Book - Text-Book of Embryology 9
| Embryology - 7 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bailey FR. and Miller AM. Text-Book of Embryology (1921) New York: William Wood and Co.
- Contents: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of Connective Tissues and the Skeletal System
All the connective or supporting tissues of the body, except neuroglia, are derived from the mesoderm. This Goes not imply, however, that all the mesoderm is transformed into connective tissues; for such structures as the endothelium of the blood vessels and lymphatic vessels, probably blood itself, the epithelium lining the serous cavities, smooth and striated muscle, and a part of the epithelium of the urogenital system are derived from mesoderm.
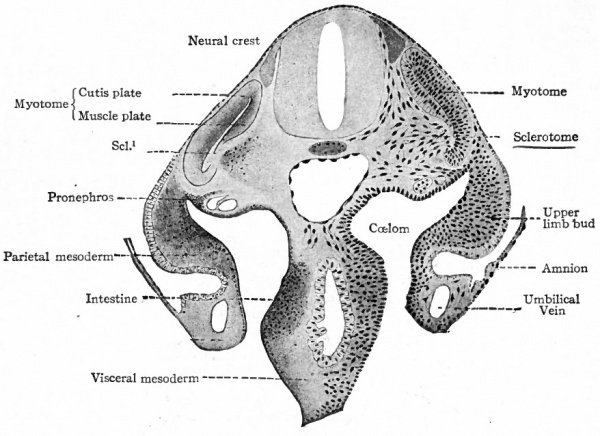
The origin of the mesoderm itself has been discussed elsewhere (p. 93). In this connection it is sufficient to recall that it is situated between the ectoderm and entoderm and consists of several layers of closely packed cells (Fig. ioo). The axial portion in the neck and body regions becomes differentiated into the mesodermic somites. At the same time a cleft (the coelom) separates the more peripheral portion into a parietal and a visceral layer (Figs. 101 and 103). In the head region where, in the higher animals, there is little or no indication of somites and ccelom, the mesoderm simply fills in the space between the ectoderm and entoderm (Fig. 102). Portions of the mesoderm in all these regions are destined to give rise to connective tissues. Each mesodermic somite! soon becomes differentiated into three parts the sclerotome, cutis plate and ( myotome (Fig. 104). Of these, only the sclero tome and cutis plate are directly concerned in the formation of connective tissues, the myotomes giving rise to striated voluntary muscle. The sclerotomes are destined to give rise to the vertebrae and other forms of connective tissue in their neighborhood, the cutis plates to a part, at least, of the corium of the skin. The parietal and visceral layers of the mesoderm (excepFthe mesothelium lining the ccelom) and the mesoderm of the head region are destined to give rise to the various types of connective tissue forming parts of the other organs of the body.
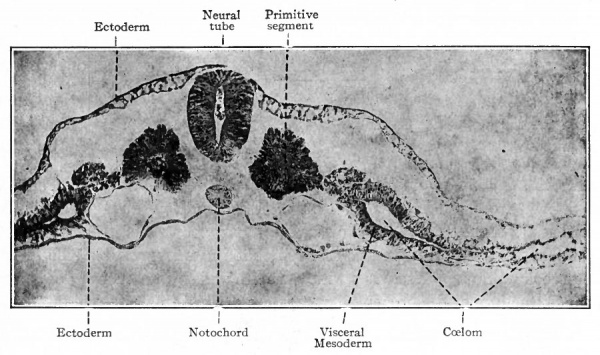
Fig. 101. Transverse section of chick embryo (2 days' incubation). Photograph. The parietal mesoderm (lying above the coelom) is not labeled. The two large vessels under the primitive segments are the primitive aortae. Spaces separating germ layers are clue to shrinkage.
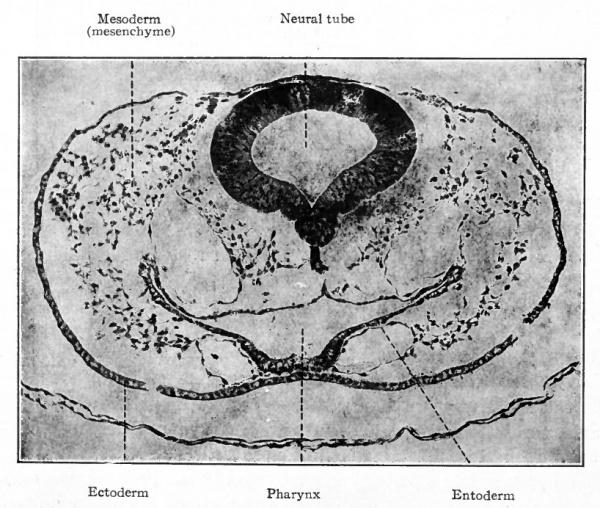
Fig. 102. Transverse section through head region of chick embryo of 42 hours' incubation. Photograph.
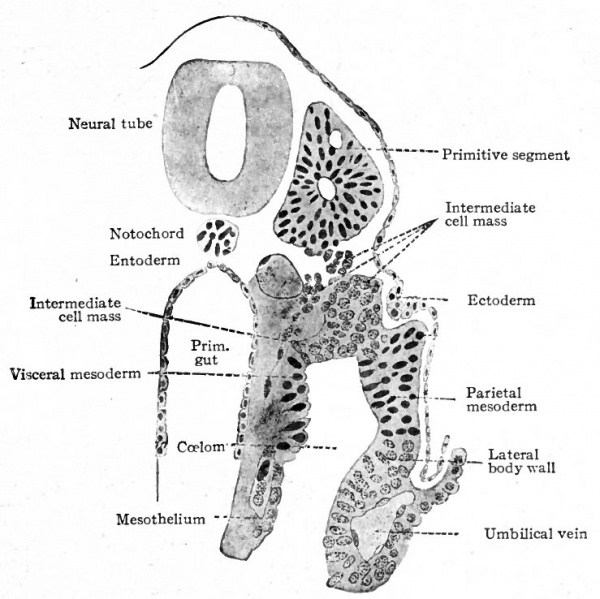
Fig. 103. Transverse section of human embryo with 13 primitive segments. Section taken through the 6th segment. Kollmann.
Histogenesis
The sclerotomes and cutis plates at first constitute parts of the mesoorermic somites, and are composed of epithelial-like cells with little intercellular substance. The intercellular substance gradually increases in amount so that the cells become more widely separated from one another, at the same time assuming oval or spindle shapes and then irregular branching forms (Fig. 106). The rest of the mesoderm, except the mesothelium, also undergoes a similar transformation so that structurally its cells are indistinguishable from those derived from the sclerotomes and cutis plates.
Fig. 104. Transverse section of human embryo of the 3rd week. Scl. 1 , Break in myotome at point where sclerotome is closely attached. Kollmann.
Fig. 105. Three primitive segments from sagittal section of human embryo of the 3rd week. Kollmann.
Thus the mesoderm at this stage is composed of irregular, branching cells, with a relatively large amount of homogeneous intercellular substance filling the interstices. The branches, or protoplasmic processes, of each cell anastomose freely with those of other cells in the immediate vicinity.
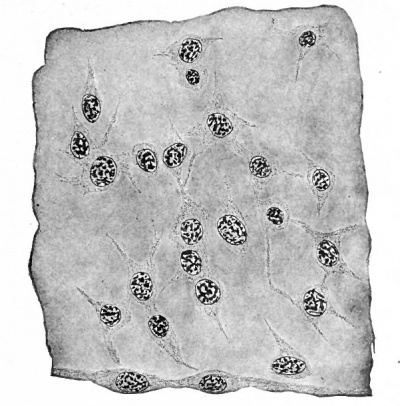
Fig. 106. Mesenchymal tissue from somatopleure of a 5 mm human embryo. Mesothelium is shown along lower border of figure.
In this manner a syncytium is formed to which the term mesenchyme is applied (Fig. 106). The mesenchyme itself lacks specialization, being what is known as an indifferent tissue, but it constitutes the structural basis upon which all the connective tissues of the adult body are built; all the forms of connective tissue (except neuroglia) develop from it.
That intercellular substance is derived originally from the cell can scarcely be denied. All the cells of the organism are derived from the fertilized ovum. As soon as two or more cells are formed by segmentation of the ovum, they are either simply in apposition or else they are united by something in the nature of a "cement" substance which must have been derived from the cells themselves. In the mesenchymal tissue this intercellular ground substance is a prominent feature, and probably represents in part nutritive materials and in part the products of cell activity.
Fibrils and Fibers
The least differentiated and perhaps the least specialized tissue derived from mesenchyme is reticular tissue, such as that found in the lymph nodes and spleen. In the peripheral part of the cytoplasm, or exoplasm, of the mesenchymal cells and their processes there arise delicate fibrils, often extending from one cell to another, which probably represent specialized parts of the spongioplasm. These fibrils maintain their intracellular position instead of becoming separated from the parent cytoplasm, so that the reticular tissue retains a marked resemblance in form to the original mesenchyme.
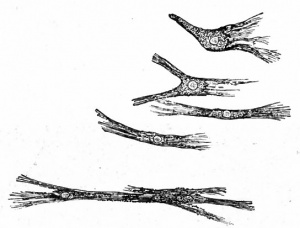
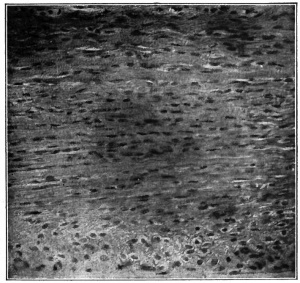
Fig. 108. Connective tissue (mesenchymal) cells from larval salamander. Flemming.
The first step in the development of the true fibrillar forms of connective tissue from mesenchyme is the formation of fibrils and fibers. While it has been held by some investigators that the fibrils arise in and from the homogeneous intercellular substance, the best substantiated view is that they arise within and from the cytoplasm of the mesenchymal cells (Figs. 107 and 108). They then become separated from the cytoplasm and lie free in the " ground" substance in bundles (fibers). These fibrillated fibers are collaginous in character. Elastic fibers, while not fibrillated, probably have a similar origin. This first step in development gives rise to a loose, delicate tissue in the embryo, known as embryonal connective tissue, from which all the adult forms, except reticular tissue, develop.
The areolar tissue of the adult retains many of the general characters of embryonal connective tissue. The fibers, both collaginous and elastic, are loosely arranged and extend in all directions. The cells (fibroblasts) are fewer, however, and while they are characterized by irregular, branching forms it is not known whether their processes anastomose.
In any fibrous tissue, such as areolar, or the denser forms (fascia, tendons, ligaments), the structure depends upon the secondary arrangement of the fibers and not upon any peculiarity of origin. In all these forms the fibers have the same origin, but in the denser fascia, tendons and ligaments they become arranged in parallel lines (Fig. 109).
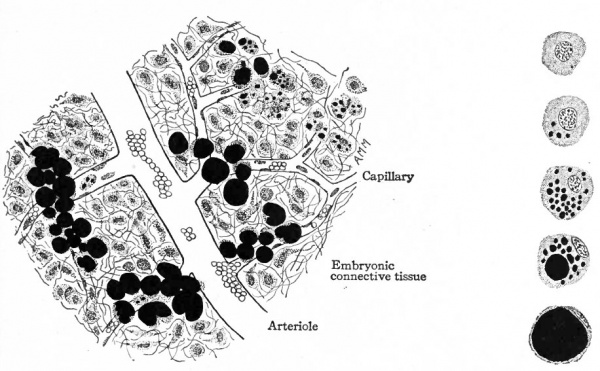
Adipose Tissue. Adipose tissue is a form of connective tissue in which the fatty element replaces to a great extent the cytoplasm in many of the embryonic connective tissue cells. It always develops in close relation to blood vessels, and first appears in the axilla and groin about the thirteenth week. It is formed in other places at later periods, even during adult life, but the mode of development is always the same. In some of the cells in the neighborhood of small blood vessels minute droplets of fat are deposited. The origin of the fat is not known. The droplets become larger, other smaller ones appear, and finally all of them coalesce to form a single large drop which practically fills the cell. The result of this is that the remaining cytoplasm is pushed outward and forms a sort of pellicle around the fat. The nucleus also is crowded outward and comes to lie flattened in the pellicle of cytoplasm (Fig. 111). At the same time the whole fat cell increases in size and forms a relatively large structure.
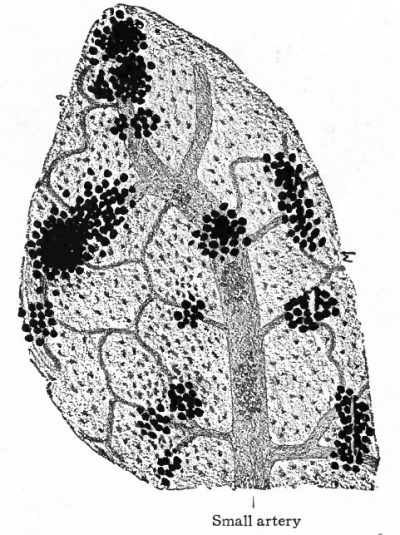
Fig. 110. Developing fat from subcutaneous tissue of pig embryo 5 inches long. Small artery breaking up into capillary network' groups of fat cells developing in embryonic connective tissue.
Fat cells usually develop in groups or masses around blood vessels (Fig. no). The neighboring groups gradually enlarge and approach each other, but do not fuse, thus leaving more or less fibrous connective tissue between them, which constitutes the interlobular tissue seen in adult adipose tissue. Among the individual cells in a lobule there is also a small amount of fibrous tissue present. From the mode of development a small artery usually affords the blood supply for each lobule.
Cartilage
In the different kinds of cartilage the matrix probably represents a modification of tne "ground substance" of the original embryonic tissue. The fibers in the matrix are probably derived from the cells in the same manner as the fibers in the fibrillar forms of connective tissue (Fig. 112).
Osseous Tissue
Here again the basis for development is embryonic connective tissue, although in one type of development cartilage precedes the bone. Two types of ossification are recognized intramembranous and intracartilaginous or endochondral. Intramembranous ossification calcium salts are deposited in ordinary embryonic connective tissue. In intracartilaginous ossification hyalin cartilage first develops in the same general shape as the future bone and the calcium salts are afterward deposited within the mass of cartilage. It is customary to speak also of another type of ossification subperiosteal in which the calcium salts are deposited under the periosteum.
Fig. 111. Developing fat from subcutaneous tissue of pig embryo 5 inches long. Fat (stained black) developing in embryonic connective tissue cells. At the right are five individual cells showing stages of development from an embryonic cell to an adult fat cell.
Intramembranous Ossification
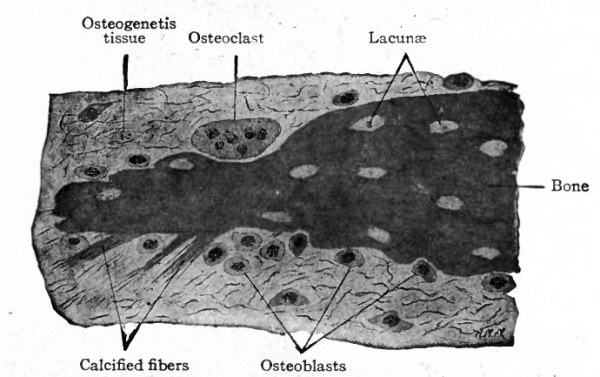
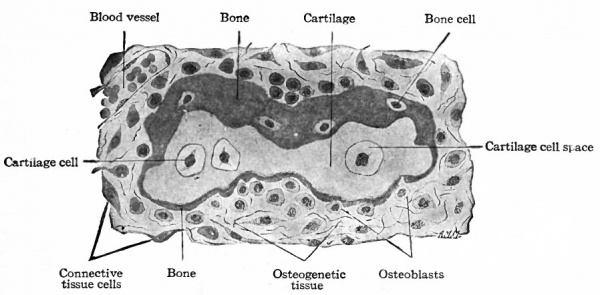
This is the type of ossification by which many of the flat bones of the skull and face are formed. The region in which these bones are to develop consists of embryonic connective tissue. At certain points in this region bundles of connective tissue fibers become impregnated with calcium salts. Such areas are known as calcification centers. In each of these areas the cells increase in number, the tissue becomes very vascular and some of the cells, becoming more or less round or oval, with distinct nuclei and a considerable amount of cytoplasm, arrange themselves in single, fairly regular rows along the bundles of calcined fibers. The differentiated cells are known as osteoblasts (bone formers) , and the whole tissue is now known as osteo genetic tissue. Under the influence of the osteoblasts a thin layer of calcium salts is deposited between the osteoblasts and the calcified fibers. In this way the first true bone is formed, and the calcification center becomes an ossification center. Successive layers or lamellae of calcium salts are laid down and some of the osteoblasts become enclosed between the lamellae to form the bone cells (Figs. 113 and 1 14). The spaces in which the bone cells lie are the lacuna. At the same time the fibers also are enclosed within the bone and give it its characteristic fibrous structure (Fig. 114).
Fig. 113. Vertical section through frontal bone of human foetus of 4 months. (Intramembranous ossification.) Photograph.
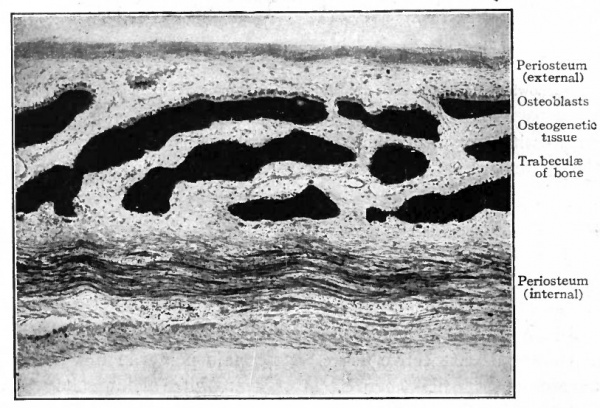
Such a process results in the formation of irregular, anastomosing trabeculae of bone. The spaces among the trabeculae are known as primary marrow spaces- and contain osteogenetic tissue (Fig. 113). This type of bone, consisting of irregular, anastomosing trabeculae and enclosed marrow spaces, is known as spongy bone. The spongy bone thus formed is covered on-ats outer side by a layer of connective tissue which from its position is called the periosteum (Fig. 113), and which represents a part of the original embryonic connective tissue membrane in which the bone was laid down. During its development the periosteum becomes an exceedingly dense fibrous membrane which is closely applied to the surface of the bone.
Fig. 114. From vertical section through parietal bone of human foetus of 4 months. Bone cells not shown in lacunae, (Intramenibranous ossification.)
In a growing embryo, provision must be made for increase in the size of the cranial cavity to accommodate the growing brain. This is accomplished in the following manner : On the inner surface of the newly formed bone, large multinuclear cells appear, which are known as osteoclasts (bone destroyers). The osteoclasts are unusually large cells with a large number of nuclei and abundant cytoplasm, and in sections can be seen lying in depressions in the bone Howslip's lacuna (Fig. 114). Whether they are the specific agents in dissolution of bone has been questioned (Arey). While the destruction of bone is going on on the inner surface, new bone is being formed on the outer surface, especially under the periosteum where the osteoblasts are most numerous. Thus the layer of bone gradually comes to lie farther and farther out and the cranial cavity is enlarged. So long as the cranial cavity continues to enlarge the new bone is of the spongy variety, but toward the end of development the trabeculae become thicker and finally come together to form the compact bone characteristic of the roof of the skull. The fact that the new bone laid down during the enlargement of the cranial cavity is laid down under the periosteum has led to the term subperiosteal ossification. The process is essentially the same as in the original intramembranous ossification.
Fig. 115. Longitudinal section of one of the metatarsal bones of a sheep embryo. (Intracartilaginous ossification.)
Intracartilaginous Ossification
(The modern term is endochondral ossification)
In this type of ossification hyalin cartilage is first formed in a shape which corresponds very closely to the shape of the future bone. For example, the femur is first represented by a piece of hyalin cartilage which develops from the original embryonic connective tissue. On the surface of the cartilage a membrane of dense fibrous connective tissue, known as the perichondrium, develops (Fig. 115). In most cases, ossification begins about the middle of the piece of cartilage, corresponding to the middle of the shaft of a long bone (Fig. 115). The cell spaces enlarge and in some cases the septa of matrix between the enlarged spaces break down, so that several cells may lie in one space. The cell spaces radiate from a common center, but a little later they come to lie in rows parallel with the long axis of the mass of cartilage. During these early changes lime salts are deposited in the matrix of the cartilage in this region, and the portion so involved is known as a calcification center.
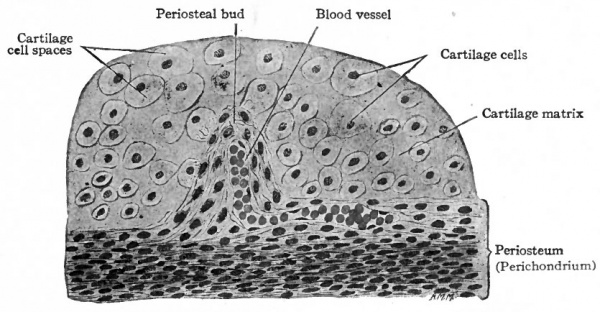
Fig. 116. From section of one of the tarsal bones of a pig embryo. Showing periosteal bud pushing into the cartilage at the ossification center. (Intracartilaginous ossification.)
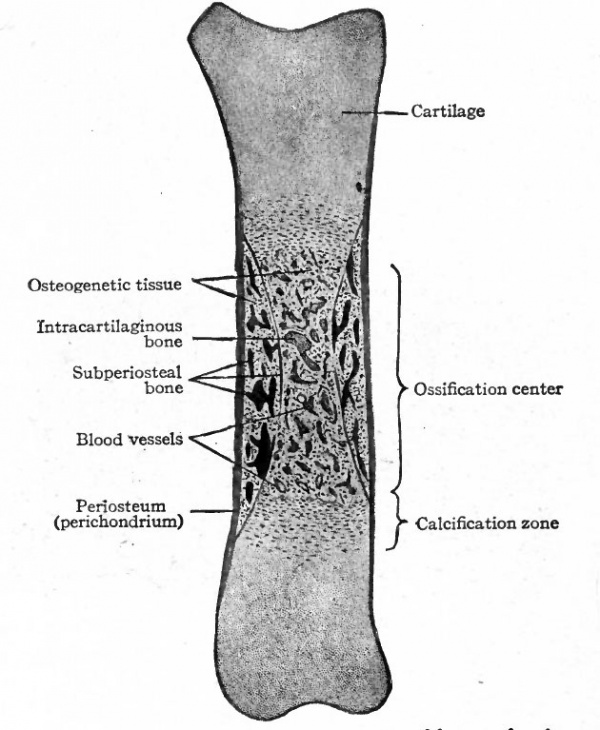
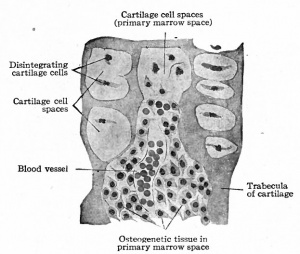
So far the process is preparatory to actual bone formation. Then small blood vessels from the perichondrium (periosteum) grow into the cartilage, carrying with them some of the embryonic connective tissue. These little ingrowths of connective tissue and blood vessels are known as periosteal buds (Fig. 1 1 6). The septa between the enlarged cartilage cell spaces break down still further, forming still larger spaces into which the periosteal buds grow. Many of the connective tissue cells are transformed into osteoblasts oval or round cells with distinct nuclei and a considerable amount of cytoplasm and with the fibers and blood vessels constitute osteogenetic tissue (Fig. 117). The cartilage cells in this region disintegrate and disappear, and the cavity formed by the coalescence of the cell spaces constitutes the primary marrow cavity (Fig. 117). From the primary marrow cavity osteogenetic tissue pushes in both directions toward the ends of the cartilage. The transverse septa between the enlarged cartilage cell spaces break down, leaving a few longitudinal septa which form the walls of long anastomosing channels which are continuous with the primary marrow cavity. The osteoblasts arrange themselves in rows along the septa of calcined cartilage and a thin layer or lamella of calcium salts is deposited between them and the cartilage. Successive lamellae are deposited in the same manner and some of the osteoblasts become enclosed to form bone cells (Fig. 118). The cartilage in the center gradually disappears. This region where bone formation is going on is known as an ossification center (Fig. 115) and the irregular anastomosing trabeculae of bone with the enclosed marrow spaces constitute primary spongy bone.
From this time on, ossification gradually progresses toward each end of the cartilage, and at the same time a special modification of the cartilage precedes it. Nearest the ossification center the cartilage cell spaces become enlarged and arranged in rows and contain cartilage cells in various stages of disintegration. Some of the septa break down, leaving larger, irregular spaces; the remaining septa become calcified (Fig. 115). Passing away from the center of ossification, there is less enlargement of the cell spaces and they have a tendency to be arranged in rows transverse to the long axis of the cartilage; there is also a lesser degree of calcification. The region of modified cartilage at each end of the ossification center passes over gradually into ordinary hyalin cartilage and is known as the calcification zone. It always precedes the formation of bone as the latter process moves toward the end of the cartilage (Fig. 115).
Along with the type of ossification just described subperiosteal ossification also occurs (Fig. 115). Beneath the periosteum (perichondrium) is a layer of connective tissue the cells of which are transformed into osteoblasts. They deposit layers of calcium salts on the surface of the cartilage in the same manner as around the trabeculae inside the cartilage.
The transformation of the spongy bone into compact bone is peculiar in that the former is dissolved and then replaced by new bone. Whether this dissolution occurs through the agency of the large multinucleated cells known as osteoclasts is not certain. By the process of dissolution the marrow spaces are increased in size and are known as Haversian spaces. Within these spaces new bone is then deposited layer upon layer, under the influence of the osteoblasts, until the Haversian spaces are reduced to narrow channels, the Haversian canals. The layers of bone are the Haversian lamella. The interstitial lamella in compact bone have two possible origins. They may be the remnants of certain lamellae of the original spongy bone which were not removed in the enlargement of the primary marrow spaces, or they may be parts of early formed Haversian lamellae which were later more or less replaced by other Haversian lamellae.
Fig. 118. From same section as Fig. 115 Showing bone deposited around one of the trabeculae of cartilage. (Intracartilaginous ossification.)
Carey, in his recent studies on certain mechanical phases of development, concludes " that cartilage and bone are not self -differentia ted, nor are they self-crystallized products," but represent " cellular responses to the varying intensity of the stresses and strains produced by resistance (pressure) counteracting the growth" of the skeleton in its blastemal stage, that is, while the cells are closely compacted prior to the appearance of the specific tissue. In his analysis of the femur, Koch has concluded that the "normal external form and internal architecture of the human femur results from an adaptation of form to the normal static demands, or normal function of the bone." It would appear therefore that in the development of bone mechanical factors play an essential part not only in the formation of the bone itself but also in the establishment of its form and internal structure.
GROWTH OF BONES. The way in which the cranial cavity enlarges has been described on page 139. While the process of enlargement is going on, the individual bones increase in size principally by the addition of new bone along their edges.
Intracartilaginous bones grow both in diameter and in length. It has already been stated that the primary spongy bone formed in cartilage is dissolved and that new bone is deposited under the periosteum. This naturally brings about an enlargement of the primary marrow cavity and at the same time an increase in the diameter of the bone as a whole. From this it is obvious that the compact bone of the shaft of a long bone is of subperiosteal origin, the intracartilaginous bone having been completely absorbed.
Fig. 119. Diagram representing growth in diameter of a long bone. from Flour ens.
The fact that the osseous tissue bordering the marrow cavity is absorbed and that new bone is deposited under the periosteum can be quite clearly demonstrated. A young growing animal is fed for a few weeks on madder, which colors all the bone formed during that time a distinct red. If the animal is then killed and sections made of the long bones, the outer part of the latter will appear a distinct red. Another growing animal is fed on madder for a few weeks, then allowed to live a few weeks longer without madder. Then if it is killed and sections made of the bones, the red bone is found to be covered with a layer of uncolored bone which was deposited after the madder feeding had been stopped. If a young growing animal is fed on madder for a time and then allowed to live long enough without madder, the red bone will be found lining the marrow cavity. (See Fig. 119.)
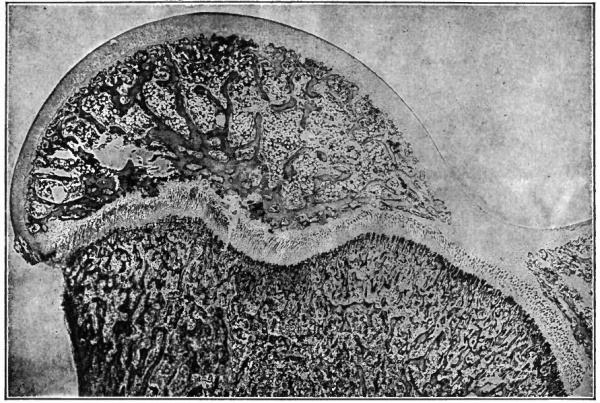
Growth in length of the long bones takes place in a different manner. The primary center of ossification is situated near the middle of the piece of cartilage, and ossification proceeds in both directions toward the ends of the cartilage to produce the diaphysis or shaft of the bone. In each end of the cartilage there appears a secondary center from which ossification proceeds in all directions to produce the epiphysis. Between the shaft and epiphysis a disk of cartilage remains, and here, so long as the bone is growing, new cartilage continues to be formed. At the same time new bone is being formed in the new cartilage, principally in the part next the shaft. This produces an elongation of the shaft, the two epiphyses being carried farther and farther apart, and consequently a lengthening of the bone as a whole. When the bone reaches the required length, the cartilage disk diminishes and finally is wholly replaced by bone, being represented in the adult only by the epiphyseal line. (See Fig. 120.) MARROW. The forerunner of marrow is the osteogenetic tissue in the primary marrow spaces, which in turn is derived from embryonic connective tissue (Fig. 117). During the development of bone, great numbers of osteoblasts are constantly being differentiated from the connective tissue cells and many of these ultimately become bone cells. When development ceases, osteoblasts cease to become differentiated. Marrow is one of the chief centers of blood cell formation in later foetal life, and in the adult is normally probably the only source of erythrocytes. An account of blood cell formation will be found in the section on "Haemopoiesis." The myeloblasts, which are probably identical with or at least closely allied to the primitive blood cells (haemoblasts), by acquiring certain types of granules in the cytoplasm become neutrophilic, acidophilic or basophilic myelocytes. During development two types of giant-cells (myeloplaxes) appear in the marrow. According to Jordan one of these is haemogenic and the other osteolytic. The former originates from enlarged hasmoblasts and may be regarded as representing centers of intense haemopoiesis, giving rise to erythrocytes. The osteolytic giant-cells (osteoclasts) arise more frequently from fused portions of the marrow reticulum, less often from fused osteoblasts, and are always multinucleated. Arey maintains in his more recent work that the so-called osteoclasts usually arise by fusion of old and basophilic osteoblasts, the cytoplasm of the syncytial mass becoming acidophilic. Arey also holds that this type of giant-cell is not a specific agent in bone resorption. In young marrow there is little or no fat present, but in later life many of the connective tissue cells are transformed into fat cells (p. 136), so that these form the greater part of the marrow. Such a process occurs most extensively in the shaft of the long bones and gives rise to "yellow" marrow. In the heads of the long bones, in the ribs, and in the short bones the marrow retains its earlier character and is known as "red" marrow.
Fig. 120. Longitudinal section from head of femur of young dog. Photograph. The head of the femur is shown in the upper part of the figure, the end of the shaft in the lower part. Between the two the lighter line represents the cartilage between the primary center of ossification (shaft) and the secondary center (epiphysis, head), and marks the site of the epiphyseal line. The lighter portion covering the head represents the cartilage bordering the joint cavity.
The Development of the Skeletal System
The Axial Skeleton
The Notochord
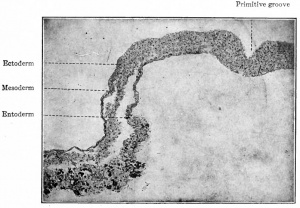
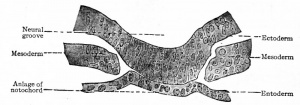
The notochord (chorda dorsalis) constitutes the primitive axial skeleton of all Vertebrates, yet it differs from the other skeletal elements in that it is a derivative of the entoderm. In man it is merely a transient structure and disappears early in foetal life, leaving but a slight trace of itself in the intervertebral disks. In embryos of 2-3 mm. the cells of the entoderm just ventral to the neural groove become slightly differentiated (Fig. 121) and then form a groove with a ventral concavity. The groove closes in, becomes constricted from the parent tissue (entoderm) and lies just ventral to the neural tube, where it soon becomes surrounded by mesodermal tissue. This structure is the notochord and constitutes a solid, cylindrical cord of cells extending from a point just caudal to the hypophysis to the caudal extremity of the embryonic body. In embryos of 17-20 mm. the mesodermal tissue around the notochord becomes modified to form the chorda! sheath. On account of its position the notochord naturally becomes embedded in the developing vertebral column, extending through the bodies of the vertebrae and the intervertebral disks. The cells are at first of an epithelial nature (Fig. 121), but those within the vertebral bodies become vacuolated and broken up into irregular, multinuclear masses which then disappear. The cord is thus first interrupted in the vertebrae, leaving only the segments within the intervertebral disks. Later these segments also undergo degenerative changes, but persist as the so-called pulpy nuclei.
While the notochord is morphologically the forerunner of the axial skeleton, and persists as a whole in Amphioxus, and in part in Fishes and Amphibia, in the higher forms it is almost exclusively an embryonic structure with little or no functional significance. It differs in origin from the true skeletal elements and becomes involved with them only to disappear as they develop.
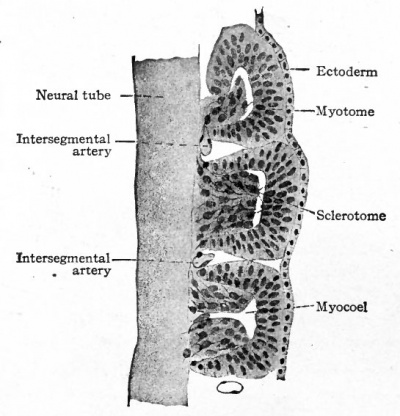
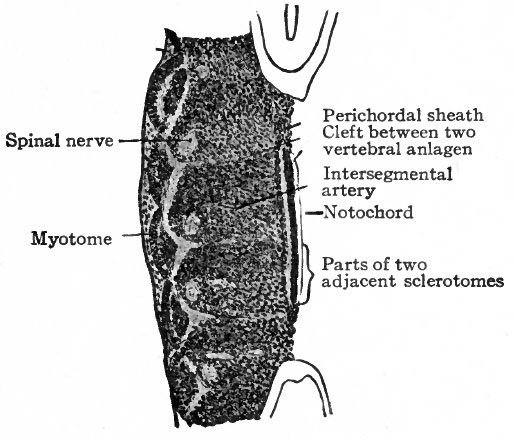
Fig. 122. Five myotomes and sclerotomes from sagittal section of human embryo of 5 mm. Bardeen.
- Each sclerotome is differentiated into a looser cephalic part and a denser caudal part, the two being separated by a cleft (fissure of von Ebner).
The Vertebrae
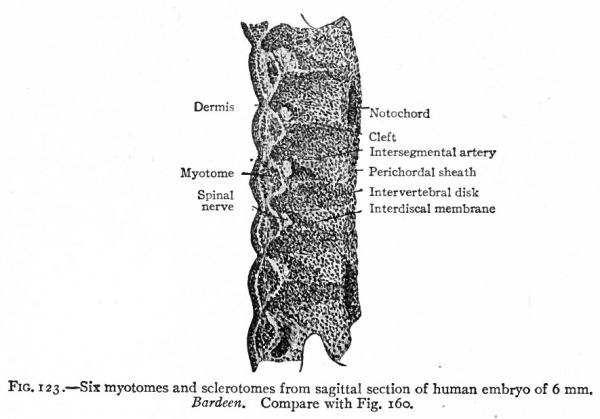
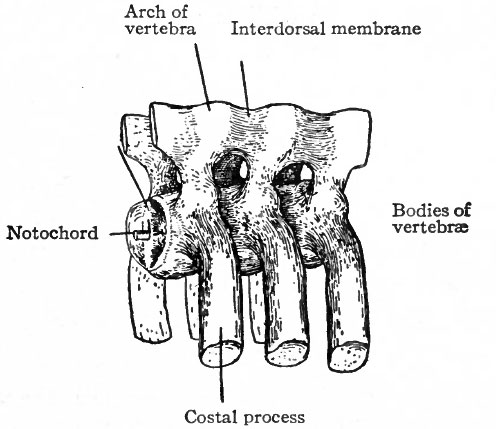
The changes which occur in the ventro-medial parts of the primitive segments to form the sclerotomes have already been described. At the same time it was stated that the vertebrae, with the other types of connective tissue around them, were derived from the mesenchymal tissue of the sclerotomes (p. 131; see also Fig. 104). The segmentally arranged masses forming the sclerotomes are separated by looser tissue in which the intersegmental arteries develop. The arteries mark the boundaries between the sclerotomes (Fig. 122). About the third week of development the caudal part of each sclerotome condenses to form a more compact mass of tissue, and a little later becomes separated from the cephalic part by a small cleft (Fig. 123). From the denser caudal part a secondary mass of tissue grows medially and meets and fuses with its fellow of the opposite side, thus enclosing the notochord. The medial mass thus formed may be considered as the anlage of the body of a vertebra. Another secondary mass also grows dorsally between the myotome and the spinal cord, forming the anlage of the -vertebral arch. A third mass grows ventro-laterally to form the costal process (Figs. 124 and 125). The looser tissue of the cephalic part of each sclerotome also sends an extension medially to surround the notochord, and fills up the intervals between the succeeding denser (caudal) parts. The looser part also forms a sort of membrane between the succeeding vertebral arches. The tissue between the denser caudal part and the looser cephalic part of each sclerotome is destined to give rise to an intervertebral fibrocartilage. While the denser tissue forming the caudal part of each sclerotome probably gives rise to the greater part of a vertebra, the looser tissue of the cephalic part is also involved in the formation of the cartilaginous body, as will be noted again in the following paragraph. The peculiar feature of the process is that the denser caudal part of a sclerotome becomes associated with the looser cephalic part of the next succeeding sclerotome, so that each vertebra is derived from parts of two adjacent sclerotomes and not from a single sclerotome. This naturally brings about an alternation of vertebra and myotomes (Fig. 123).
Fig. 123. Six myotomes and sclerotomes from sagittal section of human embryo of 6 mm. Bardeen. Compare with Fig. 160.
So far the anlagen of the vertebrae are in the so-called blastemal stage.
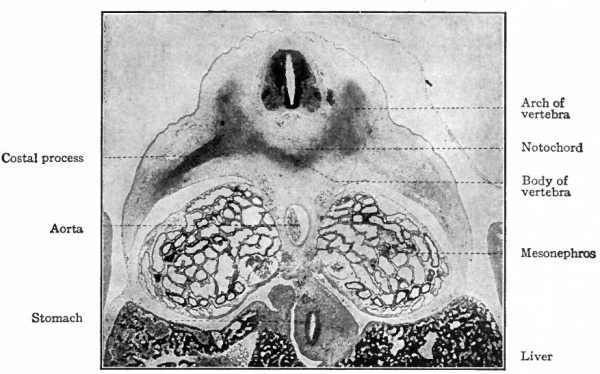
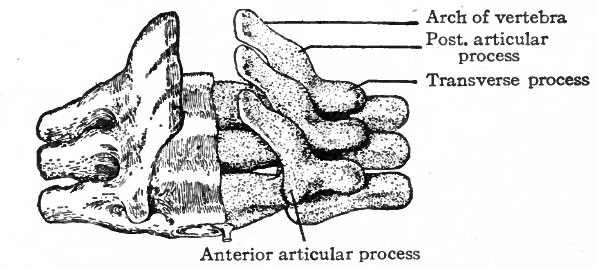
Following the blastemal stage and beginning in human embryos of about 15 mm., comes the cartilaginous stage in which the mesenchymal anlagen of the vertebrae are converted into embryonic hyalin cartilage. In the body of each vertebra a center of chondrification appears in the looser tissue of the caudal part and gradually enlarges and involves the denser cephalic part. It is to be noted that the denser tissue of the cephalic part of a vertebral body corresponds to the caudal part of a sclerotome. Two chondrification centers appear, one on (Fig. 126). All these centers then enlarge and unite to form a single mass of cartilage which corresponds quite accurately in shape to the future bony vertebra. Processes then grow out from the vertebral arch. These represent the transverse and articular processes (Fig. 127). Each half of a vertebral arch meets its fellow of the opposite side dorsal to the spinal cord, and from the point of meeting the spinous process grows out. The costal processes do each side of the medial line, but the two soon fuse around the notochord to form a single center. In addition to the center in the body of the vertebra, one also appears in each half of the vertebral arch, and one in each costal process not retain their connection with the body of the vertebra, but break away and become the rib cartilages, as will be noted again in connection with the development of the ribs.
Fig. 124. Transverse section (dorsal part) of pig embryo of 14 mm. Photograph.
Fig. 125. Models of three vertebrae in the blastemal stage from an embryo of 11 mm. Bardeen.
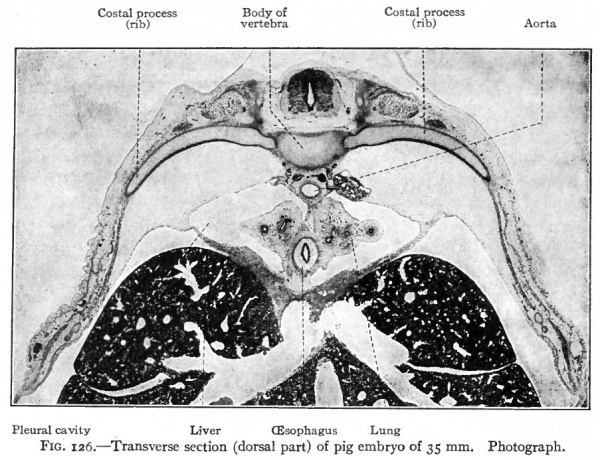 Fig. 126. Transverse section (dorsal part) of pig embryo of 35 mm. Photograph.
Fig. 126. Transverse section (dorsal part) of pig embryo of 35 mm. Photograph.
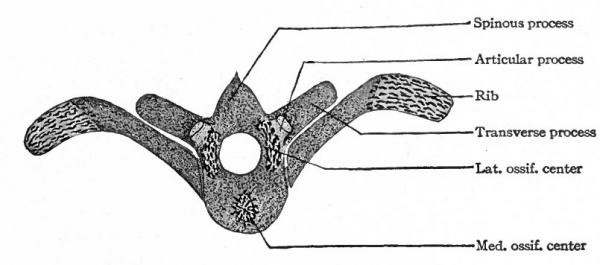
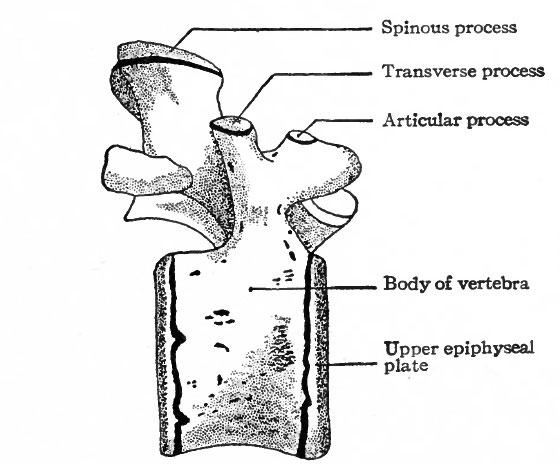
Following the cartilaginous stage is the stage of ossification in which the vertebrae become ossified and acquire the adult condition. Ossification begins during the third month of foetal life and extends over a long period, even up to the age of twenty-five years. A single center of ossification appears in the body of each vertebra, and following this a center in each half of the vertebral arch (Fig. 128). Osseous tissue then gradually replaces the cartilage. The two halves of an arch fuse dorsal to the spinal cord during the first year of postnatal life, thus completing the bony arch. The arch fuses with the body of the vertebra between the third and eighth years. Thus it is seen that the process of ossification is a slow one, and this is even more striking when one considers the formation of the secondary centers. For at about the age of puberty a secondary center appears in each of the cartilages that cover the ends of the vertebrae, producing disks of bone the epiphyses. A secondary center also appears in the cartilage on the tip of each spinous process and transverse process, and in the lumbar vertebrae one appears also on the tip of each articular process (Fig. 120). The epiphyses unite with the vertebrae any time between sixteen and twenty-five years. About the twenty-fifth year the sacral vertebrae unite to form a single mass of bone, and a similar union also takes place between the more or less rudimentary coccygeal vertebrae.
Fig. 127. Models of the 6th, 7th and 8th thoracic vertebrae of an embryo of 33 mm. (dorsal view). Bardeen. On the right the cartilage is shown, on the left the surrounding fibrous tissue.
Fig. 128. Thoracic vertebra and ribs of human embryo of 55 mm. (middle of 3rd month). Kollmann's Atlas. Cartilage indicated by stippled areas, ossification centers by irregular black lines.
Fig. 129. Lumbar vertebra (lateral view) showing secondary centers of ossification. Sappey.
While the general plan of development is practically the same in all the vertebrae, there are a few noteworthy modifications. The greatest modification is in the atlas and epistropheus (axis). The entire atlas is formed from the denser caudal part of a sclerotome. The lateral mass and the posterior (dorsal) arch represent the vertebral arch. The anterior (ventral) arch represents the hypochordal bar, a plate of cartilage which develops in all vertebrae ventral to the notochord but disappears in all except the atlas. A body also develops but instead of forming part of the atlas it unites with the body of the epistropheus to form the dens (odontoid process) of the latter.
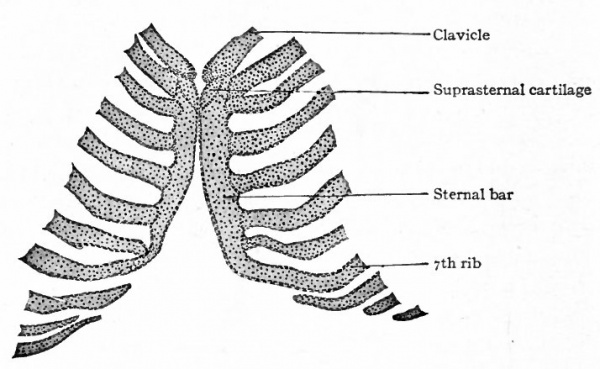
Fig. 130. Ventral view of developing sternum of human embryo of 30 mm. (beginning of 3rd month). Ruge, Kollmann's Atlas.
The various ligaments of the vertebral column are derived from the embryonic connective tissue surrounding the vertebras. The embryonic connective tissue in the clefts separating the developing vertebrae is transformed into the intervertebral fibrocartilages.
The Ribs
It has been stated in a previous paragraph that the costal processes arise as outgrowths from the denser caudal parts of the sclero tomes; that they grow in a ventro-lateral direction and consequently are at first connected with and are parts of the bodies of the vertebrae (Figs. 124 and 126). These costal processes are the anlagen of the ribs, and they continue to grow ventrally until they practically encircle the body, the ventral ends of a number of them fusing in the medial line to form the sternum. The primary junctions between the costal processes and vertebrae are dissolved, and the embryonic connective tissue in this region gives rise to the costo-vertebral ligaments. The dissolution of the junctions leaves the ribs simply articulating with the vertebrae.
A chondrification center appears in each costal process, shortly after that in the body of the vertebra, and from this point the formation of cartilage gradually extends throughout the entire rib.
Ossification begins during the third month at a center which is situated near the angle of the rib (Fig. 128). At the age of eight to fourteen years a secondary center appears in each capitulum and tuberculum and subsequently fuses with the rest of the rib at the age of fourteen to twenty-five years. As the tuberculum develops, the transverse process of the corresponding vertebra grows ventrally and caudally to meet it and form the articulation.
The ribs reach the highest degree of development in the thoracic region where one develops on each side, corresponding to each vertebra. The first seven or eight thoracic ribs extend almost to the midventral line and are attached to the sternum; the last four or five become successively shorter and are only indirectly or not at all attached to the sternum. In the cervical region the ribs do not reach a high degree of development. Their tips simply fuse with the transverse processes of the vertebrae and their heads with the bodies of the vertebrae, leaving a space the foramen transversarium through which the vertebral vessels pass. The seventh cervical rib may, however, reach a fairly high degree of development. In the lumbar region also the ribs are reduced to small pieces of bone which are firmly united with the transverse processes and form the accessory processes. In the sacral region the rudimentary ribs unite to form the lateral part (pars lateralis) of the sacral bone. After the blastemal stage there are no indications of ribs in the coccygeal region. In the blastemal stage, however, there is a small bit of tissue Fig. 131. Sternum of which probably represents the anlage of a rib, but soon fuses with the transverse process.
The Sternum
The sternum, according to Hanson's recent contribution, originates independently of the ribs. On each side, some distance from the midventral line, the sternal band or bar arises as a mesenchymal condensation in the body wall. These bars then approach the midventral line and fuse with each other to form a single cartilaginous structure. Meanwhile the ventral ends of the first seven ribs extend far enough to come into contact with and join the sternal bar (Fig. 130). Before the two bars have united a medial unpaired rudiment appears opposite their anterior ends to form the presternum with which the paired rudiments subsequently unite. The presternal component, with which the clavicles articulate, probably represents the ventral part of the primitive vertebrate shoulder girdle.
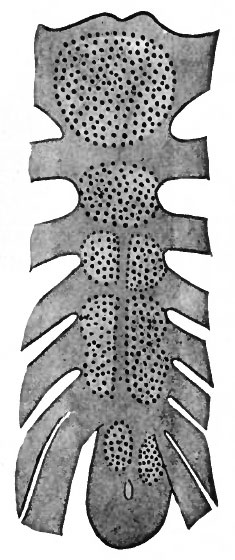
Ossification begins in the sternum about the end of the fifth month of foetal life. In the cephalic portion two unpaired centers appear; caudal to these is a series of paired centers which subsequently fuse across the midventral line. (See Fig. 131.) The paired centers perhaps reflect the paired character of the sternal bars. Sometimes, however, the centers appear as a single series, that is, with no indication of a paired character. The ossification of the most cephalic segment, along with the episternal cartilages, produces the manubrium sterni. Ossification of the following six or seven segments and their union produce the corpus sterni. The xyphoid process appears to be a caudal extension of the corpus sterni. This process remains cartilaginous for a long period, and may be single, perforated, or bifurcated, depending upon the degree of fusion between the two primary bars.
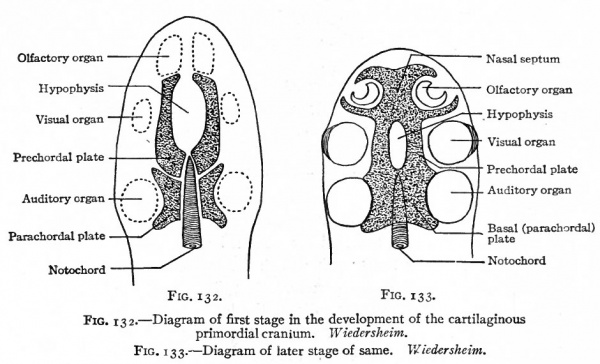
Fig. 132. Diagram of first stage in the development of the cartilaginous primordial cranium. Wiedersheim.
Fig. 133. Diagram of later stage of same. Wiedersheim.
The Head Skeleton
Topographically the skeleton of the head appears as the cephalic part of the axial skeleton. Structurally it is decidedly different, for it is adapted to different conditions. The neural tube here becomes differentiated into the brain with its many and dissimilar parts. In connection with the brain the complicated sense organs (nose, eye and ear) arise. A part of the alimentary tract and portions of the visceral arches are also inclosed within the head. The head skeleton is specially modified to accommodate these highly developed organs, and becomes extremely complicated. In general the skeleton in any part of the body adapts itself to the other structures and not the other structures to the skeleton.
The anlage of the skull is a mass of embryonic connective tissue which surrounds the cephalic end of the notochord, extends from there into the nasal region and also extends around the sides and dorsal part of the neural tube (brain). Unlike the anlage of the vertebral column, the anlage of the skull shows no distinct division into primitive segments. The only indications of a segmental character are referred to in a succeeding paragraph (small print,
The next step in the development of the skull is the appearance of cartilage in certain regions of the embryonic connective tissue. On account of the complicated arrangement of the cartilage in the human skull, it is best to consider first its more simple arrangement in the lower Vertebrates. In these there appear in the embryonic connective tissue around the cephalic end of the notochord two bilaterally symmetrical pieces of cartilage, which extend as far as the hypophysis. Then two other bilaterally symmetrical pieces appear, extending from the hypophysis to the nasal region. Subsequently all these pieces fuse into a single mass which extends from the cephalic end of the vertebral column to the tip of the nose, enclosing the end of the notochord and, to a certain extent, the ear, eye and olfactory apparatus. There is left, however, an opening for the hypophysis. From this mass of cartilage, chondrification extends into the embryonic connective tissue along the sides and roof of the cranial cavity, so that the brain and sense organs are practically enclosed. To this capsule the term cartilaginous primordial cranium has been applied. (See Figs. 132, 133, 134.)
Fig. 134. Primordial cranium of Salmo (salmon) embryo of 25 mm. Dorsal view. Gaupp Compare with Fig. 133 and note further elaboration of parts surrounding the sense organs.
In the higher Vertebrates, chondrification is limited to the basal region of the skull, while the side walls and roof are formed later by intramembranous bone.
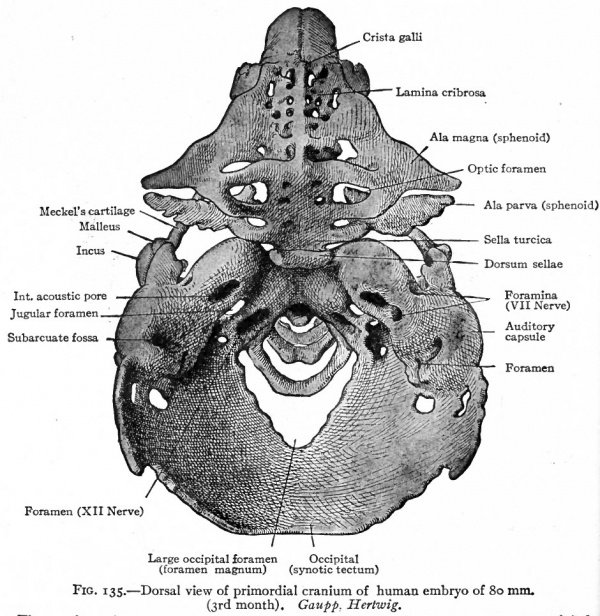
Fig. 135. Dorsal view of primordial cranium of human embryo of 80 mm. (3rd month). Gaupp. Hertwig. The membrane bones of the roof of the skull have been removed. Through the large occipital foramen can be seen the first three cervical vertebrae.
In the human embryo chondrification occurs first in the occipital and sphenoidal regions, and then gradually extends into the nasal (ethmoidal) region. A little later it spreads somewhat dorsally in the occipital and sphenoidal regions to form part of the squamous portion of the occipital and the wings of the sphenoid. At the same time cartilage develops in the embryonic connective tissue surrounding the internal ear to form the periotic capsule whicn subsequent!} unites with the occipital and sphenoidal cartilages. The pieces of cartilage thus formed constitute the chondrocranium.
In connection with the development of the caudal part of the occipital cartilage there is an interesting feature which is at least indicative of a segmental character. In some of the lower Mammals there are four fairly distinct condensations of embryonic connective tissue just cranial to the first cervical vertebra, corresponding to the first cervical nerve and the three roots of the hypo glossal. These condensations bear a general resemblance to the primitive segments and indicate the existence of four vertebrae which are later taken up into the chondrocranium. In the human embryo the condensations are less distinct, but the existence of a first cervical and a three-rooted hypoglossal nerve in this region suggests an original segmental character. If this is true, then the base of the human skull is formed from the unsegmented chondrocranium plus four vertebrae which become incorporated in the occipital region.
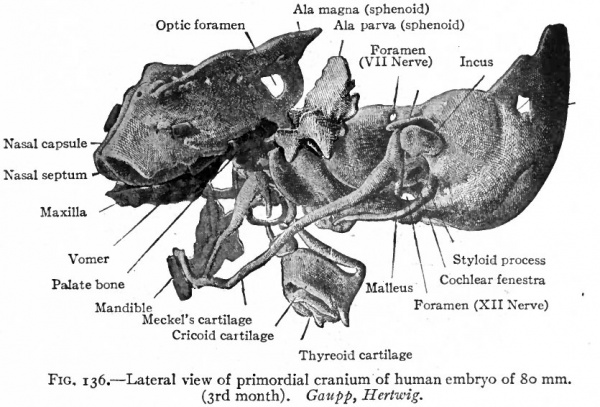
Fig. 136. Lateral view of primordial cranium of human embryo of 80 mm. (3rd month). Gaupp, Hertwig. The membrane bones of the roof of the skull have been removed. Compare with Fig. 135. The maxilla, vomer, palate, and mandible are membrane bones.
In addition to the chondrocranium, other cartilaginous elements enter into the formation of the skull, all of which are derived from the visceral arches. Not all the arches, however, produce cartilage; for in the maxillary process of the first arch, which forms the upper boundary of the mouth, cartilage does not appear, and the bones which later develop in it are of the membranous type. The mandibular process of the first arch produces a rod of cartilage Meckel's cartilage. This gives rise, at its proximal end, to a part of the auditory ossicles, but the cartilage in the jaw proper soon wholly or almost wholly disappears. The cartilage of the second arch becomes connected with the skull in the region of the periotic capsule. The cartilages of the other three arches are only indirectly connected with the skull and will be considered later.
Figs. 135 and 136 show the condition of the chondrocranium in a human embryo of 80 mm. (third month) . Although at first glance it seems exceedingly complicated, a careful study and comparison of the various parts will aid the student in his comprehension of the cartilaginous foundation upon which the skull is built.
Ossification of the Chondrocranium
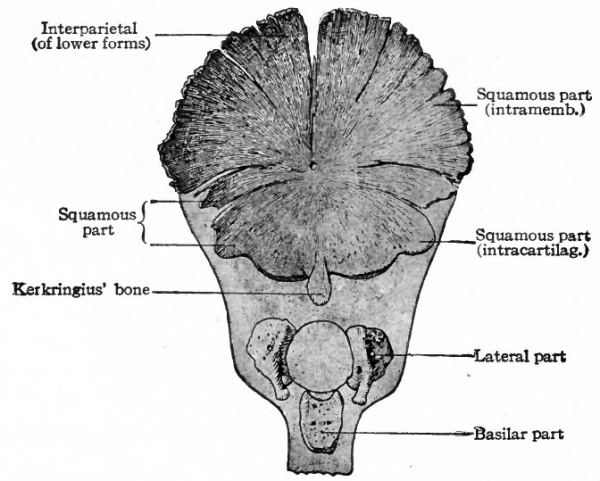
In the human foetus ossification begins in the occipital region during the third month. Four centers appear which correspond to the four parts of the adult occipital bone (Fig. 137). (i) An unpaired center situated ventral to the foramen magnum. From this center ossification proceeds in all directions to form the pars basilaris (basioccipital). (2 and 3) Two lateral centers, one on each side. From these, ossification proceeds to produce the partes laterales (exoccipital) which bear the condyles. (4) A center dorsal to the foramen magnum. This produces the pars squamosa (supraoccipital) as far as the superior nuchal line. Beyond this line the pars squamosa is of intramembranous origin. (See p. 160.) At birth the four parts are still separated by plates of cartilage. During the first or second year after birth the partes laterales unite with the pars squamosa, and about the seventh year the pars basilaris unites with the rest of the bone.
Fig. 137. Occipital bone of human embryo of 21.5 cm. Kollmann's Atlas.
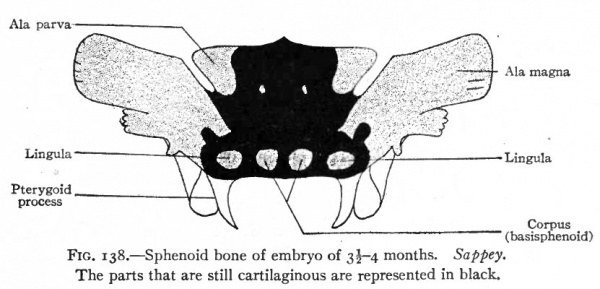
In the sphenoidal region ossification begins at a number of centers which, as in the occipital region, correspond generally to the parts of the adult sphenoid bone (Fig. 138). (i and 2) About the ninth week an ossification center appears on each side in the cartilage which corresponds to the ala magna (alisphenoid). (3 and 4) About the twelfth week a center appears on each side which corresponds to the ala parva (orbitosphenoid) . (5 and 6) A short time after this a center appears on each side of the medial line in the basal part of the cartilage, and the two centers subsequently fuse to produce the corpus (basisphenoid) . (7 and 8) Lateral to each basal center, another center appears which represents the beginning of the lingula. (9 and 10) Finally two centers appear in the basal part of the cartilage, in front of the other basal centers, and then fuse to form the presphenoid. As in the case of the occipital bone, not all of the adult sphenoid is of intracartilaginous origin; for the upper anterior angle of each ala magna is of intramembranous origin, as are also the medial and lateral laminae of the pterygoid process. The pterygoid hamulus, however, is formed by the ossification of a small piece of cartilage which develops on the tip of the medial lamina. The fusion of these various parts occurs at different times. The lateral pterygoid lamina unites with the alisphenoid before the sixth month of foetal life; about the sixth month the lingula fuses with the basisphenoid, and the presphenoid with the orbitosphenoid. The alisphenoid and medial pterygoid lamina fuse with the rest of the bone during the first year after birth. The union of the basisphenoid and basioccipital usually occurs when the growth of the individual ceases, though the two bones may remain separate throughout life.
Fig. 138. Sphenoid bone of embryo of 3-4 months. Sappey. The parts that are still cartilaginous are represented in black.
In the region of the periotic capsule, several centers of ossification appear in the cartilage during the fifth month. During the sixth month these centers unite to form a single center which then gradually increases to form the pars petrosa and pars mastoidea of the adult temporal bone. The mastoid process is formed after birth by an evagination from the pars petrosa, and is lined by an evaginated portion of the mucosa of the middle ear. The other parts of the temporal bone are of intramembranous origin, except the styloid process which represents the proximal end of the second branchial arch.
In the ethmoidal region, conditions become more complicated on account of the peculiarities of the nasal cavities, and on account of the fact that the cartilage is never entirely replaced by bone, and that "membrane" bones also enter into more intimate relations with the "cartilage" bones. The ethmoidal cartilage at first consists of a medial mass, which extends from the presphenoid region to the end of the nasal process, and of a lateral mass on each side, which is situated lateral to the nasal pit (Fig. 136). Ossification in the lateral mass on each side produces the ethmoidal labyrinth (lateral mass of ethmoid). It is perhaps not quite correct to say that ossification produces the ethmoidal labyrinth, for at first there is only a mass of spongy bone with no indication of the honey-combed structure characteristic of the adult. The latter condition is produced by at certain amount of dissolution of the bone and the growth of the nasal mucosa into the cavities so formed. By the same process of dissolution and ingrowth of nasal mucosa the superior, middle and inferior concha (turbinated bones) are formed. The medial mass of cartilage begins to ossify after birth and then only in its upper (superior) edge. It forms the lamina perpendicular is and crista galli and extends into the nose as the nasal septum. The lower (inferior) edge remains as cartilage until the vomer, which is a membrane bone (p. 194), develops, after which it is partly dissolved. The lamina cribrosa (cribriform plate) is formed by bony trabeculae which extend across between the medial mass and the lateral masses and surround the bundles of fibers of the olfactory nerve.
Membrane Bones of the Skull
Under this head we shall consider only those bones which develop a from the visceral arches, those which involve the arches being considered later. It has been seen that by far the greater parts of the bones forming the base of th skull are of intracartilaginous origin. On the other hand, those forming the sides and roof of the skull are largely of intramembranous origin. In the case of the occipital bone, two centers of ossification appear in the membrane dorsal to the supraoccipital, and the bone so formed begins to unite with the supraoccipital during the third month of foetal life. At birth the union is usually complete, though for a time an open suture may persist on each side. The bone derived from the two centers forms that part of the occipital squama which is situated above the superior nuchal line; the part below the line is of intracartilaginous origin (p. 190). The adult occipital is thus a composite bone, partly of intramembranous, partly of intracartilaginous origin.
The temporal is also a composite bone, the petrous and mastoid parts and the styloid process being of intracartilaginous origin, while the temporal squama and the tympanic part are of intramembranous origin. During the eighth week of foetal life a center of ossification appears in the membrane in the temporal region, and the bone formed from this center subsequently unites with the petrous part and becomes the temporal squama. Another center appears in the membrane to the outer side of the periotic capsule and produces a ring of bone around the external auditory meatus, which fuses with the petrous part and forms the tympanic part of the adult bone. It gives attachment at its inner border to the tympanic membrane. While the union of the different parts begins during foetal life, it is usually completed after birth.
Fig. 139. Diagram of skull of new-born child. Combined from McMurrich and Kollmann. White areas represent bones of intramembranous origin; dotted areas represent bones (not derived from branchial arches) of intracartilaginous origin; black areas represent derivatives of branchial arches.
The sphenoid bone is also composed of parts which have different origins. The body, small wings and large wings are of intracartilaginous origin, the pterygoid process of intramembranous origin. About the eighth week of development a center of ossification appears in the mesenchyme in the lateral wall of the posterior part of the nasal cavity and gives rise to the medial pterygoid lamina. On the tip of the latter a small piece of cartilage appears in which ossification later takes place to form the pterygoid hamulus (p. 159). The lateral pterygoid lamina is also of intramembranous origin and fuses with the medial lamina, the two laminae forming the pterygoid process which subsequently unites with the body of the sphenoid. (See Fig. 138.)
In the ethmoidal region, only the vomer is of intramembranous origin. An ossification center appears in the embryonic connective tissue on each side of the perpendicular plate (lamina perpendicularis) and these two centers produce two thin plates of bone which unite at their lower borders and invest the lower part of the perpendicular plate. The portion of the latter thus invested undergoes resorption.
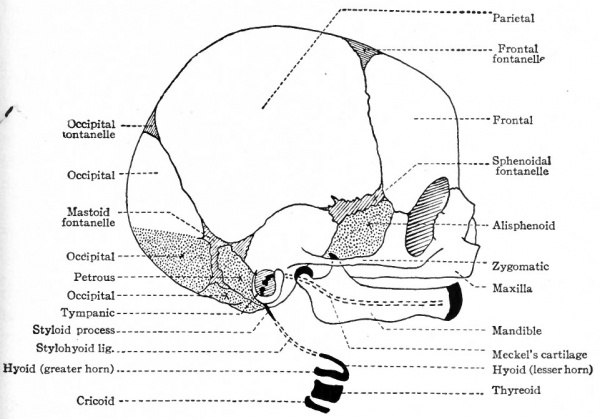
The frontal and parietal bones are purely of intramembranous origin. About the eighth week two centers of ossification, one on each side, appear for the frontal. The bones produced by these centers unite in the medial line to form the single adult bone. In the event of an incomplete union an open suture remains the metopic suture. A single center of ossification appears for each parietal bone at about the same time as those for the frontal. The union of the bones which form the roof and the greater part of the sides of the skull does not occur till after birth. The spaces between them constitute the sutures and fontanelles so obvious in new-born children (Fig. 139).
A single center of ossification appears in the embryonic connective tissue for each zygomatic, lachrymal and nasal bone, all of which are of intramembranous origin.
Bones Derived from the Branchial Arches
The first branchial arch becomes divided into two portions. One of these, the maxillary process, is destined to give rise to the upper jaw and much of the upper lip and face region. The other, the mandibular process, is destined to give rise to the lower jaw, the lower lip and chin region, and two of the auditory ossicles. The angle between the two processes corresponds to the angle of the mouth, and the cavity enclosed by the processes is the forerunner of the mouth and nasal cavities. (See Fig. 96, also p. 119.) So far as the skeletal elements are concerned, cartilage develops only in the mandibular process where it forms a slender bar or rod known as MeckeVs cartilage. Only a small part of this becomes ossified, the greater portion of the mandible being of intramembranous origin. No cartilage develops in the maxillary process. This probably indicates a condensation of development in man and the higher animals, for among the lower animals cartilage precedes the bone. In man the maxilla and palate bone also are of intramembranous origin.
The palate bone develops from a single center of ossification which appears at the side of the nasal cavity in embryos of about 18 mm. This center represents the perpendicular part, the horizontal part appearing in embryos of about 24 mm. as an outgrowth from the perpendicular and not as a separate center of ossification. The orbital and sphenoidal processes also represent outgrowths from the primary center and appear much later.
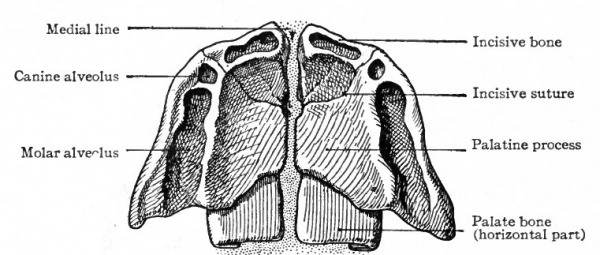
Opinions regarding the development of the maxilla are at variance. One view is that it arises from five centers of ossification. One of these centers gives rise to that part of the alveolar border which bears the molar and premolar teeth; a second center forms the nasal process and that part of the alveolar border which bears the canine tooth; a third produces the part which bears the incisor teeth; and the two remaining centers give rise to the rest of the bone. All these parts effect a firm union at an early stage, with the exception of the part bearing the incisor teeth which remains more or less distinct as the incisive bone (premaxilla, intermaxilla) . Another view arising from recent work on human embryos is that there are primarily only two ossification centers; one of these gives rise to the incisive bone, the other to the rest of the maxilla (Mall). These centers appear at the end of the sixth week (embryos of 18 mm.).
Fig. 140. Head of human embryo of 7 weeks. His. Ventral aspect of upper jaw region. Lower jaw and tongue have been removed.
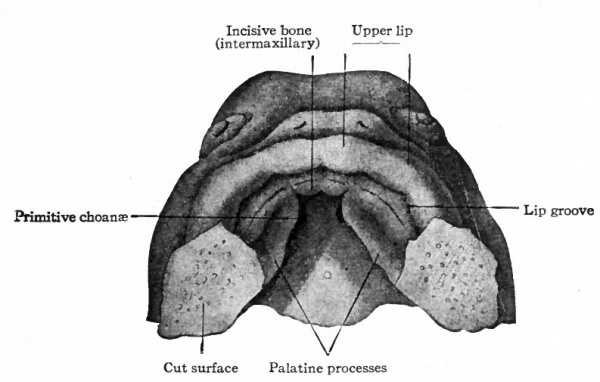
A very important feature in the development of the maxilla is its agency in separating the nasal cavity from the mouth cavity. The palatine process of the bone grows medially and meets and fuses with its fellow of the opposite side in the medial line, the two processes together thus constituting about the anterior three-fourths of the bony part of the hard palate. It should be observed, however, that the palatine processes do not meet at their anterior borders, for the incisive bone is insinuated between them (see Figs. 140, 141).
The incisive bone is probably not derived from the maxillary process of the first visceral arch, but from the fronto-nasal process. The question thus arises as to whether it is derived from both the middle and lateral nasal processes or only from the middle. According to Kolliker's view, the lateral nasal process takes no part in the formation of the incisive bone. It is derived from the middle process, hence genetically it is a single bone on each side. According to Albrecht's view the incisive bone is genetically composed of two parts, one derived from the lateral, the other from the middle nasal process. While the matter is not one of great importance merely from the standpoint of development, it has an important bearing on the question of certain congenital malformations, e.g., hare lip, and will be discussed further under that head (p. 180).
Fig. 141. Ventral aspect of hard palate of human embryo of 80 mm. Kollmann's Atlas.
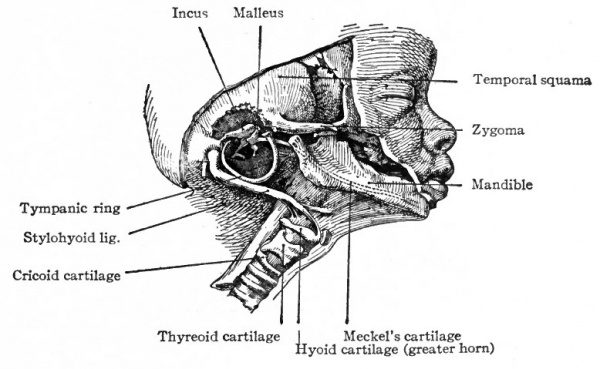
In the mandibular process of the first visceral arch, the mandible develops as a bone which is partly of intramembranous and partly of intracartilaginous origin. In the first place a rod of cartilage, known as Meckel's cartilage, forms the core of the mandibular process and extends from the distal end of the process to the temporal region of the skull, where it passes between the tympanic bone and the periotic capsule and ends in the tympanic cavity of the ear (Fig. 136). During the sixth week of foetal life, intramembranous bone begins to develop in the mandibular process. In the region of the body of the mandible the bone encloses the cartilage, but in the region of the ramus and coronoid process the cartilage lies to the inner side of the bone. Development is further complicated by the appearance of cartilage in the region of the middle incisor teeth and on the coronoid and condyloid processes. These pieces of cartilage form independently of Meckel's cartilage and subsequently are replaced by the bone which constitutes the corresponding parts of the mandible. The part of Meckel's cartilage enclosed in the bone disappears; the part to the inner side of the ramus is transformed into the sphenomandibular ligament. (See Fig. 142.) In each half of the second branchial arch a rod of cartilage develops, which extends from the ventro-medial line to the region of the periotic capsule. The proximal end of this rod is then replaced by bone which fuses with the temporal bone and forms the styloid process. The distal (ventral) end is replaced by bone which forms the lesser horn of the hyoid bone. Between the styloid process and the lesser horn, the cartilage is transformed into the stylohyoid ligament (see Figs. 139 and 142).
In each half of the third branchial arch a piece of cartilage develops and subsequently is replaced by bone to form the greater horn of the hyoid bone. The two horns become connected at their ventral ends by the body of the hyoid bone which is also a derivative of the third arch. Later the lesser horn fuses with the greater horn to bring about the adult condition (Fig. 142).
In the ventral parts of the fourth and fifth arches pieces of cartilage develop and form the skeletal elements, of the larynx. A more detailed account of these will be found under the head of the larynx.
Fig. 142. Lateral dissection of head of human foetus. Showing derivatives of branchial arches in natural position. Kollmann's Atlas.
The auditory ossicles are also derived largely from the branchial arches, the incus and malleus being derived from the proximal end of Meckel's cartilage (first arch) , the stapes having a double origin from the second arch and the embryonic connective tissue surrounding the periotic capsule. But since they form integral parts of the organ of hearing, a discussion of their formation is best included in the development of the ear.
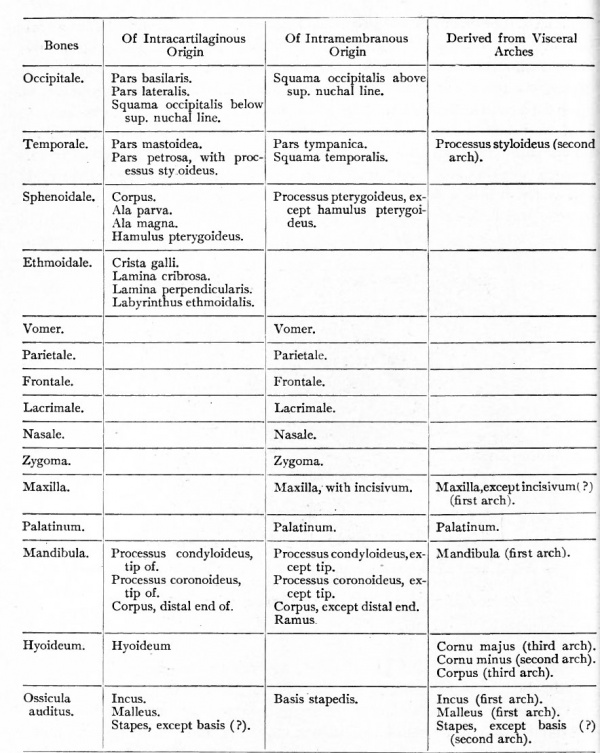
The accompanying table indicates the types of development in the different bones of the head skeleton.
The Appendicular Skeleton
The growth of the limb buds and their differentiation into arm, forearm and hand, thigh, leg and foot, along with the rotation which they undergo during development, have been discussed in the chapter on the external form of the body (p. 121). The metameric origin of the muscles of the extremities is discussed in the chapter on the muscular system (Chap. XI). It has been seen that the greater part of the axial skeleton is derived from the sclerotomes, is preformed in cartilage, and maintains its segmental character throughout life. It has also been seen that the head skeleton is in part preformed in cartilage, is in part of intramembranous origin, and shows but a trace of segmental character, and that only in the occipital region at a very early stage. The appendicular skeleton is derived wholly from the embryonic connective tissue which forms the cores of the developing extremities, and shows no trace of a segmental character. Here also, as in the axial skeleton, three stages may be recognized a blastemal, a cartilaginous (Fig. 143), and a final osseous,
Fig. 143. Cartilages of left upper extremity of a human embryo of 17 mm. Hagen.
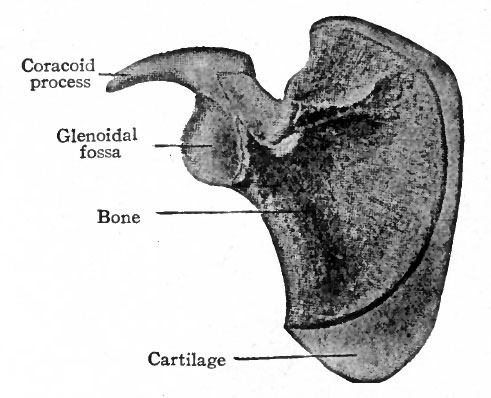
In the region of the shoulder girdle a plate of cartilage appears in the embryonic connective tissue which lies among the developing muscles dorso-lateral to the thorax. This plate of cartilage is the forerunner of the scapula, and in general resembles it in shape. During the eighth week of foetal life a single center of ossification appears and gives rise to the body and spine of the scapula. After birth certain accessory centers appear and produce the coracoid process, the supraglenoidal tuberosity, the acromion process, and the inferior angle and vertebral margin (Fig. 144). Later the supraglenoidal fuses with the coracoid and forms part of the wall of the glenoid cavity. About the seventeenth year the single center formed by the union of these two fuses with the rest of the scapula.
At the age of twenty to twenty-five years all the other accessory centers unite with the rest of the scapula to form the adult bone.
There are two views concerning the development of the clavicle: one that it is of intracartilaginous origin, the other that it is of intramembranous origin. Ossification begins during the sixth week, possibly from two centers. It is true that the cartilage that appears around the centers is of a looser character than the ordinary embryonic cartilage, but whether the centers appear in cartilage seems not to have been determined. At the age of fifteen to twenty years a sort of secondary center appears at the sternal end of clavicle and fuses with the body about the twenty-fifth year.
Fig. 144. Scapula of new-born child. Showing primary center of ossification, and cartilage (lighter shading) in which secondary centers appear. Bonnet.
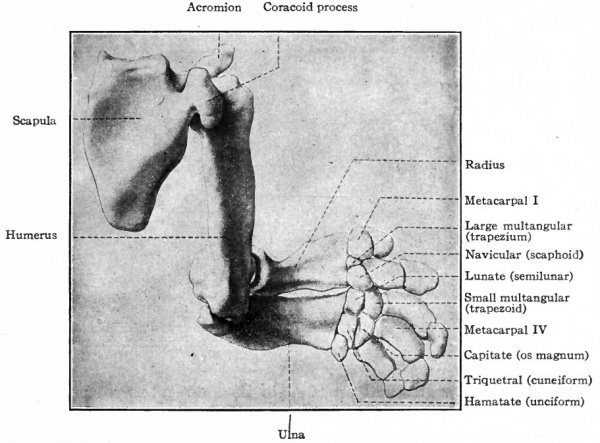
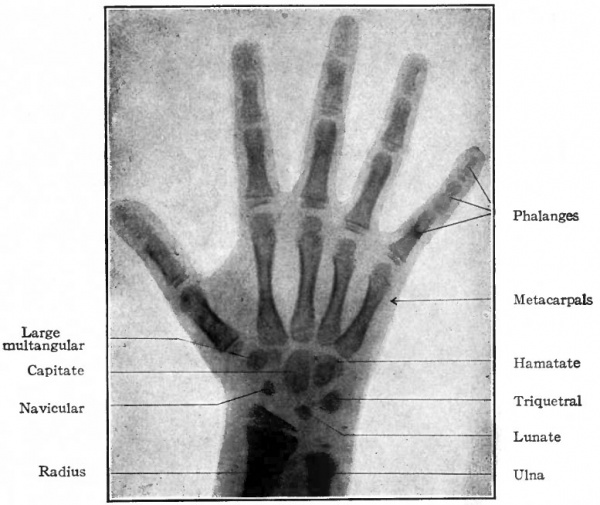
The humerus, radius and ulna are preformed in cartilage (Fig. 143) and develop as typical long bones. Ossification begins in each during the seventh week at a single center and proceeds in both directions to form the shaft. During the first four years after birth epiphyseal centers appear for the head, greater and smaller tubercles, trochlea and epicondyles. All these secondary centers unite with the shaft of the humerus when the growth of the individual ceases. In the case of the radius and ulna a secondary center appears at each end of each bone to form the epiphysis; and in the ulna another secondary center appears to form the olecranon. (For the growth of bones, see page 144) . The carpal bones are all preformed in cartilage (Fig. 143) but their development is somewhat complicated owing to the fact that pieces of cartilage appear which subsequently may disappear, or ossify and become incorporated in other bones. Primarily seven distinct pieces of cartilage develop and become arranged transversely in two rows; these represent seven of the carpal bones. The proximal row consists of three large pieces which are the forerunners of the navicular (radial, scaphoid), lunate (intermediate, semilunar) and triquetral (ulnar, pyramidal, cuneiform) . The distal row is composed of four elements which are the forerunners of the large multangular (trapezium), small multangular (trapezoid), capitate (os magnum), and hamatate or hooked (unciform). In addition to the cartilages mentioned, several others also appear in an inconstant way in different individuals. Two of these are important. One appears on the ulnar side of the proximal row and is the forerunner of the pisiform; the other is situated between the two rows and may either disappear entirely or fuse with the navicular. Ossification does not begin in the carpal cartilages until after birth; it begins in the hamatate and capitate during the third year, in the others at later periods, and is completed only when the growth of the individual ceases. The fact that the hamatate ossifies from two centers indicates that it is probably derived phylogenetically from two bones. Comparative anatomy teaches that the accessory cartilages in the human wrist are representatives of structures which are normally present in the lower forms.
Fig. 145. Diagram of right hand of 5 year old girl. (Courtesy of Dr. Edward Learning). The ossification centers are indicated by the darker areas.
The metacarpals and phalanges are preformed in cartilages which correspond in shape to the adult bones. A center of ossification appears in each cartilage and produces the shaft of the bone. Only one epiphysis develops on each metacarpal and phalanx. In each metacarpal it develops at the distal end, except in the thumb where it appears at the proximal end. In each phalanx it develops at the proximal end (Fig. 145).
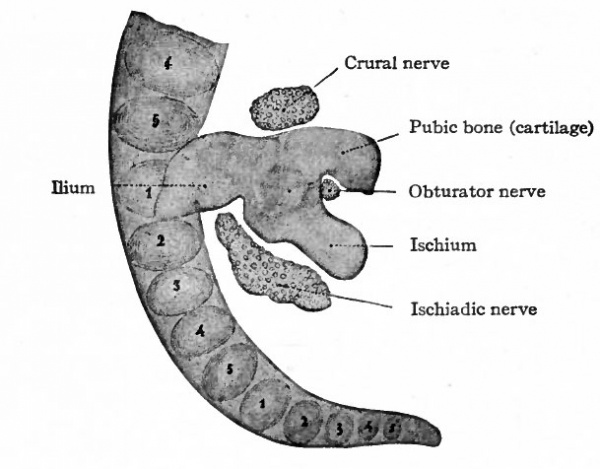
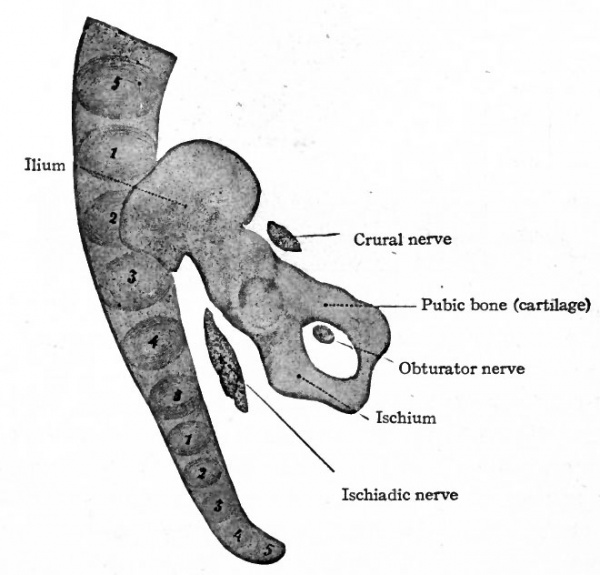
Fig. 146. Cartilage of right side of pelvic girdle of a human embryo of 13.6 mm. (5 weeks). Petersen. The numerals indicate the vertebrae; the first sacral being opposite the ilium.
Fig. 147. Cartilage of right side of pelvic girdle of a human embryo of 18.5 mm. (8 weeks). Petersen. The numerals indicate the vertebras; the first and second sacral being opposite the ilium. Compare with Fig. 146.
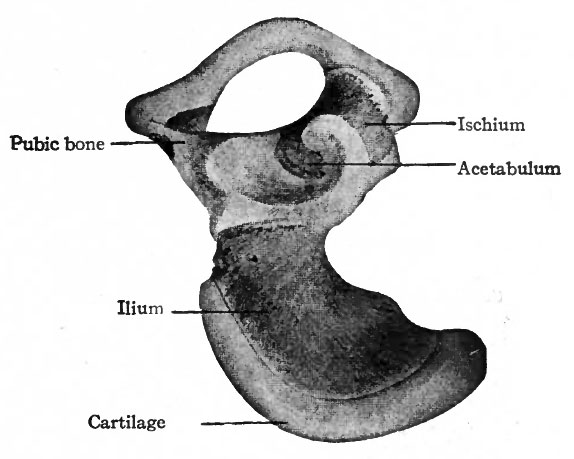
The skeletal elements of the lower extremities, including the pelvic girdle, are of intracartilaginous origin. Each hip bone (os coxae, innominate bone) is preformed in cartilage which, in a general way, resembles in shape the adult bone. The ventral part of the pubic cartilage does not at first join the ischial; but by the eighth week the junction is complete, leaving dorsal to it the obturator foramen. In the earliest stages the long axis of the cartilage is nearly at right angles to the ; vertebral column, and the ilium lies close to the fifth lumbar and first sacral | vertebrae; later (eighth week) the long axis lies nearly parallel with the vertebral l column and the whole cartilage has shifted so that the ilium is associated with I the first three sacral vertebrae (Figs. 146 and 147).
Fig. 148 Right os coxae (innominate bone) of new-born child. Bonnet. Bone is indicated by darker areas/ cartilage by lighter areas.
Ossification begins at three centers which correspond to the ilium, ischium and pubis; the center for the ilium appears during the eighth week, the centers j for the ischium and pubis several weeks later (Fig. 148). The process of ossifi: cation is slow, and is far from complete at the time of birth, for at that time the entire crest of the ilium, the bottom of the acetabulum and all the region ventral to the obturator foramen are cartilaginous. During the eighth or ninth year the ventral parts of the pubis and ischium become partly ossified, but up to the time of puberty the pubis, ischium and ilium remain separated by plates of cartilage which radiate from a common center at the bottom of the acetabulum. Soon after this, the three bones unite to form the single os coxae, leaving only the crest of the ilium, the pubic tubercle and the sciatic tuber (tuberosity of the ; ischium) cartilaginous. In each of these regions an accessory ossification center appears and finally fuses with the corresponding bone about the twentyfourth year.
The femur, tibia and fibula are preformed in cartilage. In the femur a center of ossification appears about the end of the sixth week and gives rise to the shaft; similar centers appear in the tibia and fibula during the seventh and eighth week, respectively. In the femur a distal epiphyseal center appears shortly before birth, and during the first year after birth a proximal center appears for the head. These centers do not unite with the shaft until the individual ceases to grow. The great and lesser trochanters also have accessory ossification centers. In the tibia the center of ossification for the proximal epiphysis appears about the time of birth, the one for the distal during the second year. In the fibula the epiphyseal centers appear during the second and sixth years after birth. The cartilage of the patella appears during the third or fourth month of foetal life, and ossification begins two or three years after birth.
Fig. 149. Diagram of cartilages of left leg and foot of human embryo of 17 mm. Hagen.
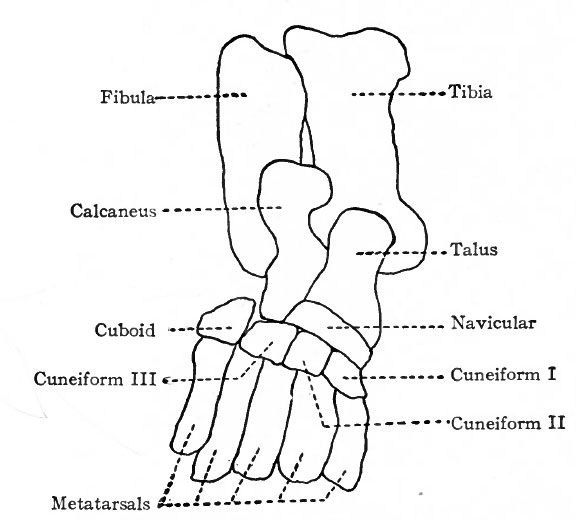
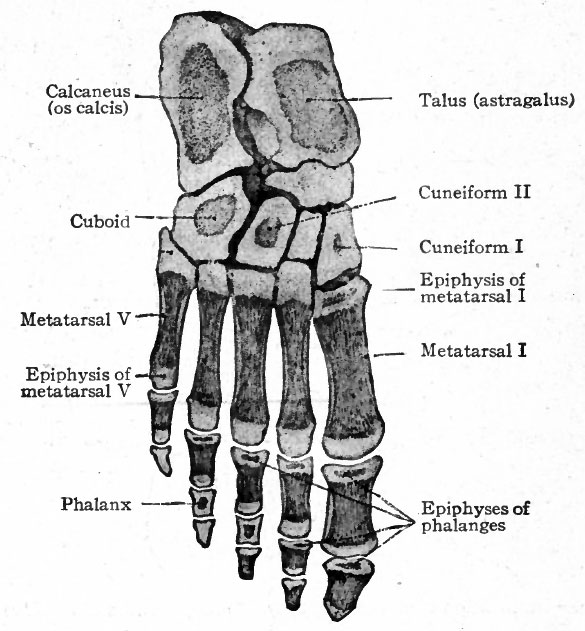
The bones of the tarsus, like those of the carpus, are preformed in pieces of cartilage which are arranged in two transverse rows. The proximal row consists of three pieces, one at the end of the tibia (tibial), one at the end of the fibula (fibular), and the third between the two (intermedial) . At an early stage the tibial and intermedial fuse to form a single piece of cartilage which corresponds to the talus (astragalus) bone. The fibular cartilage corresponds to the calcaneus (os calcis). The distal row is composed of four pieces of cartilage which correspond to the first cuneiform (internal), second cuneiform (middle), third cuneiform (external), and cuboid (Fig. 149). Between the two rows is a piece of cartilage which corresponds to the navicular (scaphoid). Ossification begins relatively late in the metatarsals. A center for the calcaneus appears during the sixth month of foetal life, and one for the talus shortly before birth. Centers appear in the cuboid and third cuneiform during the first year after birth, and in the first cuneiform, navicular and second cuneiform in order during the third and fourth years (Figs. 150 and 151). At the age of puberty ossification is nearly complete in all the metatarsals. In the talus two centers, corresponding to the tibial and intermedial, appear, but soon fuse into a single center. Occasionally the intermedial remains separate and forms the trigonum.
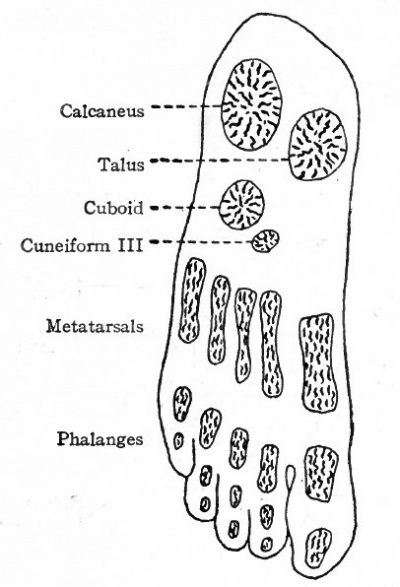
Fig. 150. Ossification centers in foot of a child 9 months old. Hasselwander. An accessory center appears in the calcaneus at the insertion of the tendon of Achilles.
The metatarsals and phalanges develop in a manner corresponding to the metacarpals and phalanges (of fingers). Ossification begins in the metatarsals about the ninth week, in the first row of (proximal) phalanges about the thirteenth week, in the second row about the sixteenth week and in the third row (distal) about the beginning of the ninth week. Epiphyseal centers appear from the second to the eighth year after birth.
Development of Joints
The embryonic connective tissue from which the connective tissues, including cartilage and bone, are developed, at first forms a continuous mass. When cartilage appears it may form a continuous mass, as in the chondrocranium, or it may form a number of distinct and separate pieces, as in the vertebral column, the pieces being united by a certain amount of the undifferentiated embryonic connective tissue.
Synarthrosis
Syndesmosis
When ossification begins at one or more centers, either in cartilage or in embryonic connective tissue, the centers gradually enlarge and approach each other, and the bone so formed comes in contact with the bone formed in neighboring centers, (a) In a case where more than one center appears for any single adult bone, they may come in contact and fuse so completely that the line of fusion becomes indistinguishable, (b) In the case of adjacent bones the fusion may not be so complete; that is, the two bones may simply articulate, leaving a visible line of junction or suture. Such joints are immovable and are represented in the sutures of the skull.
Fig. 151. Skeleton of right foot of a boy 3 years old, showing ossification centers. Toldt.
Synchondrosis
In some cases a small amount of embryonic connective tissue remains between adjacent bones, (a) In time, this embryonic connective tissue gives rise to cartilage which unites the bones quite firmly, thus producing a practically immovable joint, as in the case of the sacro-iliac joint, (b) Or the cells in the center of the cartilage disintegrate or become liquefied so that a small cavity is produced (articular cavity). This type of joint makes possible a slight degree of mobility and is exemplified by the symphysis of the pubic bones. Such a type is also represented by the joints of the vertebral column. In place of cavities, however, are the pulpy nuclei which are remnants of the notochord.
Diarthrosis
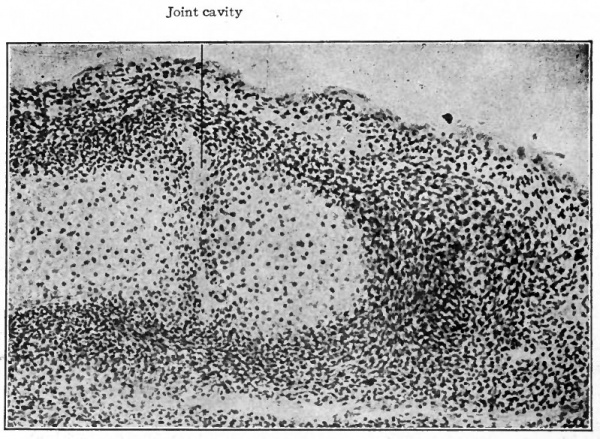
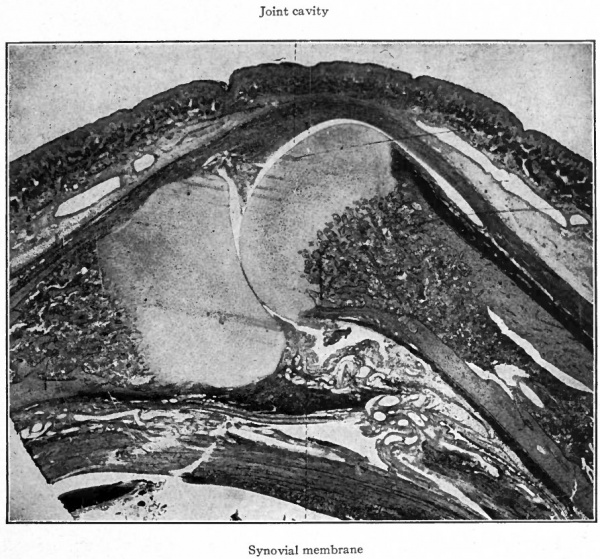
Where a great degree of mobility is necessary, the arrangement of the joint is different. The cells in the central part of the embryonic connective tissue between the ends of adjacent bones (or cartilages) (Fig. 152) liquefy so that a relatively large cavity, the joint cavity, is formed (Fig. 153). The liquefaction of the connective tissue cells may also extend for a short distance along the sides of the bones so that the joint cavity surrounds the ends of the bones (Figs. 154 and 155). The origin of the synovial fluid is not known with certainty, but it is probably in part the product of liquefaction of the connective tissue cells. The more peripheral part of the connective tissue which encloses the joint cavity is transformed into a dense fibrous tissue, the joint capsule. The cells lining the cavity become differentiated into oval or irregular cells, among which is a considerable amount of intercellular substance. By some it is held that these cells form a continuous single layer like endothelium, but the most recent researches tend to disprove this. The cells lining the cavity are the most highly differentiated, the cell bodies being large and apparently swollen, and there is gradually less differentiation as the distance from the surface increases, until finally they merge with the ordinary type of connective tissue cells of the joint capsule (Clarke). The more mobile joints of the body are all representatives of this type.
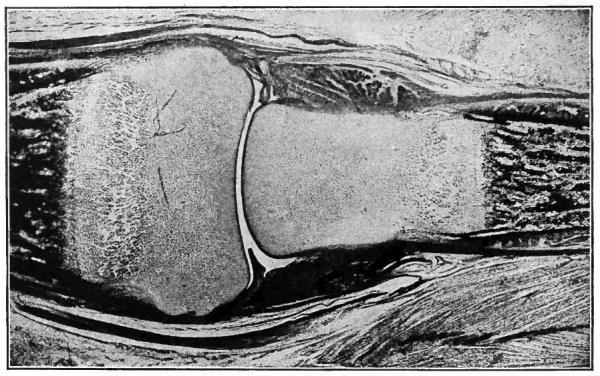
Fig. 152. Section through axilla and arm of a human embryo of 26 mm. (2 months). Photograph. Note the mesenchymal tissue between the humerus and the radius the site of the elbow joint.
Fig. 153. Longitudinal section of finger of human embryo of 26 mm. (2 months), showing beginning of joint cavity between adjacent ends of phalanges. (Photograph from preparation by Dr. W. C. Clarke.)
Fig. 154. From longitudinal section of finger of child at birth, showing developing joint cavity between adjacent ends of phalanges. The darker portion at each end of the figure indicates the ossification center in the phalanx, the end of the latter (lighter area) being yet cartilaginous. The dark bands at each side of the joint indicate developing ligaments. Photograph.
Fig. 155. From longitudinal section of finger of child at birth, showing joint cavity and synovial membrane between adjacent ends of the first metacarpal and proximal phalanx. Other description same as in Fig. 154. Photograph.
Anomalies
The Axial Skeleton
The Vertebrae
The number of cervical vertebrae in man is remarkably constant. Cases where the number is but six are extremely rare. The thoracic vertebrae may vary in number in different individuals from eleven to thirteen, twelve being the usual number. The lumbar vertebrae may vary from four to six, five being the usual number. The sacral vertebrae, fused in the adult to form the sacrum, are usually five in number, sometimes four, sometimes six. Occasionally a vertebra between the lumbar region and sacral region lumbo-sacral vertebra possesses both lumbar and sacral characters, one side being fused with the sacrum, the other side having a free transverse process. Variation occurs frequently in the coccygeal vertebrae; four and five are present with about equal frequency, more rarely there are only three.
The total number of true (presacral) vertebrae may be diminished by one or increased by one. In the former case the first sacral is the twenty-fourth vertebra, and, if the number of ribs remains normal, there are only four lumbar vertebrae. In case the total number is increased by one, the first sacral is the twenty-sixth vertebra, and there are twelve thoracic and six lumbar or thirteen thoracic and five lumbar.
From these facts it is seen that variation occurs most frequently in the more caudal portion of the vertebral column in the lumbar, sacral and coccygeal regions. According to a hypothesis advanced by Rosenberg, the sacrum in the earlier embryonic stages is composed of a more caudal set of vertebrae than those which belong to it in the adult, and during development lumbar vertebrae are converted into sacral and sacral vertebrae into coccygeal. In other words, the hip bone moves headward during development and finally becomes attached to vertebrae which are situated more cranially than those with which it was primarily associated. This change in the position of the pelvic attachment, and the corresponding reduction in the total number of vertebrae, during the development of the individual (i.e., during ontogenetic development) is believed to correspond to a similar change in position during the evolution of the race (i.e., during phylogenetic development).
According to Rosenberg, variation in the adult is due largely to a failure during ontogeny to carry the processes of reduction in the number of vertebrae as far as they are usually carried in the race, or to their being carried beyond this point.
The coccygeal vertebrae apparently represent remnants of the more extensively developed caudal vertebrae in lower forms. In human embryos of 8 to 16 mm., when the caudal appendage is at the height of its development, there are usually seven anlagen of coccygeal vertebrae. During later development this number becomes reduced by fusion of the more distally situated anlagen to the smaller number in the adult. This process of reduction varies in different individuals, so that five or four, rarely three, coccygeal vertebrae may be the result. In cases where children are born with distinct caudal appendages there is no good evidence that the number of coccygeal vertebrae is increased, although the coccyx may extend into the appendage.
The Ribs
Occasionally in the adult a rib is present on one side or on each side in connection with the seventh cervical vertebra (cervical rib), or in connection with the first lumbar vertebra (lumbar rib) . There seems to be no case on record where cervical and lumbar ribs are present in the same individual. The cervical rib may vary between a small piece of bone connected with the transverse process of the vertebra and a well developed structure long enough to reach the sternum. There are also great variations in the size of the lumbar rib. In case the number of ribs is normal, the last (twelfth) may be rudimentary.
The eighth costal cartilage not infrequently unites with the sternum. Occasionally the seventh costal cartilage fails to fuse with the sternum, owing to the shortening of the latter, but meets and fuses with its fellow of the opposite side in the midventral line.
The above mentioned anomalies can be referred back to aberrant development. Primarily costal processes appear in connection with the cervical, lumbar and sacral vertebrae. Normally these processes fuse with and finally form parts of the vertebrae (p. 153). In some cases, however, the seventh cervical or the first lumbar processes develop more fully and form more or less distinct ribs.
As an explanation of these variations in the number of ribs, it has been suggested that there is a tendency toward reduction in the total number of ribs, and that supernumerary ribs represent the result of a failure to carry the reduction as far as the normal number. In case the twelfth rib is rudimentary, the reduction has been carried beyond the normal limit. This hypothesis is a corollary to the hypothesis regarding the variations in the number of vertebrae. (See under "The Vertebrae.")
The Sternum
Certain anomalous conditions of the sternum can also be explained by reference to development. The condition known as cleft sternum, in which the sternum is partially or wholly divided into two longitudinal bars by a medial fissure, represents the result of a failure of the two bars to unite in the midventral line (p. 153, see also Fig. 130). This is sometimes associated with ectopia cordis (p. 255). The xyphoid process may also be bifurcated or perforated, according to the degree of fusion between the two primary bars
(P- I54)
Suprasternal bones may be present. They represent the ossified episternal cartilages which have failed to unite with the manubrium (p. 154). Morphologically the suprasternal bones possibly represent the omosternum, a bone situated cranially to the manubrium in some of the lower Mammals.
The Head Skeleton
The skull is sometimes decidedly asymmetrical. Probably no skull is perfectly symmetrical. The condition which most frequently accompanies the irregular forms of skulls is premature synosteosis or premature closure of certain sutures. The cranial bones increase in size principally at their margins, and when a suture is prematurely closed the growth of the skull in a direction at right angles to the line of suture is interfered with. Consequently compensatory growth must take place in other directions. Thus if the sagittal suture is prematurely closed and transverse growth prevented, increase occurs in the vertical and longitudinal directions. This results in the vault of the skull becoming heightened and elongated, like an inverted skiff, a condition known as scaphocephaly. After premature closure of the coronal suture, growth takes place principally upward and gives rise to acrocephaly. In case only one-half the coronal or lambdoidal suture is closed, the growth is oblique and results in plagiocephaly.
A suture the metopic suture sometimes exists in the medial line between the two halves of the frontal bone, a condition known as metopism. This is due to an imperfect union of the two plates of bone produced by the two centers of ossification in the frontal region (p. 162).
Certain malformations in the face region and in the roof of the mouth are brought about by defective fusion or complete absence of fusion between certain structures during the earlier embryonic stages. The maxillary process of the first branchial arch sometimes fails to unite with the middle nasal process (Kolliker's view, p. 164; see also Fig. 98). The result is a fissure in the upper lip, a condition known as hare lip, which may or may not be accompanied by a cleft in the alveolar process of the maxilla, extending as far as the incisive (palatine) foramen. The same result may be produced by a defective fusion between the middle nasal process and the lateral nasal process (Albrecht's view, p. 164; see also Fig. 98). Hare lip may be either unilateral (single) or bilateral (double), accordingly as defective fusion occurs on one or both sides, but never medial.
Occasionally the palatine process of the maxillary process fails to meet not only its fellow of the opposite side, but also the vomer (see Fig. 141) . The result is a cleft in the hard palate, a condition known as cleft palate. This malformation may be unilateral or bilateral, but not medial. Sometimes the cleft extends into the soft palate where it occupies, however, a medial position.
Cleft palate may accompany hare lip, or either may exist without the other, depending upon the degree of fusion between the processes mentioned above. In bilateral hare lip, with or without cleft palate, the incisive (intermaxillary) bone is sometimes pushed forward by the vomer and projects beyond the surface of the face, a condition known as "wolf's snout."
The causes underlying the origin of harelip and cleft palate are obscure.
The Appendicular Skeleton
The Humerus
On the medial side of the humerus, just proximal to the medial condyle, there is not infrequently a small hook-like process directed distally the supracondyloid process. This process represents a portion of bone which in some of the lower mammals (cat, for example) joins the internal condyle and completes the supracondyloid foramen, through which the median nerve and brachial artery pass.
The Carpal Bones
Occasionally an os centrale is present in addition to the usual carpal bones. It is situated on the dorsal side of the wrist between the navicular, capitate and small multangulum. In the embryo an additional piece of cartilage is of constant occurrence in this location, but usually disappears during later development; in cases where it persists, ossification takes place to form the os centrale. In some of the apes the os centrale is of constant occurrence in the adult.
The Femur
The gluteal tuberosity (ridge) sometimes projects like a comb, forming the so-called third trochanter, a structure homologous with the third trochanter in the horse and some other mammals.
The Tarsal Bones
Cases have been recorded in which the total number of tarsal bones was reduced, owing to congenital synosteosis (fusion) of the calcaneus (os calcis) and scaphoid (navicular), of the talus (astragalus) and calcaneus, or of the talus and scaphoid. Occasionally an additional bone the trigonum is present at the back of the talus. In the embryo, the talus ossifies from two centers which normally fuse at an early stage into a single center. The trigonum probably represents a bone produced by one of the centers which has remained separate.
Polydactyly
This anomaly consists of an increase in the number of fingers or toes, or both. Any degree of variation may exist from a supernumerary finger or toe to a double complement of fingers or toes. The causes underlying the origin of such anomalies are not clear. Some assign the supernumerary digits to the category of pathological growths or neoplasms, linking them with partial duplicate formations. Others explain the extra digits on the ground of atavism or reversion to an ancestral type. The latter explanation assumes an ancestral type with more than five digits. But neither zoology nor paleontology has found any vertebrate form, above the Fishes, which normally possesses more than five digits on each extremity. Consequently one must refer to the Fishes for some ancestral type to explain the existence of more than five digits. Going back so far in phylogenetic history, no certainty whatever can be attached to the origin of supernumerary digits, for it is not even known from what fins the extremities of the higher forms are derived. Still another view regarding the origin of supernumerary digits is that they are due to certain external influences among which the most important is the mechanical impression of amniotic folds or bands. This, however, could not be the sole cause of polydactylism, since such malformations are common in amphibian embryos where no amnion is present.
- Next: Vascular
References for Further Study
ADOLPHI, H. : Ueber die Variationen des Brustkorbes und der Wirbelsaule des Menschen. Morph. Jahrbuch, Bd. XXIII, 1905.
BADE, P. : Die Entwickelung des menschlichen Skeletts bis zur Geburt. Arch. f. mik. Anat., Bd. LV, 1900.
AREY, LESLIE B.: The Origin, Growth and Fate of Osteoclasts and their Relation to Bone Resorption. American Jour, of Anat., Vol. XXVI, No. 3, 1920.
BARDEEN, C. R.: Numerical Vertebral Variations in the Human Adult and Embryo. Anat. Anz., Bd. XXV, 1904.
Bardeen CR. Studies of the development of the human skeleton. (1905) Amer. J Anat. 4:265-302.
Bardeen CR. Development of the thoracic vertebrae in man. (1905) Amer. J Anat. 4: 163-174.
BARTELS, M.: Ueber Menschenschwanze. Arch. f. Anthropol., Bd. XII.
BELL, E. T.: II. On the Histogenesis of the Adipose Tissue of the Ox. American Jour. of. Anat., Vol. IX, 1909.
BOLL, F.: Die Entwickelung des fibrillaren Bindegewebes. Arch. /. mik. Anat., Bd. VIII, 1872.
BOLK, L. : Beziehungen zwischen Skelett, Muskulatur und Nerven der Extremitaten, etc. Morph. Jahrbuch, Bd. XXI, 1894.
BONNET, R.rLehrbuch der Entwickelungsgeschichte. Berlin, 1907.
BRAUS, H.: Die Entwickelung der Form der Extremitaten und des Extremitatenskeletts. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere, Bd. Ill, Teil II, 1904.
BROWN, ALFRED J.: The Development of the Vertebral Column in the Domestic Cat. Anat. Record, Vol. X, No. 3, 1916.
CAREY, EBEN J.: Studies in the Dynamics of Histogenesis. American Jour, of Anat., Vol. XXIX, No. i, 1921.
FAWCETT E.: On the Early Stages in the Ossification of the Pterygoid Plates of the Sphenoid Bone of Man. Anat. Anz., Bd. XXVI, 1905.
FAWCETT, E.: Ossification of the Lower Jaw in Man. Jour. Amer. Med. Assoc., Bd. XLV, 1905.
FAWCETT, E.: On the Development, Ossification and Growth of the Palate Bone. Jour, of Anat. and Physiol., Bd. XL, 1906.
FERGUSON, JEREMIAH S.: The Behavior and Relations of Living Connective Tissue Cells in the Fins of Fish Embryos with Special Reference, to the Histogenesis of the Collaginous or White Fibers. American Jour, of Anat., Vol. XIII, No. 2, 1912.
FLEMMING, W.: Die Histogenese der Stiitzsubstanzen der Bindesubstanzgruppe. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere, Bd. Ill, Teil II, 1901.
FLEMMING, W.: Morphologic der Zelle. Ergebnisse der Anat. u. Entwick., Bd. VII, 1897.
GAUPP, E.: Alte Probleme und neuere Arbeiten iiber den Wirbeltierschadel. Ergebnisse der Anat. u. Entwick., Bd. X, 1901.
GAUPP, E.: Die Entwickelung des Kopfskeletts. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere, Bd. Ill, Teil II, 1905.
GEGENBAUR, C.: Die Metamerie des Kopfes und die Wirbeltheorie des Kopfskeletts. Morph. Jahrbuch, Bd. XIII, 1887.
GR^FENBERG, E.: Die Entwickelung der Knochen, Muskeln und Nerven der Hand und der fur die Bewegungen der Hand bestimmten Muskeln des Unterarms. Anat. Hefte, Heft XC, 1905.
HAGEN, W. : Die Bildung des Knorpelskeletts beim menschlichen Embryonen. Arch. f. Anat. u. PhysioL, Anal. Abth., 1900.
HANSEN, C.: Ueber die Genese einiger Bindegewebsgrundsubstanzen. Anat. Anz., Ed. XVI, 1899.
HANSON, FRANK BLAIR: The Ontogeny and Phylogeny of the Sternum. American Jour, of Anat., Vol. XXVI, No. i, 1919.
HASSELWANDER, A.: Untersuchungen iiber die Ossification des menschlichen Fussskeletts. Zeitschr. f. Morphol. u. AnthropoL, Bd. V, 1903.
HERTWIG, O.:Lehrbuch der Entwickelungsgeschichte des Menschen u. der Wirbeltiere. Jena, 1906.
HUNTINGTON, G. S.: Modern Problems of Evolution, Variation, and Inheritance in the Anatomical Part of the Medical Curriculum. Anat. Record, Vol. XIV, No. 6, 1918.
JAKOBY, M.: Beitrag zur Kenntniss des menschlichen Primordialcraniums. Arch. f. mik. Anat., Ed. XLIV, 1894.
JORDAN, H. E.: A Contribution to the Problems Concerning the Origin, Genetic Relationship and Function of the Giant-cells of Hemopoietic and Osteolytic Foci. American Jour, of Anat., Vol. XXIV, No. 2, 1918.
KEIBEL, F. : Ueber den Schwanz des menschlichen Embryo. Arch.f. Anat. u. PhysioL, Anat. Abth., 1891.
KEIBEL, F.: Zur Entwickelungsgeschichte der Chorda bei Saugern. Arch.f. Anat. u. PhysioL, Anat. Abth., 1889.
Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia. Chap. XI.
KJELLBERG, K.: Beitrage zur Entwickelungsgeschichte des Kiefergelenks. Morph. Jahrbuch, Bd. XXXII, 1904.
KOCH, JOHN C.: The Laws of Bone Architecture. American Jour, of Anat., Vol. XXI, No. 2, 1917.
KOLLMANN, J.: Entwickelung der Chorda dorsalis bei dem Menschen. Anat. Anz., Bd. V, 1890.
KOLLMANN, J.: Lehrbuch der Entwickelungsgeschichte des Menschen. Jena, 1898.
KOLLMANN, J.: Handatlas der Entwickelungsgeschichte des Menschen. Jena, 1907.
Mall FP. Development of the connective tissues from the connective syncytium. (1902) Amer. J Anat. 1(3): 329-366
Mall FP. On ossification centers in human embryos less than one hundred days old. (1906) Amer. J Anat. 5:433-458.
McMurrich JP. The Development Of The Human Body. (1914) P. Blakiston's Son & Co., Philadelphia, Pennsylvania.
Paterson The sternum - its early development and ossification in man and mammals. (1900) J Anat Physiol. 35(1): 21-32 PMID 17232454
PETERSEN, H.: Untersuchungen zur Entwickelung des menschlichen Beckens. Arch, f. Anat. u. PhysioL, Anat. Abth., 1893.
RABL, C.: Theorie des Mesoderms. Morph. Jahrbuch, Bd. XV, 1889.
ROSENBERG, E.: Ueber die Entwickelung der Wirbelsaule und das Centrale carpi des Menschen. Morph. Jahrbuch, Bd. I, 1876.
SCHAUINSLAND, H.: Die Entwickelung der Wirbelsaule nebst Rippen und Brustbein. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere, Bd. Ill, Teil II, 1905.
SPULER, A.: Beitrage zur Histologie und Histogenese der Binde- und Stutzsubstanz. Anat. Hefte, Heft XXI, 1896.
THILENIUS, G.: Untersuchungen iiber die morphologische Bedeutung accessorischer Elemente am menschlichen Carpus (und Tarsus). Morph. Arbeiten, Bd. V, 1896.
Thomson A. The sexual differences of the fetal pelvis. (1899) J Anat Physiol. 33(3): 359-380.
TORNIER, G.: Das Entstehen der Gelenkformen. Arch. f. Entw.-Mechanik, Bd. I, 1895.
WALDEYER, W.: Kittsubstanz und Grundsubstanz, Epithel und.Endothel. Arch. f. mik. Anat., Bd. LVII, 1900.
WEISS, A.: Die Entwickelung der Wirbelsaule der weissen Ratte, besonders der vordersten Halswirbel. Zeitschr. f. wissensch. Zool., Bd. LXIX, 1901.
ZIMMERMANN, K.: Ueber Kopfhohlenrudimente beim Menschen. Arch./, mik. Anat., Bd. LIII, 1899.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Text-Book of Embryology: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 7) Embryology Book - Text-Book of Embryology 9. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Text-Book_of_Embryology_9
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G