet described. The embryo extended through nineteen 10 /a sections, and was cut "obliquely to the longitudinal axis." A drawing of only one section was published, and this is reproduced in Fig. 224. The cavity of the yolk-sac contains round masses of coagulum. It is bounded by a layer of entodermal cells which are not everywhere distinct. The entoderm in this section appears to be completely surrounded by mesoderm, which forms the outer layer of the wall of the yolk-sac. A strand of mesoderm extends from the yolk-sac to the chorion, bounding a space designated Sp in the figure.[1] Dorsal to the yolk-sac is the amniotic cavity, bounded above by a thin layer of amniotic ectoderm and below by the very thick embryonic shield, also ectoderm. According to von Spee, who studied Peters 's specimen (Peters, 1899), "It is impossible to speak of an isolated body-stalk which connects the embryo with the chorion, because almost the whole embryonic formation seems imbedded in a thickening of the chorionic mesoderm. Whether the first small beginning of an entodermal diverticulum (allantoic duct) has already started to grow out from the caudal end, and appears in the form of a ring of epithelioid cells arranged about a lumen (in section 11, etc.), remains to me entirely uncertain." Keibel has modelled Peters's embryo from outline drawings made upon wax plates by Selenka. He failed to find an allantois, but records that the outer surface of the yolk-sac is uneven as if blood and vessels had begun to develop in its mesoderm (Keibel and Elze, 1908). It will be noted that von Spee does not state definitely that Peters's specimen has no allantois. In describing another very young embryo he had recorded that "as compared with the embryonic shield, the allantois is remarkably long, and ought therefore to appear very early" (1896, p. 9).
Fig. 224. — Obliquely longitudinal section of Peters's embryo. (After Peters.) Cav. am. (cavum amnii), amniotic cavity; S. v. (sacculus vitellinus), yolk-sac; Ent., entoderm, and Mes., mesoderm of the yolk-sac; Mes. eh., mesoderm of the chorion; Sp., "cleft, in the exoccelom"(?).
Two other embryos, both removed from the uterus by curetting within a month after the last catamenia, may have no allantois. In one of these "an allantoic duct of the yolk-sac does not stand out clearly" (Beneke, 1904), and in the other "the body-stalk consists only of mesodermal cells" (Jung, 1908). Neither of these accounts, however, is convincing in regard to the absence of the allantois, for in the first case the statement is indefinite, and in the second the allantois is not specifically mentioned. .
Herzog's Embryo - Yolk-sac and Allantois
Herzog (1909) has recently described an embryo obtained at the autopsy of a woman who had been struck over the heart by the shaft of a swiftly moving carriage and almost instantly killed. The specimen is unquestionably normal, and is well preserved histologically, but it has suffered considerable mechanical injury, partly after being mounted. After Herzog had published and described accurate figures of twenty-two successive sections (7/* thick), including all of the embryo except a portion of the yolk-sac, he deposited the specimen in the Harvard Collection. For the privilege of studying further and modelling this embryo, the writer is under great obligation to Dr. Herzog.
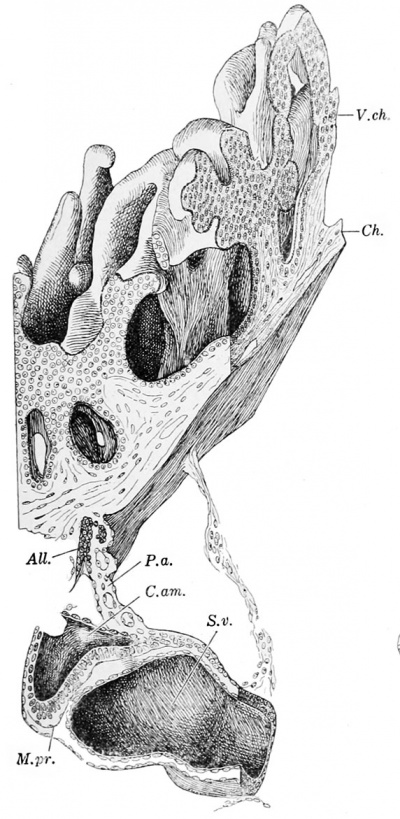
Fig. 225. — Longitudinal section of Herzog's embryo. X 215 diam. (After Herzog.) Mes.ch., mesoderm of the chorion; P. a. (pedunculus abdominalis), body-stalk; All., allantois ; Cam., amniotic cavity; Ect., ectoderm of the embryonic shield; M. pr., membrana prima, to which some cells are adherent; S. v., yolk-sac; Mes., mesoderm.
The plane of section is nearly longitudinal. In the section shown in Fig. 225 the allantois is found extending through the body-stalk toward the chorion. The entoderm of the yolk-sac is seen below, extending toward the allantois, but the connection between the two has been destroyed. It presumably occurred in this section. The ectoderm bounding the amniotic cavity consists of a thin layer above, for the most part broken away in this section, and the thick ectoderm of the embryonic shield below, which is broken into two pieces. The shield is bent upon itself, and the depression shown in the figure is transverse to the axis of the embryo. Between the ectoderm of the shield and the entoderm of the yolk-sac there is a structureless membrane such as Hensen (1875) observed in the rabbit and called the membrana prima. It is the detached basement membrane of the ectoderm of the embryonic shield, and was noted by von Spee in Peters 's specimen. A layer of mesoderm passes over the ventral surface of the yolk-sac, and anteriorly (to the left of the figure) it is in relation with the ectoderm of the shield. It does not extend between the ectoderm of the shield and the yolk-sac, and in this respect Herzog's embryo differs from Peters 's specimen as described by von Spee. In Fig. 225 the yolk-sac is cut tangentially, but it is evident that toward the allantois its cells are cuboidal. The allantois consists of similar cells and contains a lumen. Over the greater part of the yolk-sac, however, the entoderm forms a very thin layer resembling endothelium, precisely as recorded by Beneke. In the most ventral portion there are occasional cuboidal cells with large round nuclei and protoplasm which projects above the general level into the cavity of the yolk-sac. The mesoderm of the yolk-sac is also very thin, and shows neither vessels nor distinct blood islands (see Fig. 226).
Fig. 226. — Longitudinal section of Herzog's embryo, separated by twelve "/"• sections from Fig. 3. X215 diam. (After Herzog.) Ect., a mass of ectoderm bounding the amniotic cavity on the left side of the embryo. Ent., entoderm, and Mes., mesoderm of the yolk-sac S. v.
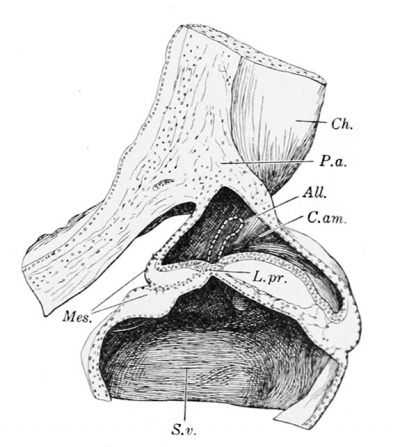
A reconstruction of Herzog's embryo, cut through at right angles with the plane of section, is shown in Fig. 227. The model will be more readily understood in comparison with a similar view of a somewhat older embryo, Fig. 228. In both cases the amniotic cavity sends a prolongation, torn in Herzog's specimen, toward the chorion; and in both the amniotic cavity is asymmetrical, extending farther toward the left of the embryo (which is on the right of the figure). In Herzog's specimen the prolongation of the amniotic cavity toward the left is at first tubular; it is then reduced to a solid clump of cells, lodged between the entoderm and mesoderm of the yolk-sac, and closely applied to the latter (see Fig. 226).
Fig. 227. — Wax reconstruction of Herzog's embryo. The plane of section is transverse to the axis of the embryo. X100 cliam. AIL, allantois; Cam., amniotic cavity; Ch., chorion; M.pr., membrana prima; P. a., body-stalk; S. v., yolk-sac; V.ch.\ (villi choriales), chorionic villi.
Fig. 228. — Wax reconstruction of Minot's embryo, showing the part corresponding with that drawn in Fig. 227. X50 diam. L.pr. (linea primitiva), primitive streak; Mes., mesoderm. Other abbreviations as in Fig. 227.
The body-stalk contains the allantois, which is apparently disintegrated near its tip. The terminal sections, however, are well preserved, and the extremity of the allantois is probably recurved. The body-stalk contains also rings of cells, some of them very near the surface. It is not certain that these represent blood-vessels — which would be the first to appear in the embryo — as Herzog interpreted them, and yet it is clear from later stages that the blood-vessels in the body-stalk arise very early. Jung has carefully described similar rings in the body-stalk of his specimen. There are occasional clefts in the mesoderm of the chorion in Herzog 's embryo, but they are of doubtful significance. As seen in the reconstruction, a strand of mesoderm extends between the yolk-sac and chorion much as in Peters 's specimen.
Von Spee's Embryo "v.H."
In 1896 von Spee published a notable contribution to human embryology, to which reference has already been made. In it he describes an embryo, designated "v. H.," which has heretofore been placed next to Peters 's specimen, but which seems older than Herzog 's for the following reasons. The axis of the embryo is said to be represented by a primitive groove (not well defined, however) which is absent in Herzog 's embryo; the mesoderm extends between the ectoderm and the yolk-sac, reaching the median line; the entoderm of the yolk-sac is not flat, but is cuboidal throughout; the mesoderm of the ventral portion of the yolk-sac is thrown into elevations by the blood islands within it. The age of "v. H.," obtained through abortion following influenza five weeks after the end of the last catamenia, has been estimated as 17 to 18 days.
Minot's Embryo - The Primitive Knot
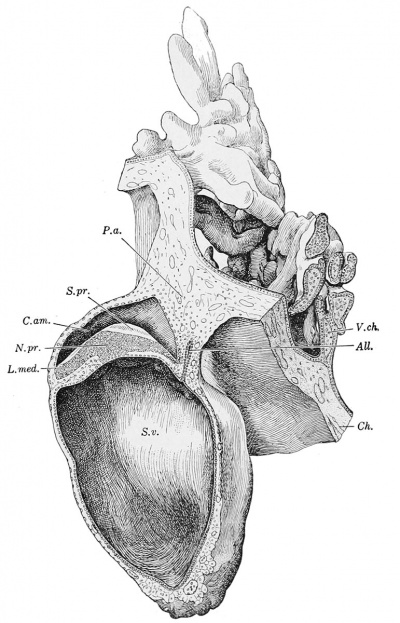
Hensen (1875), in describing the primitive streak of the rabbit, stated that anteriorly it developed a disk-shaped termination, which he designated as a "knot." He found that the layer of entoderm could be stripped from the embryo except at the knot, where it tore. There the ectoderm and entoderm are intimately blended. This primitive knot (often called Hensen 's knot) is shown in the reconstruction of a human embryo in Fig. 229. It is possible that it is represented in one section of Herzog's embryo (Fig. 11 of his publication), but von Spee did not find it in "v. H." Toward the allantois from the primitive knot, as seen in Fig. 229, the primitive groove is found, along which the mesoderm fuses with the ectoderm, as shown in the cross section, Fig. 228. Anterior to the primitive knot the thick ectoderm forms the medullary plate, but the medullary groove has not yet appeared. Beneath the medullary plate the mesoderm extends from side to side across the median line. These conditions are noted as determining the stage of development of this specimen.[2] The entodermal tract in this embryo still consists of only two parts, the yolk-sac and allantois. As compared with Herzog's specimen, the allantois has increased in length from about 0.12 to 0.20 mm. It is slightly expanded distally but is not recurved. There is a minute lumen, and the structure connects with the yolksac by a small funnel-shaped enlargement. The yolk-sac has grown more rapidly than the allantois, its transverse diameter having increased approximately from 0.25 to 0.75 mm. In the ventral portion of the yolk-sac the entoderm now consists of cuboidal cells, and the mesoderm has been curiously transformed, as shown in Fig. 230. The cells are large and extensively vacuolated, so that the protoplasm in places is reduced to strands. The nuclei are large, round, and pale, each containing a very delicate chromatic reticulum and often a single conspicuous knot of chromatin. Among these mesodermal cells blood-vessels have appeared, lined by true endothelium. They contain blood-corpuscles, characterized by finely reticular protoplasm, ill-defined cell-membranes, and nuclei which may be round, with distinct chromatin granules, or irregularly shrunken and deeply stained. Sometimes a corpuscle is closely applied to the endothelium as if arising from it.
Fig. 229. — Wax reconstruction of Minot's embryo, showing a median sagittal section. X50 diam All., allantois; Cam., amniotic cavity; Ch., chorion; L.med. (lamina medullaris), medullary plate; N.pr. (nodulus primitivus), primitive knot; P.a., body-stalk; S.pr. (sulcus primitivus), primitive groove; yolk-sac; V.ch.. chorionic villi.
Fig. 230. — A portion of the ventral wall of the yolk-sac of Minot's embryo (Harvard Collection, Series 825, Section 18). X280 diam. Co., coagulum in the yolk-sac; Coag., coagulum in the chorionic cavity; End., endothelium lining a blood-vessel containing five blood-corpuscles, one of which shows a vesicular nucleus; Ent., entoderm, and Mes., mesoderm of the yolk-sac.
In a very few places the entoderm of the ventral surface of the yolk-sac sends a prolongation into the mesoderm. In one case the outgrowth is solid, in another it contains a cavity in its outer part, and in a third a detached entodermal cyst is found near the surface of the mesoderm. These appear to be chance irregularities in the expansion of the yolk-sac.
There is granular coagulum within the yolk-sac, and also in the chorionic cavity, but there are no globular formations as in Peters 's specimen. Eternod examined the yolk-sac of a young human embryo (the age is not stated) removed by operation and still living. He states (1906, p. 256), "The very transparent liquid, which fully distended the yolk-sac, had a beautiful goldenyellow color comparable with that of the yolk in the eggs of salmon or trout. Under the influence of light, in a few moments, the color clouded and faded, becoming opalescent." In sections of very young human embryos he found entodermal cells projecting into the yolk-sac or detached and floating within it. In the Minot specimen it is very difficult to find a floating cell, but in embryos less well preserved they occur frequently. To what extent the contents of the human yolk-sac has a nutritive function is wholly undetermined.
In the Minot embryo the lining of the yolk-sac is a simple layer throughout (it is obliquely cut in Fig. 230). In the dorsal half of the sac the entodermal cells are quite flat. The mesoderm also becomes a thin layer, and the blood-vessels are very small. Apparently those in the yolk-sac do not pass into the body-stalk, which, however, contains numerous vessels. There are also many spaces in the chorion, especially near its lower surface, which are probably true vessels. Frequently these contain strands of darkly staining cells, suggesting collapsed endothelium. Similarly in the slightly older Frassi embryo there are "vessels on the yolksac, in the body-stalk, and the adjacent chorion; no vessels in the embryo proper." From the study of the specimens thus far considered it appears that the yolk-sac is not the only source of bloodvessels, but that they arise also in the body-stalk and chorion. Recently Dandy has reached this conclusion from the study of an older embryo.
Von Spee's "Gle."
Neurenteric Canal. Chordal Plate. Beginning of the Fore-gut. — At a slightly later stage than that just described, a canal develops through the primitive knot, by which the cavity of the amnion communicates with that of the yolksac. This neurenteric canal has not formed in Minot 's embryo, and in the Frassi embryo an aperture could not be demonstrated. Beneke recorded a neurenteric canal in his younger specimen, but his account is not convincing.
Von Spee, however, found a very large canal in an embryo designated "Gle" (1889). The specimen was obtained by spontaneous abortion five weeks after the end of the last catamenia, and Bryce and Teacher estimate its age as 19-20 days. A diagrammatic median section of the embryo is shown in Fig. 231, A. Before the embryo was sectioned it was made transparent with turpentine. In the position of the primitive knot (that is, between the anterior end of the primitive groove and the posterior end of the medullary groove) a ring-shaped elevation was seen, 0.13 mm. in diameter, pierced by a central aperture 0.02 mm. wide. In the series this opening was found in four sections, one of which is shown in Fig. 231, B. It will be seen that at the neurenteric canal the ectoderm is continuous with the entoderm. The mesoderm does not form any part of its wall.
Eternod (1899) has recorded two other cases of open neurenteric canals, one in an embryo very much like "Gle," measuring 1.3 mm., the other in an older specimen, measuring 2.11 mm.
The entoderm lining the yolk-sac in "Gle" resembles that in the Minot embryo, except that just beneath the medullary groove it exhibits a plate of low columnar cells (Fig. 231, C). This chordal plate gives rise to the notochord and perhaps to a portion of the intestinal epithelium. It begins at the anterior margin of the primitive knot, with which it is continuous. It extends forward as far as the yolk-sac is in contact with the medullary plate. In the anterior part of the embryo there is a slight forward prolongation of the yolk-sac, which is the beginning of the fore-gut (Fig. 231, A). The heart is developing in the fold of mesoderm just beneath it. At the opposite end of the embryo the allantois takes a somewhat zigzag course in the body-stalk. Certain portions of the mesoderm of the body-stalk, as stated by von Spee, are very rich in spaces, some of which have a smooth lining of flat cells like those of embryonic endothelium. The hind-gut has not yet appeared.
Fig. 231. — Sections of von Spee's embryo "Gle." A, median sagittal section from a model; B, transverse section through the neurenteric canal, C. n.; C, portion of a transverse section showing the chordal plate, L.ch. (After von Spee.) F.g., "fore-gut; " S. med. (sulcus medullaris), medullary groove. Other abbreviations as in preceding figures.
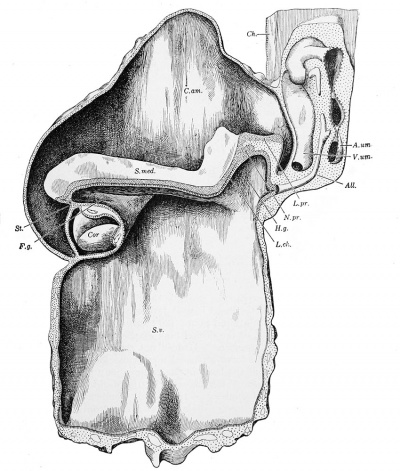
Fig. 232. — Wax reconstruction of Mall's Series 391, showing a median sagittal section of the embryo. X50 diam. All., allantois; A.um., arteria umbilioalis; C. am., amniotic cavity; Ch., chorion; Cor, heart; F.g., " fore-gut; " H .g., "hind-gut; " L.ch., chordal plate; L.pr., primitive streak; N. pr., primitive knot; S. med. (sulcus medullaris), medullary groove; St., stomodeeum; S. v., yolk-sac; V. urn., vena umbilicalis.
Mall's Series 391 - Formation of the Hind-gut
In the Mall collection there is an embryo with seven pairs of somites, measuring about 2 mm. in length, which has recently been described by Dandy. It was obtained through abortion, mechanically induced, and its age is estimated at about 24 days. Fig. 232 is from a model of the embryo, and shows a median longitudinal section. 4 The back bends sharply downward toward the cavity of the yolksac. Such flexures occur frequently, but not invariably, in embryos of about this age, and are probably due to imperfect preservation. "The fore-gut is present in thirty-two sections representing a length of 320 microns." It ends blindly in front, and, according to Dandy, it is separated by mesoderm from the ectodermal depression (or stomodaeum) which gives rise to the mouth. The presence of intervening mesoderm is, however, difficult to make out, since the tissues are somewhat fragmented in this region. The foregut shows a lateral expansion, which is the first pharyngeal pouch. Beneath the fore-gut the heart is well developed.
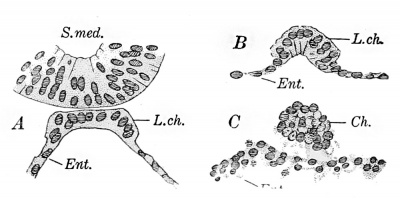
Fig. 233. — Sections showing the separation of the notochord from the digestive tract. A, from Mall's Series 391, X360 diam.; B, section in the region of the first pair of somites, and C, in the caudal region, of Low's embryo. (B and C after Low.) Ch. (chorda), notochord; Ent., entoderm of the digestive tract; L. ch., chordal plate; S. med., medullary groove.
"The hind-gut is a blind pouch 120 microns in length by sections, but on account of the dorsal kink of the embryo the actual length is somewhat greater." It does not come in contact with the ectoderm so as to form a cloacal membrane (where the anus will later appear), but the bend in the embryo may possibly have caused the separation of the layers. In the decidedly younger Frassi specimen the beginning of the cloacal membrane is said to be present. In Mall's embryo the allantois arises from the ventral surface of the hind-gut and passes into the body-stalk, accompanied by very large umbilical vessels. The allantois has a knob-like branch. The distal end of the allantois is apparently detached. In both portions a narrow lumen is found.
The chordal plate (Fig. 233, A), extending along the middorsal line of the yolk-sac, is more sharply defined than in "Gle." 4 Through the kindness of Professor Mall, the writer has been permitted to study this embryo and prepare a figure to correspond with that of the Minot specimen. This work has been greatly facilitated by Mr. Dandy's publication.
In an embryo of 13-14 somites, described by Low (1908), the chordal plate is still a portion of the wall of the yolk-sac anteriorly (Fig. 233, B), bnt it lias completely separated from it posteriorly (Fig. 233, C. See also Kollmann, 1890). In older embryos it is detached throughout, and the further history of the notochord, or chorda dorsalis, will be found in the chapter on the development of the skeletal system.
The Cloacal Membrane, Caudal Intestine, and the Later History of the Primitive Knot
In the Mall specimen the chordal plate ends posteriorly in a rounded knot of tissue in connection with the ectoderm. This is clearly the primitive knot, posterior to which is the primitive streak. There is no neurenteric canal. This region in a similar embryo (2.1 mm. long, with eight pairs of somites) has been figured by Eternod (1906 2 ), as seen in Fig. 234, A. The neurenteric canal is still present and leads anteriorly into a chordal canal, the floor of which is formed by detached cells, and the roof of which is the chordal plate. Such a chordal canal in human embryos has apparently not been found by other observers. Posterior to the neurenteric canal the primitive streak extends to the cloacal membrane, which is "composed of a mass of epithelial cells." In an embryo of 5 to 6 pairs of somites, of which Keibel and Elze have published a series of sections, it is possible that the relations are as shown in Fig. 234, B. The dorsal and ventral openings of the neurenteric canal can be found, but the middle part is not pervious. The primitive streak extends to the cloacal membrane, which is described as "the thickened ectoderm applied to the thickened entoderm." Thus the primitive streak passes around from the dorsal to the ventral side of the embryo.
Fig. 234. — Median sagittal sections through the region of the primitive knot. All X75 diam. A, Eternod's embryo with 8 pairs of somites (after Eternod); B, sketch of the "Kroemer-Pfannenstiel" embryo, based upon sections published by Keibel and Elze; C, Bremer's 4 mm. embryo; D, distal portion of the tail of a 7.5 mm. specimen. All., allantois; Can. med. (canalis medullaris), medullary tube; C. ch., chordal canal; C. n., neurenteric canal; Ch., chorda; Int. can., intestinum caudale; L. ch., chordal plate; L. pr., primitive streak; Mem. cl., membrana cloacalis; Neu. p., posterior neuropore, the last part of the medullary groove to close; N. pr., primitive knot; S. med., medullary groove; x, extension of the primitive streak (?) beyond the cloacal membrane.
Keibel and Elze believe that the primitive streak extends beyond the cloacal membrane along the body-stalk, for in several sections the ectoderm covering the body-stalk shows a local thickening which is nearly in contact with the allantoic duct. 5 The occasional occurrence of a bladder opening freely along the ventral body wall (exstrophia vesicae) may be connected with this relation. It seems probable, however, that the thickened epithelium along the body-stalk is due to a prolongation of the cloacal membrane in the urogenital area, and that it is not a part of the true primitive streak. The primitive streak is formed by a fusion of ectoderm and mesoderm, but the cloacal membrane is a fusion of ectoderm and entoderm. According to Keibel, however (1896), the cloacal membrane should be regarded as a modified part of the primitive streak, and exstrophia vesicae represents a persistent portion of the blastopore.
In an older embryo, measuring 4 mm. (Fig. 234, C), the position of the primitive knot can still be located. There the notochord ends and the primitive streak begins. It will be observed that the hind-gut has extended beyond the cloacal membrane into the tail. This prolongation is named the caudal (or postanal) intestine.
The tip of the tail of a 7.5 mm. embryo is shown in Fig. 234, D. It is still possible to recognize the primitive knot, which at this stage is commonly called the "tail-bud." The notochord terminates in this bud; the caudal intestine fuses with it ventrally, the extremity of the medullary tube dorsally, and the mesoderm laterally. In the 2.11 mm. specimen described by Eternod (Fig. 234, A ) there is a short prolongation of the chordal canal beyond the neurenteric canal, but there is apparently no other evidence that the notochord ever extends beyond the primitive knot. In a 2.1 mm. specimen described by Mall the obliterated neurenteric canal, represented by a solid cord of cells, is said to communicate with the medullary tube (1897, p. 419). "The location is opposite the twelfth muscle plate, or in the neighborhood of what will later be the position of the first rib." But the location is also at the posterior end of the notochord which will later be near the tip of the tail. It seems probable that if a neurenteric canal should persist it would be found opening externally beyond the limit of the spinal cord and its filum terminate, in the coccygeal region.[3] Marwedel (1901) has described a ease which may be interpreted as a neurenteric canal leading into the detached end of the caudal intestine. A child thirteen days old was found to have a sac, 6 cm. long, lined with mucous membrane similar to that of the large intestine and surrounded by muscle coats, opening to the surface between what were " evidently the cornua sacralia of the lowest sacral vertebra." The sac had no connection with the rectum or anus.
It should be noted that congenital cysts and sinuses in the coccygeal region are frequent (Mallory, 1892), but they are ectodermal structures, and the persistence of a neurenteric canal has not yet been satisfactorily demonstrated.
The Pharyngeal Membrane and the Prce-oral Intestine
In describing Mall's specimen with seven pairs of somites, it was stated that the anterior end of the fore-gut comes in contact with an ectodermal pouch called the stomodseum or buccal sinus. There the ectoderm unites with the entoderm to form the pharyngeal (or buccopharyngeal) membrane. This membrane is clearly shown in an embryo 2.15 mm. long, as figured by His (Fig. 235, A). The digestive tract at this stage has no anterior opening. Just in front of the pharyngeal membrane the ectoderm forms a pocket extending toward the base of the brain. Although this pocket is now generally called the hypophysis, or more precisely the anterior lobe of the hypophysis, it is often referred to embryologically as Rathke's pocket.
Fig. 235. — The pharyngeal membrane as figured by His. X37 diam. A, embryo "Lg," 2.15 mm.; B, embryo "BB," 3.2 mm. Ch., chorda; C.med., medullary tube; Mem. ph., membrana pharyngea; Ph., pharynx ("fore-gut"); R-, Rathke's pocket (anterior lobe of the hypophysis); S., Seessel's pocket; St., stomodseum.
In 1838 Rathke described it as follows : " For a long time I have observed in several animals ... a small irregularly rounded depression which belongs to the mucous membrane of the mouth, of which it is clearly a thin-walled outpocketing. . . . Finally I saw that this depression represents the first step in the formation of the pituitary gland" (p. 482). The animals studied included sheep and pig embryos.
- Professor Mall now regards this embryo of 2.1 mm. as pathological.
On the entodermal side of the pharyngeal membrane a much smaller pocket bulges toward the brain.
This was discovered by Seessel in the chick (1877). He wrote: "Shortly after the hypophyseal pocket has become distinctly formed, on about the fourth day, near and under it a second pocket-like outgrowth of the intestinal layer is seen. ... Its length compared with that of the hypophysis is as 1 : 5." Although His considered that both pockets were represented in the 2.15 mm. embryo, they are better denned in a specimen measuring 3.2 mm. (Fig. 235, B). The pharyngeal membrane has largely disappeared. "As the remains of it, there is only the prominence inserted between Ratlike 's pocket and Seessel 's accessory pocket." In sheep embryos von Kupffer (1894) found a solid entodermal outgrowth extending forward from Seessel's pocket, closely connected with the notochord. Later this mass of cells becomes detached and appears as an appendage of the notochord. It was interpreted as a rudimentary prae-oral intestine. Bonnet (1901) identified a similar structure, but with a lumen, in a dog embryo with sixteen pairs of segments, and Zimmermann (1899) found three sharply defined little cavities near Rathke's pocket in a human embryo of 3.5 mm. These cavities may have been derived from a prae-oral intestine; they are at present the only evidence of such a structure in human embryos.
Thompson's Embryo
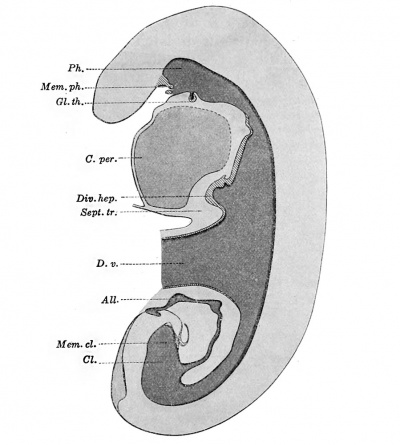
Early Stages of the Thyreoid Gland, Lungs, and Liver. — An embryo 2.5 mm. long, with 23 pairs of somites, has been modelled by Thompson (1907). The entodermal tract is shown in Fig. 236, in which the embryo is arbitrarily placed in an upright position, with its ventral surface toward the left of the figure. The yolk-sac has been cut away. It was connected with the intestine by a somewhat constricted neck, called the vitelline duct, a part of which is shown in the figure. In ventral view the connection between the duct and the intestine would appear as an elongated opening near the middle of the straight intestinal tube.
The pharynx is separated from the mouth by the pharyngeal membrane, which is already perforated. There is no trace of Rathke's pocket. Four pharyngeal pouches are present, but they are not indicated in the figure. In the median line, connected with the floor of the pharynx, there is a small, hollow, rounded diverticulum, which is the beginning of the thyreoid gland. In earlier stages it has a less constricted neck, as found by Low (1908) in a specimen with 13 or 14 pairs of somites. Posterior to the pharyngeal pouches the entodermal tube suddenly narrows. It becomes compressed laterally so that it has a cleft-like lumen. In this portion of the entodermal tract, a short distance beyond the fourth pharyngeal pouches, the lungs are indicated by a pair of lateral outgrowths (Thompson), and perhaps by the ventral swelling which is shown in Thompson's figure but not labelled.[4] Beginning with the region where the lung outgrowths are found, and extending backward as far as the liver-bud, the epithelium is markedly thickened.
The liver-bud is a median ventral knob-like outgrowth of the digestive tube, extending into the septum transversum, which is the layer of mesoderm between the pericardial cavity and the vitelline duct. The hepatic bud contains a cavity which communicates freely with the alimentary canal. There is no trace of the pancreas.
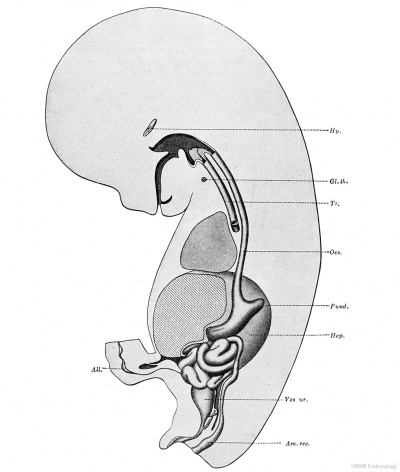
Fig. 236. — Graphic reconstruction of an embryo with 23 paired somites, , 'showing a median sagittal section of the digestive tract. X40diam. (After Thompson.) All., allantois; CL, cloaca; C. per., cavum pericardii; Div. hep. (diverticulum hepaticum), liver bud; D.v., ductus vitellinus; Gl.th., glandula thyreoidea; Mem. cl., membrana cloacalis; Mem. ph., pharyngeal membrane; PA.,lpharynx; Sept. tr., septum trans versum.
Beyond the vitelline duct is the hind-gut, with a rounded lumen. It expands at the cloaca, where it joins the allantois, and extends a short distance into the tail. The allantois is a small tubular structure with two marked dilatations and, at its distal end, ' ' a small swelling bent upon itself. ' ' Separation of the (Esophagus from the Trachea. — In an embryo which has the general shape of Thompson's specimen, but which is somewhat more advanced (Bremer, 1906), the lungs and trachea form a pear-shaped mass attached to the ventral border of the oesophagus. The lower portion of the mass, which bulges toward either side, represents the division of the trachea into the bronchi. Its cavity is still in free communication with that of the oesophagus. The trachea will become separated from the cesophagus by the down-growth of the lung-bud and the upward extension of the notch between the lung-bud and the oesophagus. The notch extends upward following a fusion of the lateral walls of the fore-gut, which begins from below (His, 1885, p. 17-18). The approximation of the lateral walls to form the tracheo-oesophageal septum is seen at the top of Fig. 237.
Fig. 237. — Wax model from Bremer's 4 mm. embryo, showing the "lung-bud," Pul., and the adjacent part of the oesophagus. X175 diam. Sept., tracheo-cesophageal septum; Sul., lateral oesophageal groove.
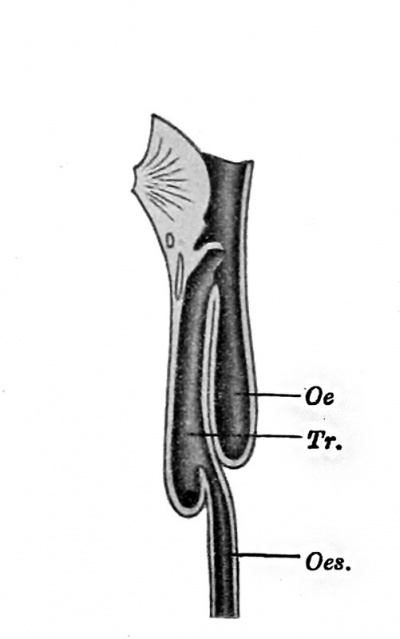
Fig. 238. — Abnormal communication between the oesophagus, Oes., and the trachea, Tr. The upper portion of the oesophagus, Oe., ends blindly below. (After Keith.)
In the most common anomaly of the oesophagus, which must arise at the stage under consideration, the oesophagus is transversely divided into two parts. The upper portion ends blindly below, and the lower portion arises from the trachea near its bifurcation (Fig. 238), or even from a bronchus, into which its lumen opens.
This malformation is detected soon after birth, since milk cannot enter the Stomach. Happich (1905) has tabulated the records of 59 cases. Sometimes the two portions of the oesophagus are connected by a strand containing smooth muscle-fibres, but " it is entirely unknown whether there is any remnant of epithelium in the interval." No epithelial connection has been recorded. Although this complex anomaly is common, a simple communication between cesophagus and trachea, when these are otherwise normal, is extremely rare. Forssner (1907) and Giffhorn (1908) have discussed the origin of the divided cesophagus and explained it with diagrams.
To produce the common form of the anomaly the lower portion of the tracheocesophageal septum must fail to develop, thus leaving the cesophagus in communication with the lower part of the trachea. In the model shown in Fig. 237 there is externally, on either side of the cesophagus, an oblique depression in the epithelium with a corresponding internal elevation. It is so situated that if the walls of the cesophagus should coalesce along this groove a ventral portion would be cut off, communicating freely with the trachea near its bifurcation. The groove does not reach the dorsal border of the cesophagus, but extends downward toward the liver. However, the part of the cesophagus dorsal to this groove has a narrower lumen than the ventral part; to produce the anomaly, this portion must become occluded. Apparently the lateral oesophageal groove, which seems correlated with the shape of the adjacent body-cavity, has not been previously described.
It has been thought that the closure of the cesophagus in the anomaly is due to the pressure of neighboring arteries, particularly the right dorsal aorta, and several cases have been found associated with the low origin of the right subclavian artery. Keith (1906) reported four cases, in three of which this abnormal artery was present and crossed to the right side between the two parts of the cesophagus. In the 4 mm. embryo, however, which is close to the stage in which the anomaly must arise, the arteries are not near this portion of the cesophagus. In the embryo of 4.9 mm. shown in Fig. 239 the separation of the cesophagus and trachea has proceeded so far that the anomaly could hardly develop.
The further history of the anterior portion of the digestive tract, including the mouth, pharynx, trachea, and lungs, will be presented by McMurrich and Grosser in separate sections of this chapter.
Ingalls's Embryo
The Formation of the Stomach and Pancreas. — Ingalls (1907) described an embryo measuring 4.9 mm., the digestive tract of which is shown in Fig. 239. Rathke's pocket is present; nothing is said of Seessel's pocket. The pharyngeal membrane has entirely disappeared. The thyreoid gland is still connected with the pharynx, as in Thompson's specimen. The trachea is quite separate from the cesophagus.
"The cesophagus is a tube, circular in cross section, which has thinner walls and is much narrower than the ventrally placed trachea. In the region of the fourth cervical segment it becomes gradually larger, its walls thicken, and at the same time it becomes flattened laterally. Thus it forms the stomach, and the digestive tube then assumes an oblique position, with its ventral border turned somewhat to the right and its dorsal border correspondingly to the left. ... At its caudal end, where it passes over into the duodenum, the stomach again becomes very narrow." (P. 549-550.)
At this stage, as shown in the figure, the stomach is a welldefined spindle-shaped enlargement of the fore-gat.
The liver has become very large. From the knob-like diverticulum, such as was seen in Thompson's specimen, a great mass of anastomosing cords of cells has grown out, invading the septum transversum. In a cross section of the embryo this mass is U-shaped, and the stomach and duodenum are lodged in the hollow of the U. In sagittal section, as in the figure, the part of the mass which crosses the median line has been cut through. It is shown in section. The inner surface of the right lobe of the liver has also been drawn.
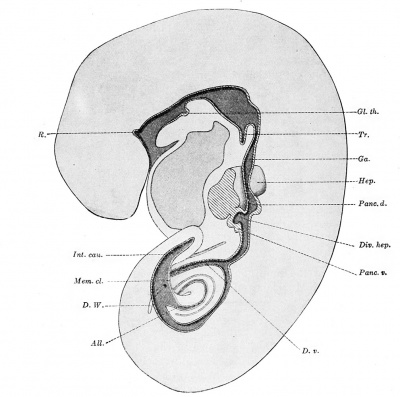
Fig. 239. — The digestive tract of an embryo of 4.9 mm., shown in median sagittal section. X24 diam. (After Ingalls.) All., allantois; Div. hep., diverticulum hepaticum; D. v., ductus vitellinus; D. W. (ductus Wolffi), Wolffian duct; Ga. (gaster, ventriculus), stomach; Gl. th., glandula thyreoidea; Hep., medial surface of the right lobe of the liver (hepar); Int. cau., intestinum caudale; Mem. cl., membrana cloacalis; Pane. d.,?pancreas dorsale; Pane, v., pancreas ventrale; R., Rathke's pocket; Tr., trachea.
The pancreas of the adult arises in the embryo as two separate organs, namely the dorsal pancreas and the ventral pancreas. In Ingalls's specimen, between the stomach and the common bile-duct, the dorsal surface of the duodenum presents a thick-walled outpocketing, which is the beginning of the dorsal pancreas. The ventral pancreas grows downward from the lower side of the hepatic diverticulum, at its junction with the intestine. It is adherent to the wall of the intestine. It contains a minute lumen, not shown in the figure, which appears to communicate with the hepatic diverticulum. (A more detailed account of the development of the liver and pancreas will be found in subsequent sections of this chapter.) The connection between the yolk-sac and the intestine in Ingalls's specimen is a slender vitelline duct, lined with a single layer of large cuboidal or cylindrical cells. The intestine bends ventrally toward its junction with the duct, and beyond this point is becomes much smaller. It then enlarges toward the cloaca and continues with a relatively large lumen into the tail. Toward the tip of the tail its wall becomes irregular in thickness.
The cloacal membrane does not consist of thickened ectoderm applied to thickened entoderm, as in a younger specimen already described. On the contrary, the ectodermal layer is here so thin that in places it can scarcely be recognized. The entodermal layer is also thinner than in the lateral walls of the cloaca. Keibel (1896) found the ectoderm of the cloacal membrane thinner than the entoderm in a 3 mm. specimen. At 4.2 mm. the layers were indistinguishable, and in discussing later stages he wrote, "The question, how much ectoderm and how much entoderm take part in the formation of the cloacal plate, must remain undecided. ' ' In Ingalls 's specimen the allantois shows two small expansions near its distal end. Proximally the allantois joins the cloaca, which is being gradually subdivided, in the cranio-caudal direction, by the growth of the cloacal septum. The Wolffian ducts empty into the ventral part of the cloaca, one on either side, and, although they are not of entodermal origin, they have been included in the accompanying figures.
Embryo of 7.5 mm
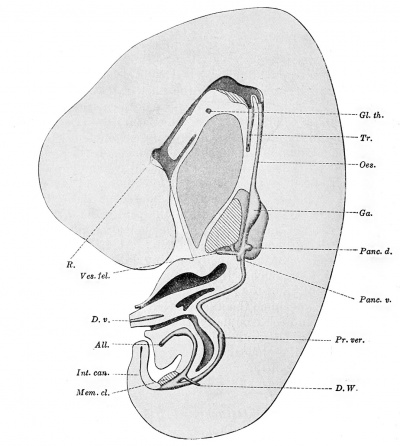
Detachment of the Yolk-sac. Origin of the Caecum and Vermiform Process. — The entodermal tract in an embryo measuring 7.5 mm. is shown in Fig. 240. Rathke's pocket still has a broad connection with the oral cavity, but the thyreoid gland has become detached. The oesophagus is much longer than in Ingalls's specimen. It becomes gradually smaller toward the stomach and then enlarges, but there is no definite boundary between stomach and oesophagus. The epithelial portion of the stomach is flattened laterally, and is so placed that its left side faces somewhat ventrally and its right side dorsally. The stomach passes gradually into the duodenum, the diameter of which is considerably greater than that of the distal part of the small intestine.
The liver consists of a large mass of anastomosing cords of cells, connected with the hepatic diverticulum by a short thick stem which represents the hepatic duct. Distal to the hepatic duct the diverticulum gives rise to the gall-bladder and cystic duct. Proximal to the hepatic duct it forms the common bile-duct (ductus choledochus) , and it connects with the ventral pancreas just before joining the duodenum. The dorsal pancreas is more sharply denned than in Ingalls's specimen.
Fig. 240. — The digestive tract of an embryo of 7.5 mm. (Harvard Collection, Series 256). X 16 diam. In addition to the structures lettered as in Fig. 239, the following are shown. Oes., oesophagus; Pr. ver., a dilatation of the lower limb of the intestinal loop, which gives rise to the processus vermiformis and the csecum, and which marks the boundary between the small intestine above and the large intestine below; Vea. fel. (vesica fellea), gall-bladder.
The slight bend of the intestinal tube toward the vitelline duct, which is seen at 4.9 mm., has increased and now forms the important primary intestinal loop. The vitelline duct has become detached from the bend of this loop, and is separated from it by a considerable interval. It lies in a prolongation of the mesentery which, together with its contents, is called the yolk-stalk. The fused vitelline veins lie in a portion of the stalk which has become separated from the rest, forming the upper subdivision shown in the figure.
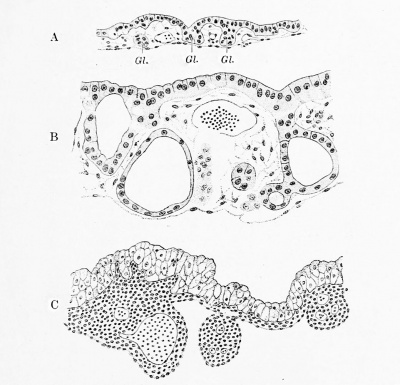
The detachment of the vitelline duct usually occurs at about this stage. A similar condition has been figured by Elze (1907) in an embryo of "about 7 mm." and by Mall (1891) in a 7 mm. specimen. But sometimes the vitelline duct, reduced to a strand of epithelial cells, retains its connection with the intestine much longer. Keibel and Elze (1908) recorded its presence in an embryo of 12.4 mm. and Thyng has found it in a specimen measuring 13.6 mm. In about 2 per cent, of adults, according to several tabulations, a persistent pouch of the intestine, 3 to 9 cm. long, marks the place where the vitelline duct formerly opened into it. This diverticulum ilei of Meckel has already been described, and the pathological importance of persistent remnants of the yolk-stalk has been noted (p. 293). The further history of the detached vitelline duct, which extends through the umbilical cord, and of the yolk-sac, which is lodged between the amnion and chorion at the distal end of the cord, may be found in vol. i, p. 173-174. The account of the yolk-sac as a jDortion of the digestive tract may be concluded with the following note concerning its entodermal layer. In Bremer's 4 mm. specimen and in a 4 mm. embryo figured by Keibel and Elze, the entoderm presents numerous solid outgrowths and hollow outpocketings (Fig. 241, A). Toward the cavity of the yolk-sac the entoderm has a wavy outline. The gland-like structures were described by von Spee as follows (1896 2 ) : "Such glands arise as little outpocketings of the entodermal lining of the distal pole of the yolk-sac, but they rapidly become elongated sacs, which branch dichotomously, and soon develop expanded club-like or vesicular end-pieces. They extend through almost the entire thickness of the wall of the yolk-sac, but their blind ends remain separated from the body-cavity by a single layer of the mesoderm. The glands are lined by a single layer of prismatic entodermal cells, the protoplasm of which contains many fine vacuoles, and, especially in later stages, an increasing quantity of fat drops blackening with osmium." In an embryo measuring 9.4 mm. (Fig. 241, B) these glandlike structures are found all over the yolk-sac. Most of them are closed cysts with walls of varying thickness. Occasionally distinct branching is seen. Von Spee believed that the yolk-sac in its glandular stage is an active organ comparable physiologically with the liver. Other investigators have questioned the glandular nature of the yolk-sac tubules, and Meyer (1904) states that, in spite of the large amount of material at his disposal, he is unable to reach any satisfactory conclusion as to the meaning of these tubules. The entodermal cells of the volk-sac have been found to contain granules which yield a typical mucin reaction (Jordan, 1907), and bundles of filaments which stain with iron haematoxylin (Branca, 1908). According to Branca, the superficial cells are provided with terminal bars, and, except within the glands, some of them show distinct cilia or brush borders. Jordan (1910) failed to find terminal bars or cilia. They do not appear in the specimens shown in Fig. 241, but the appearance which Branca described as a brush border is sometimes clearly seen. In later stages the epithelium lining the yolk-sac becomes stratified, and the diverticula and intra-epithelial cysts disappear. Thus, in an embryo of 23 mm. (Fig. 241, C) the epithelium consists of vacuolated degenerating cells. Subsequently these are lost, and the wall of the yolk-sac is then merely "a dense wavy layer of fibrous connective tissue." In this condition it is found at birth.
In the posterior half of the primary intestinal loop in the 7.5 mm. embryo (Fig. 240), there is an abrupt enlargement of the entodermal tube, which marks the boundary between spaall and large intestine. The expansion does not affect the dorsal border of the intestine, but is wholly a ventral bulging. The cavity of the large intestine extends slightly forward into the ventral swelling, so that in one section at this point there is a double lumen: the enlargement is therefore already a shallow pouch. This structure is generally considered to be the beginning of the ccecum. Keibel and Elze (1908) have noted that the caecum is indicated in embryos measuring from 6.25 to 7.0 mm. But Tarenetzky (1881) described this enlargement as the processus verrniformis. "At this stage ... a caecum is not present." He found that an actual caecum first appeared in an embryo of 65 mm. A distinction between the caecum and the vermiform process in young human embryos is not easily made, and Toldt (1894) is probably correct in referring to the primary enlargement as the common origin of both. This question is further discussed on page 328.
In the 7.5 mm. specimen the large intestine curves forward to join the allantois at the cloaca. There is still no external opening. The caudal intestine, which in Ingalls 's specimen had a wide lumen, is reduced to a strand of cells. Toward the tip of the tail a minute lumen is distinctly seen, ending in a terminal expansion (Fig. 234, D). In an embryo of 9.4 mm. (Fig. 242) the caudal intestine has disappeared, except for an isolated nodule of epithelium. Keibel and Elze have shown that it usually disappears at about this stage, but they found remnants of it in one specimen measuring 11.5 mm.
Embryo of 9 A mm Torsion of the Primary Loop of Intestine.-^-The intestinal tube in the 9.4 mm. embryo (Fig. 242) differs from that of the preceding stage chiefly through the disappearance of the yolk-stalk and the torsion of the intestinal loop. (The considerable changes in the course of the bile-ducts will be described in a separate section.) The torsion of the intestine occurs, in a general way, as follows. The large intestine at first forms part of the posterior half of the intestinal loop, and the loop is in the median plane. Then the loop becomes rotated so that its plane is transverse. The anterior half is then on the right, and the posterior half on the left. Further rotation causes the posterior half to become anterior. In side view the large intestine then crosses the small intestine, as seen in Fig. 242. At a considerably later stage the torsion is completed by the migration of the caecum to the right side of the body and down toward the pelvis. When this has occurred, the large intestine passes from right to left, ventral to the upper part of the small intestine ; it then descends to the rectum on the left of the small intestine.
Fig. 241. — Sections of the wall of the yolk-sac. X115 diam. A, embryo of 4 mm. (Harvard Collection, Series 714); B, embryo of 9.4 mm (Harvard Collection, Series 529); C, embryo of 23.0 mm (Harvard Collection, Series 192). Developing "glands," Gl., are shown in A. They have become cystic in B. In C the yolk-sac is degenerating, and the "glands" have disappeared.
In an interesting case of " imperfect torsion of the intestinal loop " reported by Reid (190S), the embryonic twisting evidently did not occur. In a man over sixty years of age he found that the caecum was within the pelvis, a little to the right of the median line. The ascending colon passed gradualty to the left, and most of it, together with all of the transverse and descending colon, was on the left side of the body. The small intestine was wholly on the right side, and was not crossed by the large intestine. Similar eases have been described by Faraboeuf (1885), Descomps (1909), and others. A reversal of the intestinal torsion, which would cause the colon to pass under the small intestine, has apparently never been seen.
Embryo of 22.8 mm The Normal Umbilical Hernia
Since 1817, when Meckel published an account of the " formation of the intestinal canal of mammals and particularly of man," it has been well known that, at a certain stage, " the greatest part of the intestinal canal is found within the umbilical cord." It had been discovered previously. Meckel observed it in the goat, sheep, cow, pig, rabbit, and man. He says, " Through a very pleasant coincidence Oken and I, at the same time and quite independently of one another, expressed the opinion that in a very early embryonic stage this position of the intestine is normal." According to His (1885), "the underlying cause of the ventral extension of the intestine is doubtless to be sought in its connection with the yolk-sac. ... As long as the yolk-stalk is present it is attached to the end of the loop extending through the umbilicus." But Mall (1898) states that " before the intestine begins to enter the cord its connection with the duct is severed." Since the liver grows downward and crowds upon the rapidly elongating intestine, Mall considers that " the intestine must escape if it has a chance, and the coelomic space within the cord naturally receives it." The loop of intestine begins to enter the cord in embryos of about 10 mm.; at 22.8 mm. (Fig. 243) the umbilical hernia is well developed.[5] It will be seen that the large intestine presents no well-marked convolutions, but that there are several bends in the course of the small intestine. This is due to the relatively rapid growth of the anterior half of the original loop. According to Mall (1898), there are six primary loops of the small intestine, first indicated in embryos of about 17 mm., and recognizable, in spite of secondary coils, even in the adult. The first loop encircles the head of the pancreas. The third loop is concave below and occurs where the small intestine passes through the narrow umbilical outlet. It is clearly shown in Fig. 243. Loop 2 is between 1 and 3, forming together with 3 an S-shaped curve. The other loops are not so easily defined, but all coils between the caecum and the place of attachment of the yolk-stalk are included in loop 6.
Fig. 242. — The digestive tract of an embryo of 9.4 mm. Harvard Collection, Series 1005). X13 diam. The lettering is the same as in Figs. 239 and 240.
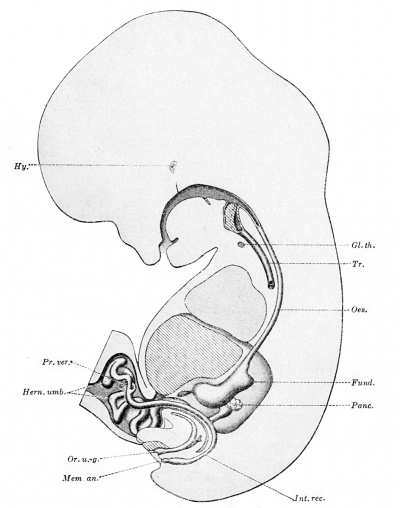
Fig. 243. — The digestive tract of an embryo of 22.S mm. Harvard Collection, Series 871). X6 diam. (Drawn by F. P. Johnson.) In addition to the structures lettered as in previous figures the following are shown. Fund., fundus of the stomach; Hern, umb., coils of intestine within the umbilical cord, forming the umbilical hernia; Hy., anterior lobe of the hypophysis, — the detached end of Rathke's pocket; Int. rec, intestinum rectum; Mem. an., membrana analis; Or. ti.-g., orificium urogenitale.
Mall considers that these coils in the umbilical cord are so fixed that it is not difficult to recognize the various loops after their return to the abdominal cavity. In the adult he finds that loops 2 and 3 make two distinct groups of coils in the left hypochondrium, loop 2 communicating with the duodenum. " After this the intestine passes through the umbilical region to the right side of the body (loop 4). Then the intestine recrosses the median line to make a few convolutions in the left iliac fossa (5), after which it fills the pelvis and lower part of the abdominal cavity between the psoas muscles (6)." He concludes that "the various loops of the adult intestine, as well as their position, are already marked in embryos of five weeks, and the position of the convolutions in the adult is as definite as the convolutions of the brain." Separation of the Intestine from the Allantois. — In embryos of the stage of Ingalls's specimen (Fig. 239) the cloaca is elongated anteroposteriorly, and the Wolffian ducts empty into its ventral portion. Later this ventral portion is split off from the dorsal part, apparently by the down-growth of the connective tissue between the allantois and rectum. The portion of the allantois 9 below the Wolffian ducts, since both the urinary and genital passages open into it, is then called the urogenital sinus.
In the 9.4 mm. embryo (Fig. 242) the urogenital sinus has been formed, but the cloaca still remains as a broad connection between the allantoic and intestinal tracts. By further downgrowth of the connective tissue, this connection becomes reduced to a slender passage called the cloacal duet. Finally the walls of the duct coalesce and the communication between the intestine and the allantois is obliterated. The connective tissue between the rectum and the urogenital sinus, where it reaches the ectoderm, constitutes the primitive perineum.
In Keibel and Elze's tables, a cloacal duct is recorded in embryos from 11 to 12.5 mm. At 15 mm. the "cloaca is still not 9 It is clearly a matter of definition whether the portion which is added to the allantois by the subdivision of the cloaca should thereafter be called allantois (see Chap. XIX).
fully divided." At 15.5 mm. the "cloaca is just divided, bul the epithelia of the urogenital sinus and of the rectum still connect; a mesodermal perineum is not yet formed." Fig. 244, A, from a specimen measuring 18.1 nun., presents this condition. The cloaca! membrane is now subdivided into the urogenital membrane ventrally and the anal mem Inane dorsal ly. The mesodermal primitive perineum is about to form, but the urogenital sinus and the rectum are apparently still connected by entoderm. At 22.8 mm. (Fig. 243) the primitive perineum is well developed. It is found at the bottom of a median sagittal ectodermal groove, known as the ectodermal cloaca. The anal membrane is also at the bottom of an ectodermal depression which may be regarded as a part of the ectodermal cloaca, but which is termed the proctodeum or anal pit. When the edges of the ectodermal cloaca coalesce in the perineal region, so as to form a raphe, the permanent perineum is produced.
Fig. 244. — Median sagittal sections to show the separation of the rectum from the urogenital sinus. A, embryo of 18.1 mm. (Harvard Collection, Series 1129), X30 diam.; B, embryo of 22 mm (Harvard Collection, Series Sol), X25 diam.; C, embryo of 32 mm. (Harvard Collection. Series 292), X 12 diam. Am. rec, ampulla recti; -4/).. anus; Mem. an., membrana analis; Mem. u.-g., membrana urogenitals; Muse., tunica muscularis (including inner circular and outer longitudinal layers); Or. u.-g., orificium urogenitale; Per., perineum; Pr., proctodeum; Rec., rectum; Sph. ex., M. sphincter ani externus; Sph, int., M. sphincter ani internus; S. u.-g., sinus urogenitalis; x, terminal bulbous enlargement of the rectum.
The urogenital tract acquires an external opening before the anal membrane is perforated, and this has occurred in the 22.8 mm specimen. The further history of the allantois and urogenital tract will be found in Chapter XIX.
The Formation of the Anus
A sagittal section through the rectum and proctodeum of a 22 mm. embryo is shown in Fig. 244, B. Just before the rectum reaches the anal membrane it forms a bulbous enlargement, seen also in Fig. 243 and in embryos of 17.5 and 18.5 mm. drawn by Keibel. Iu these specimens the circular and longitudinal muscle layers of the rectum are easily recognized. The terminal swelling of the rectum extends beyond the muscle layers, as recorded by Keibel ( L896) in a beautifully illustrated and fundamental description of the development of the human urogenital tract. In a 29 mm. specimen he described the musculature as follows (cf. Fig. 244, B and C) : "The circular muscle layer ends very abruptly at the level of the little caudal swelling of the intestine, and already it may be referred to as the beginning of the M . sphincter ani internus.
"The outer longitudinal layer of the intestinal musculature is arranged differently. It ends at the same level, but no sharp caudal limit can be recognized. The M. sphincter ani externus is clearly indicated and is relatively quite large. The cranial border of this muscle is found where the musculature of the intestine ends, therefore at the level of the little caudal entodermal enlargement. The M. sphincter ani externus is separated from the epithelium of the intestine by a rather thin layer of connective tissue, which is continuous with the connective tissue surrounding the intestine further cranially, and also with the longitudinal layer of the muscularis, from which strands of cells may be followed into it." In a 32 mm. specimen (Fig. 244, C) the anal membrane has disappeared. Along the dorsal wall of the anal canal there is a slight indication of the terminal bulbous enlargement, but it seems clear that it is a transient structure. It is probable that the elongated swelling above it gives rise to the rectal ampulla of the adult.
Otis (1905) has studied the external configuration of the embryonic anus, and has found that the development of the external sphincter produces characteristic elevations. In embryos of 21-23 mm. there is a pair of external elevations, one on either side of the anal pit. At 26 mm. these have united dorsal to the anus, thus forming a single crescentic mound. The horns of the crescent grow forward toward the perineum and finally meet, so that the mound encircles the anus.
Malformations of the Anus
The perforation of the anal membrane normally takes place in embryos of about 30 mm. It is accompanied by the formation of degenerative material staining intensely with eosine, which blocks the outlet and makes the determination of an aperture somewhat difficult. In the Harvard Collection there are embryos measuring 22 mm, 22.8 mm, and 29 mm in which perforation has occurred, and specimens of 22.8 mm. and 30 mm. in which the anus seems still impervious. Keibel and Elze's series of seven embryos measuring from 22 to 26 mm. includes only one (22.5 mm.) in which the anus is open. A persistence of the anal membrane until birth has been assumed to account for cases of atresia ani, in which the rectum ends blindly below, and the anus is represented merely by a slight depression in the skin. In these cases, however, the epithelial connection between the rectum and anal pit has been lost, so that an invasion of the anal membrane by connective tissue must have taken place. In other cases, known as atresia recti, the anal canal is present but it leads into a blind sac of intestine.
In this connection the obliteration of the lumen of the rectum, observed by Keibel in an embryo measuring 11.5 mm., is of interest. He found that " the ej)ithelium of the lower portion of the intestine blocked the lumen at two small places," but since similar conditions were not observed in other specimens he concluded that " this may well be only a chance and meaningless adhesion " (1896, p. 79). Although this observation in human embryos remains unique, Lewis (1903) has recorded a similar condition in pig and rabbit embryos, and it occurs more extensively in birds (Minot, 1900). It has been suggested that its function is to prevent the passage of the excretion of the "Wolffian bodies back into the intestine. After the cloacal duct has become obliterated, it is not needed for this purpose, and hi these later stages it is not found. It is possible that atresia recti may be due to the persistence of such an occlusion, with invasion by connective tissue.
Less common than the simple imperforate anus or imperforate rectum are cases in which the cloacal duct persists, forming a slender passage from the rectum to the raphe of the perineum, scrotum, or under side of the penis, or to the prostatic urethra or bladder. In the female such a fistula may open along the perineal raphe or into the vestibule of the vagina.
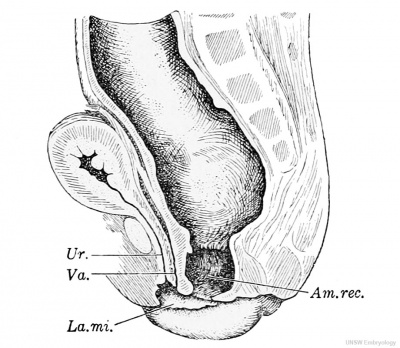
Fig. 245. — Abnormal position of the anus in a child. V. natural size. (After Mackenzie.) Am. rec., ampulla recti; La.mi. labium minus ; Ur., urethra; Va., vagina.
An interesting series of diagrams of these cases was published by Stieda (1903). The fistula may exist with a normal anus, but more frequently it is associated with an imperforate condition. Of the many cases reported two examples may be cited.
Reichel (1888) received a patient 25 years of age who complained of involuntary discharge of fseces through the vagina, beginning after her marriage three years before. The anal canal was found to be normal ; the perineum was extremely short and the vestibule strikingly deep; the labia were normal. A canal, lined with mucous membrane, was found leading from the rectum to the vestibule directly below the hymen. After discussing the possibility of mechanical injury, etc., Reichel concludes that the abnormal communication between the rectum and the vestibule was present as a slender fistula from birth, and that it was dilated following coitus. Such a condition would arise in an embryo of about 12 mm. provided that the perineal tissue should encircle the cloacal duct instead of obliterating it.
In 1906 Mackenzie reported the case shown in Fig. 245. The patient died at the age of 1 year and 11 months, having suffered from alternating attacks of diarrhoea and constipation since birth. Robinson described the condition as follows : " The anal passage runs, not in the normal direction, downwards and backwards, but downwards and forwards, and the anal orifice opens into a chamber common to it, the vagina, and the urethra; that is, the anal passage opens, not on the surface behind the genito-urinary chamber, but into a cloaca." It may be considered that the anal canal in this case, although provided with well-marked anal columns (of Morgagni), corresponds with the slender fistula in Reichel's case, and that the normal anal outlet is not represented. This is Robinson's interpretation. He says, " The entodermal cloacal chamber has never been separated into two parts : it has opened into the anterior part of the external cloacal depression, and the posterior part of that depression, if it existed, has disappeared, no trace of a proetodeal opening being discoverable." It seems possible, however, that the entodermal cloaca has been completely divided but that the primitive perineum has persisted. Thus by an imperfect development of the perineum the normal anal opening is displaced forward. Robinson rejects this interpretation.
Embryo of 42 mm
Return of the Intestines to the Abdominal Cavity.— According to Mall (1895), the return of the intestines from the umbilical cord into the body must take place very rapidly, for in embryos of 40 mm. the intestine is either in the cord or in the abdominal cavity. He found no intermediate stages. Mall was unable to determine the cause of the return, but he showed that the abdominal walls do not bulge forward so as to include the cavity of the cord within the abdomen. The intestines slip back through a rather small aperture, and the cavity in the cord is then obliterated. From a study of pig embryos Mall suggested that the increase of loops within the abdominal cavity, and their rotation, may draw upon the loops in the cord. The enlargement of the umbilical arteries on the under side of the hernia may also exert a favorable pressure. As seen in the reconstruction of a 42 mm. embryo (Fig. 246), the return has taken place, but the abdominal cavity still extends into the cord.[6] Development of the Ccecum and Vermiform Process. — The first appearance of the intestinal enlargement which is to produce the vermiform process has already been described. In the embryo of 7.5 mm. it is an entodermal swelling on the lower side of the caudal limb of the intestinal loop. After the torsion of the loop it may still project from the lower side of the intestine, as shown in the 9.4 mm. specimen (Fig. 242) and in a 13.8 mm. embryo figured by His, But in a specimen measuring 12.5 mm. His has drawn it as projecting from the upper side of the intestine, and it has been similarly figured by Keibel and Elze in an embryo of 14 mm. Mall found it projecting laterally in a 17 mm. specimen.
Fig. 246. — The digestive tract of an embryo of 42 mm. (Harvard Collection Series 838). . X4 diam. The lettering is like that in previous figures with the addition of Ves. ur. (vesica urinaria), bladder.
In all these cases, however, the apex of the projection is directed ventrally (that is, toward the small intestine). With the formation of the umbilical hernia, the vermiform process enters the cavity of the cord (Fig. 243). Later it is withdrawn into the abdomen and comes to lie against the under side of the liver.
Four stages in the development of the vermiform process are shown in Fig. 247, two of which are drawn from models and two from dissections. All are viewed from the median side. Fig. 247, A, shows the simple arrangement at 9.4 mm. To produce the condition shown in B the tip of the vermiform process must be brought toward the large intestine. Thus a U-shaped bend would result, and this U should then be twisted upon the small intestine so that its extremities extend dorsally, with the vermiform process on the right side of the colon. In Fig. 247, B, from the 42 mm. embryo, a window has been cut in the round bend made by the vermiform process and the colon, so that the outlet of the ileum is exposed. The ileum empties into the large intestine in the concavity of the bend.
Fig. 247. — Models (A and B) and dissections (C and D) to show the development of the vermiform process. A, embryo of 9.4 mm. (Harvard Collection, Series 1005), X50 diam.; B, embryo of 42 mm. (Harvard Collection, Series 838), X20 diam.; C, embryo of 95 mm., X3.5 diam.; D, embryo of 218 mm., X3.5 diam. Cae., caecum; Co., colon; II., ileum (small intestine); Ale., mesenteriolum; Mes„ mesentery; Pr. ver., processus vermiformis; Val. co., valvula coli, represented by two slight vertical swellines between which is the outlet of the ileum.
Tarenetzky (1881) has described very similar relations in a 33 mm. embryo as follows: " The processus vermiformis has assumed an elongated form ; it is no longer parallel with the ileum but forms a right angle with it. It has taken a position toward the right and obliquely above and in front of the terminal part of the ileum, so that its tip is already directed somewhat toward the colon. In this manner it forms also a right angle with the colon. The knee-shaped bend at the passage of the vermiform process into the colon is not expanded, so that at this stage no true caecum is present. The tip of the vermiform process is completely free. Along its base and middle piece there is attached a well-defined peritoneal fold, which arises from the adjacent ventral right plate of the mesenterium communeThis fold is new, and represents the mesenteriolum of the vermiform process, the chief vessels to which are contained in it.' ; The mesenteriolum is shown in Fig. 247, B, and, as Tarenetzky recorded, a dilatation to indicate the caecum is not well denned. It has already been noted that Tarenetzky first recognized the caecum in embryos of 65 mm. Toldt (1894) describes a clearer separation between the caecum and vermiform process in a "7 weeks" embryo than his figures indicate. In his drawing of a 50 mm. specimen the caecum can scarcely be distinguished. The demarcation evidently forms very gradually and at a late stage. According to Toldt, the taeniae of the caecum are present at birth, and the haustra of the caecum develop in the first half year, the smallest of them, situated nearest the vermiform process, appearing first; but the caecum does not acquire the characteristic adult form until the third or fourth year.
It was shown by Toldt that the bending of the vermiform process and caecum upon the colon gives rise to the valves of the colon. Until this bend occurs there is no indication of the valves. As a result of the bend, the end of the small intestine, where it is caught in the angle, becomes flattened by the adjacent walls of the colon and caecum respectively. At 42 mm. (Fig. 247, B) the aperture is still nearly round, but as the U-shaped bend becomes angular the flattening will result. This explanation accounts for the two lips of the valve, the labium inferius being toward the caecum, and the labium superius toward the colon. In the last fetal months and especially after birth, the relatively great expansion of the large intestine, as compared with the ileum, causes the vailves to increase in size. In this process the bulging colon and caecum still further invest the end of the ileum and adhere to it. In case the embryonic bend is not highly developed, imperfect valves may arise by the expansion of the large intestine. Toldt recorded several such cases.
Recently Parsons (1907) has reported the case of an elderly man in whom the caecum formed a straight continuation of the colon, and there was no valve whatever. He considers that the U-shaped bend had never formed in that individual. Smith (1903) described a case in which a vermiform process was present but there was a " complete absence of a properly constituted caecum " and no trace of the valvula coli.
The stage of the bend represented in Fig. 247, B, is therefore a critical one. In a 95 mm. embryo, Fig. 247, C, the vermiform process is still in contact with the liver, and the U-shaped bend is well marked, but the descent of the caecum toward the pelvis has begun. At 218 mm. (Fig. 247, D) the vermiform process has taken its final position. In this case it is coiled so as to make l 1 /? revolutions.
Form and Position of the Stomach
At first the stomach lies approximately in the median sagittal plane. It is then a flattened expansion of the digestive tube, with dorsal and ventral borders and right and left surfaces. Gradually it rotates so that its left side becomes ventral and its right side correspondingly dorsal. At the same time the dorsal border is turned to the left and the ventral border to the right. The upper portion of the stomach is displaced to the left side of the body, and the borders thus become curvatures, which are concave toward the right. The original dorsal border forms the greater curvature, and the ventral border becomes the lesser curvature. These changes in position are usually described in connection with the development of the mesenteries. They are best shown in ventral views of the embryo. It may be noted, however, that in the 7.5 mm. embryo the rotation of the stomach has partly occurred, so that the original dorsoventral axis forms an angle of 20° with the median plane; in the 22.8 mm. specimen the angle has increased to 55° ; and in the 45 mm. embryo it is 75° in the pyloric half of the stomach. The cardiac end has not rotated so much, and at 45 mm. its angle is 40°. Thus the pyloric part of the stomach is twisted across the body from left to right. .
The descent of the stomach has been described by Jackson (1909) as follows: In the 11 mm. embryo the cardia lies opposite the 3d or 4th thoracic segment, and the pylorus opposite the 7th or 8th. In the 17 mm. embryo the two ends of the stomach seem to have reached approximately their permanent positions, the cardia opposite the 10th thoracic vertebra and the pylorus opposite the 1st or 2d lumbar vertebra.
The descent is accompanied by a great elongation of the oesophagus. In a 9.4 mm. specimen the oesophagus measures 1.8 mm. At this proportion it should measure 4.3 mm. in an embryo of 22.8 mm., but its actual length is found to be 8 mm. In Jackson's paper the relations of the stomach to the adjacent viscera in early embryos have been considered.
The most notable external feature in the early development of the stomach is the formation of the fundus, which occurs in the manner described by Keith and Jones (1902). According to these authors, the fundus of the human stomach is developed, not as a general expansion of the gastric part of the fore-gut, but in the form of a localized outgrowth or diverticulum at the cardiac end of the greater curvature (dorsal border). In its manner of origin it has much in common with the caecum and vermiform process. They find that the outgrowth is best marked in embryos of the third and fourth month. After these months the diverticulum is not so well defined, since it expands and merges with the body of the stomach. The gradual development of the fundus, as a conical diverticulum is shown in the embryos of 9.4, 22.8, and 42 mm. (Figs. 242, 243, and 246).
A very .similar diverticulum was observed in pig embryos of 12 nun. by Lewis, wbo considered that it was characteristic of the pig and gave rise to the wellmarked pouch attached to the fundus of the adult. Strecker (1908) has recently called attention to a human stomach described by Luschka "in which the transition from the oesophagus to the stomach took place gradually, and beyond this the fundus possessed a conical appendage directed upward and backward, thereby, to a certain extent, resembling the form of a pig's stomach." In addition to the dilated corpus and the conical fundus, the embryonic stomach presents a third subdivision, — the tubular pars pylorica. As seen in three models of the stomach, from embryos of 16, 19, and 19.3 mm. respectively, this pyloric portion extends toward the right and slightly apward, to the pylorus. In every case the position of the pylorus is indicated by a local dilatation of the epithelial tube, such as is shown in Thyng's model from a 13.6 mm. specimen (Fig. 285, A, p. 392). The junction between the corpus and the pars pylorica, measured along the lesser curvature, occurs midway between the pylorus and the cardia. At the place of junction there is an angular bend in the lesser curvature (incisura angularis) and an abrupt change in the diameter of the tube.
Another subdivision of the human stomach is that which Luschka (1863) described as the cardiac antrum. Sometimes in the adult a bulbous enlargement is found at the junction of the oesophagus and stomach, and this is the region of the special form of glands known as cardiac glands. Strecker has studied this area, and concludes that sometimes a "Vormagen" can be recognized, but in other cases it is totally absent.
In the 22.8 mm. specimen, as seen in Fig. 243, such a subdivision is suggested, but the formation of a distinct cardiac antrum in early human embryos has never been demonstrated.
The further development of the oral cavity and its organs, and of the oesophagus, stomach, and intestine, with their folds and glands, will be considered in the following sections.
The embryology of the branchial region and respiratory system will form the concluding part of the chapter.
- ↑ Grosser, who has examined the specimen, thinks that this space may be the beginning of the cavity of the chorion, and that elsewhere the chorion is filled with loose tissue. Zentralblatt fiir Physiol., Bd. 22, Nr. 1
- ↑ The specimen, in the form of an intact chorionic vesicle already hardened and in alcohol, was placed at the writer's disposal by Professor Minot, and it may be referred to as Minot's embryo. A full account of it is in preparation, justified by its superb preservation.
- ↑ It may be noted that His described the medullary groove as extending along the body-stalk. Anatomie menschlicher Embryonen, iii, p. 224.
- ↑ According to Grosser, the pulmonary area in this embryo has been erroneously regarded as the gastric portion of the intestine by Thompson and also by Keibel and Elze (Normentafel, Embryo Nr. 7). In Thompson's embryo the stomach has not yet developed. Compare with Grosser^ description of the development of the lungs at the end of this chapter.
- ↑ The reconstruction of the 22.8 mm. embryo (Fig. 243), which has not been previously published, is the work of Mr. F. P. Johnson, and the writer is much indebted to him for permission to use it.
- ↑ In a " Supplementary Note on the Development of the Human Intestine," Mlall (1899) described a human embryo of 32 mm. "in which the intestine is in the act of returning from the coelom of the cord to the peritoneal cavity." The intestine, is said to be " sucked back " to fill the space made by the enlargement of the abdominal cavity.
Literature
Albinus, B. S.: Academicarum annotationum. Liber 1. See Tab. 1, Fig. 12. Leidae 1754. von Baer, K. E.: tjeber Entwickelungsgeschichte der Thiere. Zweiter Theiß. S. 1-315. Konigsberg 1837.
Beneke: Ein sehr juns:es raensohliches Ei. Monatssehr. f. Geb. u. Ovn. Bd. 19.cS. 771-773. 1904.
Bonnet, R. : Beitrage zur Enibryologie des Hundes. Anat. Hefte. Bd. 16, S. 233-332. 1901. Lehrbuch der Entwicklungsgeschichte. S. 1-467. Berlin 1907.
Branca, A. : Recherches sur la vesieule ombilicale de l'bomme. Ann. de Gyn. et d'Obstet. Annee 35, p. 577-607. 1908.
Bremer, J. L. : Description of a 4 mm. Human Embryo. Amer. Joum. of Anat. Vol. 5, p. 459-480. 1906.
Bryce, T. H., and Teacher, J. H. : Contributions to the Study of the Early Development and Imbedding of the Human Ovum. P. 1-93. Glasgow 1908.
Dandy WE. A human embryo with seven pairs of somites measuring about 2 mm in length. (1910) Amer. J Anat. 10: 85-109.
Dandy, W. E. : Descomps, P. : Anomalie de la torsion intestinale. Journ. de l'Anat. et de la Phys. Annee 45, p. 616-631. 1909.
Diemerbroeck : Anatome corporis humani. P. 303-304. Ultrajecti 1672. Opera omnia, p. 263—265 (as cited by Lobstein). 1687.
Elze, C. : Beschreibung eines menschlichen Embryo von zirka 7 mm. grosster Lange. Anat. Hefte. Bd. 35, S. 411^92. 1907.
Eternod, A. C. F. : II y a un canal notochordal dans l'embryon humain. Anat Anz. Bd. 16, S. 131-143. 1899. II y a un leeithophore dans l'embryon humain. Bibliogr. anat. Tome 15, p 247-258. 1906. La gastrule dans la serie animale. Bull. soc. Vaud. Sc. nat. 42, p. 197-224 1906. Fabricius ab Aquapendente : De formato foetu. 1600. (Opera omnia. Lugduni Batavorum 1738.)
Faraboeuf, M. : Arret devolution de l'intestin. Le progres medical. Annee 13, p. 411-413. 1885.
Forssner, H. : Die angeborenen Darm- und Oesophagusatresien. Anat. Hefte. Abt. I. Bd. 34, S. 1-163. 1907. Frassi, L. : Ueber ein junges menschliches Ei in situ. Arch, f . mikr. Anat. Bd. 70, S. 492-505. 1907.
Giffhorn, H. : Beitrag zur Aetiologie der kongenitalen Atresie des oesophagus mit Oesophagotrachealfistel. Arch. f. path. Anat. Bd. 192, S. 112-126. 1908.
Happich, C. : Ueber Oesophagusmissbildungen. S. 1-65. Inaug.-Diss. Marburg 1905.
Hensen, V. : Beobachtungen iiber die Befruchtung und Entwicklung des Kaninchens und Meersehweinchens. Zeitschr. f . Anat. u. Entw. S. 353-423. 1875.
Herzog MA. A contribution to our knowledge of the earliest known stages of placentation and embryonic development in man. (1909) Amer. J Anat., 9(3): 361-400.
His, W. : Anatomie menschlicher Embryonen. I. Embryonen des ersten Monats. S. 1-184. Leipzig 1880. — II. Gestalt- und Grossenentwicklung bis zum Schluss des 2 Monats. S. 1-104. Leipzig 1882. — III. Zur Geschichte der Organe. S. 1-260. Leipzig 1885. Hoboken, 1ST. : Anatomia secundinae humanae repetita. P. 37 and 217. Ultrajecti 1675. (Cited by Lonnberg.)
Ingalls, N. W. : Beschreibung eines menschlichen Embryos von 4.9 mm. Arch, f . mikr. Anat. Bd. 70, S. 506-576. 1907.
Jackson, C. M. : On the Developmental Topography of the Thoracic and Abdominal Viscera. Anat. Record. Vol. 3, p. 361-396. 1909.
Jordan, H. E. : The Histology of the Yolk-sac of a 9.2 mm. Human Embryo. Anat. Anz. Bd. 31, S. 291-303. 1907. A Microscopic Study of the Umbilical Vesicle of a 13 mm. Human Embryo. Anat. Anz. Bd. 37, S. 12-32, 56-66. 1910.
Jung, P. : Beitrage zur friihesten Ei-Einbettung beiin menschlichen Weibe. Berlin 1908.
Keibel, F. : Zur Entwickelungsgeschiehte des menschlichen Urogenitalapparates. Arch. f. Anat. u. Entw. S. 55-156. 1896. Keibel F., and Elze, C. : Normentafeln zur Entwicklungsgeschichte der Wirbeltiere. Heft 8, S. 1-314. Jena 190S. Keith, A. : Three Cases of Malformation of the Tracheo-cesophageal Septum. Journ. of Anat. and Phys. Vol. 41, p. 52-55. 1906.
Keith, A., and Jones, A. W. : A Note on the Development of the Fundus of the Human Stomach. Proc. of the Anat. Soc. of Great Britain and Ireland, p. 34-3S, in the Journ. of Anat. and Phys. Vol. 36. 1902.
Kieser, D. G. : Der Ursprung des Darmkanals aus der vesicula umbilicalis, dargestellt ini menschlichen Embryo. S. 1-31. Gottingen 1810. Kollmann, J. : Die Entwiekelung der chorda dorsalis bei dem Menschen. Anat. Anz. Bd. 5, S. 308-321. 1890.
von Kupffer, C. : Die Deutung des Hirnanhanges. Sitz.-Ber. der Gesellseh. f . Morph. und Phys. in Miinchen. S. 59-87. 1894. Lewis, F. T.: The Gross Anatomy of a 12 mm. Pig. Amer. Journ. of Anat. Vol. 2, p. 211-225. 1903.
Lobstein, J. F. : Essai sur la nutrition du foetus. Strassburg 1802. Ueber die Ernahrung des Fetus. Aus dem Franzosischen ubersetzt von T. F. A. Kästner. S. 1-214. Halle 1804. Lonnberg, J.: Studien iiber das Nabelblaschen an der Nachgeburt des ausgetragenen Kindes. S. 1-118. Stockholm 1901.
Low A. Description of a human embryo of 13-14 mesodermic somites. (1908) J Anat Physiol. 42(3): 237-51. PMID 17232769 | PMC1289161
Luschka, H. : Die Anatomie des Menschen. Bd. 2, S. 179. Tubingen 1863.
Mackenzie. F. S. : On a Specimen of the Hind-gut Opening into a Cloacal Chamber in a Child. Journ. of Anat. and Phys. Vol. 40, p. 409-411. 1906.
Mall FP. A human embryo twenty-six days old. (1891) J Morphol. 5: 459-480.
Mall, F. P. : Development of the Human Coelom. Journ. of Morph. Vol. 12, p. 395-453. 1897. Ueber die Entwiekelung des menschlichen Darms und seiner Lage beirn Erwachsenen. Arch. f. Anat. und Entw. Suppl.-Bd., S. 403-434. 1897. Development of the Human Intestine and its Position in the Adult. Bull, of the Johns Hopkins Hosp. Vol. 9, p. 197-208. 1898. Supplementary Note on the Development of the Human Intestine. Anat. Anz. Bd. 16, S. 492-495. 1899.
Mallory, F. B. : Sacro-coccygeal Dimples, Sinuses, and Cysts. Amer. Journ. of the Med. Sci. Vol. 103, p. 263-277. 1892. Marwedel, G. : Ein Fall von persistierendem Urmund beim Menschen. Beitr. z. klin. Chir. Bd. 29, S. 317-326. 1901.
Meckel, J. F. : Handbuch der pathologisehen Anatomie. Bd. 1, S. 553-597. Leipzig 1812. Bildungsgeschichte des Darmkanals der Saugthiere und namentlich des Menschen. Deutsches Arch. f. d. Phys. Bd. 3, S. 1-84. 1817.
Meyer AW. On the structure of the human umbilical vesicle. (1904) J. Anat. 3(2):155-166.
Minot, C. S. : On the Solid Stage of the Large Intestine in the Chick. Journ. of the Boston Soc. of Med. Sci. Vol. 4, p. 153-164. 1900.
Oken and Kieser: Beitrage zur vergleichenden Zoologie, Anatomie und Pliysiologie. Bamberg und Wiirzburg. Heft 1. 1806. Heft. 2. 1S07.
Otis, W. J.: Die Morphogenese und Histogenese des Analhockers. Anat. Hefte. Bd. 30, S. 201-258. 1905.
Parsons, F. G. : On the Form of the Caecum. Journ. of Anat. and Phys. Vol. 42, p. 30-39. 1907.
Peters, H. : Ueber die Einbettung des menschlichen Eies. S. 1-143. Leipzig und Wien 1S99.
Rathke, H. : Ueber die Entstehung der glandula pituitaria. Arch, f . Anat,, Phys. und wiss. Med. S. 482^85. 1838.
Reichel, P. : Die Entwickelung des Dammes und ihre Bedeutung fiir die Entstehung gewisser Missbildungen. Zeitschr. f. Geb. und Gyn. Bd. 14, S. 82 94. 1888.
Reid, D. G. : Imperfect Torsion of the Intestinal Loop. Journ. of Anat. and Phys. Vol. 42, p. 320-325. 1908.
Schlater, G. : Zur Frage vom Ursprung der Chordaten nebst einigen Bemerkungen zu den friihesten Stadien der Primaten-Embryogenese. Anat. Anz. Bd. 34, p. 33-48, 65-81. 1909.
Seessel, A. : Zur Entwicklungsgeschiehte des Vorderdanns. Arch, f . Anat. und Entw. S. 449-467. 1877. Smith, G. E. : Note on an Abnormal Colon. Journ. of Anat. and Phys. Vol. 38, p. 32-33. 1903.
von Spee, F. : Beobachtungen an einer menschlichen Keimscheibe mit offener Medullarrinne und eanalis neurentericus. Arch. f. Anat. und Entw. S. 159-176. 1889. Neue Beobachtungen iiber sehr friihe Entwickelungsstufen des menschlichen Eies. Arch. f. Anat. und Entw. S. 1-30. 1896. Zur. Demonstration iiber die Entwickelung der Driisen des menschlichen Dottersacks. Anat. Anz. Bd. 12, S. 76-79. 1896.
Stieda, A. : Ueber atresia ani congenita und die damit verbuudenen Missbildungen. Arch. f. klin. Chir. Bd. 70, S. 555-583. 1903.
Strecker, F. : Der Vormagen des Menschen. Arch. f. Anat. und Entw. S. 119 188. 1908.
Tarenetzky, A. : Beitrage zur Anatomie des Darmkanals. Mem. de l'Acad. imp. des sciences de St. Petersbourg. Ser. 7, Tome 28, No. 9, p. 1-55. 1881.
Thompson P. Description of a human embryo of twenty-three paired somites. (1907) J Anat Physiol, 41(3):159-71. PMID 17232726
Toldt, C. : Die Formbildung des menschlichen Blinddarmes und die valvula coliSitz.-Ber. der math.-naturw. Classe der k. Akad. der Wissensch. S. 41-71. Wien 1894.
Velpeau, A. A. L. M. : Embryologie on ovologie humaine. P. 1-104. Paris 1833. Die Embrvologie und Ovolog-ie des Menschen. Uebersetzt von C. Schwabe. S. 1-84. Ilmenau 1834.
Wolff, C. F. : De formatione intestinorum. In Nov. Comment. Acad. Sc. J. Petrop. 12, 1768, and 13, 1769. Ueber die Bildung des Darmkanals im bebriiteten Hiihnchen. Uebersetzt von J. F. Meckel. S. 1-263. Halle 1812.
Zimmermann, K. W. : Ueber Kopfhohlenrudimente beim Menschen. Arch. f. mikr. Anat. Bd. 53. S. 481-4S4. 1S99.