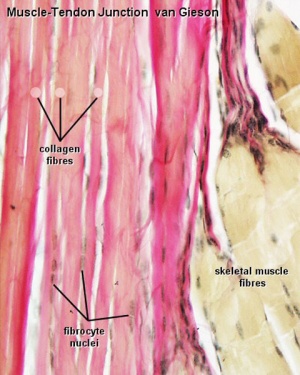
Introduction
This page describes skeletal tendon development, during formation of the connective tissue connection muscle to bone.
The syndetome is the embryonic structural origin of tendons from the somite and originates from the dorsolateral edge of the sclerotome. Expression of the basic helix-loop-helix (bHLH) transcription factor scleraxis (SCX) in early progenitor cells is thought to be key regulator in the formation of tendon and ligament tissues.[1] Scleraxis may also have additional roles in other tissues such as in early heart valve development.[2]
The origins of some muscles and tendons in the head differ from those found in the remained of the body.
See also notes Connective Tissue Development.
Some Recent Findings
- Paralogues of Mmp11 and Timp4 Interact during the Development of the Myotendinous Junction in the zebrafish Embryo[3] "The extracellular matrix (ECM) of the myotendinous junction (MTJ) undergoes dramatic physical and biochemical remodeling during the first 48 h of development in zebrafish, transforming from a rectangular fibronectin-dominated somite boundary to a chevron-shaped laminin-dominated MTJ. Matrix metalloproteinase 11 (Mmp11, a.k.a. Stromelysin-3) is both necessary and sufficient for the removal of fibronectin at the MTJ, but whether this protease acts directly on fibronectin and how its activity is regulated remain unknown. Using immunofluorescence, we show that both paralogues of Mmp11 accumulate at the MTJ during this time period, but with Mmp11a present early and later replaced by Mmp11b. Moreover, Mmp11a also accumulates intracellularly, associated with the Z-discs of sarcomeres within skeletal muscle cells. Using the epitope-mediated MMP activation (EMMA) assay, we show that despite having a weaker paired basic amino acid motif in its propeptide than Mmp11b, Mmp11a is activated by furin, but may also be activated by other mechanisms intracellularly. One or both paralogues of tissue inhibitors of metalloproteinase-4 (Timp4) are also present at the MTJ throughout this process, and yeast two-hybrid assays reveal distinct and specific interactions between various domains of these proteins. We propose a model in which Mmp11a activity is modulated (but not inhibited) by Timp4 during early MTJ remodeling, followed by a phase in which Mmp11b activity is both inhibited and spatially constrained by Timp4 in order to maintain the structural integrity of the mature MTJ."
- Jitterbug/Filamin and Myosin-II form a complex in tendon cells required to maintain epithelial shape and polarity during musculoskeletal system development[4] "During musculoskeletal system development, mechanical tension is generated between muscles and tendon-cells. This tension is required for muscle differentiation and is counterbalanced by tendon-cells avoiding tissue deformation. Both, Jbug/Filamin, an actin-meshwork organizing protein, and non-muscle Myosin-II (Myo-II) are required to maintain the shape and cell orientation of the Drosophila notum epithelium during flight muscle attachment to tendon cells. Here we show that halving the genetic dose of Rho kinase (Drok), the main activator of Myosin-II, enhances the epithelial deformation and bristle orientation defects associated with jbug/Filamin knockdown. Drok and activated Myo-II localize at the apical cell junctions, tendon processes and are associated to the myotendinous junction. Further, we found that Jbug/Filamin co-distribute at tendon cells with activated Myo-II. Finally, we found that Jbug/Filamin and Myo-II are in the same molecular complex and that the actin-binding domain of Jbug/Filamin is necessary for this interaction. These data together suggest that Jbug/Filamin and Myo-II proteins may act together in tendon cells to balance the tension generated during development of muscles-tendon interaction, maintaining the shape and polarity of the Drosophila notum epithelium."
- mTORC1 Signaling is a Critical Regulator of Postnatal Tendon Development[5] "Tendons transmit contractile forces between musculoskeletal tissues. Whereas the biomechanical properties of tendons have been studied extensively, the molecular mechanisms regulating postnatal tendon development are not well understood. Here we examine the role of mTORC1 signaling in postnatal tendon development using mouse genetic approaches. Loss of mTORC1 signaling by removal of Raptor in tendons caused severe tendon defects postnatally, including decreased tendon thickness, indicating that mTORC1 is necessary for postnatal tendon development. By contrast, activation of mTORC1 signaling in tendons increased tendon cell numbers and proliferation. In addition, Tsc1 conditional knockout mice presented severely disorganized collagen fibers and neovascularization in the tendon midsubstance. Interestingly, collagen fibril diameter was significantly reduced in both Raptor and Tsc1 conditional knockout mice, albeit with variations in severity."
- Cellular and molecular maturation in fetal and adult ovine calcaneal tendons[6] "ovine tendon morphology undergoes profound transformations during this period. Endotenon was more developed in fetal tendons than in adult tissues, and its cell phenotype changed through tendon maturation. Indeed, groups of large rounded cells laying on smaller and more compacted ones expressing osteocalcin, vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) were identified exclusively in fetal mid-stage tissues, and not in late fetal or adult tendons. VEGF, NGF as well as blood vessels and nerve fibers showed decreased expression during tendon development. Moreover, the endotenon of mid- and late fetuses contained identifiable cells that expressed several pluripotent stem cell markers [Telomerase Reverse Transcriptase (TERT), SRY Determining Region Y Box-2 (SOX2), Nanog Homeobox (NANOG) and Octamer Binding Transcription Factor-4A (OCT-4A)]. These cells were not identifiable in adult specimens. Ovine tendon development was also accompanied by morphological modifications to cell nuclei, and a progressive decrease in cellularity, proliferation index and expression of connexins 43 and 32. Tendon maturation was similarly characterised by modulation of several other gene expression profiles, including Collagen type I, Collagen type III, Scleraxis B, Tenomodulin, Trombospondin 4 and Osteocalcin. These gene profiles underwent a dramatic reduction in adult tissues. Transforming growth factor-β~1 expression (involved in collagen synthesis) underwent a similar decrease."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Tendon development | Tendon embryology |syndetome | myotendinous junction development
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin.[7] "Stem cell-based engineering strategies for tendons have yet to yield a normal functional tissue, due in part to a need for tenogenic factors. Additionally, the ability to evaluate differentiation has been challenged by a lack of markers for differentiation.... Based on scleraxis expression, TGFβ2 was tenogenic for TPCs at all stages, while loading was for late-stage cells only, and FGF4 had no effect despite regulation of other genes. When factors were combined, TGFβ2 continued to be tenogenic, while FGF4 appeared anti-tenogenic. Various treatments elicited distinct responses by axial vs. limb TPCs of specific stages. These results identified tenogenic factors, suggest tendon engineering strategies should be customized for tissues by anatomical origin, and provide stage-specific gene expression profiles of limb and axial TPCs as benchmarks with which to monitor tenogenic differentiation of stem cells."
- Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates[8] "The formation of the musculoskeletal system represents an intricate process of tissue assembly involving heterotypic inductive interactions between tendons, muscles and cartilage. An essential component of all musculoskeletal systems is the anchoring of the force-generating muscles to the solid support of the organism: the skeleton in vertebrates and the exoskeleton in invertebrates. Here, we discuss recent findings that illuminate musculoskeletal assembly in the vertebrate embryo, findings that emphasize the reciprocal interactions between the forming tendons, muscle and cartilage tissues. We also compare these events with those of the corresponding system in the Drosophila embryo, highlighting distinct and common pathways that promote efficient locomotion while preserving the form of the organism."
- Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction[9]
|
Molecular
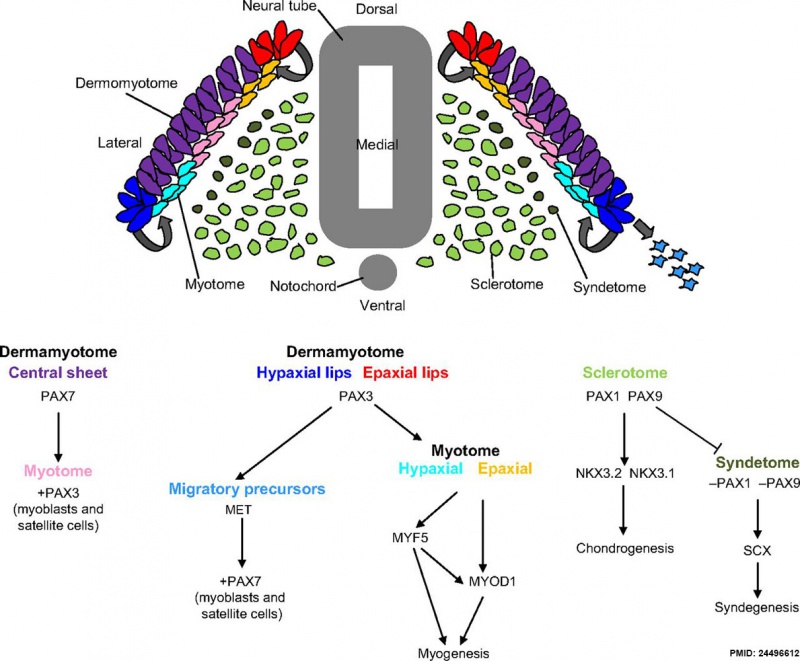
Mesoderm Development and Pax[10]
dark green - syndetome originates from the dorsolateral edge of the sclerotome, as Pax1 and Pax9 are downregulated and scleraxis (Scx) upregulation leads to syndegenesis. Pax, paired homeobox; MYOD1, myogenic differentiation antigen 1; MYF5, myogenic factor 5; NKX, NK homeobox; SCX, scleraxis.
Scleraxis
Scleraxis (SCX) is a member of the basic helix-loop-helix (bHLH) transcription factor family. It is expressed in early mesoderm progenitor cells and may regulate the formation of tendon and ligament tissues.[1]
Scleraxis may also have additional roles in other tissues such as in early heart valve development.[2]
- Cytogenetic location: 8q24.3
- Links: NCBI databases - Scleraxis | OMIM 609067
Tenomodulin
Tenomodulin (TNMD, Tnmd) is a marker of tendon differentiation, its expression has been shown to be regulated by the transcription factors Scleraxis and Mohawk.[11] May also affect the tendon stem/progenitor cells.
- Links: NCBI databases - Tenomodulin
Histology
References
- ↑ 1.0 1.1 <pubmed>11585810</pubmed> Cite error: Invalid
<ref> tag; name 'PMID11585810' defined multiple times with different content
- ↑ 2.0 2.1 Barnette DN, VandeKopple M, Wu Y, Willoughby DA & Lincoln J. (2014). RNA-seq analysis to identify novel roles of scleraxis during embryonic mouse heart valve remodeling. PLoS ONE , 9, e101425. PMID: 24983472 DOI.
- ↑ Matchett EF, Wang S & Crawford BD. (2019). Paralogues of Mmp11 and Timp4 Interact during the Development of the Myotendinous Junction in the Zebrafish Embryo. J Dev Biol , 7, . PMID: 31816958 DOI.
- ↑ Manieu C, Olivares GH, Vega-Macaya F, Valdivia M & Olguín P. (2018). Jitterbug/Filamin and Myosin-II form a complex in tendon cells required to maintain epithelial shape and polarity during musculoskeletal system development. Mech. Dev. , 154, 309-314. PMID: 30213743 DOI.
- ↑ Lim J, Munivez E, Jiang MM, Song IW, Gannon F, Keene DR, Schweitzer R, Lee BH & Joeng KS. (2017). mTORC1 Signaling is a Critical Regulator of Postnatal Tendon Development. Sci Rep , 7, 17175. PMID: 29215029 DOI.
- ↑ Russo V, Mauro A, Martelli A, Di Giacinto O, Di Marcantonio L, Nardinocchi D, Berardinelli P & Barboni B. (2015). Cellular and molecular maturation in fetal and adult ovine calcaneal tendons. J. Anat. , 226, 126-42. PMID: 25546075 DOI.
- ↑ Brown JP, Finley VG & Kuo CK. (2014). Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech , 47, 214-22. PMID: 24231248 DOI.
- ↑ Schweitzer R, Zelzer E & Volk T. (2010). Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development , 137, 2807-17. PMID: 20699295 DOI.
- ↑ Gilsohn E & Volk T. (2010). Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development , 137, 785-94. PMID: 20110313 DOI.
- ↑ Blake JA & Ziman MR. (2014). Pax genes: regulators of lineage specification and progenitor cell maintenance. Development , 141, 737-51. PMID: 24496612 DOI.
- ↑ Miyabara S, Yuda Y, Kasashima Y, Kuwano A & Arai K. (2014). Regulation of Tenomodulin Expression Via Wnt/β-catenin Signaling in Equine Bone Marrow-derived Mesenchymal Stem Cells. J Equine Sci , 25, 7-13. PMID: 24834008 DOI.
Reviews
Articles
Brent AE, Schweitzer R & Tabin CJ. (2003). A somitic compartment of tendon progenitors. Cell , 113, 235-48. PMID: 12705871
Search PubMed
Search Pubmed: Tendon Development | myotendinous junction development
Additional Images
Terms
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Musculoskeletal System - Tendon Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Musculoskeletal_System_-_Tendon_Development
- What Links Here?
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G