K12 Thalidomide
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
K12 Professional Development 2016
| Teacher Note |
|---|
| 50px|left]] This is currently only a draft designed to help K12 students understand the drug thalidomide.
Below are links to more detailed pages that are designed for university level students, that would also be valuable for teacher reference. These collapsible tables are additional information and the pages can also be printed out with these sections collapsed, so students do not see the contents. Note that this is currently a draft page still under development.
K12 Professional Development 2016 | K12 Professional Development 2014 |
What is the history of thalidomide?
Thalidomide is a drug that was introduced on to the market on October 1, 1957 in West Germany as "contergan". When taken, mainly in first world countries, early in a pregnancy children were born with limb and other defects. In the late 1950's and early 1960's these children became known as "thalidomide babies".
Originally intended to be prescribed as a general "sleeping pill", once its effects on suppressing sickness (nausea) were identified, it soon became a drug also prescribed to pregnant women to combat the symptoms associated with morning sickness.
| Teacher Note |
|---|
| Thalidomide is the "chemical name" of the drug, and like many other drugs released today was known by doctors and patients by its "commercial| or "trade". This commercial name can also differ between countries, even though the active chemical remains the same.
Thalidomide Commercial (trade) names
|
Morning sickness
| Around half to two-thirds of all pregnant women will experience nausea and vomiting of pregnancy, typically called "morning sickness". This occurs mainly in the first trimester of pregnancy (the first 3 months) due to pregnancy changes in hormone levels, blood pressure fluctuations, and changes in carbohydrate metabolism.
Therefore drugs which could "suppress" this feeling of nausea were, and still are, in high demand. |
<html5media width="352" height="240">https://www.youtube.com/embed/https://youtu.be/xg3zhW-4_B0</html5media> |
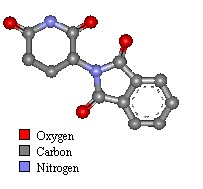
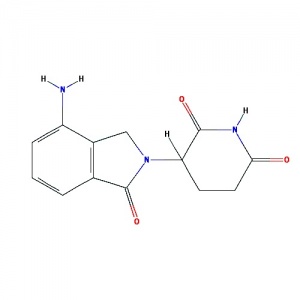
What does thalidomide look like?
| Teacher Note |
|---|
| Thalidomide two chemical format with the same molecular structure are called an "enantiomer isomer mix" (laevo+ and dextro-).
Both have the physical properties, but are non-superimposable mirror images of each other and rotate plane-polarized light (+/−) by equal amounts but in the opposite directions (laevo+ and dextro-). |
Who discovered the danger of this drug?
It was identified initially as dangerous by two doctors who saw serious abnormalities appearing more frequently in newborn children.
| Teacher Note |
|---|
| This is an example for students of inadequate drug testing and a lack of understanding of environmental effects on human development. This is often cited today as a reason to have significant testing of drugs before release and classification of drugs based upon their affects on development. |
Why do you think that this drug affected 1000's of children in mainly first word countries?
Why did this drug affect development?
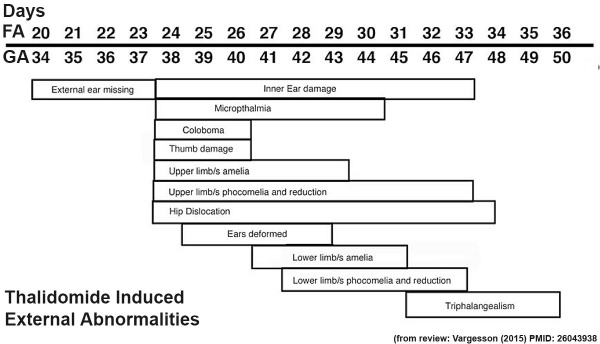
The drug generally affected the growth of blood vessels. It was also time specific in its effects.
|
|
|
|
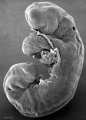
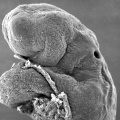
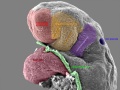
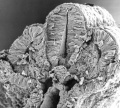
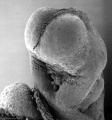
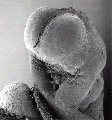
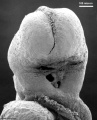
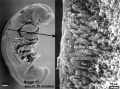
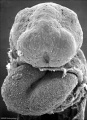
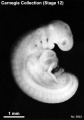
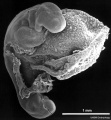
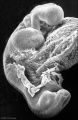
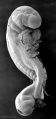
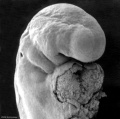
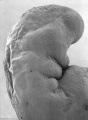
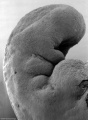
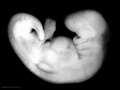
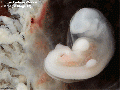
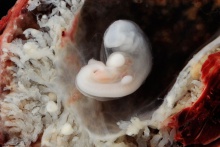
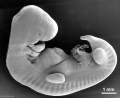
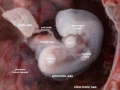
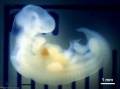
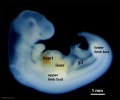
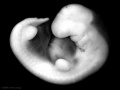
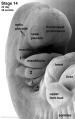
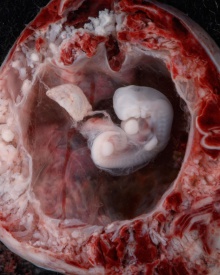
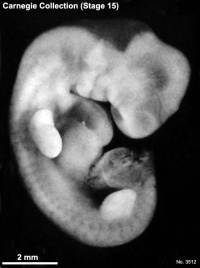
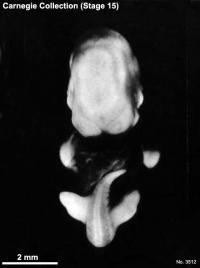
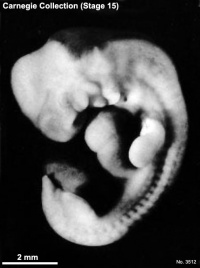
Carnegie stage 15
35 - 38 days
How do we test drugs today?
Problems associated with the historic introduction of drugs, like thalidomide, that a strict and lengthy process is required for each new drug.
| Drug Testing |
|---|
| Typical testing of new drug compound today involves a lengthy series of animal and human studies.
Animal studies Usually tested in at least two mammalian species (rats and guinea pigs) using both single and repeated doses. For determining reproductive effects, tests on both male and female animals with dosing begins 4 weeks prior to mating are conducted to determine effects on fertility in both sexes, on embryogenesis, and on fetal malformation. Human Clinical trials Following animal studies to determine dose, efficacy and apparent safety, human studies can commence. Clinical trials are carried out under very strict conditions, set by international regulatory bodies in agreement with the principles espoused in the Declaration of Helsinki. There are four phases to the trials.
After phase I to III the pharmaceutical company compiles all study data for independent assessment by government regulatory authorities in each country:
Regulatory Agency (MHRA) in the UK, and Health Products and Food Branch (HPFB) in Canada. |
| Declaration of Helsinki |
|---|
| The Declaration of Helsinki was developed by The World Medical Association (WMA) as a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data. The Declaration is intended to be read as a whole and each of its constituent paragraphs should not be applied without consideration of all other relevant paragraphs. It is widely regarded as the cornerstone document on human research ethics. It is named after the location of its initial adoption in Helsinki, Finland, in June 1964. |
How do we classify drugs?
Read the Australian Drug Categories, and then think about how would you classify thalidomide?
| Australian Drug Categories |
|---|
| Legal drugs are classified, usually by each country's appropriate regulatory body, on the safety of drugs during pregnancy. In Australia, the Therapeutic Goods Authority has classes (A, B1, B2, B3, C, D and X) to define their safety. In the USA, drugs are classified by the Food and Drug Administration (FDA) into classes (A, B, C, D, and X) to define their safety. (More? Australian Drug Categories)
|
Is thalidomide still being used?
(CC-5013, Revlimid) A derivative of thalidomide introduced in 2004, initially intended as a treatment for a cancer of blood plasma cells (multiple myeloma). Myeloma cells are made in the bone marrow. Each year in Australia around 1,500 people are diagnosed with myeloma.
Has also shown efficacy in the haematological disorders, myelodysplastic syndromes.
Cite this page: Hill, M.A. (2024, April 27) Embryology K12 Thalidomide. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/K12_Thalidomide
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G