Musculoskeletal System - Cartilage Development
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The musculoskeletal system consists of skeletal muscle, bone, and cartilage and is mainly mesoderm in origin with some neural crest contribution.
The mesoderm forms nearly all the connective tissues of the musculoskeletal system. Each tissue (cartilage, bone, and muscle) goes through many different mechanisms of differentiation. Recent studies[1] show that Sox9 acts as a key regulator of early chondrocyte differentiation.
The intraembryonic mesoderm can be broken into paraxial, intermediate and lateral mesoderm relative to its midline position. During the 3rd week the paraxial mesoderm forms into "balls" of mesoderm paired either side of the neural groove, called somites. Somites appear bilaterally as pairs at the same time and form earliest at the cranial (rostral,brain) end of the neural groove and add sequentially at the caudal end. This addition occurs so regularly that embryos are staged according to the number of somites that are present. Different regions of the somite differentiate into dermomyotome (dermal and muscle component) and sclerotome (forms vertebral column). An example of a specialized musculoskeletal structure can be seen in the development of the limbs.
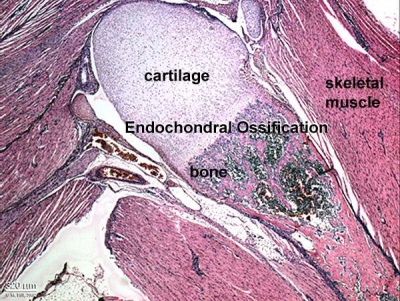
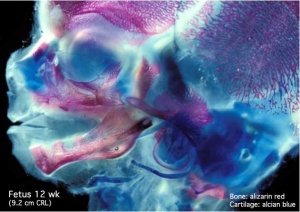
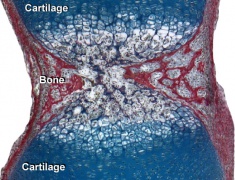
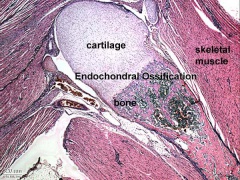
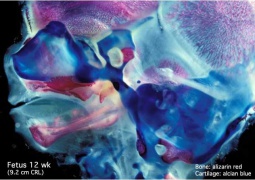
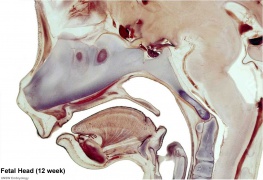
Skeletal muscle forms by fusion of mononucleated myoblasts to form mutinucleated myotubes. Bone is formed through a lengthy process involving ossification of a cartilage formed from mesenchyme. Two main forms of ossification occur in different bones, intramembranous (eg skull) and endochondrial (eg limb long bones) ossification. Ossification continues postnatally, through puberty until mid 20s. Early ossification occurs at the ends of long bones.
Musculoskeletal and limb abnormalities are one of the largest groups of congenital abnormalities.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cartilage Development | Cartilage Embryology | Chondrogenesis |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Textbooks
- The Developing Human: Clinically Oriented Embryology (8th Edition) by Keith L. Moore and T.V.N Persaud - Moore & Persaud Chapter 15 the skeletal system
- Larsen’s Human Embryology by GC. Schoenwolf, SB. Bleyl, PR. Brauer and PH. Francis-West - Chapter 11 Limb Dev (bone not well covered in this textbook)
- Before we Are Born (5th ed.) Moore and Persaud Chapter 16,17: p379-397, 399-405
- Essentials of Human Embryology Larson Chapter 11 p207-228
Objectives
- Identify the components of a somite and the adult derivatives of each component.
- Give examples of sites of (a) endochondral and (b) intramembranous ossification and to compare these two processes.
- Identify the general times (a) of formation of primary and (b) of formation of secondary ossification centres, and (c) of fusion of such centres with each other.
- Briefly summarise the development of the limbs.
- Describe the developmental abnormalities responsible for the following malformations: selected growth plate disorders; congenital dislocation of the hip; scoliosis; arthrogryposis; and limb reduction deformities.
Development Overview
Below is a very brief overview using simple figures of 3 aspects of early musculoskeletal development. More detailed overviews are shown on other notes pages Mesoderm and Somite, Vertebral Column, Limb in combination with serial sections and Carnegie images.
Mesoderm Development

|
Cells migrate through the primitive streak to form mesodermal layer. Extraembryonic mesoderm lies adjacent to the trilaminar embryo totally enclosing the amnion, yolk sac and forming the connecting stalk. |
|
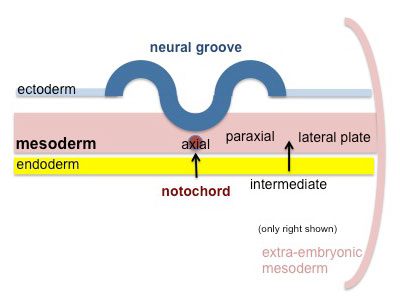
Paraxial mesoderm accumulates under the neural plate with thinner mesoderm laterally. This forms 2 thickened streaks running the length of the embryonic disc along the rostrocaudal axis. In humans, during the 3rd week, this mesoderm begins to segment. The neural plate folds to form a neural groove and folds. |
|
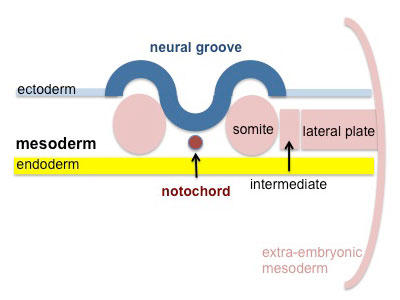
Segmentation of the paraxial mesoderm into somites continues caudally at 1 somite/90minutes and a cavity (intraembryonic coelom) forms in the lateral plate mesoderm separating somatic and splanchnic mesoderm.
Note intraembryonic coelomic cavity communicates with extraembryonic coelom through portals (holes) initially on lateral margin of embryonic disc. |
|
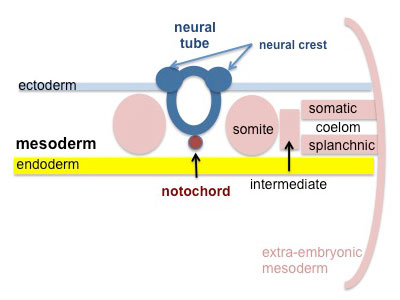
Somites continue to form. The neural groove fuses dorsally to form a tube at the level of the 4th somite and "zips up cranially and caudally and the neural crest migrates into the mesoderm. |
Somite Development

|
Mesoderm beside the notochord (axial mesoderm, blue) thickens, forming the paraxial mesoderm as a pair of strips along the rostro-caudal axis. |
|
Paraxial mesoderm towards the rostral end, begins to segment forming the first somite. Somites are then sequentially added caudally. The somitocoel, is a cavity forming in early somites, which is lost as the somite matures. |
|
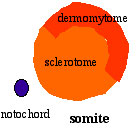
Cells in the somite differentiate medially to form the sclerotome (forms vertebral column) and dorsolaterally to form the dermomyotome. |
|
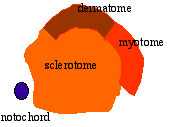
The dermomyotome then forms the dermotome (forms dermis) and myotome (forms muscle).
Neural crest cells migrate beside and through somite. |
|
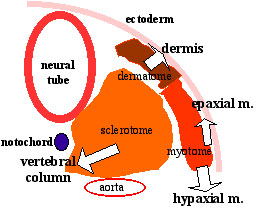
The myotome differentiates to form 2 components dorsally the epimere and ventrally the hypomere, which in turn form epaxial and hypaxial muscles respectively. The bulk of the trunk and limb muscle coming from the Hypaxial mesoderm. Different structures will be contributed depending upon the somite level. |
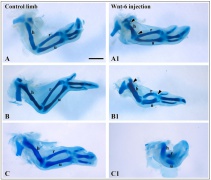
Limb Development

|
Chondroblast
Chondrocyte
References
- ↑ 1.0 1.1 Umeda K, Oda H, Yan Q, Matthias N, Zhao J, Davis BR & Nakayama N. (2015). Long-term expandable SOX9+ chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Reports , 4, 712-26. PMID: 25818812 DOI.
- ↑ Atsuta Y, Tomizawa RR, Levin M & Tabin CJ. (2019). L-type voltage-gated Ca2+ channel CaV1.2 regulates chondrogenesis during limb development. Proc. Natl. Acad. Sci. U.S.A. , 116, 21592-21601. PMID: 31591237 DOI.
- ↑ Rhodes CS, Matsunobu T & Yamada Y. (2019). Analysis of a limb-specific regulatory element in the promoter of the link protein gene. Biochem. Biophys. Res. Commun. , 518, 672-677. PMID: 31470976 DOI.
- ↑ Kobayashi T & Kozlova A. (2018). Lin28a overexpression reveals the role of Erk signaling in articular cartilage development. Development , 145, . PMID: 30042178 DOI.
- ↑ Buchtova M, Oralova V, Aklian A, Masek J, Vesela I, Ouyang Z, Obadalova T, Konecna Z, Spoustova T, Pospisilova T, Matula P, Varecha M, Balek L, Gudernova I, Jelinkova I, Duran I, Cervenkova I, Murakami S, Kozubik A, Dvorak P, Bryja V & Krejci P. (2015). Fibroblast growth factor and canonical WNT/β-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage. Biochim. Biophys. Acta , 1852, 839-50. PMID: 25558817 DOI.
- ↑ Michigami T. (2014). Current understanding on the molecular basis of chondrogenesis. Clin Pediatr Endocrinol , 23, 1-8. PMID: 24532955 DOI.
- ↑ Cheng A & Genever PG. (2010). SOX9 determines RUNX2 transactivity by directing intracellular degradation. J. Bone Miner. Res. , 25, 2680-9. PMID: 20593410 DOI.
- ↑ Hattori T, Müller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bösl MR, Hess A, Surmann-Schmitt C, von der Mark H, de Crombrugghe B & von der Mark K. (2010). SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development , 137, 901-11. PMID: 20179096 DOI.
Reviews
Takigawa M. (2013). CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal , 7, 191-201. PMID: 23794334 DOI.
Kawakami Y, Rodriguez-León J & Izpisúa Belmonte JC. (2006). The role of TGFbetas and Sox9 during limb chondrogenesis. Curr. Opin. Cell Biol. , 18, 723-9. PMID: 17049221 DOI.
Goldring MB, Tsuchimochi K & Ijiri K. (2006). The control of chondrogenesis. J. Cell. Biochem. , 97, 33-44. PMID: 16215986 DOI.
Shum L & Nuckolls G. (2002). The life cycle of chondrocytes in the developing skeleton. Arthritis Res. , 4, 94-106. PMID: 11879545 DOI.
Articles
Search PubMed
Search April 2010
- Musculoskeletal System Development - All (44637) Review (5065) Free Full Text (6601)
- Musculoskeletal Development - All (44637) Review (5065) Free Full Text (6601)
Search Pubmed: Cartilage Development | chondroblast Development | chondroclast Development
Additional Images
- Cartilage Images
Terms
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Musculoskeletal System - Cartilage Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Musculoskeletal_System_-_Cartilage_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G