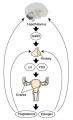
Menstrual Cycle
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
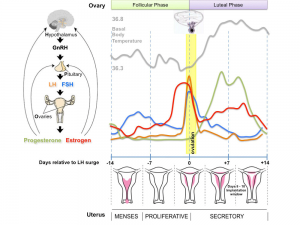
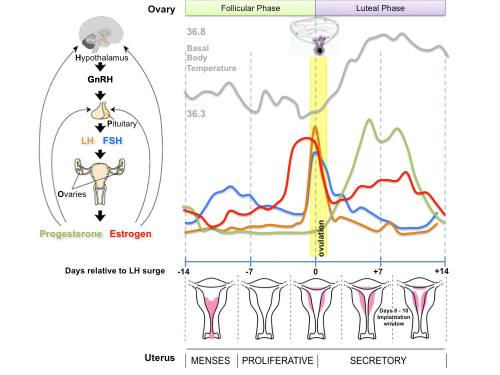
The menstrual cycle describes the female human reproductive cycle. This is a cyclic endocrine regulated change in female anatomy and physiology that occur over 28 days (4 weeks, a lunar month) during reproductive life (between puberty and menopause). Endocrine changes during pregnancy block the menstrual cycle, which normally would shed the functional layer of the uterine lining each cycle. A common misunderstanding is that development of the follicles occurs within a single cycle, in fact humans require at least 3 menstrual cycles to occur in the development of an ovulating follicle.
- The average menstrual cycle is 28 days with ovulation (egg release) occuring approximately the middle of the cycle.
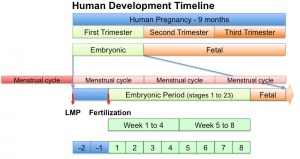
- The last menstrual period (LMP) is used clinically in determining developmental ages.
- Menstruation phase (menses, period) is the loss of the uterus epithelial functional layer and occurs if fertilization and implantation have not occurred before the end of the current cycle.
- Menstrual cycle stages can be characterised by histological analysis, first devised by Papanicolaou in 1933.[2] (see also Menstrual Cycle - Histology)
This cycle differs from other non-primate female vertebrates (eg rats, mice, horses, pig) that have a reproductive cycle called the estrous cycle (oestrous, British spelling).
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Menstrual Cycle | Progesterone | Estrogen | Leutenizing Hormone |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Menstrual Parameters
There is a broad variability in the parameters of the adult human menstrual cycle. The data below is based upon normal mid-reproductive years and using simplified terminology, Table IV from 2007 international agreement discussion.[8] The same group also recommended replacing confusing clinical terms such as amenorrhea, menorrhagia, metrorrhagia, hypermenorrhea and dysfunctional uterine bleeding.
| Clinical dimensions of menstruation and menstrual cycle | Descriptive terms | Normal limits (5th - 95th percentiles) |
|---|---|---|
| Frequency of menses (days) | Frequent | < 24 |
| Normal | 24 - 38 | |
| Infrequent | > 38 | |
| Regularity of menses (days) (cycle to cycle variation over 12 months) |
Absent | |
| Regular | Variation ± 2 to 20 days | |
| Irregular | Variation greater than 20 days | |
| Duration of flow (days) | Prolonged | > 8.0 |
| Normal | 4.5 - 8.0 | |
| Shortened | < 4.5 | |
| Volume of monthly blood loss (ml)[9] | Heavy | > 80 |
| Normal | 5 - 80 | |
| Light | < 5 |
Follicular Phase
Follicular Phase (ovary) or Proliferative Phase (uterus).
Luteal Phase
Luteal Phase (ovary) or Secretory Phase (uterus).
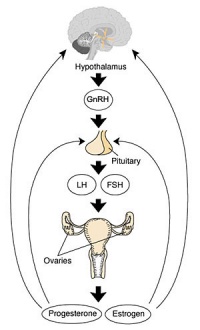
Progesterone
| The progesterone (progestin) hormone is produced by the granulosa cells of the ovarian follicles at different levels during the menstrual cycle and at high levels by the luteal cells (P4) of the corpus luteum.
|
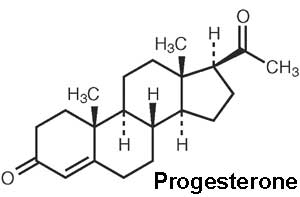
Progesterone molecular structure |
A recent study has shown that maternal first trimester serum progesterone levels does not associated with either a reduction of length of gestation or increased risk of preterm delivery.[14]
Estrogen
| The estradiol (estrogen, oestrogen) hormone is a steroid sex hormone expressed in both male and female.
estrogenic activity in human placental extracts was due to the presence of at least three compounds: estriol, estrone, and 17β-estradiol. In the female, this hormone together with progesterone regulate changes that occur each menstrual cycle. During female development the fetal adrenal gland cortex synthesises DHEA (and DHEA/S), an oestrogen precursor (see image Fetal adrenal gland steroidogenesis), converted by the placenta into estrogen compounds; estriol, estrone, and 17β-estradiol. During puberty, ovarian estrogen production is responsible for development of the secondary feminine sex characteristics. In the male, Leydig cells produce estrogen into the rete testis fluid at variable levels in different species. During male embryonic development exposure to high levels of estrogen can lead to genital abnormalities. |
Estradiol |
| Diethylstilbestrol | |
|---|---|
Diethylstilbestrol (DES or diethylstilbetrol) was a drug prescribed to women from 1938-1971 to prevent miscarriage in high-risk pregnancies. Is as a potent estrogen (mimics the natural hormone) and therefore acts as a potential endocrine disruptor. Banned by the USA FDA in 1979 as a teratogen, previously used as livestock growth promoter.
Diethylstilbestrol induces vaginal abnormalities (vaginal adenosis) by inhibiting the BMP4/Activin A-regulated vaginal cell fate decision through a down-regulation of RUNX1.[15] Has also been shown to induces autophagy in thymus thymocytes through epigenetic modulation.[16] |
Diethylstilbestrol |
Iron Depletion
See also the article on iron depletion by whole-blood donation harms menstruating females.[17] "The collection of 450 or 500 mL of whole blood, plus an additional 30 to 50 mL for blood tests, results in 480 to 550 mL of blood loss per whole-blood donation. These losses leads to a 60- to 88-g loss of hemoglobin (Hb) per whole-blood donation in women, based on a Hb range of 12.5 to 16.0 g per dL, and 204 to 299 mg of iron loss, based on 3.4 mg of iron per gram of Hb. This iron loss is 9 to 13 percent of the total body iron in an average woman (2300 mg), and it is 66 to 97 percent of the total stored iron in an average menstruating woman (309 mg). Therefore, whole-blood donation is an iron depletion event that causes significant iron loss in women."
- prevalence of iron deficiency in 20- to 49-year-old women before blood donation is 12 percent
- prevalence of iron deficiency in 16- to 69-year-old men is 2 percent
(based on data from the National Health and Nutrition Examination Survey (NHANES 1999-2000)
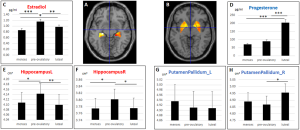
Changes in Brain Size
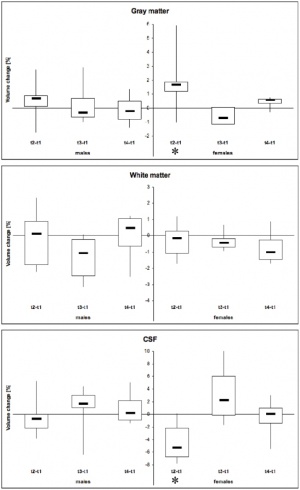
A recent MRI study[18] of women during the normal menstrual cycle correlated with hormone and ultrasound determination of ovulation has shown "that brain morphology varies during the menstrual cycle, with a (grey matter) volume peak at time of ovulation which can be estimated to be ~13,5 ml for a “standard” brain." They also identified "significant grey matter volume peak and CSF loss at the time of ovulation in females. This volume peak did not correlate with estradiol or progesterone hormone levels."
Environmental Effects
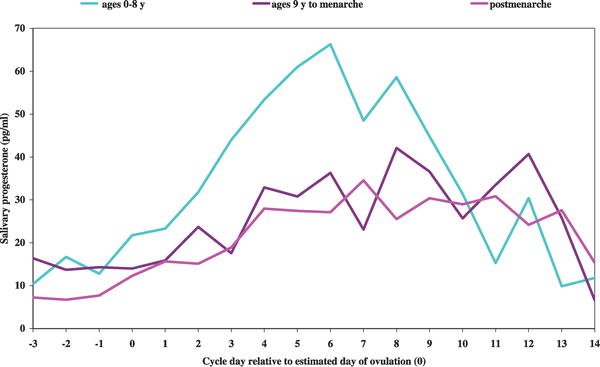
A recent paper has also demonstrated that the human menstral cycle can be modulated postnatally by environmental conditions as measured by changes in progesterone based upon the age of migration from a relatively poor environment (Bangladesh) to a relatively better environment (UnitedKingdom).[19]
Luteal Progesterone Profiles by Age at UK Migration. Women who migrated during infancy and early childhood (ages 0 to 8 years) had a significantly earlier age at menarche. Women who migrated after menarche, length of time spent in the UK had no significant impact on luteal progesterone levels.
Last Menstrual Period
The Last Menstrual Period (LMP), the menstrual period (menses) that occurs before a pregnancy, has been widely used clinically as a date to calculate clinical pregnancy development (GA, gestational age). Note that in humans this is approximately two weeks different from embryonic development, which begins at fertilisation around the mid-point of the menstrual cycle.
The interval between the beginning of the LMP and fertilisation can have a wide range (7 to 25 days). This variation can be due to both maternal (menstrual cycle timing and ovulation) and fetal (blastocyst implantation) effects. The calculation also requires an accurate maternal recall of LMP and can be affected by irregular menses, first-trimester vaginal bleeding, unrecognized spontaneous abortions, oral contraceptive use.
Measurement of fetal size by ultrasound has been used more recently to accurately calculate pregnancy development. The ultrasound measurement tends to be more accurate in early development staging, by the third trimester there can be some individual variations in fetal growth and the effects of abnormalities or fetal growth restriction. Serial ultrasound measurements may identify these abnormal growth effects.
Gestational age GA is the clinical term given in week to describe human development timed from the first day of the last menstrual period (LMP).[20] For gestational age in assisted reproductive technology pregnancy 2 weeks are added to the fertilisation date. Age therefore differs by approximately two weeks from research materials timed from fertilisation (conceptional age), this term is generally not used clinically.
Menstruation is also called menstrual bleeding, menses, catamenia or a period.
- Links: Gestational Age | Pregnancy Test | Timeline human development | Birth
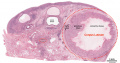
Menstrual Cycle Histology
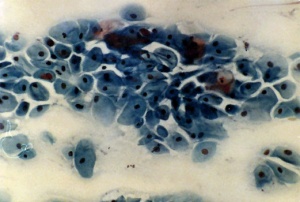
The different stages of the menstrual cycle can be monitored by the cellular appearance of vaginal smears Menstrual Cycle - Histology.
A more invasive technique is dilate and curettage (DnC), which allows sampling of the functional layer of the uterine endometrium Menstrual Cycle - Histology.
Decidualization
Decidualization is the process of converting endometrial stromal cells into decimal cells and requires at least 8–10 days of hormone stimulation.
- initiated during the mid-secretory phase of the menstrual cycle
- in response to elevated progesterone levels
- acts mainly through progesterone receptor (PR) PR-A (other isoform is PR-B)
Molecular
PMID: 21546446 Prokineticin 1 (PROK1) signalling via prokineticin receptor 1 (PROKR1) regulates Dickkopf 1 (DKK1) expression, a negative regulator of canonical Wnt signaling.
- Links: Placenta - Maternal Decidua
Human Oocyte Numbers
There is continuing debate as to whether the human ovary has the ability to generate, or continues to generate, new functional follicles and oocytes postnatally.[21][22] (More? Ovary Development)
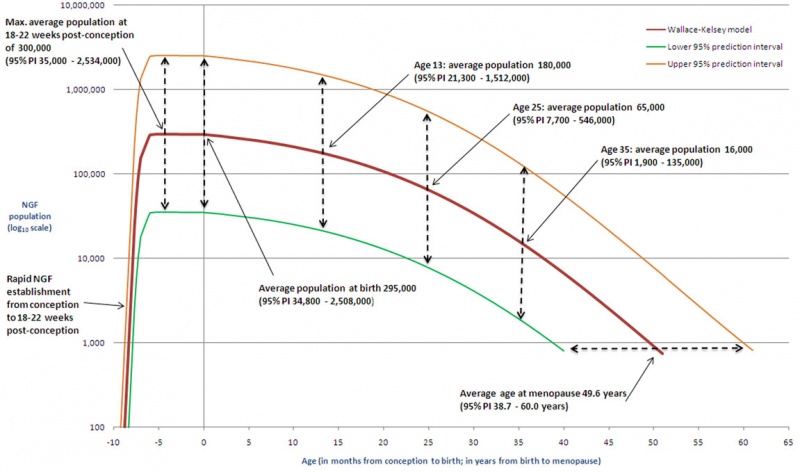
- The graph shows a model of non-growing follicle numbers[23] based upon several histological studies of the human ovary.
- The maximum follicle number occurring around birth.
- These numbers decrease through childhood by apopotic cell death.
- At puberty there remain only about 180,000 remain.
- Only a small percentage will be released through reproductive life.
- At menopause only about 1,000 remain.
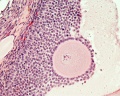
Follicle Development
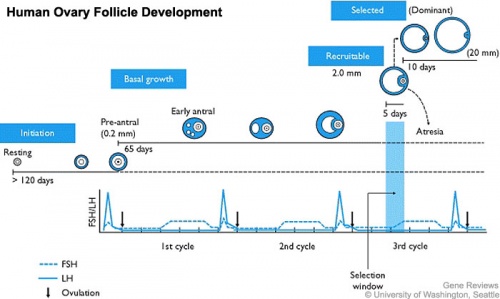
A common misunderstanding is that development of the follicle containing the oocyte occurs within a single cycle. In fact humans require at least 3 menstrual cycles to occur in the development of a single ovulating follicle. Most of the other follicles will degenerate in a process described as atresia.
Oocyte Development
Primary Oocyte
- arrested at early Meiosis 1
- diploid: 22 chromosome pairs + 1 pair X chromosomes (46, XX) autosomes and sex chromosome
- Oogenesis- pre-antral then antral follicle (Graafian follicle is mature antral follicle released)
Secondary Oocyte
- 1 Day before ovulation completes (stim by LH) Meiosis 1
- haploid: 22 chromosomes + 1 X chromosome (23, X)
- nondisjunction- abnormal chromosome segregation
- begins Meiosis 2 and arrests at metaphase
- note no interphase replication of DNA, only fertilization will complete Meiosis 2
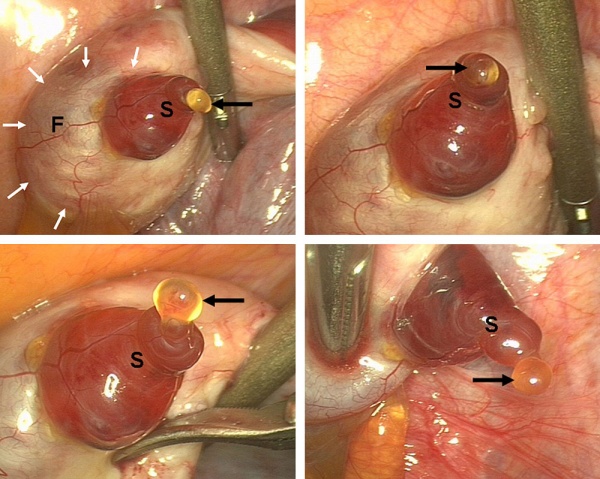
Ovulation
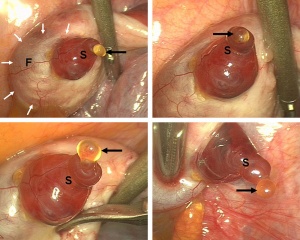
Laparoscopic observation of human ovulation.[1]
Associated with follicle rupture is movement of the the ampulla region of the uterine horn.
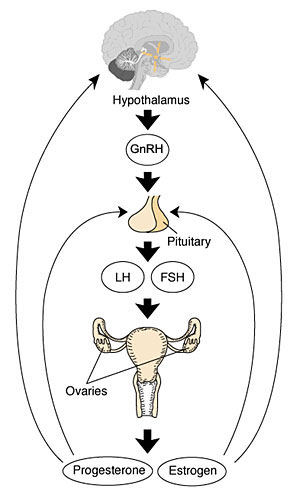
- Hypothalmus releases gonadotropin releasing hormone (GRH, luteinizing hormone–releasing hormone, LHRH) -> Pituitary releases follicle stimulating hormone (FSH) and lutenizing hormone (LH) -> ovary follicle development and ovulation.
- release of the secondary oocyte and formation of corpus luteum
- secondary oocyte encased in zona pellucida and corona radiata
Rabbit ovulation also available as a movie.
Ovulation Movies
Left or Right Ovulation
In humans, it is assumed that about equal numbers of ovulations occur from each of the ovaries. Whether ovulation in a succeeding cycle occurs ipsilaterally (same ovary; right/right or left/left) and contralaterally (opposite ovary; left/right or right/left) has also been studied. A shorter follicular phase length (less than 13 days) has been identified to correlate with a greater number of contralateral ovulations, while a follicular length greater than 14 days has a random ovulation.[24]
- right-sided ovulation has been shown to favour pregnancy more than left-sided ovulation[25]
- See also the review[26]
- contralateral ovulation, ovulation occurring alternately from one ovary to the other in two consecutive cycles, has been shown to be inversely correlated with age, greater in younger than older women.[27]
- both ovaries appear to respond equally to clinical ovulation induction.[28]
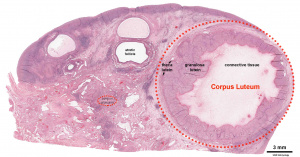
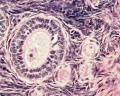
Corpus Luteum
Following ovulation, the ovulating follicle forms a unique endocrine structure, the corpus luteum. The corpus luteum functions to produce both progesterone and estradiol, with maximum function about 6 days following ovulation.[29]
A study of blood flow during corpus luteal development[30] identified:
- active angiogenesis occurs after the ovulatory LH surge
- becomes one of the most highly vascularized organs in the body
- provides luteal cells with large amounts of cholesterol (for progesterone synthesis)
- delivers this progesterone to the circulation
Follicular Waves
Follicular waves is a term referring to the growth of follicles in coordinated groups or waves, in humans this occurs either 2 to 3 times between ovulations. These waves have previously been described in several other mono-ovulatory species, such as the horse (equine) and cow (bovine).
Ovarian Stimulation
A variety of drug based techniques are used to stimulate maternal oocyte development, called ovarian stimulation, for many assisted reproductive technology (in vitro fertilization) procedures. The recommended for technique will vary for some procedures and also from clinic to clinic and between countries.
An example of ovarian stimulation (based on PMID20953827)
- Gonadotrophin releasing hormone agonist (GnRHa) triptorelin acetate (Decapeptyl (0.1 mg/day) treatment started on the 22nd day of the preceding menstrual cycle.
- Human menopausal gonadotrophin (HMG) and/or follicular stimulating hormone (FSH) was carried out daily 12 to 15 days later.
- Dosage may vary dependent upon patient response and can be monitored by hourmone levels (oestradiol) and transvaginal ultrasound (follicular size).
- The resulting ovulatory wave generates large follicles (greater than 18 mm in diameter).
- Human chorionic gonadotrophin (HCG) is then administered (36 to 38 h later)
- Clinical transvaginal puncture is used to collect from these follicles cumulus-oocyte complexes.
- Cumulus-oocyte complexes can be processed to isolate oocytes.
Fertility Window
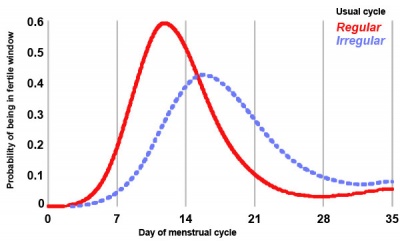
Probability of women with regular or irregular cycles being in their fertile window. |
Clinical guidelines have typically identified the "fertile window" between days 10 and 17 within the typical 28 day menstrual cycle.
Data from a large USA NIEHS - Early Pregnancy Study (1982-86) identified the timing of the “fertile window” within a range of different menstrual cycles.[31]
|
- Links: Fertilization
Ultrasound
Ultrasound may be used in monitoring the menstrual cycle, both endometrial and ovarian cyclical changes, see review.[32]
- Links: Ultrasound
Menopause
Menopause onset is defined clinically as the final menses, confirmed after 1 year without menstruation, about 10% of the general female population is postmenopausal at age 45.
A biological term describing the physiological changes that accompany the age related loss of fertility.
There is a decrease in ovarian follicle numbers, gradually elevated FSH levels, onset of cycle irregularity leading to the final cessation of menses.[33] A recent review[34] has looked at genetic factors that could affect the age at natural menopause and identified from linkage analyses (9q21.3 and chromosome 8 at 26 cM) and association studies genomic regions (19q13.42 and 20p12.3), containing two promising candidate genes (Bruck syndrome 1, BRKS1) and Menopause quantitative trait locus 3 (MENOQ3).[35]
See this recent review article putting forward a theory for the evolutionary origin of human menopause[36]
Premature Ovarian Failure
Premature Ovarian Failure (POF)[37] a clinical term describes the absence of normal ovarian function due to the depletion of the primordial follicle pool before 40 years of age a range of factors (autoimmune, iatrogenic, infections, genetic defects). This occurs in approximately 1% of women below 40 years of age.
Premature Ovarian Failure (POF) can be primary or secondary based on puberty.
- primary - absence of puberty, development and primary amenorrhea, generally caused by ovarian dysgenesis (45XO, Turner syndrome).
- secondary - normal puberty, usually present with the later disappearance of menstrual cycles.
Oral Contraceptives
A recent 2016 Danish study[38] births from Danish registries between 1997 and 2011 identified that "Oral contraceptive exposure just before or during pregnancy does not appear to be associated with an increased risk of major birth defects."
See also a paper describing the changing composition of the contraceptive pill.[39]
- Links: Abnormal Development
Abnormalities
Infrequent Menstrual Cycle
An infrequent menstrual cycle (oligomenorrhea) can be due to a wide range of endocrine and other causes. Abnormal adrenal development, congenital adrenal hyperplasia, can be associated with both menstrual and genital changes.
Congenital Adrenal Hyperplasia
| Congenital Adrenal Hyperplasia | |||
|---|---|---|---|
| Type | Enzyme Deficiency | Female | Male |
| classic virilizing adrenal hyperplasia | 21-hydroxylase, 11-beta-hydroxylase, or 3-beta-hydroxysteroid dehydrogenase |
ambiguous genitalia at birth - complete or partial fusion of the labioscrotal folds and a phallic urethra to clitoral enlargement (clitoromegaly), partial fusion of the labioscrotal folds, or both | normal genitalia, present at age 1-4 weeks with salt wasting (salt-wasting adrenal hyperplasia) |
| simple virilizing adrenal hyperplasia | mild 21-hydroxylase | identified later in childhood because of precocious pubic hair, clitoral enlargement (clitoromegaly), or both, often accompanied by accelerated growth and skeletal maturation | early genital development (pubic hair and/or phallic enlargement) accelerated growth and skeletal maturation |
| nonclassic adrenal hyperplasia | milder deficiencies of 21-hydroxylase or 3-beta-hydroxysteroid dehydrogenase |
present at puberty or adult with infrequent menstruation (oligomenorrhea), abnormal hair growth (hirsutism), and/or infertility | |
| 17-hydroxylase deficiency syndrome | 17-hydroxylase deficiency or 3-beta-hydroxysteroid dehydrogenase |
rare, phenotypically female at birth do not develop breasts or menstruate in adolescence and may have hypertension | steroidogenic acute regulatory (StAR) deficiency have ambiguous genitalia or female genitalia, at puberty may lack breast development and may have hypertension |
Endometriosis
Endometriosis occurs wheh endometrial tissue is located in other regions of the uterus or other tissues. This misplaced tissue develops into growths or lesions which respond to the menstrual cycle hormonal changes in the same way that the tissue of the uterine lining does; each month the tissue builds up, breaks down, and sheds.
References
- ↑ 1.0 1.1 Lousse JC & Donnez J. (2008). Laparoscopic observation of spontaneous human ovulation. Fertil. Steril. , 90, 833-4. PMID: 18440526 DOI.
- ↑ Papanicolaou GN. The sexual cycle in the human female as revealed by vaginal smears. (1933) Amer. J Anat. 52: 519–637.
- ↑ 3.0 3.1 Pletzer B, Harris T & Hidalgo-Lopez E. (2018). Subcortical structural changes along the menstrual cycle: beyond the hippocampus. Sci Rep , 8, 16042. PMID: 30375425 DOI.
- ↑ Hidalgo-Lopez E, Mueller K, Harris T, Aichhorn M, Sacher J & Pletzer B. (2020). Human menstrual cycle variation in subcortical functional brain connectivity: a multimodal analysis approach. Brain Struct Funct , , . PMID: 31894405 DOI.
- ↑ Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S & Azizi F. (2016). Menstrual Cycle Irregularity and Metabolic Disorders: A Population-Based Prospective Study. PLoS ONE , 11, e0168402. PMID: 27992506 DOI.
- ↑ Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA & Adams GP. (2012). The nerve of ovulation-inducing factor in semen. Proc. Natl. Acad. Sci. U.S.A. , 109, 15042-7. PMID: 22908303 DOI.
- ↑ Baird DD & Steiner AZ. (2012). Anti-Müllerian hormone: a potential new tool in epidemiologic studies of female fecundability. Am. J. Epidemiol. , 175, 245-9. PMID: 22247047 DOI.
- ↑ Fraser IS, Critchley HO, Munro MG & Broder M. (2007). Can we achieve international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding?. Hum. Reprod. , 22, 635-43. PMID: 17204526 DOI.
- ↑ Hallberg L, Högdahl AM, Nilsson L & Rybo G. (1966). Menstrual blood loss--a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand , 45, 320-51. PMID: 5922481
- ↑ Butenandt A. Neure Ergebnisse auf dem Gebiet der Sexualhormone. Wien Klin Wochenschr. 1934;47:936.
- ↑ Slotta KH, Ruschig H, Fels E. Ȕber der Hormon aus dem Corpus-luteum. Ber Chem Ges. 1934;67:1270.
- ↑ Hartmann M, Wettstein A. Ein krystallisiertes Hormon aus Corpus-luteum. Helv Chim Acta. 1934;17:878.
- ↑ Allen WM & Wintersteiner O. (1934). CRYSTALLINE PROGESTIN. Science , 80, 190-1. PMID: 17817057 DOI.
- ↑ Shen SY, Chen QZ, Zhang LF, He JR, Lu JH, Li WD, Xiao WQ, Zhou ZH, Morse AN, Keung Cheng K, Mol BWJ, Xia HM & Qiu X. (2020). Association between serum progesterone concentration in early pregnancy and duration of pregnancy: a cohort study. J. Matern. Fetal. Neonatal. Med. , 33, 2096-2102. PMID: 30474453 DOI.
- ↑ Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S & Kurita T. (2013). Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Müllerian duct epithelium. Dev. Biol. , 381, 5-16. PMID: 23830984 DOI.
- ↑ Singh NP, Miranda K, Singh UP, Nagarkatti P & Nagarkatti M. (2018). Diethylstilbestrol (DES) induces autophagy in thymocytes by regulating Beclin-1 expression through epigenetic modulation. Toxicology , 410, 49-58. PMID: 30153466 DOI.
- ↑ Harlow SD & Ephross SA. (1995). Epidemiology of menstruation and its relevance to women's health. Epidemiol Rev , 17, 265-86. PMID: 8654511
- ↑ 18.0 18.1 Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW & Gaser C. (2011). Changes in brain size during the menstrual cycle. PLoS ONE , 6, e14655. PMID: 21326603 DOI.
- ↑ Núñez-de la Mora A, Chatterton RT, Choudhury OA, Napolitano DA & Bentley GR. (2007). Childhood conditions influence adult progesterone levels. PLoS Med. , 4, e167. PMID: 17503960 DOI.
- ↑ Engle WA. (2004). Age terminology during the perinatal period. Pediatrics , 114, 1362-4. PMID: 15520122 DOI.
- ↑ Bukovsky A, Caudle MR, Svetlikova M & Upadhyaya NB. (2004). Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod. Biol. Endocrinol. , 2, 20. PMID: 15115550 DOI.
- ↑ Begum S, Papaioannou VE & Gosden RG. (2008). The oocyte population is not renewed in transplanted or irradiated adult ovaries. Hum. Reprod. , 23, 2326-30. PMID: 18596027 DOI.
- ↑ Wallace WH & Kelsey TW. (2010). Human ovarian reserve from conception to the menopause. PLoS ONE , 5, e8772. PMID: 20111701 DOI.
- ↑ Fukuda M, Fukuda K, Andersen CY & Byskov AG. (1996). Contralateral selection of dominant follicle favours pre-embryo development. Hum. Reprod. , 11, 1958-62. PMID: 8921071
- ↑ Fukuda M, Fukuda K, Andersen CY & Byskov AG. (2000). Right-sided ovulation favours pregnancy more than left-sided ovulation. Hum. Reprod. , 15, 1921-6. PMID: 10966987
- ↑ Shazly SA, Badee AY, Ali MK, Sobh AM & Aleem AA. (2013). The laterality of ovulation: how far does it matter?. Eur. J. Obstet. Gynecol. Reprod. Biol. , 167, 8-13. PMID: 23140993 DOI.
- ↑ Fukuda M, Fukuda K, Andersen CY & Byskov AG. (2001). Characteristics of human ovulation in natural cycles correlated with age and achievement of pregnancy. Hum. Reprod. , 16, 2501-7. PMID: 11726566
- ↑ Thomson AJ, Gazvani MR, Wood SJ, Meacock SC, Lewis-Jones DI & Kingsland CR. (2001). Comparison of ovarian response in right and left ovaries in IVF patients. Hum. Reprod. , 16, 1694-7. PMID: 11473965
- ↑ Baerwald AR, Adams GP & Pierson RA. (2005). Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol , 25, 498-507. PMID: 15846762 DOI.
- ↑ Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ & Schwall RH. (1998). Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. , 4, 336-40. PMID: 9500609
- ↑ Wilcox AJ, Dunson D & Baird DD. (2000). The timing of the "fertile window" in the menstrual cycle: day specific estimates from a prospective study. BMJ , 321, 1259-62. PMID: 11082086
- ↑ Matijevic R & Grgic O. (2005). Predictive values of ultrasound monitoring of the menstrual cycle. Curr. Opin. Obstet. Gynecol. , 17, 405-10. PMID: 15976548
- ↑ Broekmans FJ, Soules MR & Fauser BC. (2009). Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. , 30, 465-93. PMID: 19589949 DOI.
- ↑ Voorhuis M, Onland-Moret NC, van der Schouw YT, Fauser BC & Broekmans FJ. (2010). Human studies on genetics of the age at natural menopause: a systematic review. Hum. Reprod. Update , 16, 364-77. PMID: 20071357 DOI.
- ↑ Stolk L, Zhai G, van Meurs JB, Verbiest MM, Visser JA, Estrada K, Rivadeneira F, Williams FM, Cherkas L, Deloukas P, Soranzo N, de Keyzer JJ, Pop VJ, Lips P, Lebrun CE, van der Schouw YT, Grobbee DE, Witteman J, Hofman A, Pols HA, Laven JS, Spector TD & Uitterlinden AG. (2009). Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat. Genet. , 41, 645-7. PMID: 19448619 DOI.
- ↑ Takahashi M, Singh RS & Stone J. (2016). A Theory for the Origin of Human Menopause. Front Genet , 7, 222. PMID: 28111590 DOI.
- ↑ Beck-Peccoz P & Persani L. (2006). Premature ovarian failure. Orphanet J Rare Dis , 1, 9. PMID: 16722528 DOI.
- ↑ Charlton BM, Mølgaard-Nielsen D, Svanström H, Wohlfahrt J, Pasternak B & Melbye M. (2016). Maternal use of oral contraceptives and risk of birth defects in Denmark: prospective, nationwide cohort study. BMJ , 352, h6712. PMID: 26738512
- ↑ Regidor PA. (2018). The clinical relevance of progestogens in hormonal contraception: Present status and future developments. Oncotarget , 9, 34628-34638. PMID: 30349654 DOI.
Books
- Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990. Birth Control | Figure 174.1 A 28-day menstrual cycle. Not all cycles are 28 days long. It is the phase before ovulation that varies in length.
- Endocrinology: An Integrated Approach. Nussey S, Whitehead S. Oxford: BIOS Scientific Publishers; 2001. Box 6.42 The human menstrual cycle | Box 6.43 Feedback control of the hypothalamo-pituitary-ovarian axis
Reviews
Benton MJ, Hutchins AM & Dawes JJ. (2020). Effect of menstrual cycle on resting metabolism: A systematic review and meta-analysis. PLoS ONE , 15, e0236025. PMID: 32658929 DOI.
Andersen CY, Kelsey T, Mamsen LS & Vuong LN. (2020). Shortcomings of an unphysiological triggering of oocyte maturation using human chorionic gonadotropin. Fertil. Steril. , , . PMID: 32654823 DOI.
Messinis IE, Messini CI & Dafopoulos K. (2014). Novel aspects of the endocrinology of the menstrual cycle. , 28, 714-22. PMID: 24745832 DOI.
Tingen C, Kim A & Woodruff TK. (2009). The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol. Hum. Reprod. , 15, 795-803. PMID: 19710243 DOI.
Diaz A, Laufer MR & Breech LL. (2006). Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. , 118, 2245-50. PMID: 17079600 DOI.
Zakar T & Hertelendy F. (2007). Progesterone withdrawal: key to parturition. , 196, 289-96. PMID: 17403397 DOI.
Articles
Pritschet L, Santander T, Taylor CM, Layher E, Yu S, Miller MB, Grafton ST & Jacobs EG. (2020). Functional reorganization of brain networks across the human menstrual cycle. Neuroimage , 220, 117091. PMID: 32621974 DOI.
Järvelä IY, Ruokonen A & Tekay A. (2008). Effect of rising hCG levels on the human corpus luteum during early pregnancy. Hum. Reprod. , 23, 2775-81. PMID: 18694877 DOI.
Wilcox AJ, Dunson D & Baird DD. (2000). The timing of the "fertile window" in the menstrual cycle: day specific estimates from a prospective study. BMJ , 321, 1259-62. PMID: 11082086
Dunson DB, Colombo B & Baird DD. (2002). Changes with age in the level and duration of fertility in the menstrual cycle. Hum. Reprod. , 17, 1399-403. PMID: 11980771
Fukuda M, Fukuda K, Andersen CY & Byskov AG. (1996). Contralateral selection of dominant follicle favours pre-embryo development. Hum. Reprod. , 11, 1958-62. PMID: 8921071
Search Pubmed
Search Pubmed Now: Menstrual Cycle | corpus luteum | Images- menstrual cycle
Additional Images
Terms
- arteric corpora lutea -
- corpora - (singular corpus) a number of bodies.
- corpus - (plural corpora) body, aggregate, or mass.
- corpus heamorrhagicum - (corpus hemorrhagicum, bloody body) ovulating (Graffian) follicle immediately following ovulation, also described as an early corpus luteum.
- corpus luteum - (Latin, yellow body)
- hypermenorrhea - (heavy periods, excessive menstrual bleeding) Clinical term describing heavy bleeding during the menstrual cycle (menses). This excessive bleeding can be due to a number of different physiological and pathological conditions.
- metrorrhagia - Clinical term used to describe uterine bleeding at irregular intervals between the normal menstrual cycle periods (menses).
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Menstrual Cycle. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Menstrual_Cycle
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G