Book - Manual of Human Embryology 13
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Mall FP. XIII. Coelom and diaphragm in Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia.
XIII. Coelom and Diaphragm
by Franklin P. Mall.
The small ovum described recently by Bryce and Teacher is hardly two millimetres in diameter, and is covered with a reticular mass of syncytium, true villi with mesodermal cores not being present. Within there is an extremely small embryo anlage embedded in a delicate cellular reticulum. The mesenchymatous tissue shows no signs of cleavage into a parietal and a visceral layer nor has it arranged itself into a denser layer around the wall of the vesicle. The general appearance of the mesoderm is shown in Fig. 10, Chapter IV, and in Fig. 93, Chapter VII. It is there seen that no exoccelom is present. In comparing this specimen with Peters^ ovum, which is somewhat larger, we must imagine a destruction of some of the mesodermal tissue, either in the centre of the ovum or near the embryo, in order to form the primitive exoccelom. In fact this interpretation can be given to the form of the exoccelom in Peters' ovum, as is shown in Figs. 96 and 97, Chapter VII. The first figure is taken from Peters' plate, and the second is an interpretation of this figure by Professor Grosser, who has compared the section from which the Peters drawing was made with the drawing itself. It is seen, therefore, that the exoccelom is not present in an ovum two millimetres in diameter, and that it is well formed in an ovum somewhat larger, that is, at the beginning of the third week of pregnancy.
All the other young human ova which have been studied by embryologists, possibly including that by Peters and those by Graf Spee, have within them a large cavity lined entirely with a layer of mesoderm. The precocious development of this space, the exoccelom, appears to be peculiar to man and monkeys, for it has not been observed in other mammalian ova which have been studied with great care in a large number of species. Han^ng freely within this cavity, but attached to one side of the chorion, there is always found in normal ova a relatively small embryonic mass composed of a closed amnion and an umbilical vesicle joined together by the anlage of the embryo, which in the youngest specimens contains only the three primitive blastodermic membranes. In general the diameter of the embryonic mass is but one-fifth of that of the exoccelom, which indicates that the latter began to form by a splitting of the mesoderm when the ovmn was very small unless we assume that the embryonic mass becomes absolutely smaller while the exoccelom is forming. In the Peters ovum the embryonic mass measures about 0.2 mm. in diameter, and that of the exoccelom, which is egg-shaped, averages about one mm.
Until the embryo is 2 mm long the amnion hugs the embryo closely and does not encroach markedly upon the exoccelom. As the embryo grows a little larger some anmiotic fluid accumulates around it, which naturally causes the embryonic mass to grow much more rapidly than the embryo. During this time the embryo £dso grows relatively faster than the exocoelom and therefore the embryo and amnion soon begin to obliterate the exocoelom. At the time the embryo is 4 mm. long the ratio in size between the embryonic mass and the exocoelom is about 5, and somewhat later it is but 3. When the embryo measures 7 mm. and the ovum is 18 mm. in diameter, the ratio is 2, and by the beginning of the eighth week the exoccelom is obliterated entirely. Now the amnion, lines the entire chorion with the exception of a small region around the umbilical vesicle which lies inunediately below the chorion and is surrounded by a small space, the last remnant of the exocoelom.
The exocoelom is filled with an albuminous fluid which is held together by a delicate network of fibrils and gives it a jellylike consistency. This mass, the magma reticule of the older authors (Velpeau), is also well marked in the exocoelom of monkeys' ova, and is probably more marked in normal ova than is generally believed (Keibel). In case the fibrils of the magma are scanty or beginning to disintegrate, or in case they are greatly increased in number, dense enough partly to obliterate the embryo, the specimen is certainly pathological. Magma which is transparent and contains just enough reticulum to hold it together is to be viewed as the normal constituent of the exocoelom.
The nature of magma fibrils has not been definitely settled, but they appear to be connected with cells in some way as indicated in the ova described by Bryce and Teacher and Peters. Some embryologists are inclined to consider them the product of coagulation. However, they are present in the freshest specimens, and they cannot be stained by Weigert's fibrin stain. I have found reticular magma present in normal ova which were preserved in strong formalin immediately after their abortion. according to Eetzius, the magma reticule contains fibrils of a muco-fibrillar nature which become transparent in dilute acetic acid.
Before the circulation of blood is established between the embryo and the chorion, — that is, before the embryo is 2 mm. long, — the nutrition of the embryo and its umbilical vesicle must pass from the chorion through the magma. This may be the reason why any alteration in the nutrition of the embryo, which affects its normal development, first manifests itself in a change in structure of the magma reticule. As the exoccelom is gradually obliterated the magma is pushed ahead of the advancing amnion and finally forms a delicate membrane of fibrils between the amnion and chorion.
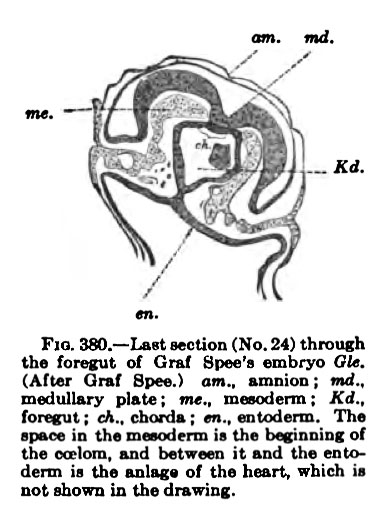
The coelom of the embryo arises independently of the exoccelom in embryos between 1.5 and 2 mm. long. In specimens less than 1.5 mm. long studied by Peters, Graf Spee, and Minot, there is no trace of either blood-vessels or body cavity within the embryo, but in Graf Spee's well-known embryo Gle. (1.54 mm. long) traces of the beginning of the heart and its Coelom are present.
Fig. 380. which is through the head of this embryo, contains on either side of the body a few scattered cells between the mesoderm and entoderm. These Kd.
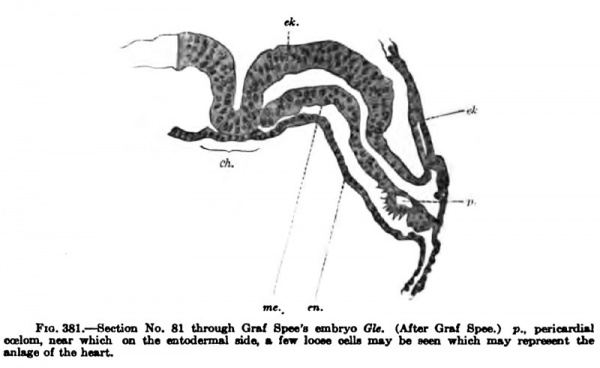
Graf Spee believes to be the endothelial anlage of the heart. In this same section there is a split in the mesoderm of the embryo, which continues through other sections and marks the beginning of the pericardial coelom. It may be noted that the endothelial anlage of the heart and its coelom arise after the foregut is well formed the c«iom, Md between it and tho fnio— that is, after the head of the embryo is separated from the yolk-sac. The different sections show that the coelom is of irregular form and size and it is surrounded with numerous dividing cells (Fig. 381).
Since no other human embryo has been studied which follows immediately upon Graf Spee's Gle., we must fill out the gap by observations upon other mammalian embryos; these are very complete and have been made with great care. They do not contradict one another nor any of the observations made upon the development of the human Coelom. For these reasons much confidence can be jilaced upon facts derived from comparative embryology upon the early formation of the pericardial Coelom and their bearing upon the same in human embryos.
Bonnet's careful study of the development of the Coelom in the embryo sheep fills out perfectly the gap between Spee's Gle. and older human embryos. In the sheep t!ie pericardial coelom appears as irregular spaces on either side of the head much as in Graf Spee's embryo Gle. The spaces arise on both sides of the embryo, but are so irregular in position and form that their arrangement can not possibly be considered metameric Next the spaces unite, thus forming in a relatively short time large spaces on either side of the body, which soon unite with each other at the extreme anterior end of the embryo, forming a horseshoeshaped canal in the mesoderm on the ventral side of the head. Throughout its development the pericardial Coelom is closed on its lateral sides and does not communicate with the exoccelom, with the exception of its later and indirect communication through the peritoneal coelom. It may also be noted that in the sheep the whole pericardial coelom is formed hand in hand with the invagination of the foregnt and before the endothelial anlage of the heart has arranged itself into vascular tubes, much as is the condition found in Graf Spee's embryo Gle.
Fig. 381. Section No. 81 throuch Graf Spee's embryo Template:Gla. (Aftar Qrul Spn wBlmk, near which on the «ntad(niul nda, m fair looM mlla nuy be wen nhii^ i ■nlege of the hou-t
That the pericardial Coelom arises very early and independently of the exoccelom is farther proved by the work of Strahl and Carius, who studied its development in the guinea-pig and the rabbit and confirmed fully the earlier observations of His upon the first formation of the pericardial coelom (Parietalhohle). In the rabbit the pericardial Coelom ends in two dorsal and two ventral recesses, all four of which connect subsequently with the peritoneal Coelom. However, only the dorsal recesses break into the peritoneal coelom in the human embryo, and it is this recess or canal which later on encircles the lung and probably forms the main anlage of the pleural Coelom.
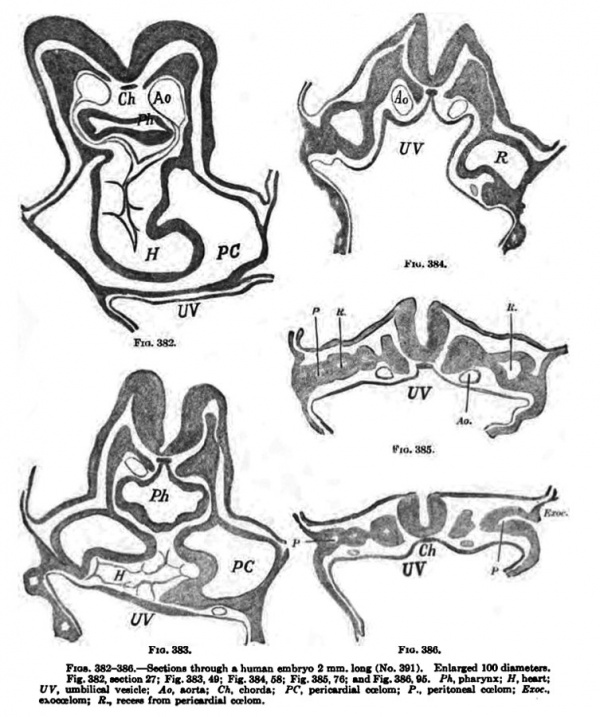
We can now return to the human embryo, and the next stage after Graf Spee's Gle. which bears upon this point is found in a well-preserved normal human embryo 2 mm. long. The embryo came from a self-inflicted mechanical abortion and was soon preserved in formalin. Before transferring it to alcohol it measured about 2 mm. in length, but due to its shrinkage while being embedded it produced but 160 transverse sections each being 10 um thick. The embryo has 7 myotomes. The figures (382-386) show that the pericardial coelom encircles the heart on its ventral side and reaches down into a recess which communicates with the peritoneal spaces and through these with the exoeoelom. The pericardial coelom is entirely closed on all sides. The peritoneal Coelom is forming at numeroua points in the mesoderm of the trunk of the emhryo, not in any regular fashion ; some of the spaces connect irregularly with one another, others connect with the exoccelom {Fig. 386), as is found to be the case in the sheep. Dandy has studied this embryo with great care, and a more detailed account of it, containing also a reconstruction of the coelom, may be found in his publication.
Fig. 382-386. — Section through human embryo 3 mm long (No. Template:CE361). Enlarged 100 diam. Fig. 382, section 27; Fig. 383, 49; Fig. 384. 68; Fig. 385, 76; ud Fig. 386. fi. /'A. pharynx; H, tmrt; UV, umbilioil v«ick; Aa. »on»: C*. chord*; PC. periouduU ealom; P., perilone«l CoFlom: Ejoc.. exoccelom; A., recao rrom pericardial coelom.
Quite recently Keibel and Elze described an embryo nearly 2 mm. long in which the pericardial Coelom is separated from the exoccelom by a pronounced bridge of tissue. This specimen is of prime importance in the study of the coelom in the human embryo, for through it we can connect the development of the Coelom in older and younger stages with one another, as well as with the condition found in the rabbit and in the sheep.
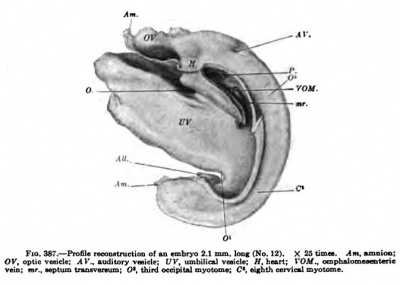
Fig. 387. — Profile reconstruction o( an embryo 2.1 mm long (No. 12). X 25 times. Am, ajnoicm: OK, opUc vesicle: Al'., audilory veaick; t/F. umbilical vnide; ff, heart; I'OJtf., omphalomesenteric veioi nr„ septum tratuvenuni: O*. third occipital myotome; C, eisbth cervical myotome.
Another embryo slightly older shows that the pericardial Coelom communicates freely with the peritoneal Coelom and this in turn with the exoccelom (Fig. 387). Probably this specimen is not altogether normal, because the brain is not well developed and the spinal cord behind appears to be too wide open. The liver and thyroid are beginning to form and two branchial pockets are well developed from the pharynx. In its caudal shifting the amnion has passed the heart, leaving the ventral body wall covered with ectoderm. The attachment of the umbilical vesicle to the body is conBtricting from all sides, receding before the amnion in front, at O, and from the allantots behind, 0'. Somewhat later, when the constriction is more marked and the amnion comes to iie upon the ombilical stalk, VV, the points O and O* will be the points of communication between the peritoneal coelom of the two sides of the body.
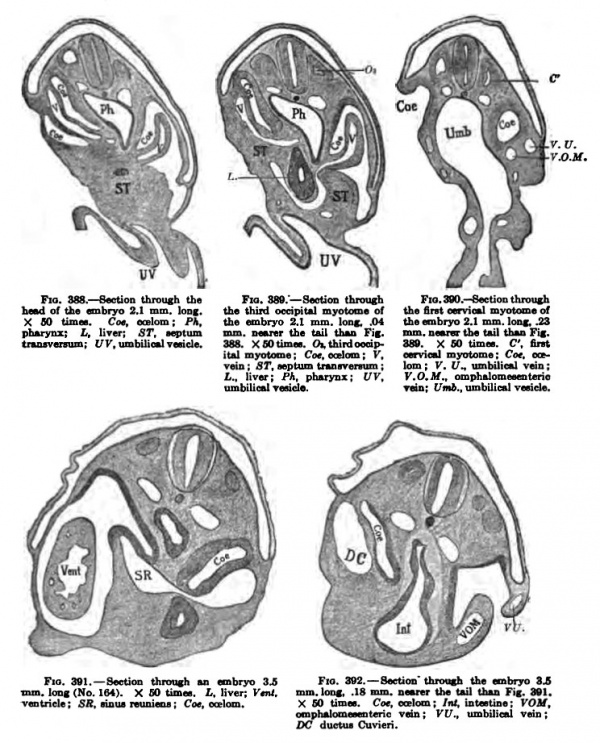
Fig. 388-392. — Section through an embryo 3.S Fig. 392. — Section' Ihrouah the embryo 3JS mm. loDf (No. 164). X GO timm. L. liven f*"'. mm. lone 18 mm. amtnt Uw tail ihaQ Fig. 391. ventricle ; SR, Noua reunina ; Cot. eat\am. X GO tim«. Cot, ontom ; Int. intntine ; VOM, omph^onieeentcric vein ; VV,, umbilical vein : DC ductiu Cuvieri. Other sections of this embryo are shown in Fig. 229, Chapter XI. Vol. I.— 34
In front the yolk-vein, VOM, connects freely with the capillaries of the yolkvesicle, and as it enters the heart it is bounded on its ventral side by a recess, Fig. 388, Coe, which, however, does not communicate directly with the peritoneal Coelom or with the exoccelom. Capillary veins also arise in the septum transversum, around the liver bud. Fig. 389. More caudal, Fig. 390, VU, a branch of the vein extends to the lateral side of the body, which is no doubt the anlage of the umbilical vein. These veins are more pronounced in an embryo slightly older. Figs. 391 and 392, which shows the ductus Cuvieri, the umbilical vein, the omphalomesenteric vein, and a vein running from the head — the jugular vein. All these veins unite in the sinus reuniens, which in turn enters the heart.
Septum Transversum
It is seen from the description just given that the body cavities arise from two primary spaces, as was first well shown by His, and that each of these in turn is bilateral in origin. In addition to these there is the exoccelom as well as the cavities of the myotomes which in the human embryo soon disappear ; they communicate in part with the rest of the body cavity (Keibel and Elze). The two pericardial coelom cavities soon unite at the front end of the head, and this space later on encircles the whole ventral surface of the heart. Next, two pockets arise from it, extend dorsally, and unite with the two peritoneal cavities. These diverticula barely exist as separate cavities, but blend immediately, as soon as they begin to form, through the peritoneal cavity with the exocoelom. By the end of the fourth week, as represented in Fig. 387, the five primitive cavities form a single coelom, from which at last seven body cavities arise. The primitive and most fundamental septum by which the common body cavity is broken into compartments was discovered by His, which he described as the septum transversum. Figs. 383 and 384 show that the umbilical vesicle is probably tied to the heart by means of two blood-vessels, which are well embedded in a mass of tissue of the embryo as shown in Fig. 387. Within this mass the blood-vessels go to the heart and into it the liver grows. To the extent in which the pericardial coelom (Parietalhohle) does not communicate with the peritoneal coelom, its floor is formed by a mass of tissue which unites the two sides of the trunk of the body with each other, and also binds the sinus venosus to the foregut. It is this mass of tissue which His terms the primitive diaphragm or the septum transversum. From it the principal part of the permanent diaphragm is formed, and by its extension the primary pericardium and the primary diaphragm are completed.
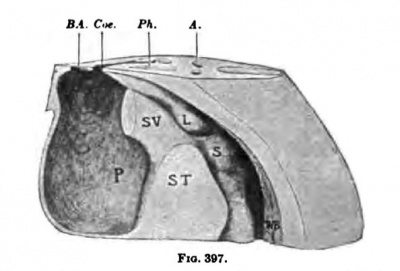
In an embryo slightly larger than. the one just described, the septum transversum is well defined, as may be seen in Pig. 394. The lungs have jnst begun to sprout (Fig. 393), and on the right side there is a diverticulum from the peritoneal coelom, which marks the beginning of the lesser peritoneal cavity. The liver is beginning to ramify within the septum and protrudes dorsally into the embryonic peritoneal cavity. In another embryo of about the same age the septum transversum is practically complete and in its form and position corresponds exactly with the description of it given by His (Fig. 395). The pericardial, pleural, and peritoneal Coeloms communicate as freely as they ever will, for there is as yet practically no sign of the formation of secondary septa. A reconstruction of the region of the septum of this embryo, showing the Coelom,is given in Figs. 396 and 397. The pericardial cavity lies completely on the ventral side of the septum, from which it extends in the form of a U-shaped canal around the sep
Fig. 3m.— nimugh an turn on either side of the lung, stomach. Muw^vrT a. and intestine. The pleural coelom — that is, ?? the coelom which surrounds the lung buds —is much the same in form on both sides of the body, but the peritoneal coelom shows marked bilateral inequalities due to the changes which have taken place with the shifting of the stomach towards the left side of the body.
Fig. 393. —
Fig. 3»a.— .->wIioii <hnius]imn eTiibr)o4.3min.
Fig. 394. — S«tion through the tmbtyo 1.3 longiNo.1481. X2!H\aua>. ri.fimt thur«cicmyomm. long, A mm, dHper than Fig. 393. X 2S ach-.B. bmn'chui-; H. heart: T.. thyrmd; i'C', periliver; V, 'ventriele : B^ , bulb of the «urla : Am.'. nntial cavity: L, liver; f IF .. foramen of Winxlow. wtiDiDo: [TV. umbiliol vein.
Fig. 397. Right uid Mt vi timet. X..aorU; i>A.,pli*r7Sx; fi^.,buib ITfi, Wolffiu body: S.itomMh; FW. (on
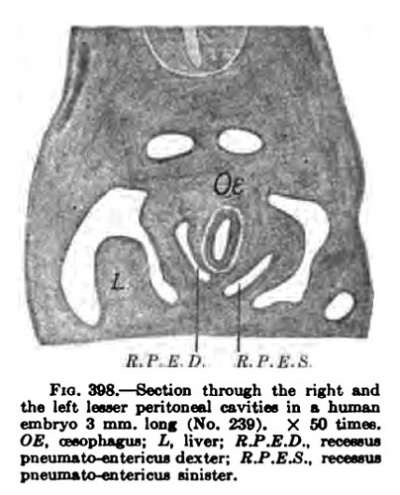
In Fig. 396, below the letters FW, there is a marked depression, the recessus mesenterico-entericus, which corresponds with the shifting of the stomach to the left. (This is well shown in Pig. 405.) Above FW a sac or invagination passes on the dorsal median side of the lung to form the recessus pnenmato-entericus dexter. This space also borders upon the liver and forms a third recess, the recessus hepato-entericus, over the region of the lobus Spighelii. These spaces together form the lesser peritoneal cavity and were recognized as such by His in 1880. From this time onward it has been described by nmnerous embryologists, and the most satisfactory study of it in the human embryo is by Broman, whose terminology I have adopted.
There is but little to be said about the left pleural recess except that its configuration is that of the Inng over which it passes. However, between the lung and the stomach (Fig. 397, L and 8) there is a slight depression. There is present at tliia point in reptiles and birds a marked pocket, or "left lesser peritoneal cavity," which has also been described in maoamalian embryos by Ravn. Ravn's discovery was doubted by a number of embryologists, but recently Broman has found it in all mammalian embryos examined, including the human. according to Broman the left bursa appears in human embryos about 3 mm. long, and vanishes immediately, that is, before they are 4 mm. long. The left recessus pneumato-entericus, as Broman calls it, is decidedly smaller than the right, does not encroach upon the left lung as much as the right does upon the right lung; in fortunate transverse sections they appear to be in symmetrical and equal (Fig. 398). Up to the present stage the septum transversum, which arose has gradually receded until its dorsal end has fallen to the region of the fifth cervical nerve. This stage is of importance, for it shows how the phrenic nerve enters the septum transversum (Fig. 399, Ph). This section shows that the nerve grows at first between the subclavian and cardinal veins along the lateral wall of the pleural Coelom towards the ductus Cuvieri. Subsequently, when the pericardial Coelom is separated c. V. from the pleural by the growth of the pleuroperieardial membrane from the wall of the ductus Covieri to the root of the lung, it leaves the nerve on the lateral edge of this separating membrane. Later, when the lung burrows to extend around to the side and the front of the heart (Fig. 414), the nerve is pushed into the pleuroperieardial membrane between the heart and the lung. This additional rotation m the development nerve the viscera moses this nerve the most important landmark by which we retain our conception of the relation of these organs.
— Section through the right uid m the region of the head,
Fig. 398. — . X 26 tinws. Ct fifth CoTviou tioual
In addition to the rotation of the organs aromid the phrenic nerve in the transverse plane of the body, there is another and greater shifting of the organs in their descent from the region of the head down into the thorax. This well-known descent of the diaphragm during development was first observed by von Baer, and later was more completely studied by His, Uskow, and myself. The accompanying scheme, Fig. 400, illustrates this process. On the right side of the schema, which is in the form of an embryo six weeks old, the numbers of the segments of the body are given. The space marked Co represents the communication between the pleural and the peritoneal Coeloms. The rectangular blocks in front represent the position of the septura transversum in embryos of various sizes which are indicated by the accompanying numbers. Thus, the block in the head region represents the position of the septum in an embryo 2 mm. long and that in the lower thoracic region the position of the finished diaphragm in an embryo 24 mm. long. The arrows indicate that the septum is moving more rapidly where they are located than elsewhere. In general there are two main foci around which the diaphragm rotates :
(1) in the upper cervical region at the dorsal end of the septum, whi<'h is its position in embryos 4 to 7 mm. long, and (2) in the thoracic region at the ventral end of the septum, which is brought about by the growth of the heart and lungs within the thorax.
Up to the end of the fiftli week there is no sign whatever of a separation between the various portions of the eiplom, but now a line of separation makes its appearance between the pericardial and pleural cosloms, due to a constriction of its walls along the course of the ductus Cuvieri, or rather the ductus lies in a ridge of tissue encircling the canal of communication between the pleural and the pericardia] Coelonis. This ridge is not present in one embryo 5 mm. long, but in another of this size there is an elevation on the dorsal wall of the pleural Coelom. Fig. 401, which encircles the lung and joins the dorsal end of the septum transversum. This ridge is one of the pillars of Uskow, or the beginning of the pulmonary ridge, as I have termed it. It gradually widens to cover the whole lung; on its cephalic end it gives rise to the pleuro-pericardial membrane of Uskow, and on its caudal end to the pleuroperitoneal membrane of Brachet. For the present, while it still represents two structures, it should carry a single name, and I believe pulnionary ridge to be a suitable one. In later stages, when the pulmonary ridge gives rise to the plearopericardial and the pleuroperitoneal membranes, the name should be dropped.
Separation of the Pericardial Pleural and Peritoneal Cavities
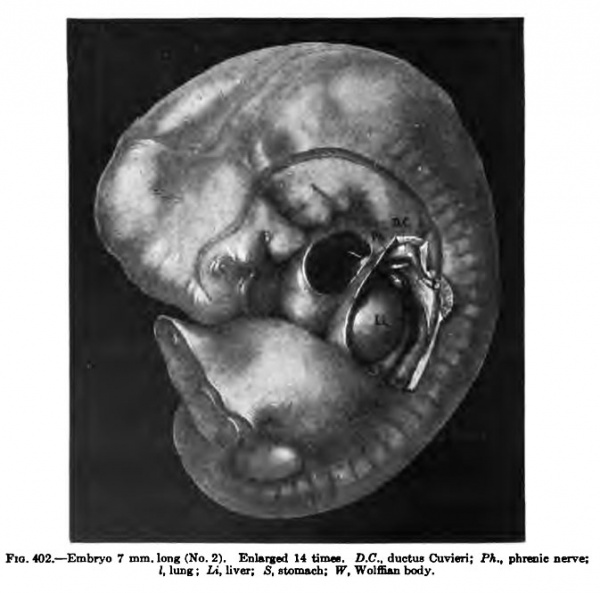
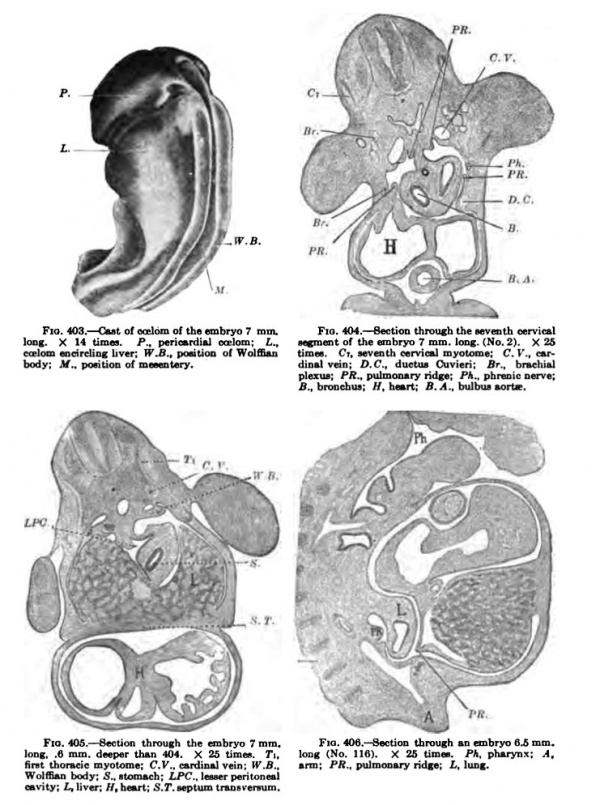
The first steps required to bring about a separation of these cavities have been taken and are well under way in an embryo 7 mm. long (Figs. 402-i05). The embryo is well kinked upon itself and the septum transversum in its wandering is entering the thorax, as Figs. 400 and 402 show. The communications between the pericardial and pleural eceloms have been reduced to narrow slits, as indicated by the arrow in Fig. 402 and in the cast of the coelom as shown in Fig. 403. A section through the lung region. Fig. 404, shows the two ridges cut across four times. The reconstruction, Fig. 402, gives the relation of the pulmonary ridge to the surrounding organs and to the structures of the body in its immediate neighborhood. This ridge is well shown in the His Atlas, embryos A and B, as well as in Piper's reconstruction, and it may be seen in an; human embryo of this stage. The relation of the ridge to the phrenic nerve, as well as its form in older embryos, makes of it the anlage of both plenropericardial and pleuroperitoneal memhranes. It lies in the sagittal plane of the body in this embryo in the region of the fourth cervical nerve, immediately over the iraig bud, and connects the dorsal end of the septum transversum with the Wolffian body. In sagittal sections of embryos of this stage tbe ridge may also be cut twice, as Fig. 406 shows.
Fig. 402. — Embryo 7
Fig. 403-406.
The embryos just described are kinked to their maximum, and in the next stage, with the disappearance of the sinus pnecervicalis and the beginning of the development of the neck, the head begins, to erect itself and the pulmonary ridge widens and spreads both towards the heart and the stomach, as Fig. 407 shows. The connection between the pleural and pericardial cavities is reduced to a small narrow slit, which is guarded on the pericardial side by a valve-like membrane, the pleuroperieardial membrane.
As the pulmonary ridge widens to encircle most of the lung, the dorsal end of the septum transversum sinks into the thorax more rapidly than does its ventral end. By this process the septum gradually turns a half revolution. The side that was its ventral surface in an embryo 7 mm. long (Fig. 402) has become its dorsal surface in an embryo 11 mm long (Fig. 414). All this is well shown in the three reconstructed figures as well as in the diagram (Fig. 400). As the pulmonary ridge widens, the lung buds become buried deeper and deeper in the body walls of the embryo, and as the liver gradually shifts with the rotation of the septum, the lungs are forced, step by step, to the side of the septum opposite the liver {Fig. 414). So, therefore, the lung which was in contact with the liver is shifted to the upper side of the septum by the widening of the pulmonary ridge and by the more rapid rotation of the dorsal end of the septum which carries the liver ventral
It or oieIodi of the embryo 7 mm.
Fig. 404, Bection through t1 a. P., p«ric«fdUl oceIoed; Z,„ stoment of the embryo 7 mm. k liver: W.B., pontion o\ Wolffian timn. Ct. seventh cervical my< dinalvdn; D.C.. cIueUu Cuvii plexua; PR., pulmonary ridge; , fi.. broDcfaus: //.heart: B.A.,\
through the embryo T mm.
Fig. 406. Section through an embryo fl.S m 1 404. X 25 limn. T\. long (No. IIS). X 26 times. Ph. pharynx: .V., ardiual vein: tr.fi.. arm; PA., pulmonary ridge; £, Iudr. h; LPCJeeserpehloneal S.r.eeptum iraneveisum.
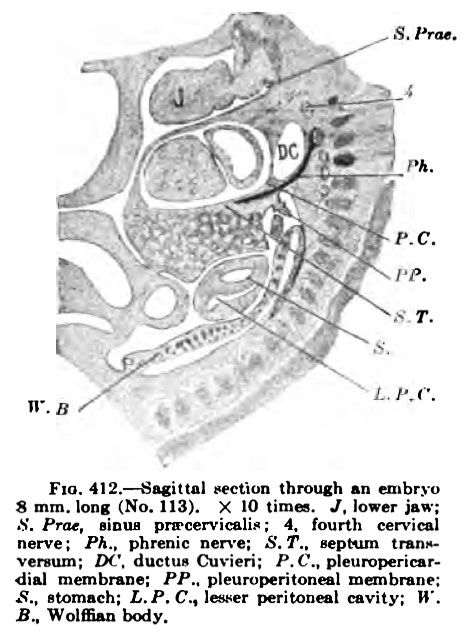
Fig. 412. Sagittal Hction ihnnigh ui ffubryu Fia. 413. — Section through 8 mm. long (No. 113). X 10 mag. J, lower ja»: which Fig. 412 is taken, nearer K. Prat. liDiu pivcervicalio : 4. fourth cervical veraum: IM'. ducliu Cuvicri; P,C.. pleuropericsrdial mcmbnne: PP.. pleuruperitonfal membranp: .S.. alomnch; i.P.C, leswr peritoneal cavity; »'. S^ Wolffian body.
The sections of the embryo pictured in Fig. 407 are shown in Figs. 408-411. They do not include the communication between the pleural and pericardial coeloms. However, they show the position of the phrenic nerve, and this, because of its later position, indicates the extent of the pleuropericardial membrane. In Fig. 409 a cavity is seen dorsal and lateral to the pleural coelom, and this burrowing continues until it approaches the pericardial coelom, where a new membrane is formed, the membrana pericardiaccperitonealis. This membrane is farther emphasized when the liver begins to separate from the septum transversum, leaving but a thin membrane between the pericardial and peritoneal coeloms.
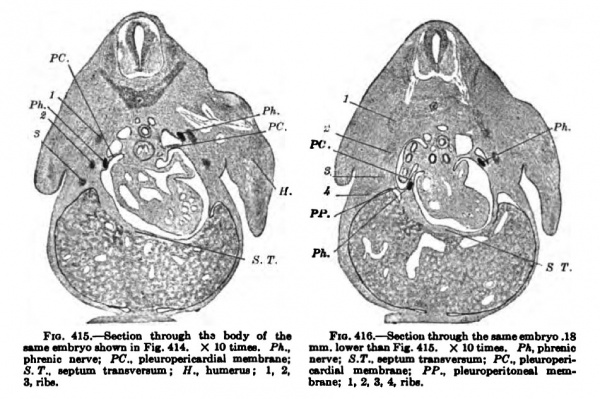
Figs. 412 and 413 are from sagittal sections of an embryo about as large as the one pictured in Fig. 407. The phrenic nen'e is shown throughout its whole course, from the fifth cervical nerve to the pleuropericardial membrane. Hanging from the pleiiropericardial membrane a section of the pleuroperitoneal may be seen {Fig. 412), which extends around the lung and unites with the dorsal body wall (Fig. 413) immediately in front of (he WolflSan body. About this time that portion of the pulmonary ridge destined to become the pleuropericardial membrane unites with the root of the lung and separates the pericardial space completely from the pleural. All this has just taken place in an embryo 11 mm. long and is shown in Figs. 414, 415, and 416. The plane of the pi eurojieri cardial membrane is now practically that of tlie septum transversum, the two together being transverse to the long axis of the embryo. The course of the ductus Cuvieri is along the edge of the pleuropericardial membrane, and that of the phrenic nerve is well within it (Fig, 416).
The relation of the ductus Cuvieri to the point of closure between the pleural and pericardial cavity was first pointed out by His in 1881, and since then little has been added to our knowledge of the process by which the separation is made.
At the time of the closure the small ridge of tissue called the pleuropericardial membrane (Uskow) is very insignificant,its extension being due to a subsequent rapid growth of the lung. It is, however, to the credit of Braehet to have shown that the canal connecting the pleural and pericardial cavities is oijy constricted by the ductus Cuvieri, and that its complete closure is due to the active growth of the aulage of the pleuropericardial membrane.
After the pericardial cavity is fully isolated the pulmonary ridge spreads out rapidly over the lung buds (Fig. 414) and forms an incomplete membrane at its lower tip, through which the pleural and peritoneal cavities communicate for some time longer. This membrane — the pleuroperitoneal membrane of Braehet — soon forms a-<-shaped figure, with the pleuropericardial membrane above and the septum transversum on its ventral side. On its dorsal it is closely related to the Wolffian body, whose descent it follows closely. Before this membrane is completed there must be added to it the ridge of tissue from the septum transversum to the Wolffian body, described by Uskow as the dorsal pillars of the diaphragm. The true nature of these pillars and their relation to the permanent diaphragm was finally determined by Ravn and Brachet, and by Swaen for the human embryo.
Fig. 415-416. — Section through the body of the Fia.416. — Section through the embryo. 18
MinievnbrvosbinrniD Fir 414, X lOtimn. Ph., mm.low«thuFi|. 41G. X lOtimai. PA.phnoio phroilii nerve; PC., pleuropericudial membnue: nerve; S.T., septum tnDBvanum: PC., plmiroperi5.7*.. septum tnnsvereum; H.. bumenu; 1. 2. wrdiftl membnuie; PP., ideuroperitoneal mau3. rib*. brane; I. 2. 3, 4, nbs.
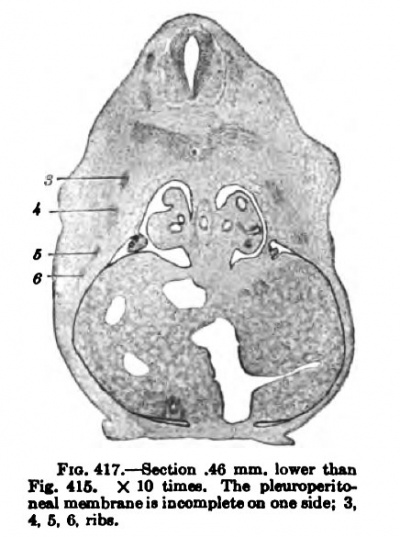
Fig. 417.
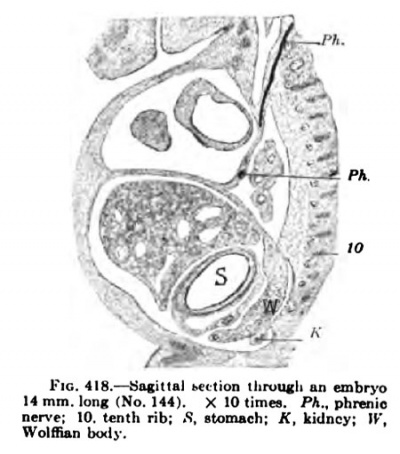
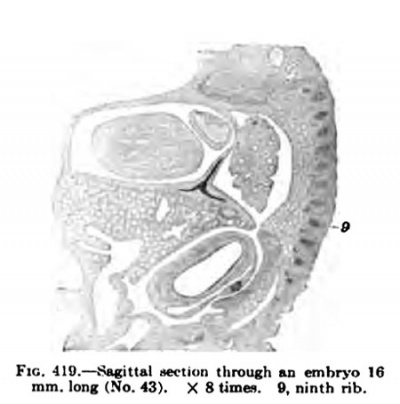
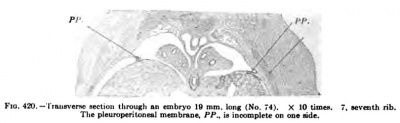
In a human embryo 11 mm. long, immediately after the completion of the pleuroperieardial membrane, it is seen that the rotation of the liver and septum transversum is accelerated, and that the pleuroperitoneal membrane extends rapidly down into the thorax as the Wolffian body recedes. (Compare Figs. 407 and 414.) The -^-shaped section of the septum transversum and pleuroperieardial and peritoneal membranes (Fig. 414) is soon changed by the growth of the lung and the shifting of the diaphragm (Figs. 417 and 418), which gradually places the pleuroperieardial membrane at right angles to the diaphragm. The opening behind the pleural and peritoneal cavities gradually becomes smaller and smaller (Fig. 419), closes first on the right and then on the left side (Fig. 420) ; usually both membranes are complete in embryos 19 nam. long.
Fig. 418.
Fig. 419.
Fig. 420.
As the lungs invade the thorax in the wandering of the diaphragm they must carry their surrounding pleural space with them, which calls for a radical shifting of the mesenchyme between the parietal pleura and the ribs. In fact there is a great mass of mesenchyme just at this point (Figs. 417 to 420), which in embryos at this stage has many unusually large spaces in it indicating that they are normal tears. After the diaphragm has reached its permanent position and the lungs begin to grow relatively larger, they encroach upon this tissue and it is reduced in quantity to make room for them. The lung also burrows between the pleuropericardial membrane and the main body wall, increases the extent of the membrane, and pushes it with its inclosed phrenic nerve to the medial side of the lung, between it and the heart.
To what extent the permanent diaphragm is formed from the pleuroperitoneal membrane is difficult to determine. It is probable that the portion dorsal to the attachment of the pleuropericardial membrane of the septum transversum is formed by the pleuroperitoneal membrane. At any rate, the point of entrance of the phrenic nerve may be viewed as the most fixed point, one difficult to shift, for it is closly associated with the muscle of the diaphragm which invades the septum transversum when it is still high in the neck of the embryo. However, this kind of reasoning is not altogether sound and should be taken with some reserve. The shifting of large masses of organs, their power to burrow and extend as the lung does around the heart, and the fact that the liver grows into the pleuroperitoneal membrane (Fig. 418) while it is being separated from the septum transversum on its ventral side (Fig. 417), should make us somewhat reserved in our statements regarding the origin of the dorsal and ventral diaphragms. In reality we have gotten but a little further than to confirm His, who stated that the septum transversum is extended dorsally and separates the pleural from the pericardial and peritoneal cavities. Defining the septum transversum as he did, proved to be the foundation-stone of all subsequent study regarding the separation of the body cavities.
The coelom cavities of the myotomes are in general independent of the peritoneal cavity in man, with the exception of the second, which, according to Keibel and Elze, communicate with this cavity in an embryo 1.38 mm. long. These cavities of the myotomes are well developed in embryos 2 mm. long, but after this stage they soon disappear.
The diverticulum of the coelom into which the testis wanders begins to form as the Wolffian body atrophies during the third month of pregnancy. At first there is an evagination of the abdominal wall in the inguinal region^ forming the inguinal bursa which is lined by a sac of peritoneum, the vaginal process. This in turn sinks into the embryonic scrotum, which has formed independently. Soon the bursa is partly filled again by a marked thickening of its apex to form the conus inguinalis or gubemaculum, which continues into the genito-inguinal ligament to the testis. During the seventh month the final descent of the testis takes place. At this time the bursa becomes markedly enlarged, the conus retracts, and the testis moves into the embryonic tunica vaginalis, which becomes completely separated from the peritoneal cavity at about the time of birth.
A similar but much less marked process takes place in the female. In the case of the ovary the migration is slight, although provision for the descent has been made in the formation of both inguinal bursa and ligament. The lumen of the vaginal process usually disappears, but may remain open to form what is known as the diverticulum of Nuck.
In not all cases does the testis enter the inguinal bursa, but instead remains in the abdominal cavity or within the inguinal canal. In other cases the processus vaginalis does not close after the descent of the testis and some of the abdominal viscera may enter the canal, forming congenital inguinal hernia. A similar hernia of the diaphragm may occur when the communication between the pleural and peritoneal coelom is not completely cut off. This kind of anomaly is much more common in the left side of the body than on the right, probably on account of the corresponding unequal growth of the liver on the two sides of the body. Congenital hernia may also occur in the umbilical cord when the coelom of the cord is not obliterated after the intestine returns from it into the abdominal cavity. In case the opening with the exoccelom of the cord is very large, most of the abdominal viscera — liver, spleen, large and small intestines — may extend into it. In such cases the extruded viscera are covered only by a thin membrane composed of peritoneum and amnion.
There remains still one very fundamental pocket of the peritoneal coelom; it is, the pocket which forms the lesser peritoneal cavity. This pocket, the bursa omentalis, was first recognized in the embryo by His, and later Ravn discovered that it developed not only on the right side of the body but on the left side also, and that the cephalic tip of the right cavity separated and formed one of the serous spaces which comes to lie in the region of the right lung. More recently the development of the lesser peritoneal cavity in man has been studied by Swaen and by Broman. Broman's admirable study is comparative and includes numerous human embryos; he confirms for the human embryos Bavn's discoveries in the rabbit, mentioned above.
In an embryo 3 mm. long there are two peritoneal pockets on either side of the oesophagus, the right being somewhat larger than the left. The left is of but short duration in the human embryo; it evaginates, Broman believes, but the right, the reeesaus pneumato-entericus dexter, is plosely associated with the lesser peritoneal cavity. Just below this recess there is another depression (Fig. 396, below FW), the recessus mesentericoenterieus of Swaen, which soon extends between the stomach and liver and later gives rise to the omental bursa.
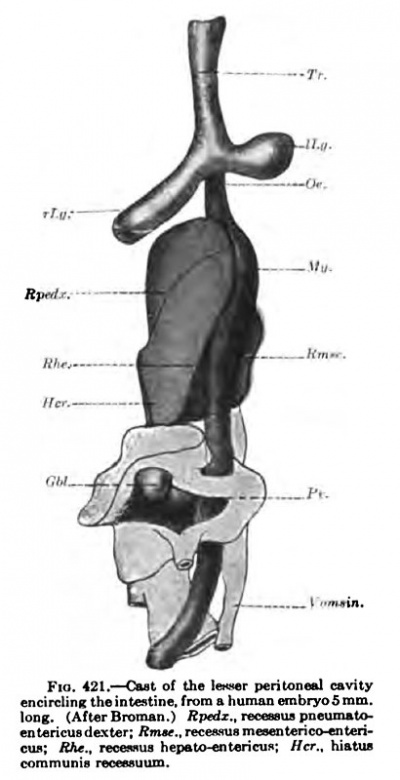
Fig. 421. Cast of the lesser peritoneal cavity encircling the intestine, from a human embryo 5 mm long (After Broman)
A reconstruction of the right recess, in an embryo 5 "' mm. long, is shown in Fig. 421. The upper recess (Bpedx) reaches to the lung bud of the right side, the dorsal recess extends into the mesentery of the stomach, and the ventral recess, recessus hepato-entericus, borders on the liver and later encircles the Spighelian lobe. Below they communicate with the peritoneal cavity through the hiatus communis recessuum (Her) or the primitive foramen of Winslow. Sections through different stages in Figs. 393, 405, and 411. As the stomach recewus hepew-entericus: ffcf.. hLMiM grows and bends to the left, it gradually gives form to the lesser peritoneal cavity, as is shown by a cast of it from an embryo 11.7 mm. long {Fig. 422). It is easily seen that the hiatus of Fig. 421 has become the true foramen of Winslow, the recessus hepato-entericus has become the cavity of the lesser omentum (Bomin), and the recessus mesenterico-entericus has become the cavity of the greater omentum (Bomaj). The cavity of the greater omentum is formed, according to Swaen, by a burrowing of the cavity into the wall of the stomach, and not by a simple bend of its mesentery, as is indicated, for instance, in Fig. 411, according to the B.N.A., the bursa omentalis is divided into a vestibulum and a recessus superior, which form together Broman's bursa omenti minoria, and a recessus inferior, which is the same as the bursa omenti majoris. In my opinion, it would be better to apply the terms of the B.N.A. to these spaces in the embryo as soon as their fate is clear. For the same reason I have used the term pericardia] cavity in describing the smallest embryos, for the relation of this space to the heart enables us to identify it. Additional unnecessary names only complicate the subject.
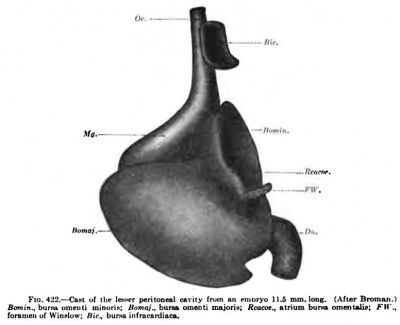
Fig 422. Cast of the lesser peritoneal cavity from an embryo 11.5 mm long (After Broman)
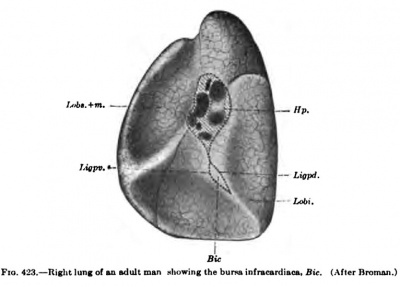
According to Broman, the recessus pneumato-entericus of Eavn extends upward in tlie human embryo to the bifurcation of the lungs. By the time the embryo is 11 mm long, the recessus pneumato-entericus begins to separate from the lesser peritoneal cavity and is soon pinched off to form the bursa infraciirdiaca, as sliown in Fig. 422. From now on, it can be found in all embryos as a closed sac lying between the right side of the cesophagus and the diaphragm. It gradually grows in size and is about one centimetre in diameter at birth; in a specimen from an adult man (Fig. 423, Bic) it measures 42 x 20 mm. This third pleural cavity, very well developed in all animals which have an infracardial lobe of the lung, is therefore found in all human embryos and probably also in most adults. Its frequency will have to be established by statistics.
Fig. 423. Right lung of an adult man showing the bursa infracardia
The various body cavities communicate freely with one another in all vertebrate embryos. In later stages this primitive cavity is divided into several compartments by the formation of the septa described above. Only in Myxine does it remain single, while in Petromyzon the division takes place at the time of meta
In selachians the pericardial cavity becomes separated completely from the remaining peritoneal cavity by the septum pericardiaccperitoneale. This membrane is present also in ganoids imd teleosts, but as yet it has not been studied carefully,
Independent pleural cavities are not found in amphibians nor in most reptiles; in birds, however, there is always a complete pleuroperitoneal membrane present. The process of the subdivision of the Coelom is far more complex in mammals than in the remaining vertebrates. In them the septum pericardiaeoperitoneale is called the septum transversum, from the dorsal end of which both the plenropericardial and pleuroperitoneal membranes arise, A chief difference between mammals, on the one hand, and birds, reptiles, amphibia, and fishes, on the other, is the very early formation of the septa in the former and their late appearance in the latter.
Literature
Bonnet: Beitriige ziir Embryologie der Wiederkauer, Arch, fiir Anat. und Phyaiol,, Anat. Abl., 1889.
Bbaciiet: Die Eiitwicklung der grossen Korperfaohlen und ihre Trennung vonein ander, Ergebnisse der Anat. und Entwicklungsgescb., Bd. 7, 1897.
- Bulletin de I'Aead^mie Royale de M^decine. Vol, lix. Broxelles, 1906.
Broman : Beschreibimg eines menschlichen Embryo von beinabe 3 mm. Lange, Morpbol. Arbeiten, Bd. 5, 1894.
- Entwickliing des Zwerchfelles beim Menscben, Verhandl. d. anat. Gesellscb., 1902. Die Entwickluiigsgeschichte der bursa omentalis, Wiesbaden, 1904.
Bryce and Teacher: Contribution to the Study of the Early Development and Imbedding of the Human Ovum, Glasgow, 1908.
Dandy: A Human Embryo with Seven Pairs of Somites, Measuring About 2 mm. in Length, Amer. Jouni. of Anatomy, vol. 10, 1910.
Herzog : Contribution to Our Knowledge of the Earliest Known Stages of Placentation and Embryonic Development in Man, Amer. Joum. of Anatomy, 1909.
His: Anatomic der menschl. Embryonen, Heft 1, 1880. Mitteilung zur Embryologie der Siiugetiere und des Menschen, Arch. f. Anat. u. Physiol., Anat. Abt., 1881.
Hochstetter: Die Entwicklung des Blutgefass-Systems, Hertwig's Handb. d. vergleich. und exper. Entwickl. d. Wirbeltiere, Bd. 3, 1906.
Keibel : Menschenaflfen, IX. Lieferung von Selenka's Werk, Wiesbaden, 1906.
Keibel und Elze: Normentafeln zur Entwickl. d. Wirbeltiere (Mensch), Heft 8, 1908.
Klaatsch : Uber den descensus testiculorum, Morph. Jahrbuch, Bd. 6, 1890.
Kolliker: Entwicklungsgeschichte des Menscben, Leipzig, 1879.
Mall FP. A human embryo twenty-six days old. (1891) J Morphol. 5: 459-480.
Mall FP. Development of the human coelom. (1897) J Morphol. 12: 395-453.
Mall FP. Supplementary note on the development of the human intestine. (1899) Anat. Anz. 16: 492-495.
Mall FP. A contribution to the study of the pathology of early human embryos, (1900) Johns Hopkins Hosp. Rep., 9: 1-68.
Mall FP. Normal plates of the development of vertebrates. Anat. Rec, (1908).
Mall: Development of the Lesser Peritoneal Cavity in Birds and Mammals, Joum. of Morphol., vol. 11, 1891.
- Development of the Human Coelom, Journ. of Morphol., vol. 12, 1897.
- Supplementary Note on the Development of the Human Intestine, Anat. Anz., Bd. 16, 1899.
- Contribution to the Study of the Pathology of Human Embryo, Johns Hopkins Hospital Rep., vol. 9, 1900, and Joum. of Morph., vol. 19, 1908.
- On the Development of the Human Diaphragm, Johns Hopkins Hospital Bulletin, vol. 12, 1901; and Proceedings of Amer. Assoc. Anatom., vol. 5, Washington, 1901.
Mazilier: Contribution de Pembryologie du diaphragma, Lille, 1907.
M15LLER, E. : Beitrage der Anatomic des menschlichen Fetus, Kgl. Schwedisebe Akademie, Bd. 29, 1897.
Muller, L: Uber den Ursprung der Netze, Meckel's Arch., 1830. Piersol: Human Anatomy, Philadelphia, 1907.
Piper: Tiber ein im Ziegler'schen Atelier hergestelltes Modell eines menschlichen Embryos von 6.8 mm. Nackenlinie, Anat. Anz., Bd. 21, 1902.
Ravn: Uber die Bildung der Scheidewand zwischen Brustund Bauchhohle in Saugetierembryonen, Arch. f. Anat. u. Physiol., Anat. Abt., 1889.
Retzius : Biolog. Untersuch., Bd. 1, 1890.
Spee, Graf v.: Beobachtungen an einer menschlichen Keimscheibe mit offener Medullarrinne und canalis neurentericus, Arch. f. Anat. u. Physiol., Anat. Abt., 1889.
Strahl : Beitrage zur Entwicklungsgeschichte des Herzens und der Korperhohlen, Arch. f. Anat., 1889.
Swaen: Recherehes sur le developpement du foi, Joum. d'Anat. et de Physiol., vol. 33, 1897.
- Note sur la Topographic des Organs abdominaux, Bibliog. Anat., 1899.
Toldt: Uber die Geschichte der Mesenterien, Verhandl. d. Anat. Gesellsch., 1893.
UsKOw: Uber die Entwicklung des Zwerchfells, des Pericardiums und des Coloms, Arch. f. mikr. Anat., 1883.
Velpeau : Embryologie on Ovologie humaine, etc., Paris, 1833.
Waldeyer: Lehrbuch der topographisch-chirurgischeu Anatomie, Bonn, 1899.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Manual of Human Embryology 13. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Manual_of_Human_Embryology_13
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G