Fetal Cells in Maternal Blood
| Embryology - 30 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
This current page is a general starting point for the topic of fetal cells and DNA in maternal blood as a new potential source for prenatal diagnosis. It turns out that the fetal cells in maternal blood are extremely difficult to isolate and analyse, so Non-Invasive Prenatal Testing (NIPT) of cffDNA is the more common method being employed.
As far back as 1997 fetal DNA was identified in maternal blood.[1] it has taken some time for technologies to develop to a point where now just the fetal genetic material can be analysed as a noninvasive prenatal test (NIPT).
The links below are to resources that give more specific information about some diagnostic techniques available at different stages of pregnancy. When abnormal development is identified this can be due to genetic, environmental, unknown causes or a combination of these effects. (More? Abnormal Development) These tests currently being developed allow detection of some genetic abnormalities. A trend in developed countries of an increasing maternal age, long associated with increased genetic abnormalities, has emphasised the need for good diagnostic low risk tests that allow informed decisions early in a pregnancy. (More? Genetic).
The topic of Preimplantation Genetic Screening (PGS) of blastocysts, associated with Assisted Reproductive Technologies (ART), is not discussed here.
- This Embryology site is a developmental educational resource, it does not provide specific clinical details, you should always refer to a health professional.
| Assisted Reproductive Technology | In Vitro Fertilization | Journal - Prenatal diagnosis
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Fetal Cells in Maternal Blood | Fetal DNA in Maternal Blood |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
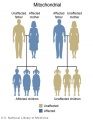
Microchimerism
Microchimerism (Mc) is a term used to describe when a usually small population of foreign cells or DNA harboured by one individual that derive from a genetically distinct individual. May occur in pregnancy when cells exchange between the fetus and mother or mother and fetus. Microchimerism may also be seen in twinning and transplants. Maternally acquired cells in the fetus may also persist postnatally.[9]
- Links: Twinning
Genetic Testing
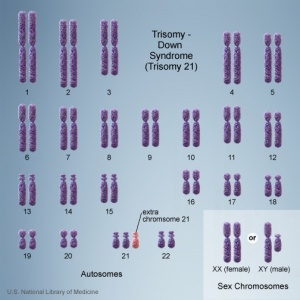
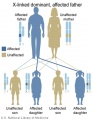
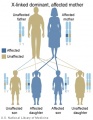
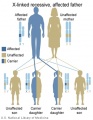
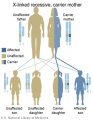
There are clinically more and more tests becoming available as we learn more about the genetic basis of some diseases. The most common diagnostic test relates to the current trend in an increasing maternal age, which has long been associated with an increase in genetic abnormalities, the most frequent of these is trisomy 21 or Down syndrome.
Inheritance Genetics
- Links: Genetic risk maternal age | Trisomy 21
Australia
A recent publication from NHMRC Medical Genetic Testing: information for health professionals (2010). This paper covers background information on all types of genetic tests, not just those associated with prenatal diagnosis.
Types of genetic tests
- Somatic cell genetic testing involves testing tissue (usually cancer) for non-heritable mutations. This may be for diagnostic purposes, or to assist in selecting treatment for a known cancer.
- Diagnostic testing for heritable mutations involves testing an affected person to identify the underlying mutation(s) responsible for the disease. This typically involves testing one or more genes for a heritable mutation.
- Predictive testing for heritable mutations involves testing an unaffected person for a germline mutation identified in genetic relatives. The risk of disease will vary according to the gene, the mutation and the family history.
- Carrier testing for heritable mutations involves testing for the presence of a mutation that does not place the person at increased risk of developing the disease, but does increase the risk of having an affected child developing the disease.
- Pharmacogenetic testing for a genetic variant that alters the way a drug is metabolised. These variants can involve somatic cells or germline changes. Even if these variants are heritable (that is germline changes), the tests are usually of relevance to genetic relatives only if they are being treated with the same type of medication.
USA
A new site developed by NIH "GeneTests" provides medical genetics information resources available at no cost to all interested persons. It contains educational information, a directory of genetic testing laboratories and links to other databases such as OMIM.
- Links: GeneTests | Medline Plus - Genetic Testing
Comparative Genomic Hybridization
This new test under development is based upon microarray-based comparative genomic hybridization (array CGH).
All fetal cells should have complete copies of maternal and paternal genomes. The test compares regions of fetal DNA that deviate from this "pattern" due to either too much or too little DNA, alterations reflect regions of the genome that are either copied or deleted. These genetic changes may therefore cause disease.
Ethics of Testing
Major developmental abnormalities detected early enough can be resolved far more easily than those discovered late in a pregnancy.
What are the ethical questions that are raised by prenatal testing? Future individual rights or parents rights? But what about diseases, like Huntington's, where a diagnostic test can be made but there are no current treatments for the postnatal (95% of cases adult onset) disease?
Huntington's disease
Guidelines for the molecular genetics predictive test
- Recommendation 2.1 "the test is available only to individuals who have reached the age of majority."
- Recommendation 7.2 "the couple requesting antenatal testing must be clearly informed that if they intend to complete the pregnancy if the fetus is a carrier of the gene defect, there is no valid reason for performing the test."
References
- ↑ Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW & Wainscoat JS. (1997). Presence of fetal DNA in maternal plasma and serum. Lancet , 350, 485-7. PMID: 9274585 DOI.
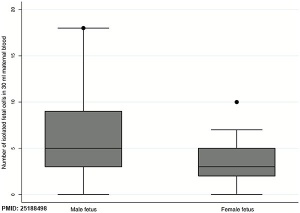
- ↑ 2.0 2.1 Schlütter JM, Kirkegaard I, Petersen OB, Larsen N, Christensen B, Hougaard DM, Kølvraa S & Uldbjerg N. (2014). Fetal gender and several cytokines are associated with the number of fetal cells in maternal blood--an observational study. PLoS ONE , 9, e106934. PMID: 25188498 DOI.
- ↑ Van De Looij A, Singh R, Hatt L, Ravn K, Jeppesen LD, Nicolaisen BH, Kølvraa M, Vogel I, Schelde P & Uldbjerg N. (2020). Do fetal extravillous trophoblasts circulate in maternal blood postpartum?. Acta Obstet Gynecol Scand , , . PMID: 32323316 DOI.
- ↑ Taneja PA, Prosen TL, de Feo E, Kruglyak KM, Halks-Miller M, Curnow KJ & Bhatt S. (2017). Fetal aneuploidy screening with cell-free DNA in late gestation. J. Matern. Fetal. Neonatal. Med. , 30, 338-342. PMID: 27124739 DOI.
- ↑ Bianchi DW, Parsa S, Bhatt S, Halks-Miller M, Kurtzman K, Sehnert AJ & Swanson A. (2015). Fetal sex chromosome testing by maternal plasma DNA sequencing: clinical laboratory experience and biology. Obstet Gynecol , 125, 375-82. PMID: 25568992 DOI.
- ↑ Norwitz ER & Levy B. (2013). Noninvasive prenatal testing: the future is now. Rev Obstet Gynecol , 6, 48-62. PMID: 24466384
- ↑ Kitzman JO, Snyder MW, Ventura M, Lewis AP, Qiu R, Simmons LE, Gammill HS, Rubens CE, Santillan DA, Murray JC, Tabor HK, Bamshad MJ, Eichler EE & Shendure J. (2012). Noninvasive whole-genome sequencing of a human fetus. Sci Transl Med , 4, 137ra76. PMID: 22674554 DOI.
- ↑ Guo X, Bayliss P, Damewood M, Varney J, Ma E, Vallecillo B & Dhallan R. (2012). A noninvasive test to determine paternity in pregnancy. N. Engl. J. Med. , 366, 1743-5. PMID: 22551147 DOI.
- ↑ Eikmans M, van Halteren AG, van Besien K, van Rood JJ, Drabbels JJ & Claas FH. (2014). Naturally acquired microchimerism: implications for transplantation outcome and novel methodologies for detection. Chimerism , 5, 24-39. PMID: 24762743
Articles
Liston R, Sawchuck D & Young D. (2007). Fetal health surveillance: antepartum and intrapartum consensus guideline. J Obstet Gynaecol Can , 29, S3-56. PMID: 17845745
Search Pubmed
Search Pubmed: Fetal Cells in Maternal Blood | Fetal DNA in Maternal Blood
- ART - Assisted Reproductive Technology a general term to describe all the clinical techniques used to aid fertility.
- blastomere biopsy - An ART preimplantation genetic diagnosis technique carried out at cleavage stage (day 3), excluding poor quality embryos, detects chromosomal abnormalities of both maternal and paternal origin. May not detect cellular mosaicism in the embryo.
- blastocyst biopsy - An ART preimplantation genetic diagnosis technique carried out at blastocyst stage (day 4-5), removes several trophoblast (trophoderm) cells, detects chromosomal abnormalities of both maternal and paternal origin and may detect cellular mosaicism.
- cell-free fetal deoxyribonucleic acid - (cfDNA) refers to fetal DNA circulating and isolated from the plasma portion of maternal blood. Can be performed from GA 10 weeks as a first-tier test or as a second-tier test, with women with increased probability on combined first trimester screening offered cfDNA or diagnostic testing.
- false negative rate - The proportion of pregnancies that will test negative given that the congenital anomaly is present.
- false positive rate - The proportion of pregnancies that will test positive given that the congenital anomaly is absent.
- free β human chorionic gonadotrophin - beta-hCG subunit of hCG used as a diagnostic marker for: early detection of pregnancy, Trisomy 21, spontaneous abortion, ectopic pregnancy, hydatidiform mole or choriocarcinoma.
- multiples of the median - (MoM) A multiple of the median is a measure of how far an individual test result deviates from the median and is used to report the results of medical screening tests, particularly where the results of the individual tests are highly variable.
- negative predictive value - The probability that a congenital anomaly is absent given that the prenatal screening test is negative.
- Non-Invasive Prenatal Testing - (NIPT) could refer to ultrasound or other imaging techniques, but more frequently used to describe analysis of cell-free fetal DNA circulating in maternal blood.
- polar body biopsy - (PB biopsy) An ART preimplantation genetic diagnosis technique that removes either the first or second polar body from the zygote. As these are generated by oocyte meiosis they detects chromosomal abnormalities only on the female genetics.
- positive predictive value - The probability that a congenital anomaly is present given that the prenatal screening test is positive.
- pre-implantation genetic diagnosis - (PGD, pre-implantation genetic screening) a diagnostic procedure for embryos produced through Assisted Reproductive Technology (ART, in vitro fertilisation, IVF) for genetic diseases that would generate developmental abnormalities or serious postnatal diseases.
- prenatal screening sensitivity - (detection rate) The probability of testing positive on a prenatal screening test if the congenital anomaly is present.
- prenatal screening specificity - The probability of testing negative on a prenatal screening test if the congenital anomaly is absent.
- quadruple test (maternal serum testing of a-fetoprotein Template:AFP, free B-hCG or total hCG, unconjugated estriol, and inhibin A) is a fetal chromosomal anomaly test usually carried out later in pregnancy (GA 14 to 20 weeks).
- single nucleotide polymorphisms - (SNPs) the variation in a single DNA nucleotide that occurs at a specific position in the genome.
- triple test - (maternal serum testing of a-fetoprotein Template:AFP, free B-hCG or total hCG, and unconjugated estriol) is a fetal chromosomal anomaly test usually carried out later in pregnancy (GA 14 to 20 weeks).
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Australian and New Zealand
- Royal Australian College of General Practitioners Australian Family Physician (2014)
- College of Obstetricians and Gynaecologists Intrapartum Fetal Surveillance Clinical Guidelines - Second Edition (June 2011)
- Canada - Society of Obstetricians and Gynaecologists of Canada
- USA
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 30) Embryology Fetal Cells in Maternal Blood. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Fetal_Cells_in_Maternal_Blood
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G