Book - Text-Book of Embryology 12
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bailey FR. and Miller AM. Text-Book of Embryology (1921) New York: William Wood and Co.
- Contents: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Alimentary Tube and Appended Organs
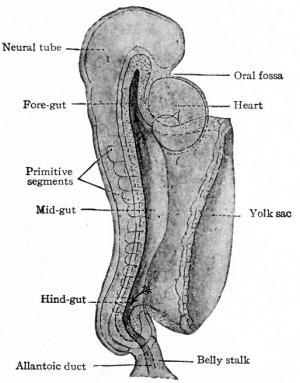
The embryonic disk, composed of the three germ layers, primarily lies flat upon the yolk sac (see p. 107; also Fig. 75). A little later the axial portion of the embryo is indicated by the primitive streak, the neural groove (subsequently the neural tube), the notochord, and the primitive segments (Fig. 71). Then along each side of the axial portion and at the cephalic and caudal ends, the germ layers bend ventrally and medially and finally meet and fuse in the midventral line (p. 109) . The portion of the entoderm ventral to the notochord is bent into a tube which, for the most part, becomes pinched off from the parent entoderm and is suspended in the embryonic coelom by the common mesentery (Figs. 103 and 104). This entodermal tube is the primitive gut. At first it ir but slightly elongated and is closed at both ends. On the ventral side, however it opens widely into the yolk sac (Figs. 244 and 245). The primitive gut, therefore, has no communication with the exterior. It communicates at its caudal end with the central canal of the spinal cord through the neurenteric canal (Fig. 76; compare with 77).
As development proceeds, this simple tube elongates rapidly and becomes differentiated into distinct regions. The cephalic end, in connection with the branchial arches and grooves, becomes the dilated pharyngeal region. Caudal to and continuous with this, is the short, narrow cesophageal region which in turn passes over into the slightly dilated stomach region. The portion of the gut caudal to the stomach is the intestinal region. During the differential changes, the communication with the yolk sac becomes relatively smaller, forming the yolk stalk which joins the intestinal portion a short distance caudal to the stomach (Figs. 246 and 247).
Fig. 245. Ventral view of human embryo of 2.4 mm. His, Kollmann.
- Note the opening in the ventral wall of the gut. This indicates the communication between the gut and the yolk sac. The latter has been removed. Compare with Fig. 244.
The Mouth
At a very early period the primary fore-brain region bends ventrally almost at a right angle to the long axis of the body to form the naso-frontal process.
As the first branchial arch develops, it grows ventrally until it meets and fuses with its fellow of the opposite side in the midventral line, thus forming the mandibular process. From the cephalic side of the first arch a secondary process maxillary process develops and fills in the space between the arch itself and the naso-frontal process. These various structures thus bound a distinct depression on the ventral side of the head. This depression is the oral pit, the forerunner of the oral and nasal cavities (Fig. 245; compare with Figs. 244 and 85). The groove in the midventral line between the mandibular processes marks the symphysis of the lower jaws. The groove on each side between the maxillary process and the mandibular process marks the angle of the mouth. The groove between the maxillary process and the naso-frontal process is the naso-optic furrow, at the dorsal end of which the eye develops. The bottom of the oral pit is formed by a portion of the ventral body wall, which separates the oral cavity from the cephalic end of the gut, and which is composed of ectoderm and entoderm, with a small amount of mesoderm between. This closing plate, the pharyngeal membrane, which is still present in I embryos of 2.15 mm., soon becomes thinner and finally breaks away, leaving I the oral pit and the gut in direct communication (Fig. 247). Since the oral pit , is lined with ectoderm, the epithelial lining of the mouth or oral cavity is largely of ectodermal origin. In the medial line of the roof of the oral cavity, near the pharyngeal membrane, the epithelium (ectoderm) evaginates to form Rathke's pocket. This comes in contact with an evagination from the floor of the brain and with it forms the pituitary body.
Fig. 246. Alimentary tube of a human embryo of 4.1 mm. His Kollmann.
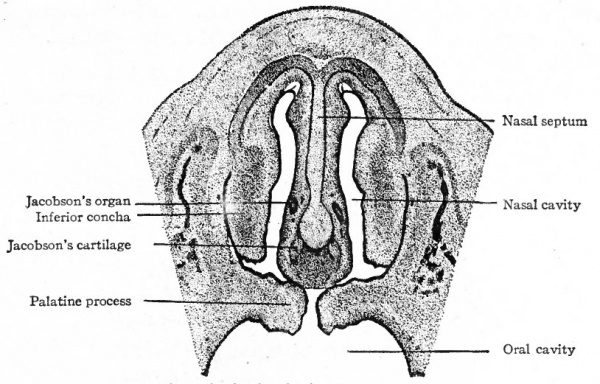
The further development of the mouth consists of an elaboration of the structures which primarily bound the oral pit and the growth of certain new structures such as the teeth and the tongue. The first branchial arch fuses with its fellow of the opposite side in the midventral line to form the symphysis of the lower jaws, giving rise also to the lower lip and chin region. As the nasofrontal process continues to grow, two depressions appear on its ventral border, one on each side, a short distance from the medial line. These depressions are the nasal pits which indicate the beginning of the external openings of the nasal passages. The part between the nasal pits is destined to give rise to the nasal septum and the medial part of the upper lip (Fig. 98). The primary oral cavity is divided into the oral cavity proper and the nasal cavity by outgrowths from the maxillary processes. From the medial side of each maxillary process a plate-like structure grows across the primary oral cavity toward the medial line (Fig. 140). These two plates, or palatine processes, meet and fuse with the lower part of the nasal septum (Fig. 248) . (For further details of this fusion, see page 121 and page 163). The palatine processes thus form the palate, or the roof of the mouth, which separates the mouth cavity from the nasal cavity. The palate does not extend far enough backward, however, to separate the posterior part of the nasal cavity from the pharynx. Thus the posterior nares and pharynx are left in communication. Externally the maxillary processes extend medially, separate the nasal pits from the oral cavity, and form the lateral portions of the upper lip (Fig. 99).
Fig. 247. Sagittal section of reconstruction of a human embryo of 5 mm. His, Kollmann.
Fig. 248. From a section through the head of a human embryo of 28 mm. Showing the nasal septum, the nasal cavities, the oral cavity, and the palatine processes. Peter.
The Tongue
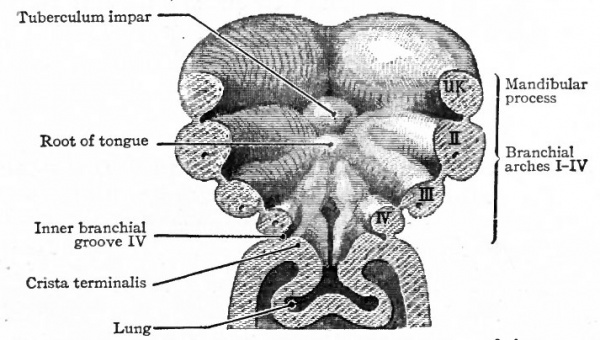
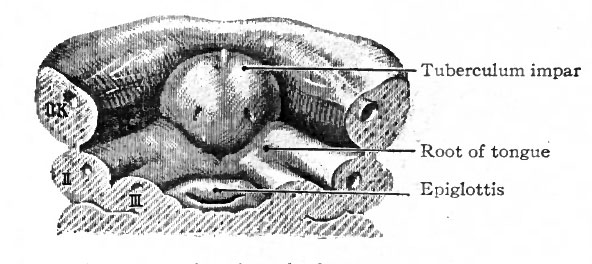
The tongue develops from three separate anlagen which unite secondarily. In embryos of about 3 mm a slight elevation appears on the floor of the pharynx in the region of the first branchial arch. This is the tuberculum impar, being, as the name indicates, unpaired, and is destined to give rise to the tip and body of the tongue (Fig. 249). Soon afterward two bilaterally symmetrical elevations appear on the floor of the pharynx, which are destined to give rise to the root of the tongue (Fig. 250). These paired elevations, arising in, the region of the second and third branchial arches, gradually enlarge and unite with each other and with the tuberculum impar, leaving between the latter and themselves, however, a V-shaped groove (Fig. 251). At the apex of the groove there is a depression the foramen cecum lingua which is the external opening of the thyreoglossal duct (see p. 301). The groove later disappears, but its position is indicated in the adult by the vallate papillae.
Fig. 249. Floor of the pharyngeal region of a human embryo of about 3 weeks. Wilhelm His (1831-1904).
According to Hammar, the tuberculum impar is a transitory structure and does not give rise to the tip and body of the tongue. The tip and body are derived from a much more extensive elevation in the floor of the pharynx.
The tongue as a whole enlarges and grows from its place of origin toward the entrance to the primary oral cavity. For a time it practically fills the cavity. When the palate develops it recedes and finally comes to lie on the floor of the oral cavity proper, as in the adult. The growth of the tongue involves the epithelial lining of the pharynx and oral cavity and also the underlying mesenchymal tissue. The latter produces the connective tissue and at least a part of the intrinsic muscle fibers of the tongue. The papillae involve the epithelium and connective tissue, while the glands and taste buds are derived from the epithelium alone.
Fig. 250. Floor of pharyngeal region of a human embryo of 12.5 mm. His.
The portion of the lingualis muscle innervated by the facial (VII) nerve is probably derived from the mesenchymal tissue in the tongue anlage. The rest of the muscle is innervated by fibers from the hypoglossal (XII) nerve, indicating a possible derivation from certain rudimentary segments in the occipital region which correspond to the three roots of the nerve. This would make it appear that during phylogenesis a part of the lingualis muscle has grown into the tongue from a region caudal to the last branchial arch.
The lingual papilla begin to develop during the third month. Their development is limited to the dorsum of the tongue and to the portion derived from the tuberculum impar. In other regions slight elevations may appear, but not in the form of distinct papillae. The fungijorm and filiform papillae appear as pointed elevations in the connective tissue, which push their way into the epithelium, the latter at the same time being raised above the surface over these points. Gradually the little masses of connective tissue assume the shapes characteristic of fungiform or filiform papillae. During the fifth month the epithelium between the papillae apparently degenerates to some extent, thus leaving them projecting still farther above the surface. The formation of papillae probably goes on for some time after birth, since at birth their form, size, number and arrangement are not the same as at later periods. It is an interesting fact that the filiform papillae lose many of their taste buds after the child is weaned.
The anlage of the vallate papillae appears as a ridge along the V-shaped line of fusion between the paired and unpaired portions of the tongue. The ridge is apparently formed by the ingrowth of a solid mass of epithelium along each side, although the connective tissue between the masses may grow toward the surface to some extent. Later the ridge is broken up into the individual papillae by the ingrowth of the epithelium at certain points. The more superficial cells of the masses then degenerate, thus leaving each papilla surrounded by a trench and wall.
Fig. 251. Dorsal view of the tongue of a human embryo of 20 mm. His, Bonnet.
The development of the lingual glands is confined for the most part to the root and inferior surface and to the region of the vallate papillae. The glands begin to develop during the fourth month as solid ingrowths of epithelium, the mucous glands appearing first, the serous somewhat later. The epithelial masses acquire lumina and grow deeper into the tongue, where they usually branch and coil to form the secreting portions. The latter open to the surface through the original ingrowths which become the ducts. Ebner's glands develop from the bottoms of the trenches around the vallate papillae.
The Teeth
The development of the teeth involves the ectoderm and mesoderm, the former giving rise to the enamel, the latter to the dentine and pulp. In human embryos of 12-15 mm. (thirty-four to forty days), before the lip groove is formed, a thickening of the epithelium (ectoderm) takes place along the edges of the processes that bound the slit-like entrance to the mouth. When the lip groove appears (Fig. 140), the epithelial thickening comes to lie along the edge of the jaw, or in other words, along the edge of the gums. It then grows into the mesenchymal tissue (mesoderm) of the jaw obliquely toward the lingual surface to form the dental shelf. A little later the dental groove appears on the edge of the jaw, along the line where the ingrowth of epithelium took place.
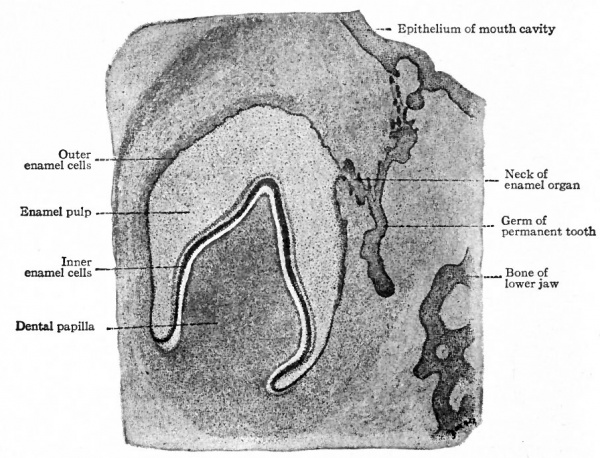
Fig. 252. Section of developing tooth from a 3 months human fetus. Szymonowicz.
Note the portion of the original dental shelf connecting the developing tooth with the epithelium of the mouth cavity.
The dental shelf is at first of uniform thickness, but in a short time five enlargements appear in it in each upper and lower jaw, indicating the beginnings of the milk teeth. When the embryo reaches a length of 40 mm. (an age of eleven to twelve weeks) the mesenchymal tissue on one side of these enlargements (above and to the inner side in the upper jaw, below and to the inner side in the lower jaw) becomes condensed and pushes its way into the epithelium. Each of these mesenchymal ingrowths is a dental papilla. Thus at this stage the anlage of each tooth is a mass of epithelium fitting cap-like over a mesenchymal papilla. The epithelium is the forerunner of the enamel organ; the papilla is destined to give rise to the dentine and pulp. The anlagen are connected with one another by intermediate portions of the dental shelf, and with the surface by the original ingrowth of epithelium.
The Enamel
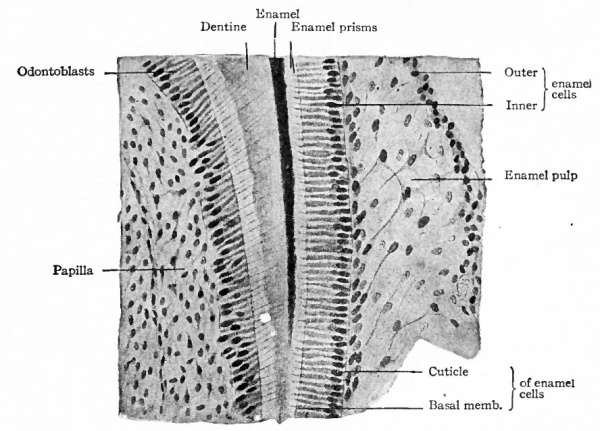
The epithelial cells nearest the dental papilla become high columnar in shape, forming a single layer. Those in the interior of the mass become separated and changed into irregular, stellate, anastomosing cells, with a fluid intercellular substance, constituting the enamel pulp. Those farthest from the papilla become flattened (Fig. 252 ; compare with Fig. 253). Calcification begins in the basal ends of the columnar cells, or in the ends next the papilla, and in the intercellular substance, and gradually progresses throughout the cells, the latter at the same time becoming much more elongated. Thus the cells are transformed into enamel prisms which are held together by the calcined intercellular substance (Fig. 253).
Fig. 253. Section through the border of a developing tooth of a new-born puppy. Bonnet.
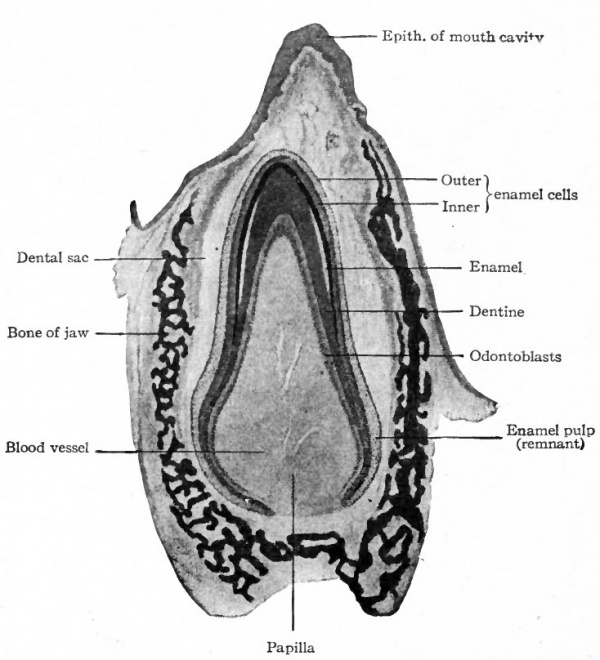
The formation of enamel begins in the milk teeth toward the end of the fourth month and probably continues until the teeth break through the gums. The enamel organ at first surrounds the entire developing tooth except where the papilla joins he underlying mesenchymal tissue (Fig. 252). Later the deeper part of the organ disappears as such, and the enamel is formed only on that part of the tooth which eventually becomes the crown. The enamel pulp increases in amount for a time, but subsequently disappears as the tooth grows into it (Fig. 254). Its function is not fully understood. It may serve as a. line of least resistance in which the tooth grows, and it may convey nourishment to the enamel cells, the enamel organ being non-vascular.
The Dentine and Pulp
At first the dental papilla is simply a condensation of mesenchyme, but later it is converted into a sort of connective tissue penetrated by blood vessels and nerves (Fig. 254). The cells nearest the enamel organ become columnar and arranged in a single layer, with the nuclei toward their inner ends. The outer ends are blunt, while the inner ends are continued as slender processes that extend into the pulp and probably with other cell processes. These columnar cells are the odontoblasts, under the influence of which the lime salts of the dentine are deposited, and which are coi parable with the osteoblasts in developing bone.
Fig. 254. Longitudinal section of a developing tooth of a new-born puppy. Bonnet.
Toward the end of the fourth month the odontoblasts form a membrane like structure, the membrana preformativa, between themselves and the enamel. This membrane is first converted into dentine by the deposition of lime salts, after which the process of calcification progresses from the enamel toward the pulp. During calcification slender processes of the odontoblasts remain in minute channels, or dentinal canals, forming the dentinal fibers which anastomose with one another (Fig. 253). In the peripheral part of the dentine certain areas apparently fail to become calcified and form the inter globular spaces. The same cells that are originally differentiated from the mesenchyme probably persist throughout development as the odontoblasts and produce the entire amount of dentine in a tooth. Even in the fully formed tooth there is a layer of odontoblasts bearing the same relation to the dentine and pulp as in the developing tooth. The chief difference between dentine formation and bone formation is that in the latter the osteoblasts become enclosed to form bone cells, while in the former the odontoblasts merely leave processes enclosed as the cell bodies recede.
The pulp of the tooth is of course derived from the mesenchymal tissue in the interior of the dental papilla (compare Figs. 252 and 254). The blood vessels and nerves grow in from the underlying connective (mesenchymal) tissue.
At an early stage the mesenchymal tissue around the anlage of the tooth, including the enamel organ, condenses to form a sort of sheath, the dental sac, which is later ruptured when the tooth breaks through the gum (Fig. 254). The cement is formed around the root of the tooth from the tissue of the dental sac in the same manner as subperiosteal bone is formed from osteogenetic tissue (p. 142). In fact, cement is true bone without Haversian systems.
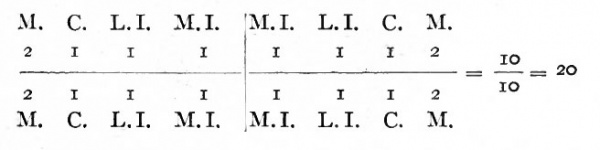
The milk teeth, which are the first to develop and the first to appear above the surface, are represented by the medial incisors, lateral incisors, canines, and molars, to the number of ten in the upper and ten in the lower jaw. They may be indicated graphically thus:
Milk teeth
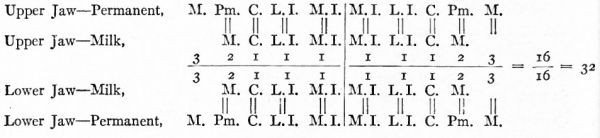
In describing the formation of the dental shelf, it was noted that the papillae of the milk teeth grow into corresponding thickenings of the epithelium (p. 292). The growth takes place from the side, thus leaving the edge of the shelf free to grow farther toward the lingual side of the jaw. In this free edge other tooth germs arise, which mark the beginnings of the permanent teeth (Fig. 252). In addition to the germs that correspond in position to the milk teeth, three others arise in each jaw, representing the true molars of the adult. The latter arise in a part of the dental shelf which has grown toward the articulation of the jaws without coming in contact with the surface epithelium. The first papilla of the permanent dentition to appear is that of the first molar. It appears immediately behind the second milk molar at a time when the milk teeth are well advanced (embryos of 180 mm., about seventeen weeks). The permanent incisors and canines appear about the twenty-fourth week; the premolars, which correspond to the milk molars, about the twenty-ninth week. The second molar does not appear till after birth (six months), and the third molar, or wisdom tooth, begins to develop about the fifth year.
The formation of the anlagen of the permanent teeth and the development of the enamel, dentine and pulp take place in precisely the same manner as in the milk teeth. The true molars grow out through the gums in the same way as the milk teeth. Those permanent teeth which correspond in position to milk teeth grow under the latter, exert pressure on their roots and thus loosen and finally replace them.
The two sets of teeth may be graphically represented thus:
Milk teeth
Permanent teeth
Normally all the epithelium of the dental shelf, except the parts directly concerned in the development of the teeth, disappears at times which vary in different individuals. Occasionally, however, remnants of this epithelium give rise to cystic structures (developmental tooth tumors) .
Fig. 255. From a transverse section through the tongue and oral cavity of a mouse embryo. Goppert.
The Salivary Glands
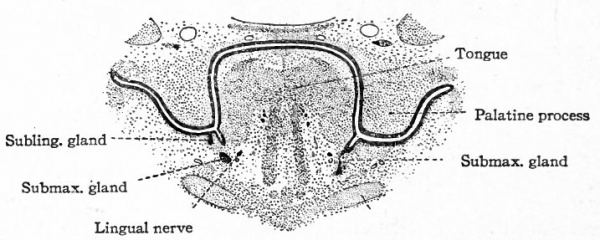
The anlage of the submaxillary gland appears, in embryos of 10 to 12 mm., as a flange of epithelium directed ventrally from the portion of the lingual sulcus just caudal to the crossing of the lingual nerve. The flange grows into the mesenchyme of the lower jaw, and at an early period becomes triangular with its longest side free and a free vertical caudal border. Cell proliferation begins at the angle of union of the two borders and gradually progresses cephalad along the longest border, thus producing a solid ridge-like thickening of the latter.
The main portion of the gland is produced by a sprouting of the epithelium from the angle of union of the two free borders of the flange and grows deep into the mesenchyme along the mesial side of the ramus of the mandible. The sprouts branch repeatedly in the course of their development, thus laying the foundation for the division of the gland into lobes and lobules.
The distal end of the duct of the submaxillary (Wharton's) is formed from the ridge-like thickening of the free margin of the flange through a dissolution of the greater part of the flange between the lingual sulcus and the thickened margin itself, thus freeing this portion of the duct from the sulcus. By a continuation of the growth which produced the ridge along the free border of the original flange an extension of this same ridge is produced along the bottom of the lingual sulcus forward toward the chin region. This portion of the ridge is progressively constricted off from the sulcus from cehind forward, until finally the attachment of the duct reaches its definitive position at the side of the frenulum linguae.
The anlage of the Bartolinian element of the suUingual gland appears as a smaller flange attached to the lateral border of the submaxillary flange near the crossing of the lingual nerve and prolonged forward by an interrupted crest along the lingual sulcus. Its later development is similar to that of the submaxillary.
A small medial flange also on the submaxillary flange gives rise to a sprout in much the same manner as the other anlagen. While the history of this anlage is not complete in the human embryo, it probably gives rise to the anterior lingual gland (gland of Bland in and Nuhn). The alveolingual elements arise from a keel attached to the alveolingual sulcus (the groove between the floor of the mouth and the alveolar process of the lower jaw).
The parotid gland originates from the buccal sulcus in essentially the same way as the submaxillary arises from the lingual sulcus. The anlage then continues to grow through the mesenchyme of the cheek across the masseter muscle, the distal end branching freely to form the secreting portion of the gland. The outgrowths are at first solid, but later become hollow, the proximal portion of the original outgrowth forming the parotid (Steno's) duct, the more distal portions forming the smaller ducts and terminal tubules.
The histogenetic changes in the salivary glands probably continue until the child takes solid food, when the glands become of greater functional importance. In the parotid gland, which is serous in man, the original, undifferentiated epithelial cells undergo changes in form and arrangement so that by the twenty-second week the larger ducts are lined with a two-layered epithelium, the smaller ducts with a simple cuboidal epithelium, and the terminal tubules with a single layer of high columnar cells. The two-layered epithelium in the larger ducts persists. The ducts lined with the cuboidal epithelium become the socalled intermediate tubules, the cells changing to a flat type. The high columnar cells of the terminal tubules become the serous secreting cells.
Quite similar changes also occur in the submaxillary, but in foetuses of eight to nine months the crescents of Gianuzzi appear as masses of darkly staining cells forming the ends or sides of the terminal tubules. The crescents at first border on the lumina, but later, probably by a process of evagination, come to lie on the surface of the tubules.
The beginning of the secretory function may be detected by a diminution in the affinity of the cells for stains.
The Pharynx
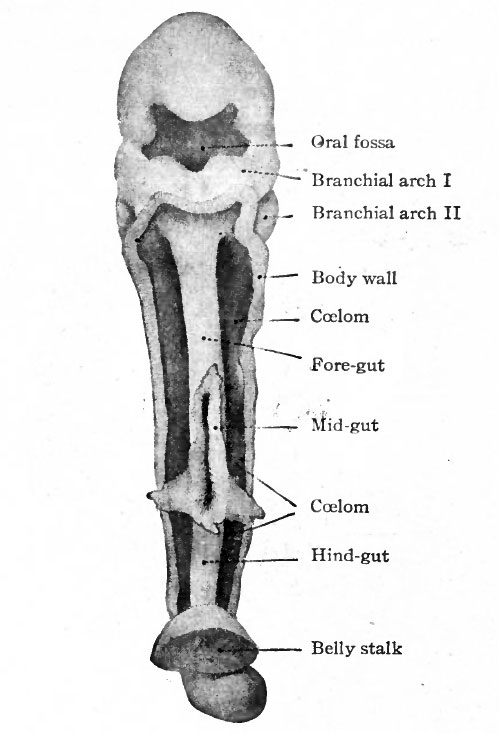
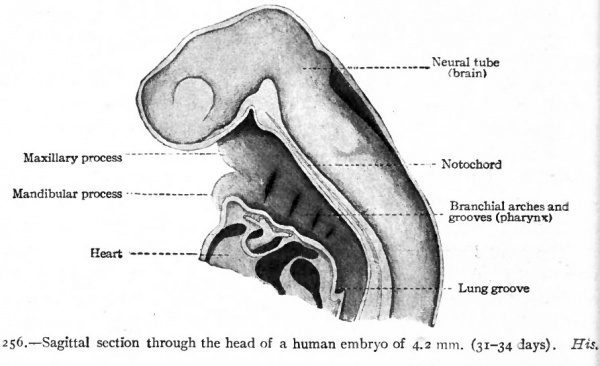
The pharynx develops from the cephalic end of the primitive gut. This part of the gut is primarily of uniform diameter, is broadly attached by mesoderm to the dorsal body wall, and ends blindly (Fig. 247). When the branchial arches and grooves develop in this (the cervical) region, they affect the gut as well as the periphery of the body. The arches form ridges on the surface of the body (Fig. 85) and at the same time form ridges on the wall of the gut. The grooves form pockets which alternate with the arches (Fig. 256). The pock in the pharyngeal cavity, or inner branchial grooves, are directed outward toward corresponding outer branchial grooves (Fig. 249). The arches are covered externally with ectoderm, internally with entoderm, and are filled with mesoderm. Between the arches, or in the grooves, the ectoderm and entoden are in contact or nearly so. Thus the pharynx is not surrounded by a coelomic cavity.
Fig. 256. Sagittal section through the head of a human embryo of 4.2 mm (31-34 days). His
Since the branchial arches develop in such a way that they are successively smaller from the first to the fourth, the pharyngeal cavity becomes funnelshaped (Fig. 256). It also becomes somewhat flattened in the dorso-ventral direction, and in the earlier stages when the arches and grooves are fully formed, the pharynx constitutes approximately one-third the entire gut (Fig. 247). Primarily the pharyngeal cavity is separated from the oral cavity by the pharyngeal membrane (see p. 287 ; also Fig. 244). When this ruptures and disappears (during the fourth week ?) the two cavities are in open communication. What point in the adult represents the attachment of the pharyngeal membrane is not known; but the glosso- and pharyngo-palatine arches (pillars of the fauces) are usually considered as the boundary between the mouth and pharynx. The caudal limit of the pharynx is the opening of the larynx (Figs. 247 and 256).
Thus in the early stages the general adult character of the pharynx is established. While the branchial arches and grooves undergo profound changes, the pharyngeal cavity retains the same relation to the mouth and to the oesophagus and respiratory tract. The cavity becomes relatively shorter, however, and the alternating ridges and pockets in its walls are lost as the arches and grooves are transformed into other structures. The metamorphosis of the arches and grooves is considered elsewhere (p. 118).
The Tonsils
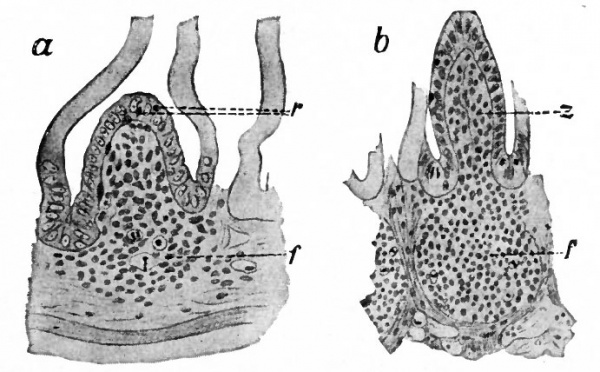
The tonsils arise in the region of the ventral part of the Lsecond inner branchial groove. During the third month the epithelium (entoderm) grows into the underlying connective (mesenchymal) tissue in the form of a hollow bud. From this, secondary buds develop, which are at first solid, but later (during the fourth or fifth month) become hollow by a disappearance of the central cells and open into the cavity of the primary bud, thus forming the crypts. Lymphoid cells wander from the neighboring blood vessels, or are derived directly from the' epithelium- (Retterer), and with the connective tissue form a diffuse lymphatic tissue under the epithelium (Fig. 257). By the eighth month the cells become more numerous in places, and by the third month after birth form distinct lymph follicles with germinal centers. The formation of follicles goes on slowly and is probably not complete until some time after birth.
The Lingual Tonsils
The lymphatic tissue of the tongue develops in relation to the lingual glands. During the eighth month lymphoid infiltration occurs around the ducts of the glands, and the connective tissue acquires the reticular character. True follicles probably do not appear until the child is at least five years old.
The Pharyngeal Tonsils
During the sixth month small folds appear in the mucous membrane of the roof of the pharynx and become diffusely infiltrated with lymphoid cells. This occurs first in the posterior part of the roof, but later (seventh or eighth month) it extends forward and along the sides of the nasopharygeal cavity. By the end of foetal life the ridges become quite large. Follicles may appear before birth or not until one or two years later. After puberty the ridges almost completely disappear, but the adenoid tissue remains wholly or in part.
The bursa pharyngea is an evagination from the roof of the pharynx about the upper border of the superior constrictor muscle, and is apparent in embryos of eleven weeks. It probably has no genetic relation to the hypophysis. Its significance is not known.
Fig. 257. Section through the middle of the developing tonsil of a human embryo of 5 months. Stohr.
- 6, Epithelial buds (secondary outgrowths) from the epithelium lining the primary crypt (c) ; L, lymphoid infiltration of the connective (mesodermal) tissue.
The Branchial Epithelial Bodies
The Thyreoid Gland
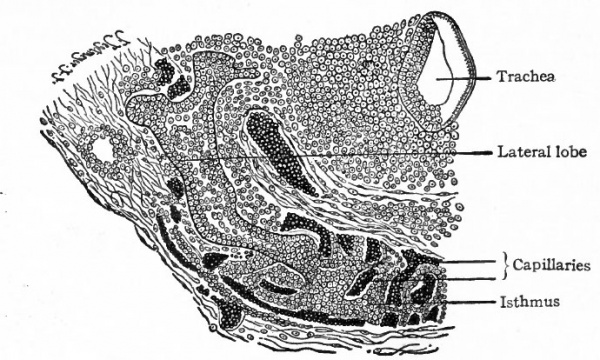
The thyreoid arises, after the manner of ordinary glands, as an evagination from the epithelium of the pharynx. It appears in embryos of 3 to 5 mm as a ventral outgrowth of epithelium in the floor of the pharynx, at the point where the tuberculum impar and the two paired anlagen of the tongue join (Fig. 258). This point is the foramen caecum linguae which has already been mentioned in connection with the development of the tongue (p. 290) . The evagination grows into the mesodermal tissue in the ventral wall of the neck, and forms a transverse mass of epithelium. The latter breaks up into irregular cords of cells which, by a further process of budding, grow cau dally along the ventral surface of the larynx. The cords of cells are from the first surrounded by connective tissue and later also become surrounded by networks of capillaries (Fig. 259). They ultimately break up into smaller masses which become hollow and form the alveoli. Colloid secretion begins toward the end of fcetal life or soon after birth.
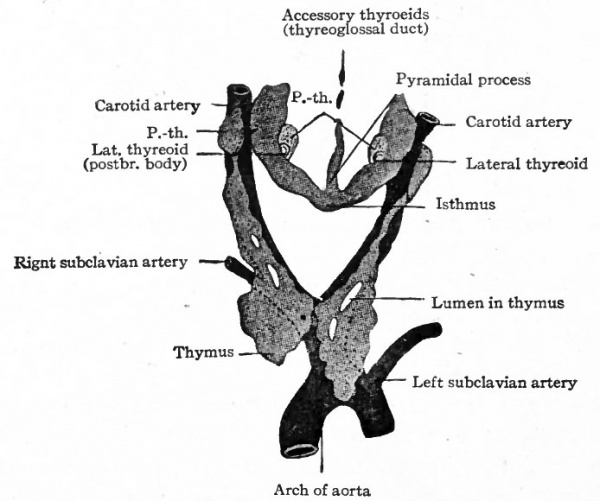
As the gland grows toward its final position it becomes enlarged laterally into the two lateral lobes, which remain connected by the isthmus (Fig. 260). The Pyramidal process represents either a secondary outgrowth from the isthmus or one of the lobes, or a remnant of the original connection with the tongue, that is, of the thyreoglossal duct. The duct usually disappears for the most part, but certain structures sometimes found in the adult in the line of the duct are possibly remnants of it. They have been variously named, according to their position, accessory thyreoid , suprahyoid, and prehyoid glands (Fig. 260).
A pair of structures, appearing first in embryos of 8 to 10 mm., arise as evaginations from the ventral ends of the fourth inner branchial grooves. They grow into the mesodermal tissue and then caudally along the ventro-lateral side of the larynx, where they come into close relation with the lateral lobes of the thyreoid (Fig. 260). They have been called the lateral thyreoids, and acquire the thyreoid structure.
Fig. 258. Transverse section through the region of the 3d branchial groove of an echidna embryo. Maurer. i.= Pharynx, below which are the paired anlagen of the tongue.
Considerable confusion has arisen in regard to the lateral thyreoids. The earlier investigators held that they were derived from the fourth groove and united with the medial portion, which appeared at the foramen caecum, to become integral parts of the thyreoid. Further researches among the lower Vertebrates led others to deny that the thyreoid arose other than as a medial anlage, and that the so-called lateral thyreoids in the embryo were the postbranchial bodies which never assumed the thyreoid structure, but atrophied and disappeared. More recently it has been thought that, although the postbranchial bodies do not function in the lower Vertebrates, they may in the higher Mammals and man unite with the medial thyreoid and secrete colloid.
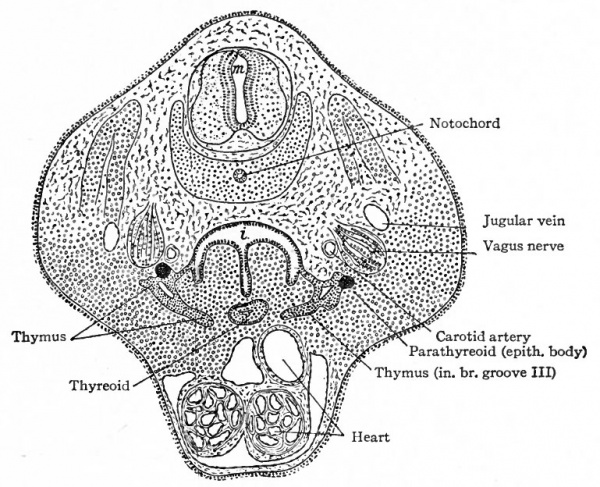
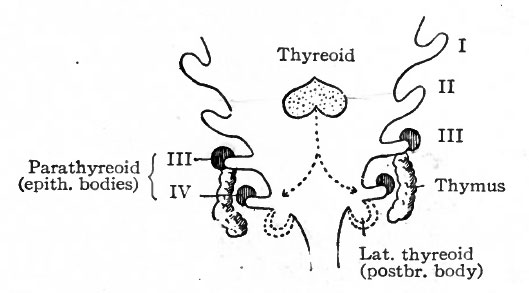
The parathyreoids or epithelial bodies also come into close relation with the thyreoid. They arise as paired evaginations from the cephalic sides of the third and fourth grooves, dorsal to the thymus and the lateral thyreoid evaginations (Figs. 258 and 261). As the thyreoid grows caudally from its point of origin, these bodies come to lie close to it or may even become embedded in it (Fig. 260). They acquire a structure which resembles that of the suprarenal gland and not that of the thyreoid. Their relation to the latter organ seems to be purely topographical.
Fig. 259. Section of the right half of the thyreoid gland of a pig embryo of 22.5 mm. Born.
Fig. 260. Branchial groove derivatives of a rabbit embryo of 16 mm. P.-th., parathyreoid or epithelial body. Verdun, Bonnet.
The Thymus
The thymus appears in embryos of about 6 mm. as an entodermal evagination from the ventral part of the third branchial groove on each side (Fig 258) . The outgrowths are at first hollow and communicate with the pharyngeal cavity; later they become solid and (in embryos of 14 mm.) lose their connection with the parent epithelium. They elongate and grow caudally in the mesodermal tissue until (in embryos of 16 mm.) their caudal ends lie ventral to the carotid arteries (Fig. 260). In embryos of 29 mm. their caudal ends rest upon the cephalic surface of the pericardium, their cephalic ends reaching to the isthmus of the thyreoid. The two parts eventually fuse to a considerable extent, but the gland as a whole always consists of two distinct lobes.
The gland continues to enlarge, at the same time becoming lobulated by the ingrowth of connective tissue, until the child is two or three years old. At this time it is situated in the anterior mediastinum, usually in the medial line. After this it begins to atrophy and becomes a mass of fibrous and fatty tissue through the growth of the interlobular septa and their encroachment upon the lobules. The classical view that the thymus begins to atrophy after the second or third year and is quite degenerated in the adult has recently been somewhat offset by the view that comparatively slight changes take place in it until puberty. According to the latter view, degeneration goes on after puberty at a rate which varies widely in different individuals, and the thymus may persist as a functional organ up to the age of sixty years.
Fig. 261. Diagram of the branchial groove derivatives in man. Verdun.
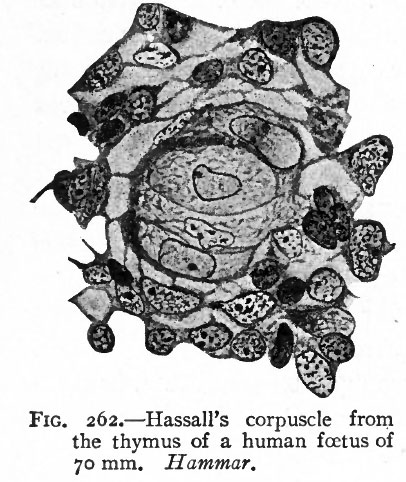
The histogenesis of the thymus has been a subject of much study and controversy, not only in regard to its origin, but also in regard to its change from an epithelial to a lymphoid structure and the regressive changes in the latter. It has almost certainly been proven to be of entodermal origin. It is at first an epithelial mass which later becomes broken up into lobules by the ingrowth of connective tissue. In regard to the histological changes which it undergoes, the older views are in general that a " pseudomorphosis " takes place; that is, the epithelial elements are replaced by lymphoid cells which wander in from the neighboring blood vessels, Hassall's corpuscles being remnants of the epithelium. Later other investigators looked upon the changes as a "transformation," asserting that the epithelial cells were transformed into lymphoid cells in situ, and that Hassall's corpuscles were remnants of epithelium and disintegrating blood vessels. Some went even so far as to assert that the thymus was the first place of origin of the leucocytes. More recent researches furnish very strong evidence that no lymphoid cells are derived from the epithelial cells (Maximow), but that the epithelium is transformed into the reticular tissue of the thymus, in which the lymphoid cells undergo mitotic division, Hassall's corpuscles possibly representing compressed parts of the reticulum (Hammar) (Fig. 262).
The Glomus Caroticum
The early formation of the glomus caroticum (carotid FIG. 262. Hassall's corpuscle from gland) has not been observed in the human ZftfO^Z** embryo. From observations on lower animals it has not been made clear whether it is derived from the entoderm of a branchial groove or from the adventitia of the carotid artery.
The Esophagus
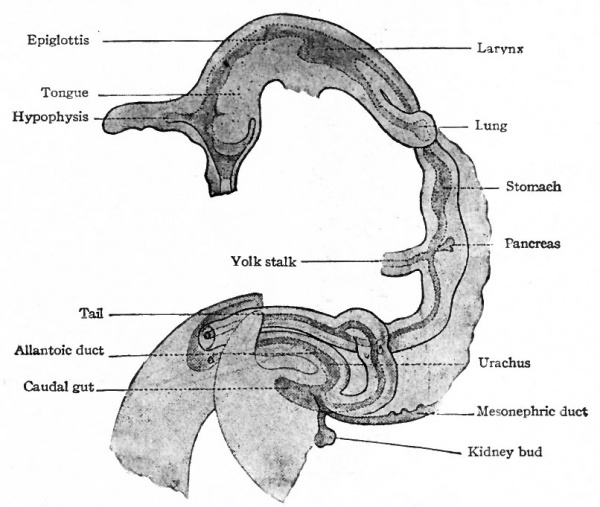
When the primitive gut becomes differentiated into* distinct regions (p. 286), the cesophageal region forms a comparatively short: tube, of uniform diameter, extending from the pharynx to the stomach (Fig. 247). In embryos of about 3 to 4 mm. the anlage of the respiratory system arises from the cephalic end of the tube (see p. 330). The latter is lined with entoderm and broadly attached to the dorsal body wall by mesoderm (Fig. 247). During later stages it becomes relatively longer as the heart recedes into the 1 thorax (p. 214), but maintains its uniform diameter.
Further development produces no marked changes in the relative position; of the oesophagus. It remains broadly attached to the dorsal body wall! throughout the life of the individual. In other words, there is never a distinct! mesentery. The entoderm gives rise to the epithelial lining and the glands, the: surrounding mesoderm to the connective tissue and muscular coats.
The Stomach
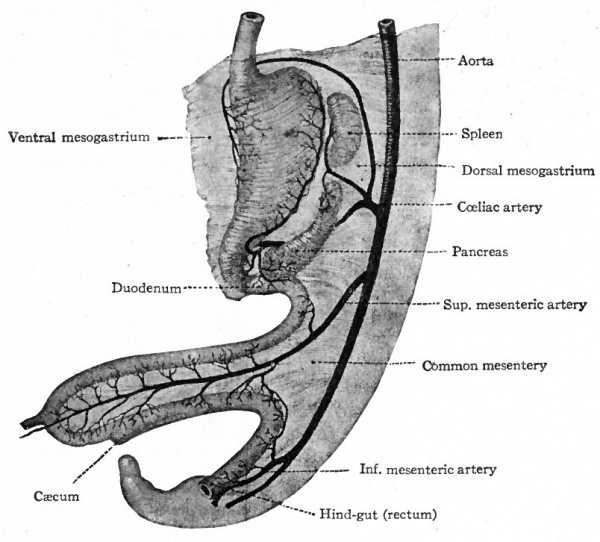
The anlage of the stomach can be recognized in embryos of about 5 mm as a slight spindle-shaped enlargement of the primitive gut ai short distance cranial to the yolk stalk (Fig. 246). The dilatation goes on more rapidly on the dorsal than on the ventral side, thus producing the greater and^ lesser curvature respectively. The greater curvature is attached to the dorsaU body wall by the dorsal mesogastrium which is a part of the common mesentery.
The lesser curvature is connected with the ventral body wall by the ventral mesogastrium (Fig. 263).
In further development, apart from histogenesis, the greater curvature becomes much more prominent and the organ as a whole changes its position, the latter process beginning in embryos of 12 to 14 mm. The cephalic (cardiac) end moves toward the left side of the body, the pyloric end toward the right At the same time the stomach rotates, the greater curvature turning caudally from its dorsal position and the lesser curvature cranially from its ventral position. The result is that the organ comes to lie in an approximately transverse position in the body, with the cardiac end to the left, the pyloric end to the right, the greater curvature directed caudally, and the lesser curvature directed cranially (compare Figs. 247 and 263 with Figs. 276 and 304).*
Fig. 263. Gastrointestinal tract and mesenteries of a human embryo of 6 weeks. Toldt.
- These changes may be more easily understood if the student will hold a closed book in the sagittal plane in front of him, with the back of the book toward, and the open edge away from him. The back represents the greater curvature, the open edge the lesser curvature. The upper end of the book represents the cardiac end of the stomach, the lower end the pylorus. Turn the upper (cardiac) end to the left, the lower (pyloric) end to the right, at the same time allowing the back of the book (the greater curvature) to drop downward on the side toward the body. The changes in the position of the book represent the changes in the position of the developing stomach.
It is obvious that the lower end of the oesophagus is carried toward the left side of the body with the cardiac end of the stomach, and at the same time twisted so that the side which originally faced the left comes to face ventrally. The changes in the mesentery which accompany the changes in the stomach are described elsewhere (p. 348).
The torsion of the stomach also produces an asymmetrical condition of the vagi nerves. The latter reach the stomach before it changes its position. As the changes take place, the left nerve is carried around to the left and ventrally so that in the adult it passes through the diaphragm ventral to the oesophagus and extends over the ventral surface of the stomach. The right nerve passes over the dorsal surface of the stomach.
The Intestine
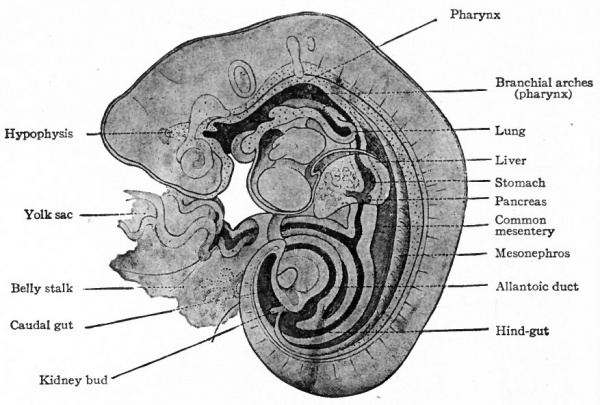
When the primitive gut is differentiated into recognizable regions (p. 286) the intestinal region forms a simple tube, of uniform diameter, extending from the stomach to the caudal end of the embryo where it ends blindly. The yolk stalk is attached to the intestine a short distance from the stomach. Near the caudal end the allantoic duct arises (p. 582). The lumen of the yolk stalk and of the allantoic duct is continuous with that of the intestine (Fig. 247). In embryos of 2 to 3 mm. the liver anlage arises from the ventral side of the intestine near the stomach, that is, from that part of the intestine which is to become the duodenum. In embryos of 3 to 4 mm. the pancreas anlage arises in the same region, in part from the liver evagination and in part from the dorsal side of the intestine (Fig. 247).
The intestine as a whole is suspended in the abdominal cavity by the dorsal mesentery which is attached to the dorsal body wall and which is continuous with the dorsal mesogastrium. A ventral mesentery, continuous with the ventral mesogastrium, is present only at the cephalic end of the duodenum (Fig. 263).
The further development of the intestine, apart from histogenesis, consists very largely of the formation of loops and coils, due to an enormous increase in the length of the tube. The abdominal cavity at the same time enlarges to accommodate the increased bulk. As the stomach changes its position (p. 305) , the duodenum comes to lie obliquely across the body and forms a curve with the concavity directed dorsally (Fig. 263). The rest of the intestine forms a loop which extends ventrally and caudally as far as the umbilicus. The arms of the loop are almost parallel and the cephalic arm lies a little to the left of the caudal. The apex of the loop extends into the umbilical ccelom and is attached to the yolk stalk. From the dorsal end of the caudal arm the intestine extends directly to the caudal end of the body (Fig. 263).
Soon after the loop is formed a small evagination appears on its caudal arm, not far from the apex. This is the anlage of the cecum and marks the boundary between the small and large intestine (Fig. 263). At this stage, therefore, all the great divisions of the intestinal tract are distinguishable, viz. : the duodenum with the ducts of the liver and pancreas; the mesenterial small intestine with the yolk stalk; and the colon extending from the caecum to the caudal end. There are, however, practically no differences between the regions, either in structure or in size.
In further development the duodenum comes to lie more nearly transversely across the body, thus assuming its adult position. Its mesentery fuses with the peritoneum of the dorsal body wall and the duodenum thus becomes a fixed portion of the intestinal tract (p. 350; also Fig. 301). It enlarges a little more rapidly than the rest of the small intestine and acquires a greater diameter. In embryos of 12 to 13 mm. the lumen becomes obliterated by an overgrowth of the mucous membrane caudal to the ducts of the liver and pancreas. In embryos of about 15 mm., however, the lumen reappears. It seems difficult to find a cause for this peculiar growth of the mucosa.
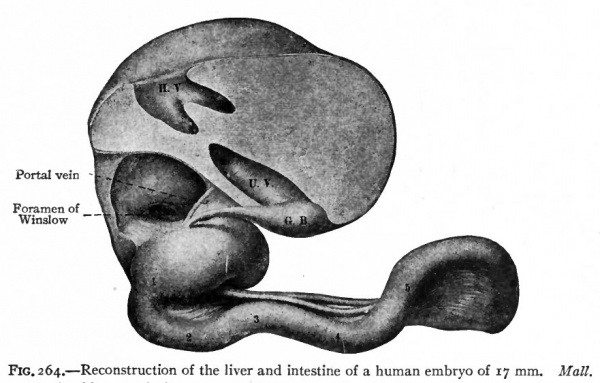
Fig. 264. Reconstruction of the liver and intestine of a human embryo of 17 mm. Mall. G.B., gall bladder; H. V., hepatic vein; U.V., umbilical vein; 1 -6, primary bends in the long intestinal loop; 1 represents the duodenum.
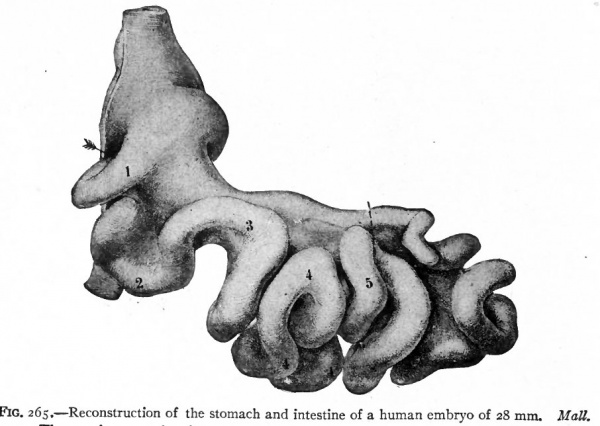
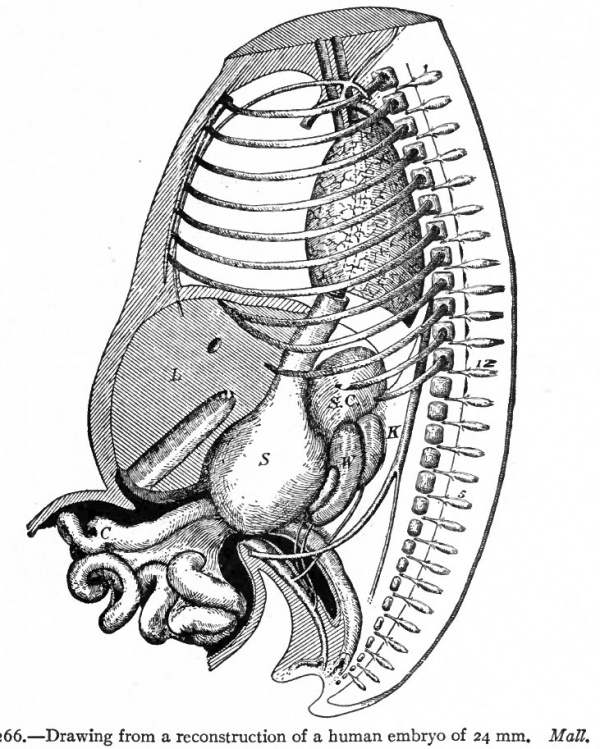
Very shortly after the formation of the long loop in the intestine, six bends become recognizable in the portion between the stomach and the apex of the loop (Fig. 264). These bends later form distinct loops which are destined to become definite parts of the small intestine. The first loop is the duodenum, the development of which has already been considered, and which maintains practically its original position. The other five loops continue to elongate and form secondary loops, all of which push their way into the umbilical coelom where they remain until the embryo reaches a length of 40 mm. (compare Figs, 265 and 266). Then they return very quickly to the abdominal cavity proper.
After their return, the primary loops, with the secondary loops derived from them, come to occupy fairly constant positions. The second and third move to the left upper part of the abdominal cavity; the fourth crosses the medial line and occupies the right upper part. The fifth crosses back and lies in the left iliac fossa; the sixth lies in the pelvis and lower part of the abdominal cavity (Fig. 267).
Certain variations may occur but are usually not considered as abnormal. The most frequent variation is one in which the fourth coil, along with the second and third, lies on the left side, its usual position on the right being occupied by the ascending colon. Not uncommonly the positions of the fourth and the second and third are reversed. Less commonly extra loops are formed.
Fig. 265. Reconstruction of the stomach and intestine of a human embryo of 28 mm. Matt.
- The numbers are placed on the coils derived from the primary bends as shown in Fig. 302; 1 represents the duodenum.
Usually the proximal part of the yolk stalk disappears during foetal life. In a few cases, however, it persists as a blind sac of variable length, known as Meckel's diverticulum (see also p. 581).
Even before the loops return to the abdominal cavity the colon or large intestine increases in diameter more rapidly than the small intestine. After the return, the caecum is carried across to the right side and comes to lie just caudal to the liver. From the caecum the colon extends across the abdominal cavity, ventral to the duodenum, forming the transverse colon. It then descends on the left side as the descending colon which passes over into the sigmoid colon (Fig. 299). The transverse, the descending and the sigmoid portions of the colon are recognizable in the third month. Up to the time of birth the sigmoid portion is disproportionately long; after birth the other portions grow relatively faster. After the fourth month the portion to which the caecum is attached grows downward in the right side of the abdominal cavity, thus forming the ascending colon (Fig. 304).
Fig. 266. Drawing from a reconstruction of a human embryo of 24 mm. Matt. The intestinal coils lie for the most part in the umbilical coelom. C, caecum; K, kidney; L, liven S, stomach; S. C., suprarenal gland; W, mesonephros; 12, twelfth thoracic nerve; 5, fifth lumbar nerve.
The caecum, which appears in very early stages as an evagination at the junction of the small and large intestines, for a time continues to increase uniformly in size. Then the proximal end increases more rapidly than the distal, and forms the caecum of adult anatomy. The distal end, failing to keep pace in development, remains more slender and forms the vermiform appendix (Fig. 267).
As has already been mentioned, the primitive gut ends blindly in the caudal end of the embryo (Fig. 246). The anal opening is a secondary formation, On the ventral side of the caudal end of the body there is formed a depression known as the anal pit. The mesoderm at the bottom of the pit becomes thinner until the ectoderm comes in contact with the entoderm on the ventral side of the gut, thus forming the anal membrane. The area of contact is not at the extreme end of the gut, but a short distance toward the allantoic duct. In the meantime, the urogenital ducts come to open into that portion of the gut which lies just cranial to the anal membrane. The gut enlarges in this region to i/form the cloaca. The latter becomes separated by the urorectal fold into a portion, the rectum, and a ventral portion, the urogenital sinus (Figs. 323 and 325). At about the time of separation (embryos of about 14 mm. or thirty-six to thirty-eight days) the anal membrane ruptures and the anal opening is formed. The portion of the gut caudal to the anus, known as the caudal gut, normally disappears.
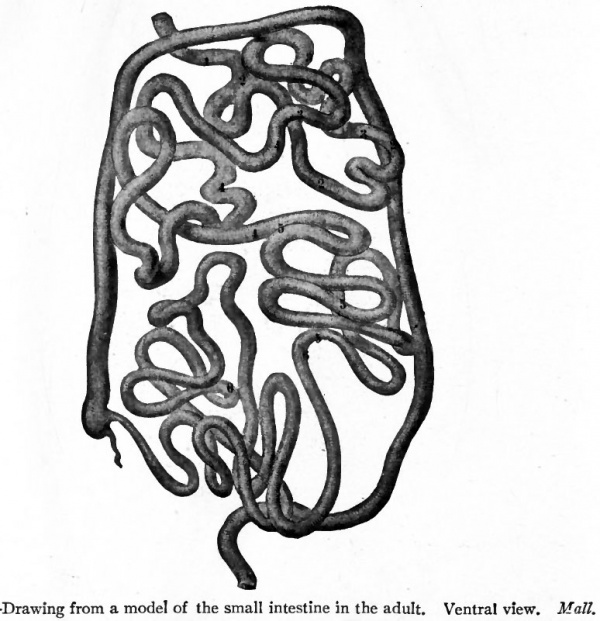
Fig. 267. Drawing from a model of the small intestine in the adult. Ventral view. Mall.
- The intestinal coils are shown in the usual relative position. The numbers indicate the coils derived from the primary bends in the foetus as shown in Figs. 264 and 265.
Histogenesis of the Gastrointestinal Tract
The wall of the primitive gut is composed of two layers the entoderm which lines the lumen, and the splanchnic mesoderm which borders on the ccelom or body cavity. While the germ layers are still flat, the entoderm is a single layer of flat cells with bulging nuclei, but after the closure of the gut the cells become columnar. The splanchnic mesoderm is composed of two layers the mesothelium bordering on the ccelom, the cells of which gradually change from flat to rather high, and a number of indifferent, branching mesenchymal cells lying between the mesothelium and entoderm. The entoderm is destined to give rise to the general epithelial lining of the gastrointestinal tract and to all the glands connected with it. The mesothelium around the gut forms a part of the general mesothelial lining of the ccelom, its cells apparently changing back to a flat type. The mesenchymal tissue is destined to give rise to all the connective tissue and smooth muscle of the tract. The circular layer of muscle appears first, the longitudinal next, both appearing during the third and fourth months, and last of all the muscularis mucosae (Fig. 268).
Fig. 268. Transverse section of the small intestine of a pig embryo of 32 mm. Bonnet.
The Mucous Membrane
The mucous membrane is formed by the epithelium (entoderm) and the subjacent mesenchymal tissue. In its development there are two factors to be considered: (i) The formation of folds to increase the absorbing surface and (2) the formation of secreting organs or glands. As to the relation between these two factors there is a difference of opinion. Some hold that both kinds of structures are the result of the same formative process, that is, that the glands are simply the depressions or pits formed by the intersection of folds at various angles, and that the folds are produced primarily by the growth of the epithelium and mesenchymal tissue into the lumen of the gut. Others maintain that although the folds may be produced by the growth of the epithelium and mesenchymal tissue into the lumen, the glands arise as independent growths of the epithelium into the subjacent tissue. The latter view is supported by the fact that in some Amphibia the glands appear before the folds (Fig. 269). Recent work on Mammals also favors this view.
The development of the folds and glands begins in the different parts of the gastrointestinal tract at different times. It begins first in the stomach, then in the duodenum, then in the colon, and then whence it progresses slowly into the ileum. In the stomach it is uncertain whether the crypts and glands are depressions left among projections of the mucous membrane, or the glands represent evaginations of the epithelium into the underlying tissue. In the case of the large intestine the same uncertainty exists. If the so-called glands are depressions among villous projections that grow-in to the lumen of the intestine, they are not true glands from an embryological point of view.
Studies of the development of the villi in the human small intestine have led to the conclusion that they are formed primarily as growths of the mucosa into the lumen. In embryos of 19 mm. the mucosa of the cephalic end is thrown into a number of longitudinal folds (Fig. 270). These then develop progressively toward the caudal end. Beginning in embryos of 50 to 60 mm. the longitudinal folds become broken transversely into conical structures, the villi. The intestinal crypts (of Lieberkiihn) possibly represent outgrowths of the epithelium from the bottoms of the intervillous spaces.' The duodenal (B runner's) glands are possibly to be considered as a continuation of the pyloric glands of the stomach. They apparently grow as evaginations from the intervillous crypts.
The epithelial lining of the gastrointestinal tract is from the beginning a single layer of cells, although the individual cells are altered in shape and structure and acquire different functions in different regions. There is still some dispute as to whether the mucous cells are continuously being derived from the other epithelial cells or, when once formed, reproduce themselves by mitosis. As a matter of fact, mitosis has been observed in the mucous cells of the stomach.
Fig. 270. From a reconstruction of the small intestine of a human embryo of 28 mm. Berry. Showing the longitudinal ridges which eventually become broken transversely to form the villi.
The Lymph Follicles
In the development of the lymph follicles in the gastrointestinal tract the same question arises as in the case of the tonsils and thymus. Are the lymphoid cells of mesodermal or of entodermal (epithelial) origin? Evidence at present favors the mesodermal origin. In the case of Peyer's patches, collections of lymphoid cells appear near the blood vessels in the stroma and neighboring parts of the submucosa. These increase in extent, the lymphoid cells dividing actively, and grow into the bases of some of the villi and deeper into the submucosa (Fig. 271). Germinal centers appear in many of the follicles, and the surrounding stroma becomes densely infiltrated with the lymphoid cells. Individual follicles may develop, in the manner described, in any part of the gastrointestinal tract. The appendix especially is the seat of extensive lymphatic tissue formation. It is stated in the section on the lymphatic system that lymph glands may arise at any time in any region as the result of unusual conditions (p. 251), and this also holds true in the case of lymph follicles in the digestive tract.
Fig. 271. Sections through the wall of the caecum of (a) a rabbit 2.5 days and (b) 5 days after birth, showing the development of the lymph follicles. Stohr.
- Lymphoid infiltration in the stroma; r, wandering cells in the epithelium; 2, lymphoid cells in the core of a villus.
The Development of the Liver
The liver is the first gland of the digestive tract to appear. In embryos of about 3 mm. a longitudinal ridge-like evagination develops from the entoderm on the ventral side of the gut a short distance caudal to the stomach, that is, in the duodenal portion of the gut (Figs. 247, 272, 273). The cephalic part of the evagination is solid and, being destined to give rise to the liver proper, is called the pars hepatica. The caudal part is hollow, its cavity being continuous with the lumen of the gut, and is destined to give rise to the gall bladder, whence it is called the pars cystica. Beginning at both the cephalic and caudal ends, the evagination as a whole becomes constricted from the gut until (in embryos of about 8 mm.) its only connection with the latter is a narrow cord of cells which is the anlage of the ductus choledochus. The pars hepatica by this time has enlarged considerably and remains attached to the ductus choledochus by a short cord of cells, the anlage of the hepatic duct. The pars cystica has also become larger, its distal portion being somewhat dilated, and is connected with the ductus choledochus by the anlage of the cystic duct (Figs. 274 and 275). The pars cystica grows into the ventral mesentery and thus becomes surrounded by mesodermal tissue. The proximal portion continues to elongate to form the cystic duct and the distal portion becomes larger and more dilated to form the gall bladder.
Fig. 272. Transverse section of a human embryo of 5 mm. Showing the liver evagination and the breaking up of the omphalomesenteric veins by the hepatic cylinders. Photograph.
Fig. 273. From a model of the duodenum and the primary evaginations of the liver and pancreas in a 5 mm sheep embryo. Stoss.
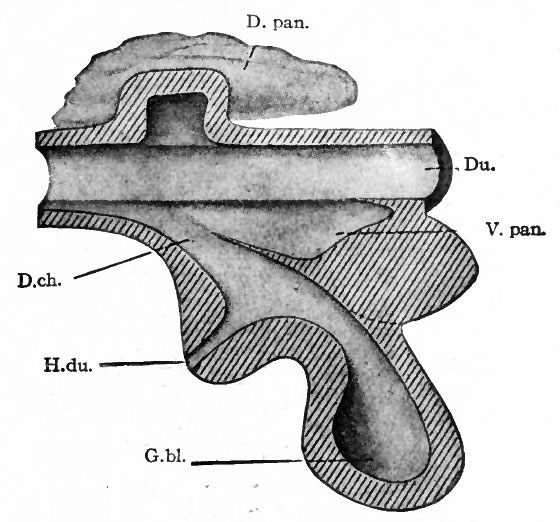
- D.pan., Dorsal pancreas; Du., duodenum; D. ch., ductus choledochus; G. bl., gall bladder; H. du., hepatic duct.
The pars hepatica, or anlage of the liver proper, also grows into the ventral lesentery, thus becoming surrounded by mesodermal tissue. As stated in connection with the development of the diaphragm, the portion of the mesentery into which the liver grows is involved in the formation of the septum trans versum (p. 344). Thus the developing liver becomes enclosed in the septum (Fig. 292). The mesodermal tissue gives rise to the fibrous capsule of Glisson and to the small amount of connective tissue within the gland.
Although the liver develops as a series of outgrowths from the original evagination, there are certain features in its development which distinguish it from glands in general. The outgrowths come in contact with the omphalomesenteric veins which are situated in the ventral mesentery (p. 229). They push their way into and through the veins, breaking them up into smaller channels (Fig. 272). They anastomose freely with one another, and the veins send off branches which circumvent them. Thus there is formed a network of trabec ulse of liver cells, called hepatic cylinders, the meshes of which are filled with blood vessels. Therefore the liver is distinguished from other glands in general in that the hepatic cylinders, which are comparable with the smaller ducts and terminal tubules of other glands, anastomose, and in that the blood vessels are broken up by the growth of these cylinders.
Fig. 274. From a reconstruction of the anlagen of the liver and pancreas and a part of the stomach and duodenum of a human embryo of 4 weeks. Felix.
Fig. 275. From a reconstruction of the anlagen of the liver and pancreas and the stomach of a human embryo of 8 mm. Hammar.
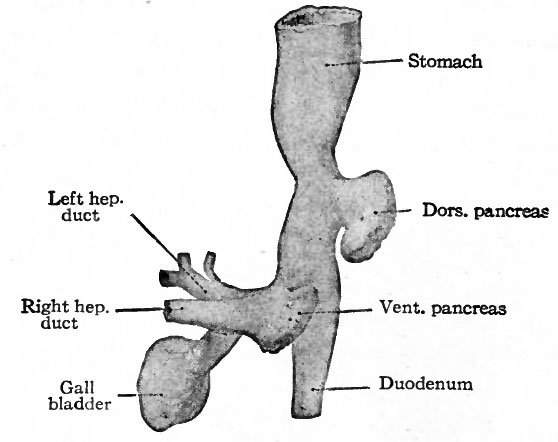
- D.P., Dorsal pancreas; Du., duodenum; D. F., ductus venosus; G.B., gall bladder; R.I., right lobe of liver; S. t stomach; V. P., ventral pancreas.
This mode of development establishes what is known as a sinusoidal circulation, which differs from the ordinary capillary circulation. The sinusoids are produced by the growth of the trabeculae of the developing organ into large vessels and the breaking up of the latter into smaller vessels. It is obvious that a sinusoidal circulation is purely venous or purely arterial. Furthermore, development of this nature leaves comparatively little connective tissue within the gland, another feature characteristic of the liver.
All the blood carried to the liver by the omphalomesenteric veins must follow the tortuous course of the sinusoids before being collected again and passed on to the heart. When the umbilical veins come into connection with the liver they also join in the sinusoidal circulation. Subsequently, however, a more direct channel the ductus venosus is established and persists for a short time. This is probably due to the large volume of blood brought in by the umbilical veins. Finally the ductus venosus disappears and the sinusoidal circulation remains as the permanent form. (For the development of the veins in the liver see p. 228.)
Fig. 276. Tranverse section of a 14 mm pig embryo, through the region of the stomach. Photograph. The arrow points into the bursa omentalis.
The lobes of the liver develop in a general way in relation to the great venous trunks which at one time or another pass into or through the gland. The anlage of the organ grows into the ventral mesentery, subsequently becoming enclosed in the septum transversum. In so doing it encounters the omphalomesenteric veins, and forms, in relation to the latter, two Incompletely separated parts which have been called the dorso-lateral lobes. When the umbilical veins enter the liver a more ventral, medial mass is formed. This becomes incompletely separated into two parts which give rise to the permanent right and left lobes. The right becomes the larger. The right umbilical vein loses its connection with the liver (p. 230). After birth the left, which lies between the right and left lobes, degenerates into the round ligament of the liver. The other lobes arise secondarily as outgrowths from the right primary dorsolateral lobe, the caudate (lobe of Spigelius) from its inner (medial) surface, the quadrate from its dorsal surface.
The liver as a whole grows rapidly and by the second month is relatively large. During the third month it fills the greater part of the abdominal cavity. After the fifth month it grows less rapidly and the other intraabdominal organs overtake it, so to speak, although at birth it forms one-eighteenth the total weight of the body. After birth it actually diminishes in size. The right lobe is from the beginning larger than the left, and after birth the predominance increases.
Histogenesis of the Liver. The hepatic part (pars hepatica) of the liver anlage is derived from the entodermal lining of the gut and constitutes a mass of cells with no lumen. From this mass, solid bud-like evaginations grow into the mesentery, break up the omphalomesenteric veins into smaller channels and form trabeculae, or hepatic cylinders (p. 316). The latter anastomose freely with one another and are composed of polyhedral, darkly staining cells with vesicular nuclei (Fig. 277, A). Lumina begin to appear in the cylinders about the fourth week as small cavities which communicate with the cavity of the gut.
The hepatic cylinders are the forerunners of the hepatic cords or cords of liver cells. There are two views as to the manner of transformation. The older view is that the cylinders gradually become stretched, the number of cells in cross-section becoming less until it is reduced to two. Between these two lies the lumen of the cord or the so-called "bile capillary" (Fig. 277, B). The other view is that branches from the sinusoids grow into the cylinders and subdivide them into hepatic cords.
As stated above, the hepatic cylinders are at first composed of darkly staining, polyhedral cells with vesicular nuclei. These are the liver cells proper. Later other small spherical cells, with dense nuclei, appear and during the fourth month become very numerous (Fig. 277, A). From this time on, they grow less in number and at birth have practically disappeared. Earlier investigators considered them as developing liver cells. Further study on the development of the blood, however, has led others to consider them as erythroblasts (p. 239). Since they are inside of the hepatic cylinders, they either wander in from the intertrabecular blood vessels or lie in intratrabecular vessels. The latter supposition accords with the view that the cylinders are broken up into hepatic cords by the ingrowth of branches from the sinusoids.
The development of the lobules of the liver, producing the peculiar relations between the parenchyma of the gland and the blood vessels, has not been clearly and completely demonstrated. In young embryos the branches of the hepatic veins are surrounded by comparatively little connective tissue. The branches of the portal vein are surrounded by a considerable amount which subdivides the liver into lobules but not in the same manner as in the adult. The trabeculae possess no radial character and there are several so-called central veins in each lobule. The changes by which these primary lobules are subdivided into the permanent ones do not take place until after birth. The branches of the portal vein, with the surrounding connective tissue, invade the primary lobules and divide them into a number of secondary lobules, corresponding to the original number of central veins. At the same time the hepatic cords (which have been formed meanwhile) become arranged radially around the central veins in the characteristic manner. The hepatic artery grows into the liver secondarily and its branches follow the course of the branches of the portal vein.
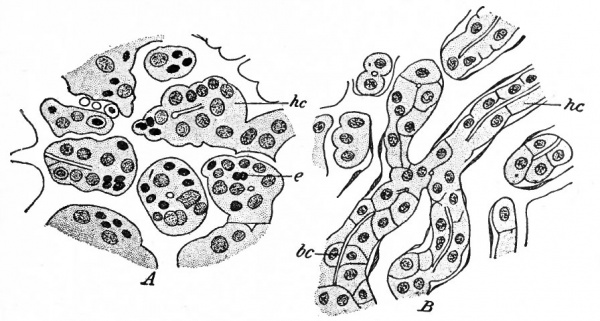
Fig. 277. Sections of the liver of (^4) a human foetus of 6 months and (B) a child of 4 years. Toldt and Zuckerhandl. McMurrich. be, Bile "capillary"; e, erythroblast; he, hepatic cylinder (in A), cord of liver cells (in B).
Degeneration of the liver cells occurs in the region of the left triangular ligament, the gall bladder and the inferior vena cava. The bile ducts may, however, withstand the degenerative processes and persist as the vasa aberrantia of the liver. The cause of the degeneration is possibly the pressure brought to bear by other organs.
The Development of the Pancreas
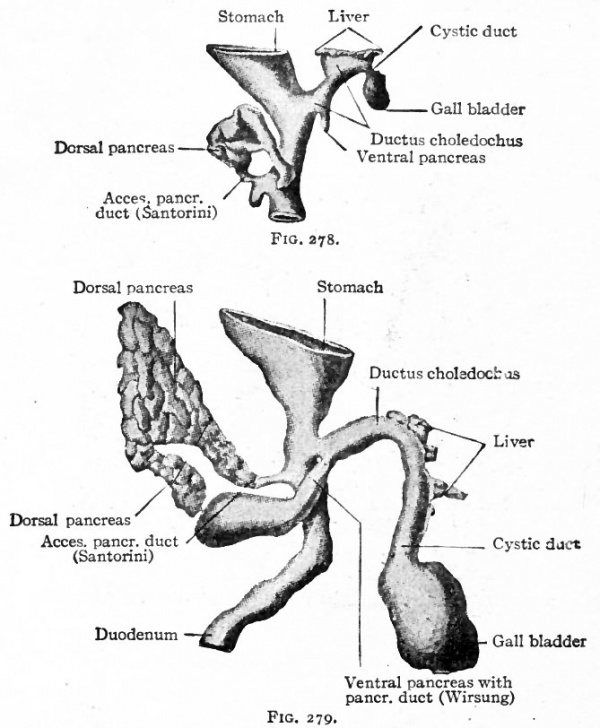
The epithelium of the pancreas, like that of the liver, is a derivative of the entoderm. It arises from two (or three) separate anlagen, one dorsal and one (or two) ventral. The dorsal anlage appears first as a ridge-like evagination from the dorsal wall of the gut, slightly cranial to the level of the liver (Figs. 273 and 274). It appears about the same time as the liver or a little later. The mass of cells grows into the dorsal mesentery and becomes constricted from the parent epithelium except for a thin neck which becomes the duct of Santorini (Fig. 278). A little later two other diverticula appear, one from each side of the common bile duct. It is uncertain whether only one or both of these take part in the formation of the pancreas, but it seems most probable that the left one disappears entirely. The right diverticulum continues to develop and becomes constricted from the parent epithelium, leaving only a thin neck which becomes the duct of Wirsung.
Figs. 278 and 279. From models of the developing liver and pancreas of rabbit embryos of 8 mm. and 10 mm, respectively. Both seen from the right side. Hammar, Bonnet.
The smaller ventral pancreas grows to the right and then dorsally in the mesentery (Fig. 260), passing over the right surface of the portal vein, until it meets and fuses with the proximal part of the larger dorsal pancreas. The fusion takes place in the sixth week, and the two anlagen then form a single mass. A communication is established between the two ducts, and the dorsal duct (Santorini) usually disappears, leaving the ventral (Wirsung) as the permanent duct opening into the ductus choledochus. In a general way it may be said that the ventral anlage gives rise to the head, the dorsal anlage to the body and tail of the pancreas (compare Figs. 278 and 279).
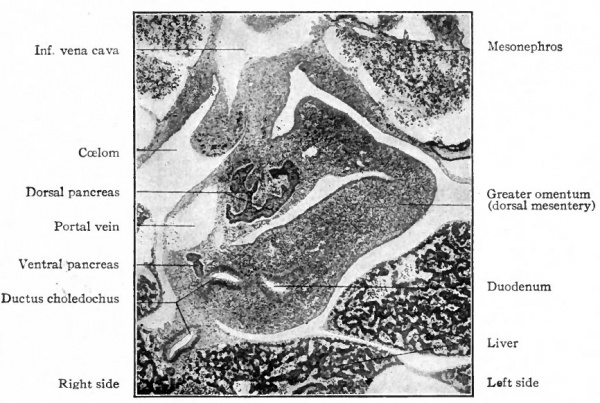
As the pancreas grows into the dorsal mesentery it comes to lie in the dorsal mesogastrium between the greater curvature of the stomach and the vertebral column, and since the dorsal mesogastrium at first lies in the medial sagittal plane, the pancreas is similarly situated. After the sixth week, however, as the stomach changes its position (p. 305) , the pancreas is carried along with the mesogastrium and comes to lie in a transverse plane, with its head to the right and embedded in the bend of the duodenum, and its tail reaching to the spleen on the left. The organ as a whole is at first movable along with the mesentery, but when it assumes its transverse position it lies close to the dorsal abdominal wall. The mesentery then fuses with the adjacent peritoneum (see p. 350), and the pancreas is firmly fixed.
Fig. 280. From a transverse section through the region of the duodenum of a pig embryo of 14 mm. Photograph.
The connective tissue of the pancreas is derived from the mesodermal tissue of the mesentery. As the processes or buds which form the ducts and terminal tubules grow out from the primary masses, they penetrate the mesodermal tissue and are surrounded by it. Groups of tubules form lobes and lobules, and the entire gland is surrounded by a capsule of connective tissue.
Histogenesis of the Pancreas. The masses of entodermal cells forming the anlagen of the pancreas develop further by a process of budding, which goes on until finally a compound tubular gland is produced. According to some investigators the primary evaginations are hollow, their lumina beinj continuous with the lumen of the gut. According to others they are solid al first and acquire their lumina secondarily. The same uncertainty exists regard to the later outgrowths or buds.
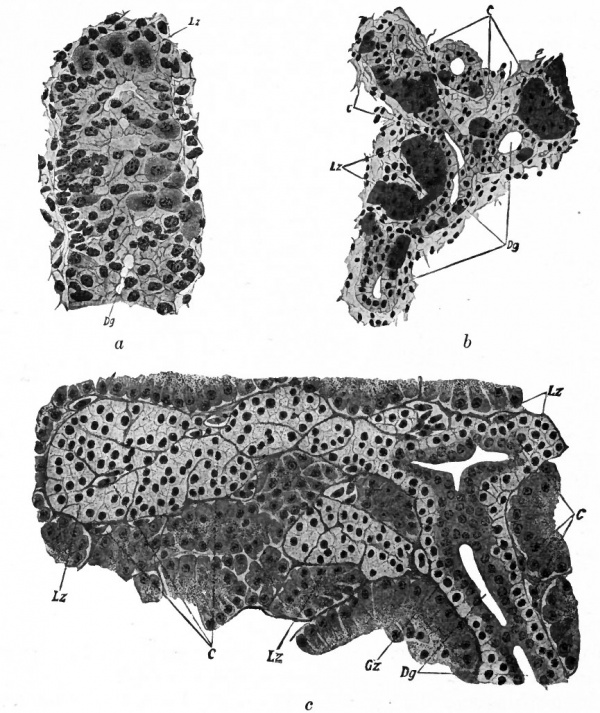
Fig. 281. Sections of the developing pancreas of a guinea-pig embryo of 12 mm. (a); of 33 mm. (&) ; of Torpedo marmorata (c) . Hetty.
- c t Capillaries; Dg, ducts; Gz, duct cells; Lz, Langhans' cells. The cells in c show distinct zymogen granules
The early entodermal cells proliferate, and the resulting cells change according to their position in the gland. Those lining the larger ducts become high columnar, with more or less homogeneous cytoplasm; those lining the intermediate (intercalated) ducts become low; those lining the terminal secreting tubules become pyramidal and more highly specialized, and also acquire certain constituents the zymogen granules (Fig. 281, c) which vary with the functional activities of the gland. The centro-tubular cells in the terminal tubules are probably to be explained on a developmental basis. While a few maintain that they are "wandering" cells, it is quite generally accepted that they are simply continuations of the flat cells lining the intermediate ducts, the result being that the cells of the terminal tubules seem to spread out over the ends of the intermediate ducts in the form of cap-like structures.
It was once thought that the islands of Langerhans were derived from the mesodermal tissue. Recently it has been pretty clearly demonstrated that they are derived from entoderm. In guinea-pig embryos of 5 to 6 mm., at a time when the dorsal pancreas has merely begun its constriction from the gut, certain cells in the mass appear darker and slightly larger than the others. They show darker areas of cytoplasm around the nuclei, and later the darker areas extend throughout the cells and the nuclei become larger and more vesicular. When lumina appear in the outgrowths or buds, these cells occupy a position on or near the surface of the buds (Fig. 281, a). In further development they tend to separate themselves from the buds and collect in clumps (Fig. 281, b). Capillaries then penetrate the clumps and break them up into the trabeculae of cells characteristic of the islands of Langerhans (Fig. 281, c). Studies on the development of the islands in the human pancreas indicate a similar origin and mode of development.
Anomalies
One of the most striking anomalies of the organs of alimentation is found in connection with a more general anomalous condition known as transposition of the viscera (situs viscerum inversus) . The transposition may be so complete that the minor asymmetries normally present on the two sides are all repeated in reverse order, the functions of the organs being unimpaired. As regards the alimentary tract, this means that the position of the stomach is reversed in the abdominal cavity; that the duodenum crosses from left to right; that the various coils of the jejunum and ileum occupy positions opposite to the normal; that the caecum and ascending colon are situated on the left side and the descending colon on the right; and that the larger lobe of the liver lies on the left side. The other visceral organs are transposed accordingly, the heart being inclined toward the right side, the left lung consisting of three lobes and the right of two, the left kidney being lower than the right, etc. Such cases are not uncommon, two hundred being on record.
Various theories as to the causes of transposition of the organs have been advanced. In the most plausible of these the anomalous condition is considered as due to the influence of the large veins in the embryo. It seems best, therefore, to consider first the transposition of the heart (dextrocardia, referred to on page 255).
After the tvvo anlagen unite in the midventral line, the heart constitutes a simple straight tube which lies in a longitudinal direction in the primitive pericardial cavity, and which is joined caudally by the two omphalomesenteric veins and cranially by the ventral aortic trunk (p. 197) . Normally the left omphalomesenteric vein is the*larger and pours a greater quantity of blood into the heart tube than the right. This condition is regarded as the primary factor in the deflection of the tube toward the right side (p. 199; also Fig. 158). If the conditions were reversed, that is, if the right omphalomesenteric vein were the larger and poured the greater quantity of blood into the heart tube, the primary bend of the latter would be toward the left side. Consequently the heart would continue to develop in the transposed position and eventually come to lie on the side opposite to the normal.
Although dextrocardia is very frequently associated with transposition of the abdominal organs, it is not necessarily so, for there are cases of the latter in which the heart occupies the normal position. Consequently it seems that further influences must be present to account for transposition of the abdominal organs when the thoracic organs are normal. A number of investigators have emphasized the importance of the influence of the large venous trunks in the abdominal region, especially on the position of 'the liver and stomach.
Primarily the omphalomesenteric veins pass cranially through the mesentery. Later they form two loops or rings around the duodenum. Then the left half of the upper ring and the right half of the lower disappear, the common venous trunk thus following a spiral course around the duodenum (p. 231 ; also Fig. 201). This primary relation of the omphalomesenteric vein is retained in the relation of the portal vein to the duodenum. The stomach lies to the left of the portal vein. After the allantoic (placental) circulation is established the umbilical veins pass cranially in the lateral body walls. After the veins come into connection with the liver, the right atrophies and the left increases in size and becomes the single large umbilical vein of later stages (p. 230; also Fig. 202). The right lobe of the liver becomes the larger.
If, as is maintained by some investigators, the usual position of the stomach and liver is due to the persistence of the left venous trunks, a persistence of the right venous trunks would afford a plausible explanation of the transposition of these organs. It is not unreasonable to attribute also the transposition of the other abdominal organs directly or indirectly to the persistence of the right venous trunks. Certainly a reversal in the position of the stomach would cause a reversal in the position of the duodenum.
If these conditions are the real ones, the fact that the thoracic organs can be transposed without a transposition of the abdominal organs, or vice versa, is accounted for. The primary bend of the heart tube occurs at a very early period, before the changes in the vessels in the region of the liver. Consequently a reversal of the conditions of the omphalomesenteric at a very early stage only would be likely to affect the heart. The principal changes in size of the venous trunks in the abdominal region take place after their channels have been broken up in the liver. In other words, the modifications in the veins in the liver occur after the definite relations of the heart have been established. Therefore the transposition of the abdominal organs may take place after the heart has begun to develop normally.
The Mouth
Anomalies in the mouth region, due to defective fusion of the processes that bound it, have been considered elsewhere (p. 180).
Anomalies of the tongue sometimes arise as the result of imperfect development of one or more of its anlagen. Imperfect development of the tuberculum impar results in total or partial lack of the anterior part. Defects in the root are probably due to imperfect development of one or both of the paired anlagen (p. 289). Malformations of the lower jaw (micrognathus, agnathus) are usually accompanied by malformations of the tongue, both structures being derived largely from the first pair of branchial arches.
The Pharynx
The pharynx is the seat of cysts, fistulae and diverticula which have been considered in connection with the anomalies in the region of the branchial arches and grooves (Chap. XX).
The thyreoid gland is not infrequently the seat of certain anomalies that arise as the result of abnormal development. Persistent portions of the thyreoglossal duct, the upper end of which is indicated by the foramen caecum linguae, may give rise to cystic structures extending to the region of the hyoid bone. Persistent portions of the duct may even give rise to accessory thyreoid (suprahyoid, prehyoid) glands (p. 301; also Fig. 260). Considerable variation also exists in the isthmus and lateral lobes of the thyreoid, due to variation in the manner of development of the medial anlage.
Impaired development of the thymus gland sometimes leads to cysts which come to lie in the anterior mediastinum.
The Oesophagus
Very rarely the oesophagus is entirely lacking, being represented by a mere cord of tissue. More frequently it is defective in certain parts. Tne atresia may begin just below the pharynx or just above the stomach, the intermediate portion being composed of a cord of fibrous tissue. Occasionally the non-atretic portion opens into the trachea. Possibly this represents an imperfect separation between the primitive gut and the anlage of the respiratory system (p. 330).
The Stomach
Occasionally the stomach is smaller than the normal. It may even be a narrow tube resembling the other portions of the gut, owing to lack of dilatation. Other congenital malformations, apart from transposition (p. 323), are very rare.
The Intestines
One of the most common anomalies is the persistence of the proximal end of the yolk stalk, forming MeckeVs diverticulum (see p. 581). This usually is attached to the ileum about three feet from the caecum. In exceptional cases it retains its lumen and, when the stump of the umbilical cord disappears, forms a congenital umbilical fistula. Usually, however, the diverticulum is shorter and ends blindly. Occasionally it becomes constricted from the intestine and forms a cystic structure. (See also Chap. XX.)
Congenital stenosis and atresia may occur in different regions of the intestine, the duodenum being the most common site. Normally the lumen of the duodenum becomes closed for a brief period during development (p. 307) , and congenital closure of the lumen may represent a persistence of the early embryonic condition.
A conspicuous malformation is the persistence of the cloaca. The septum which normally separates the latter structure into rectum and urogenital sinus fails to develop, thus leaving a common cavity (see Figs. 323 and 324). In addition to this the cloacal membrane may fail to rupture and the cloaca become much distended. More often the septum develops in part, leaving only a small opening between the rectum and urogenital sinus. After the latter undergoes further development, the rectum comes to open into the urethra or bladder, or into the vagina or uterus.
Atresia of the anus is not infrequently met with. The cloacal (or anal) membrane fails to rupture and the rectum ends blindly. In other cases the rectum opens into the urogenital sinus, as described in the preceding paragraph. Occasionally the lumen of the rectum is closed atresia recti and the gut ends blindly some distance from the surface, being connected with the anal region by a cord of fibrous tissue.
Variations in the position of the intestinal loops, apart from transposition (p. 323), are of frequent occurrence. It is not customary to include these variations among malformations (see p. 308) . The caecum (and appendix) and colon present some striking variations. The caecum may be situated high up in the abdominal cavity, the ascending colon being absent. Or it may be situated at any intermediate point between that and its usual position in the right iliac fossa. These variations are due to different degrees of development of the ascending colon (p. 309).
The Liver
Congenital malformations of the liver are rare. The most frequent, apart from transposition, include anomalies in the size and number of lobes. Accessory lobes may occur within the falciform ligament. One case of lack of development of the gall bladder has been observed. Stenosis of the bile passages is occasionally met with.
The Pancreas
Occasionally accessory glands are found in the intestinal or gastric wall. These probably represent aberrant portions of the main gland, and may give rise to cystic structures. Very recently, however, a number of intestinal diverticula have been observed in certain mammalian embryos and also in human embryos. Although the history of these unusual diverticula has not been traced, their presence may offer a clue to the origin of accessory pancreatic structures. The ducts of the pancreas are subject to distinct variations, which, however, are not usually considered as anomalies. Not infrequently the duct of the dorsal anlage (duct of Santorini) persists and opens directly into the duodenum. It may persist along with the duct of the ventral anlage (duct of Wirsung), or the latter may disappear (p. 321; compare Figs. 2 78 and 279).
- Next: Respiratory
References for Further Study
BADERTSCHER, J. A. : The Development of the Thymus in the Pig. I, Morphogenesis. II, Histogenesis. Am. Jour, of Anat., Vol. XVII, 1915.
Bardeen CR. The critical period in the development of the intestines. (1914) Amer. J Anat. 16: 427 – 445.
Bell ET. The development of the thymus. (1905) Amer. J Anat. 5: 29-62.
BERRY, J. M.: On the Development of the Villi of the Human Intestine. Anat. Anz., Bd. XVI, 1900.
BONNET, R.: Lehrbuch der Entwickelungsgeschichte. Berlin, 1907.
BORN, G.: Ueber die Derivate der embryonalen Schlundbogen und Schlundspalten bei Saugetiere. Arch.}, mik. Anat., Bd. XXII, 1883.
BRACKET, A. : Die Entwickelung und Histogenese der Leber und des Pancreas. Ergebnisse der Anat. u. Entwick., Bd. VI, 1897.
CHIEVITZ, J. C.: Beitrage zur Entwickelungsgeschichte der Speicheldriisen. Arch. f. Anat. u. Physiol., Anat. Abth., 1885.
CHORONSCHITZKY: Die Entstehung der Milz, Leber, Gallenblase, Bauchspeicheldruse und des Pfortadersyssems bei den verschiedenen Abteilungen der Wirbeltiere. Anat. Hefte, Bd. XIII, 1900.
Fox, H.: The Pharyngeal Pouches and their Derivatives in the Mammalia. Am. Jour, of Anat., Vol. VIII, No. 3, 1908.
FUSARI, R.: Sur les phenomenes, que Ton observe dans la muqueuse du canal digestif durant le developement du fcetus humain. Arch. ital. Biol., T. XLII, 1904.
GOPPERT, E.: Die Entwickelung des Mundes und der Mundhohle mit Driisen und Zunge; die Entwickelung der Schwimmblase, der Lunge und des Kehlkopfes der Wirbeltiere. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere. Bd. II, Teil I, 1902.
HAMMAR, J. A.: Einige Plattenmodelle zur Beleuchtung der friiheren embryonalen Leberentwickelung. Arch.f. Anat. u. Physiol., Anat. Abth., 1893.
HAMMAR, J. A.: Allgemeine Morphologic der Schlundspalten beim Menschen. Entwickelung des Mittelohrraumes und des ausseren Gehorganges. Arch. f. mik. Anat., Bd. LIX, 1902.
HAMMAR, J. A. : Das Schicksal der zweiten Schlundspalte. Zur vergleichenden Embryologie und Morphologic der Tonsille. Arch.f. mik. Anat., Bd. LXI, 1903.
HELLY, K.: Studien iiber Langerhanssche Inseln. Arch. f. mik. Anat., Bd. LXVII, 1907.
HERTWIG, O. : Lehrbuch der Entwickehmgsgeschichte der Wirbeltiere und des Menschen. Jena, 1906.
HENDRICKSON, W. F.: The Development of the Bile Capillaries as Revealed by Golgi's Method. Johns Hopkins Hosp. Bull., 1898.
His, W.: Anatomic menschlicher Embryonen. Leipzig, 1880-1885.
His, W.: Die Entwickelung der menschlichen und tierischen Physiognomien. Arch, f. Anat. u. Physiol., Anat. Abth., 1892.
JACKSON, C, M.: On the Development and Topography of the Thoracic and Abdominal Viscera. Anat. Record, Vol. Ill, 1909.
Johnson FP. The development of the mucous membrane of the oesophagus, stomach and small intestine in the human embryo. (1910) Amer. J Anat., 10: 521-559.
JOHNSON, F. P.: The Development of the Mucous Membrane of the Large Intestine and Vermiform Appendix in the Human Embryo. Am. Jour. of. Anat., Vol. XIV, 1903
JOHNSON, F. P. : The Development of the Rectum in the Human Embryo. Am. Jour. of Anat., Vol. XVI, 1914.
KINGSBURY, B. F.: The Development of the Human Pharynx. I, The Pharyngeal Derivatives. Am. Jour, of Anat., Vol. XVIII, 1918.
KOHN, A.: Die Epithelkorperchen. Ergebnisse der Anat. u. Entwick., Bd. IX, 1899.
KOLLMANN, J.: Die Entwickelung der Lymphknotchen in dem Blinddarm und in dem Processus vermiformis. Die Entwickelung der Tonsillen und die Entwickelung der Milz. Arch.f. Anat. u. Physiol., Anat. Abth., 1900.
KOLLMANN, J.: Lehrbuch der Entwickelungsgeschichte des Menschen. Jena, 1898.
KOLLMANN, J.: Handatlas der Entwickelungsgeschichte des Menschen. Jena, 1907.
MALL, F. P.: Ueber die Entwickelung des menschlichen Darmes und seiner Lage beim Erwachsenen Arch.f. Anat. u. Physiol., Anat. Abth. Suppl., 1897.
MAURER, F.: Die Entwickelung des Darmsystems. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere., Bd. II, Teil I, 1902.
McMuRRiCH, J. P. : The Development of the Human Body. Third Ed. Philadelphia, 1907.
MUMMERY, J. H.: The Microscopic Anatomy of the Teeth, 1919.
Norris EH. The early morphogenesis of the human thyroid gland. (1918) Amer. J Anat. 24(4): 443-
Pearce RM. The development of the islands of Langerhans in the human embryo. (1903) Amer. J Anat. : 446-455.
PIERSOL, G. A.: Teratology. In Wood's Reference Handbook of the Medical Sciences, Vol. VII, 1904.
POLZL, A.: Zur Entwickelungsgeschichte des menschlichen Gaumens. Anat. Hefte, 1905
ROSE, C.: Ueber die Entwickelung der Zahne des Menschen. Arch. f. mik. Anat., Bd. XXXVIII, 1891.
STEIDA, A.: Ueber Atresia ani congenita und die damit verbundenen Missbildungen. Arch.], klin. Chir., Bd. LXX, 1903.
STOHR, P.: Ueber die Entwickelung der Darmlymphknotchen und iiber die Riickbildung von Darmdriisen. Arch. f. Anat. u. Physiol., AnaL Abth., 1898.
TANDLER, J.: Zur Entwickelungsgeschichte des menschlichen Duodenum in friihen Embryonalstadien. Morph. Jahrb., Bd. XXIX, 1900.
TOLDT und ZUCKERHANDL Ueber die Form und Texturveranderungen der menschlichen Leber wahrend Wachsthums. Sitzungsber. d. kaiser. Akad. d. Wissensch., Wien. Math.-Naturwiss. Klasse., Bd. LXXII, 1875.
TOURNEUX ET VERDUN: Sur les premiers developpements de la Thyroide, du Thymus et des glandes parathyroidiennes chez I'homme. Jour. de. I' Anat. et. de la Physiol., T. XXXIII, 1897
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Text-Book of Embryology: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Text-Book of Embryology 12. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Text-Book_of_Embryology_12
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G