Book - Contributions to Embryology Carnegie Institution No.48
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Macklin CC. the skull of a human fetus of 43 millimeters greatest length. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ., 48, 10:59-102.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Skull of a Human Fetus of 43 millimeters Greatest Length
By Charles C. Macklin, Associate Professor of Anatomy, Johns Hopkins University. (1921)
With 5 plates containing 47 figures.
Contents
|
I. Introduction II. Description 1. The skull as a whole
3. Otic region
|
5. Ethmoidal region
|
6. Cartilaginous branchial-arch skeleton
7. Membrane bones
III. Summary IV. Bibliography VI. Abbreviations |
Introduction
Since the publication of my work on the skull of a human fetus of 40 mm. (Macklin, 1914) I have had the opportunity of studying and modeling a very satisfactory specimen of a somewhat younger stage. This is human fetus No. 886 of the collection of the Carnegie Institution of Washington. The sections were cut in paraffin in the frontal plane at a thickness of 100 micra and stained with alum cochineal. The technical work was all excellently done, and the series is practically perfect.
The models, of which there are 28, were made by the plaster-of-paris method of Lewis (1915), which reproduces the structures with almost absolute accuracy. The skull as a whole was first reconstructed at a magnification of 10 diameters and the details were then worked out in separate models, most of which were made at a magnification of 20 diameters. In the few instances where smaU details were lost, these were made good by making new models. Each model was very carefully checked by comparing each separate plate fine, as the model was being painted, with the bromide photographs of the sections. The models are, I believe, as nearly exact reproductions of the original structures as it is possible to obtain. The bones were modeled on the right side only.
The parietal and frontal bones were too delicate to be modeled except in outline, and in drawing their texture the method of profile reconstruction was employed. The same method was used to check up the general outline of the skull and to obtain the relation of the external form and of the brain to the skull.
The drawings were accurately made by Mr. J. F. Didusch. The method of geometric projection was used, which insures an accurate representation of the original.
The study of No. 886 gave an opportunity for comparison of this specimen 'ndth the skull of a 40 mm. fetus from Professor McMurrich's collection, known as "I" Toronto," and hereinafter referred to as "la," which I previously modeled. In la the measurement was crown-rump, and in No. 886 it was greatest length, and this accounts for the fact that No. 886 is considerably younger though of greater linear dimension. An opportunity was also afforded to compare the skull of No. 886 with that of No. 460 of the Carnegie collection, which has recently been modeled by Lewis (1920) and which shows the condition of the skull in a human fetus of 21 mm.
Young cartilage and precartilage, although not abundant at this stage, were included, and are mentioned where they occur.
Terms of direction in the following description are all related to the basal plate in a horizontal position. Accordingly they are, at times, at variance with the terms of orientation applying to the adult skull, particularly in the ethmoidal region, since this is usually described with the basal plate almost vertical. For the same reason the terms are often different from those used in the description of la.
Description
The Skull as a Whole
The skull of No. 886, although considerably younger than that of la, resembles it quite closely, so that I have not been put to the task of writing such a detailed account as would have been necessary had my former article not been available. I have endeavored to avoid a repetition of my former description, and to make this article largely a comparison of No. 886 with la.
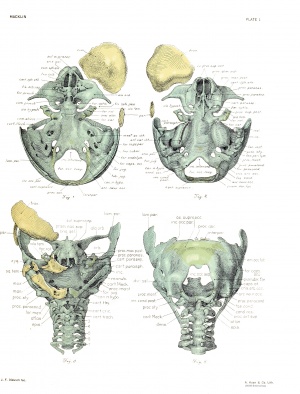
Figure 1 presents the most favorable view from above. The basal plate is not exactly horizontal, the cranial end being a Uttle closer to the eye of the observer than the caudal. The nasal capsules are seen from an oblique direction, and hence a true concept of their length is not obtained, as will be realized when the parts are regarded from the side.
By imagining the frontal and parietal bones as having been inserted on the left side, as well as on the right, and by filHng the gaps between the elements of the outer border, it is seen that the contour of the cranial cavity, from this point of view, is oval, and a little wider anteriorly, in the region of the frontal bones, than posteriorly, at the widest part of the parietal plates and otic capsules. Posteriorly this contour is made up of the cartilaginous walls of the future posterior cranial fossa; anteriorly, however, it is composed of membrane and membrane bone. The chondrocranium is divided into two unequal portions in the region of the body of the sphenoid by the superior orbital fissure — an extension of the sphenoparietal fissure. Projecting into this fissure from the body of the sphenoid is the short temporal wing.
If this figure be compared with figure 1 of la we note obvious signs of advancement in the latter, perhaps the most marked being the more developed state of the anterior end of the skull. Lewis (1920) has commented upon the relatively more rapid growth which must take place in the prechordal, as contrasted with the chordal, portion of the skull, following the stage of 21 mm. which he studied, and others have expressed themselves similarly. From a comparison with No. 460, on the one hand, and with la on the other, it is very apparent that in No. 886 development of the anterior end of the skull is going on more rapidly than that of the posterior end.
Certain features of la, such as the sharp bending of the otic capsule, suggest that the specimen from which the model was made was laterally compressed and somewhat shrunken.
In figure 2 we have the most favorable aspect of the skull base. In it the basal plate is almost horizontal, the caudal end being a little closer to the eye of the observer. The view is thus not directly antipodal to figure 1, and hence the outer contours of the entire skull, and of the chondrocranium, are slightly different. The ethmoidal region is viewed from the anterior end, making impossible an adequate appreciation of its length and that of the fissura basalis, which separates the ectethmoid from the mesethmoid. The lower jaw has been entuely cut away.
A frank view of the face is seen in figure 3, which also includes partial views of the larynx and cervical vertebrae. The gaping mouth and orbits are conspicuous features.
The skull is so placed in figiure 4 that the eye looks squarely into the foramen occipitale magnum. The vertebral column is tilted a little so that its lower end is slightly nearer to the eye than the upper. The skull is not absolutely symmetrical and there is a slight deflection of the axis of the basal plate to the left, as the figure shows.
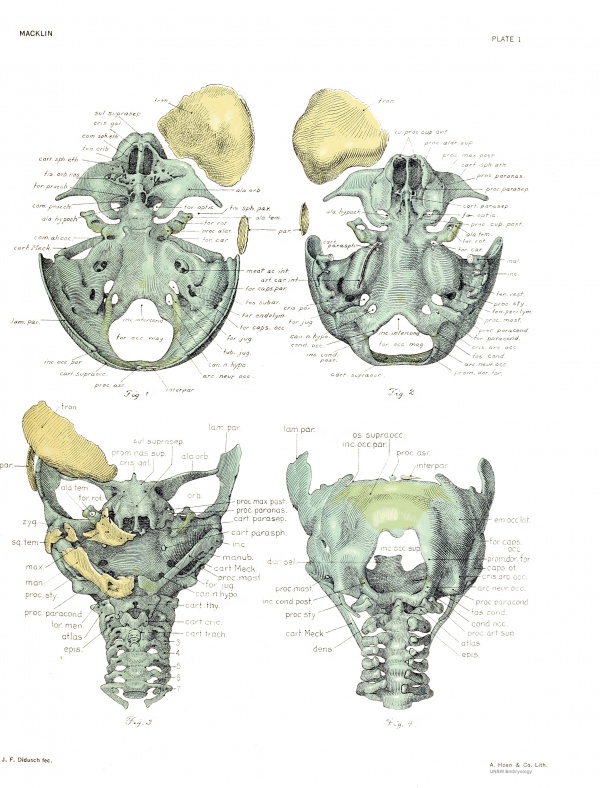
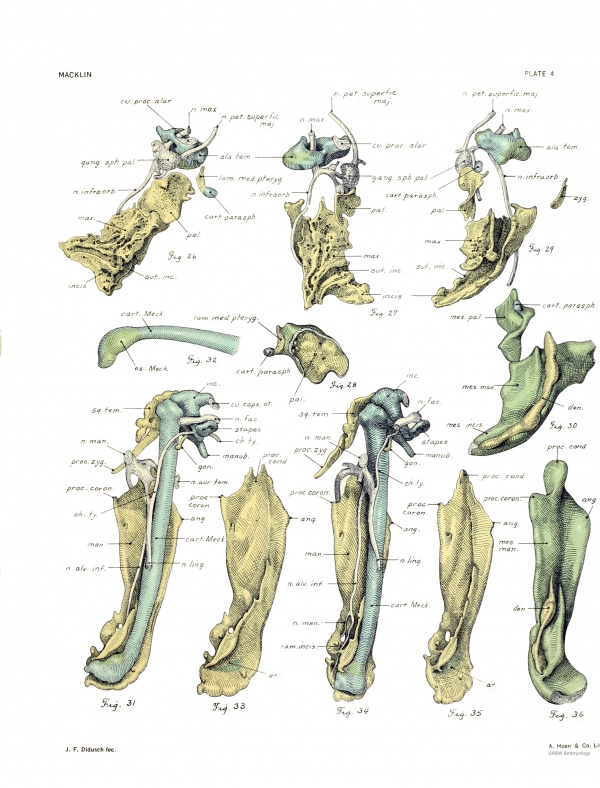
Side views of the skull are afforded by figures 5 and 6, the former including the membrane bones. The depth of the posterior cranial fossa is appreciated by looking at the dorsal portion of the chondrocranium. The hyoid, thyroid, cricoid, and four upper tracheal rings are seen. The contours of the external form, brain, frontal and parietal bones, and chondrocranium are seen from the right side in figure 7 in their normal relationship. This figure was made by profile reconstruction. The other figures show various details of the chondrocranium, and will be referred to in the description.
In general form the skull of No. 886 resembles closely the 28 mm. stage of Levi (1900), which seems to be a little farther advanced than the 30 mm. stage of Jacoby (1895).
Central Stem
The central stem of the chondrocranium is seen from the side in figure 10, with the cut surfaces of its adnexa indicated. Its chordal and prechordal limbs meet in the region of the hypophyseal fossa, where the stem appears to have been twisted through an angle of 90°, as well as strongly bent. The chordal limb represents the basal plate of the skull, while the prechordal limb contains the body of the sphenoid, with the interorbital and nasal septa. The lower contour lines of the two limbs, as seen in figure 10, meet at an angle of 115°, as in la. The prechordal limb in No. 460 is relatively shorter, and the corresponding angle, as measured by me on Lewis's figure 5, is 125°.
The basioccipital and basisphenoidal elements of the basal plate are united by continuity of cartilage at the undefined spheno-occipital commissure. Here the plate is thiimest, as figm-e 11, of the midsagittal section, shows. In this figure, too, it is apparent that the anterior end is much thicker than the posterior and that the upper sm-face presents a deep antero-posterior concavity, located in advance of the center.
At the junction of its anterior and middle thirds the plate is narrowed, due to the encroachment of the cochleae. The caudal end of the curved basicochlear commissm-e projects outward beyond the cranial. The cut edge of this union is seen on the basal plate in figure 10, and on the cochlea in figm-e 15. The dorsal and ventral basicochlear grooves are not so deep as those of la.
The dorsal surface of the basal plate (figure 1) is shallowly concave from side to side throughout almost its entire extent. Anteriorly, however, this concavity becomes very narrow and indistinct as the surface rises upon the basisphenoid, and disappears at the root of the dorsum sellae. The ventral surface presents a corresponding low transverse convexity, which falls away laterally into the ventral basicochlear grooves, flanked by the protruding cochlear portions of the otic capsules. The ventral suiiaces of the cochleae and of the basal plate is practically in the same level and thus combine to give to the ectal surface of the base of the skull a flattened appearance in contrast to the roomy concavity of the corresponding ental surface. Posteriorly, the diverging limbs pass uninterruptedly into the primordia of the exoccipitals.
Notochord
The notochord was modeled in reUef upon the midsagittal section of the basal plate and epistropheus and is illustrated in figure 11. Commencing below, it proceeds through the body of the epistropheus and dens, emerging from the apex of the latter. The cartilage of the dens projects a little farther forward than the point of exit of the chorda and upon this cartilaginous tip the chorda rests; it then springs across the very narrow gap between the dens and the adjoining caudal edge of the basioccipital and here shows some thickening. It now proceeds along the dorsal surface of the basioccipital, in the midline, for 900 micra, being buried in perichondrium. The anterior end of this part is compressed dorsoventrally. It then traverses the plate as shown in the figure, here perforating the anterior end of the beginning ossification center for the basioccipital. Within the cartilage it is a very slender thread and at its point of exit it loses its continuity, there being here a break of something less than 100 micra. Shortly after emerging below the basal plate it comes into contact with the long, attenuated pharyngeal bursa and follows a course upon the dorsal edge of this. Especially at the extremities of the bursa it is contorted and varicose. Leaving the pharyngeal bursa, the chorda lies immediately above the epithelial roof of the pharynx for a considerable distance. It then turns sharply, as an attenuated and somewhat contorted cord, to approach the body of the sphenoid, which it enters almost at right angles to the surface. Finally, it curves forward to terminate in the body of the sphenoid near its dorsal surface and some distance short of the crista transversa. This basisphenoidal portion of the chorda is a little wider than that just outside the cartilage and its terminal end is somewhat nodular and irregular in duection.
In its course the notochord of No. 886 thus resembles closely that in Ruber's (1912) human embryo J, No. 47, 32 mm. long, shown in his figure 10.
Occipital region
The occipital region is a homogeneous mass of cartilage whose caudal boundary is formed by the margin of the primitive foramen magnum. The cranial boundary is marked by cartilaginous unions with the otic region and the foramina separating these. Proceeding from the front late ally and backward, we note the following commissures: spheno-occipital, basicochlear, capsule- occipital, and occipitoparietal. Behind the
basicochlear commissure a section of the boundary is formed by the posterior margin of the jugular foramen. The lateral half of this, which is thin and forms the concave lip of the sigmoid sulcus, runs almost directly outward and so makes an angle with the medial thicker and more rounded half. It is at this angle that we find the anterior end of the jugular tubercle. The boundary is then continued as the capsulo-occipital commissure, which curves upward and forward around the canaHcular part of the otic capsule, to become directly continuous with the capsulo-parietal commissure. It is interrupted by the conspicuous capsulo-occipital foramen (figs. 5 and 6). Figure 14 shows the surface where the commissure has been severed.
Occipito-parietal Groove
The next section of the boundary is formed by the occipito-parietal commissure, which meets the preceding at an acute angle. It joins together the squama occipitalis and parietal plate. The latter bends inward a little upon the former to make the shallow occipito-parietal groove (fig. 14), which marks the position of the commissure upon the ental surface. The sections show that the cartilage is very much thinner here, especially at the dorsal end, though not materially different in quality as compared with that above and below. As in 7a, there is no trace of a corresponding groove upon the ectal surface.
The ventral end of this groove is not very well defined, but may be placed just above the capsulooccipital foramen. On the right side there is here a very small foramen. The dorsal hmit of the groove is marked by the conspicuous occipitoparietal notch which separates the pointed dorsomedial termination of the parietal plate from the underlying supraoccipital element (fig. 14). Between these extremities the groove pursues a course almost directly backward, upward, and inward.
The occipito-parietal groove has been noted by Kernan (1916) as early as the 20 mm. stage. In la it was well marked and presented two foramina upon the right side and one upon the left. These foramina are not represented in No. 886, nor are they noted in No. 460 or in Kernan's specimen. Furthermore, in la the occipito-parietal notch was considerably deeper and, indeed, terminated as a narrow slit. What appeared to be degenerating cartilage cells were found in la on the right side (the sections did not include the corresponding region on the left side), uniting the parietal plate with the squama in the region of this cleft. From this it would seem that there is a gradual disunion of these two plates in progress at the posterior end of the occipito-parietal commissure during this developmental period.
The groove for the endolymphatic sac, which Lewis mentions having found upon the "mastoscjuamal plate," corresponds to the anterior end of the occipito-parietal groove. In No. 886 the attenuated prolongation from the endolymphatic sac lies immediately medial to the anterior end of the groove, but separated from it by 0.5 mm. In la it was similarly situated. It is not impossible that this groove may be related historically to the endolymphatic sac, for it leads backward and inward to a point quite close to the processus ascendens; and in the lizard, as has been pointed out by Gaupp (1900) and others, the endolymphatic sac hes just lateral to the processus ascendens. The region between the otic capsule and the tectum posterius has undergone an enormous amount of extension in the mammals, and particularly in man, and the endolymphatic sac has accordingly become removed from its original position above the tectum posterius, because of its connection with the otic capsule.
Processus Ascendens
Of the cranial boundary there remains to be completed only that part lying between the aforementioned occipito-parietal notches. This hne, which marks the thin upper border of the supraoccipital cartilage, is transverse, with a backward bowing (fig. 1). Projecting upward from this, in the midhne, is a single small nodule of rather young cartilage. Upon either side of it are the fundaments of the interparietal bone, which are seen as thin strips lying close to the upper edge of the supraoccipital. This nodule is spheroidal and its anterior face projects forward a little. It is directly continuous below with the supraoccipital cartilage. This process corresponds to the "short process" of Fawcett (19106), which he found in a 30 mm. human embryo. He states that it "seems to correspond exactly with the ascending process of the tectum synoticum of reptiles and amphibia as figured in Hertwig's Handbook of Embryology." In this homologization I agree. Bolk (1904) shows such a process in his figure 1, plate 6, projecting upward from the "Knorpelspange," but does not describe or name it. This figure was drawn from a human skull, apparently of about the same stage of development as No. 886. The "Knorpelspange" of Bolk, be it remarked, is merely the upper edge of the tectum posterius, which remains uncalcified, and so may be stained by the methylene blue of the van Wihje method which he uses, while the calcified cartilage below it, which Bolk erroneously interprets as membrane, is not stained in his preparations.
In the pig's chondrocranium Mead (1909) has described a single free noduleof cartilage just above the tectum posterius in the midline, which seems to correspond to the processus ascendens of No. 886. He thought it might possibly be the homologue of the processus ascendens of the tectum posterius of the reptiles. In the lizard the processus ascendens has been described by Gaupp (1900) and by Rice (1920). Rice states that in the lizard it gives protection to the endolymphatic sacs, which lie upon either side of it. Such a function is, of course, out of the question in the skull of homo, since the endolymphatic sacs are very remote from this position.
In the skull of la I described a small free nodule, with a very minute cartilaginous fragment beside it. These I termed the posterior cranial cartilages and suggested that they might represent ttie unpaired, elongated, transverse, free nodule of cartilage described by Bolk in human chondrocrania, which lay in the membrane in the midline some distance above the tectum posterius (and ascending process) in the position of the future interparietal bone and which seemed to be undergoing regi'ession in his second stage, shown in his figure 2. On account of the fact that the sections of 1a were missing just behind these nodules I was unable to ascertain their exact relationship to the tectum. In No. 886 there 's no cartilage corresponding to this single nodule of Bolk.
Basioccipital Cartilage
The basioccipital cartilage has been described with the basal plate. Its ossification center (figs. 1 and 2) is already indicated in a single area of cartilage undergoing the change preliminary to ossification. This area occupies practically the entire thickness of the plate, as shown in figure 11, and extends for about 700 micra antero-posteriorly. Its extremities, however, are indefinite and the cartilage for some distance in front of and behind it is of an older type than that toward the sides of the plate. Its innermost portion shows the greatest degree of change in the cartilage cells and there is a gradual transition toward the normal condition peripherally. Its anterior end (fig. 11) is traversed by the notochord, which is here much attenuated.
Exoccipital cartilage
Traced backward, the exoccipital cartilage undergoes a progi'essive widening together with a twisting and bending, the outer edge becoming tilted upward and the upper surface looking more and more directly inward and forward. The hypoglossal foramen shows no partition on either side. Its large and stout medial border is directly continuous behind with the thickened margin of the foramen occipitale magnum. This bar is bent downward to form the rounded occipital condyle, best seen in side views of the skull. The lateral border of the foramen is comparatively slender and is placed at a considerably higher level. It joins the anterior end of the jugular tubercle.
The jugular tubercle (fig. 1), which separates the hypoglossal foramen from the jugular cave lying laterally, is best marked anteriorly, where it is thin and high. As Terry (1917) remarks for the cat skull, it "presents much more the form of a ridge than of a tubercle." Passing almost directly backward, it mounts upon the rising surface of the exoccipital cartilage, where it becomes lower and broader. It terminates by curving inward and is lost upon the rounded margin of the foramen occipitale magnum at the posterior condylar notch. Later, in the occipital bone, the jugular tubercle comes to overlie the hypoglossal canal, and even reaches forward beyond it. Throughout its extent the ridge exhibits characteristic preossification changes, and we have here the beginning ossification center of the exoccipital fundament.
This center is shown in figm-e 1. Its posterior half is somewhat wider than the anterior and evidences the greatest amount of change in the cartilage. Here, too, in contrast to the anterior part of the center, the process involves the entire thickness of the plate, the area in the posterior condylar notch in figure 2, and better still in figure 4, showing it upon the ectal surface. Here the process has extended to the border of the foramen occipitale magnum, at the posterior condylar notch (figs. 5 and 6), thus implicating the neural arch of the occipital vertebra. In la the preossification change was farther advanced.
Medial to the jugular tubercle, the surface slopes down to the foramen magnum — more steeply behind than in front — and passes over upon the condylar surface, which here projects medially into the contour of the foramen (fig. 1). Just behind the hypoglossal foramen there is a hollow for the hj-poglossal nerve.
Lamina alaris. — Lateral to the jugular tubercle is the lamina alaris, deeply grooved to form the sigmoid sulcus. Its outer edge, confluent above with the neighboring otic capsule, does not extend so far forward as the jugular tubercle. Ventrally the plate terminates as a thin concave lip, over which the sigmoid sinus empties into the jugular foramen. The edge of this lip, slightlj' convex anteriorly, shows a down-turned tip (fig. 10) which strikingly resembles the corresponding formation in the osseous condition. The ventral part of the lamina alaris is formed by the paracondyloid process.
The floor of the sigmoid sulcus rises steeply behind, thus bounding the jugular recess posteriorly. This steep area corresponds to the deep condylar fossa of the ectal surface. The recess is partially ovcrhunj; anteriorly by the conspicuous posterior ampullary prominence, which gives to the region a cave-like appearance.
As in 7a there is on the left side, but not on the right, a small paracondyloid foramen (200 micra in antero-posterior diameter and rather less in transverse) which pierces the thin lamina alaris just lateral to the jugular tubercle. It contains only connective tissue. The corresponding area on the right side is very thin.
Paracondyloid process. — The paracondyloid process, less stout than in Ja, is a prominent object in figure 2, appearing as a ridge passing outward and slightly upward and forward from the condyle, and becoming more sharply marked laterally. It ends in a point which projects outward and a little downward (fig. 10), and which lies just lateral to and below the outer limit of the jugular foramen. I have already expressed myself (Macklin, 1914) as agreeing with Levi (1900) and Voit (1909) in their identification of the corresponding structure of their specimens as the forerunner of the jugular process of the occipital bone, and Lewis (1920) is in accord with this idea. Kernan (1916, p. 626) asserts that this process unde goes absorption.
It is at the transverse ridge formed by the condylar and paracondyloid processes that the occipital cartilage makes its sharp bend upward, as is seen in side views of the skull (figs. 5, 6, 10). In this way the ectal surface is divided into anterior (or basal) and posterior (or nuchal) areas. It is of interest that, at this stage, the paracondyloid process reaches laterally far beyond the joined transverse and costal processes of the underlying atlas, as is seen well in figure 4. This contrasts most strongly with the condition in the adult, where the reverse relation holds, the transverse process of the atlas overreaching the jugular process.
Kernan describes the tip of the paracondyloid process in his 20 mm. human embryo as separate from the cartilage medial to it, but attached to the basioccipital through a thin process of cartilage which passes medially and cranially in front of the hypoglossal foramen. This "thin process" (which is what I have described as the posterior border of the jugular foramen, and which is bounded behind, on the left side, by the paracondyloid foramen) he represents to be the independent costal process of the second occipital vertebra (not of the first, as I suggested), and the paracondyloid process is considered to be the caudal end of this. Thus, according to Kernan, the paracondyloid
process does not represent the transverse process (and possibly the costal process) of the occipital vertebra, as I suggested (Macklin 1914). However, it should be noted that the paracondyloid process of Kernan is but the lateral free tip of the structure which I described under the same name. In Kernan's 20 mm. stage the paracondyloid process is very small as compared with its condition in No. 886 and 7a. Even if the tip of it does belong to the second vertebra, it would still seem that the main mass of it is to be looked upon as having been derived from the transverse and possibly the costal process of the occipital vertebra. Kernan notes that there is no independent costal element of the occipital vertebra.
The paracondyloid process of Kernan's specimen is directly continuous above with the lateral portion of the lamina alaris, which appears upon the ectal surface as the lower end of the formation which I have termed the crescentic ridge. Kernan erroneously speaks of this as "the inferior nuchal line of the ex-occipital portion of the adult bone." This lateral portion of the lamina alaris shows, according to Kernan, alternating interruptions and junctions with the cartilage lying medial to it and is looked upon as a costal bar, the junctions representing the bases of transverse processes of vertebra;. He suggests that "in the lamina alaris are represented the costal and transverse processes of several vertebrae. The outer bar represents the fused costal elements and may be termed the costal bar." He claims to be able to discern parts of three vertebrae in the lamina alaris.
Lewis, who describes a stage of the human embryo (21 mm.) almost identical with that of Kernan, says nothing as to this comphcated structure of the lamina alaris nor as to the independence of the tip of the paracondyloid process. He states (p. 317): "The occipital transverse process forms part of the caudal and lateral margins of the jugular foramen and continues up into the squama and alar lamina without line of demarcation." Further (p. 318) he says: "The transverse or jugular process springs from the occipital hemiarch at the junction of the roots and lamina and projects laterally back of the jugular foramen. We have akeady noted its serial relationship with the vertebral transverse processes. The lateral extremity of the jugular process has a knob-like enlargement, and into this are inserted the rectus capitis lateralis muscle and the occipito-mastoid muscle."
It should be remarked that the structure which Kernan in his plate 3 labels as "Processus costalis et transversus, occipital vertebra" is labeled by Lewis as "occipital condyle" in his figure 6. I have examined the Lewis model and agree with his labehng of the occipital condjle. It would seem that Kernan has placed his direction line for the occipital condyle much too near the midline and that the occipital condyle is to be found at or near the point designated by him as the "Processus costalis et transversus, occipital vertebra." The relation of the articular surface of the atlas in Kernan's sections seems to favor this view. It is quite obvious, too, that in his plate 3 Kernan has mislabeled the hypoglossal foramen, calling it the "foramen jugulare." If his labeling is correct, then the "rib element, 2nd occipital vertebra" passes in front of the jugular foramen — a relationship which is evidently impossible.
In his description and figures of the cat's chondrocranium, Terry (1917) has apparently included the "costal bar" of Kernan with the paracondyloid process, considering the process to be even more extensive than I have conceived it to be.
Supraoccipital Cartilage
Behind the lamina alaris the squama occipitalis, thfe right side of which is seen from within in figure 14, becomes progressively wider to the region lying between the upper extremity of the foramen magnum and the anterior end of the occipitoparietal commissure; after this it becomes somewhat narrower. Its surfaces are fah-ly smooth, but there are certain markings which should be mentioned.
Just within the capsulo-occipital commissure is the medial capsulo-occipital groove, deepened in front by the posterior end of the otic capsule, around which it curves. Posteriorily this groove is bordered, in places rather indefinitely, by a low ridge, which becomes confluent below with the jugular tubercle. Just behind the capsulooccipital foramen this ridge is quite well marked. The gi'oove contains the transverse sinus which, when traced from above, descends until it reaches the sigmoid sulcus, where it turns sharply and runs forward for a short distance before plunging downward again into the jugular foramen. The corresponding lateral capsulo-occipital groove is indefinite.
The lateral occipital eminence (figs. 4, 5, 6) is not so conspicuous as in 7a, nor is the cartilage of the plate here so thick. Behind it the ectal surface shows a wide, shallow but distinct groove, Hmited below by the crescentic ridge, bordering the U-shaped area of cartilage around the superior incisure; the latter, in tm-u, protrudes slightly, as figure 4 shows. Tiii, ends of this low ridge are confluent with the extremities of the crescentic ridge in the region of the dorsal foraminal prominences. Both shallow groove and low ridge are represented upon the ental surface in reverse, as shown in figure 14.
The crescentic ridge or crista arcuata occipitalis (figs. 2, 4, 5, 6) sweeps upward, backward, and inward from the tip of the paracondyloid process to end rather indefinitely in the region of the dorsal foraminal prominence. Its structure is very similar to that of la. The ventrolateral end is narrow, raised, and distinct, but as the ridge passes backward it broadens and flattens out.
Judging from his plate 2, Kernan has divided the structure which I termed in la the crescentic ridge into two parts, the dorsomedial of which he labels "crescentic ridge" and the ventrolateral, "costal bar, lamina alaris." I used the term "crescentic ridge" (as I have done in No. 886) to apply to the entire ridge, beginning at the tip of the paracondyloid process and ending upon the border of the foramen occipitale magnum. Kernan saj's that this structure is the inferior nuchal line. I think it will be obvious to anyone who examines the models of the chondrocranium showing these ridges and compares them with the mature skull, that this interpretation is not correct, for, as is well known, the inferior nuchal lines, although arising anteriorly from the same region, viz, the jugular process, yet meet dorsally upon the external occipital crest at a point some distance behind the posterior margin of the foramen occipitale magnum. In the models, on the contrary, the ridges do not meet, but end upon the margin of the foramen, at the dorsal foraminal prominences, which are situated some distance anterior to the posterior limit of the primitive foramen. Now, if the primitive foramen closes by the approximation of the dorsal foraminal prominences (which represent the tips of the neural hemiarches of the occipital vertebra, as described in my former paper), then these crescentic ridges will meet upon the posterior border of the foramen, and not some distance behind it, as do the inferior nuchal lines. If, on the other hand, the dorsal foraminal prominences do not fuse in the final closure of the foramen, then these crescentic ridges will never meet, but will end upon the foraminal margin. Thus their posterior extremities can not be made to coincide with those of the inferior nuchal lines. It does not seem possible that in the closure of the primitive foramen more of its area will be taken up than that of the wide superior occipital incisxire (lying above and behind the dorsal foraminal prominences), judging from my later stage and that of Hertwig. Hence it would seem quite likely that the crescentic ridges never come to occupy the jiosition of the inferior nuchal lines, and hence they can not represent them.
In the mature occipital bone the crescentic ridges are represented rather imperfectly, but are nevertheless recognizable as the lateral delimitations of the condj'lar fossa. Their lateral extremities are here fairly distinct, reaching the jugular tubercle, but their posterior extremities are lost in the region of the posterior margin of the foramen magnum.
The paraforaminal area, or condylar fossa, is the depressed area bounded by the crescentic ridge, the paracondyloid process, and the occipital neural hemiarch. Its size here is relatively much greater than that of the corresponding region of the mature bone. As yet the superior articular process of the atlas does not lie far enough out, as figure 4 shows, to reach the fossa. Its floor is unperforated, but is very thin. The corresponding convexity upon the ental surface forms the posterior boundary of the jugular recess.
The supraoccipital fundament includes the dorsomedial parts of the two squamae, joined together above the superior occipital incisure by the tectum posterius. The tectum is practically plane and its ental sm-face looks almost directly forward. Its lower border presents a descending process, which projects into the superior occipital incisure (figs. 4, 14). It is of a younger type of cartilage than that of the tectum above it. In a 30-mm. human embryo a similar process has been described by Fawcett (19106). The ascending process has been described. The cartilage of the tectum is very thin, as is seen in the midsagittal section shown in figure. 14.
The ossification center for the supraoccipital is single at this stage. Its right half is seen from the front in figure 14, and figure 4 gives a view of it from the rear. It is somewhat butterfly-shaped and teiTninates laterally in sharp down-turned points which do not pass beyond the occipitoparietal commissure. From these lateral extremes the outer border curves downward and inward to the dorso-lateral angles of the superior incisure of the foramen magnum. The ascending and descending processes are not included in the ossification, which, liowever, involves the entire remaining tectum posterius with the exception of a narrow edge along the superior border. No actual bone has yet been deposited, but there has evidently been considerable calcification of the cartilaginous matrix, judging by the staining reaction.
It is quite evident that this area of calcified cartilage has remained unstained in Bolk's (1904) van Wihje preparations of the occipital region of the human skull, and he has accordingly misinterpreted this region as membrane. This failure to recognize an imperfection in his method has led Bolk into a number of errors. Thus he is in error when he states that it is highly improbable that the supraoccipital ossification is of the true endochondral type. It is as distinctly endochondral as any ossification could be. This region is unique in that the calcification of the cartilage takes place very early. From his preparations Bolk gains the impression that bonj' development begins in the center of the supraoccipital before cartilaginous development is accomplished here, the failure to chondrify being due to the rapid development of the brain. He does not explain why this rapid brain development should not also have a similar effect in retarding the development of the "Knorpelspange" just above, which is merely the uncalcified upper margin of the tectum posterius, as I have pointed out.
It is of interest, however, that this upper margin does remain uncalcified and unossified for such a long period for, as Bolk has shown, it is present throughout his series of four human chondrocrania. In the last, however, it is becoming thinner and more attenuated. I would suggest that this cartilaginous edge may be retained to favor growth of the supraoccipital here. A similar reason may underlie the persistence of the twin nodules of cartilage which are present at the apex of the superior occipital incisure, in Bolk's preparations, and which he states agree in position, in one case at least, with the bones of Kerckring. These nodules, in Bolk's figure 1, are in the same position as the descending process in No. 886, apparently. It seems to me quite likely that they are not isolated masses of cartilage situated in membrane, but that they are connected with the calcified cartilage of the tectum above.
It is of interest to observe that Fawcett (19106) questions Bolk's findings when he says (p. 306) : "1 must confess the appearances in his figures scarcely explain what is seen in this cranium." Some other recent authors have not been quite so critical.
Occipital Vertebra
The neural hemiarch of the occipital vertebra does not stand out so distinctly as that of la. Behind the condyle it is bent to form the posterior condylar notch. In figure 4 it is quite distinctly outlined, terminating in the dorsal foraminal prominences. The ental surface shows, just lateral to the hemiarch, a groove which is rather indefinitely marked except just behind the jugular tubercle. The dorsal foraminal prominences are much farther apart than in la, and the foramen occipitale magnum is correspondingly larger. There is, throughout the entire extent of the hemiarch, a direct connection of its cartilage with that of the adjoining squama. In the region of the aforementioned preossification center its material is of a distinctly more advanced type than that of the neighboring squama.
It is of interest to note that Lewis has found the tip of the arch, in a 21-mm. human embryo, separated from the squama, this tip projecting dorsally into the mesenchyme. Kernan (1916) does not mention this separation in his 20-mm. human embryo. In three other human embryos examined by Lewis, of 20 mm. length, there was no separation of the occipital hemiarch from the squama, though there was a difference in the character of the cartilage of these parts, the squama being of a younger type. Even in the 19 mm. stage, Lewis found a greater amount of fusion between these structures than in No. 460, so that there seems to be some variation here. No. 460, it may be noted, was a negi-o skull. Because of this separation of the occipital neural hemiarch from the squama in No. 460, and of the difference in the character of the cartilage here and in the other embryos examined, together with his observation that "there is also a more gradual transition as regards the degree of differentiation from the cartilage of the transverse process into the squama than from that of the lamina," Lewis favors the view that the squama arises by upward extension from the transverse process of the occipital vertebra rather than from the occipital neural hemiarch, as I formerly suggested.
Foramen Occipitale Magnum
The foramen occiptale magnum is relatively larger than in la. Its lateral contour shows the dorsal and ventral foraminal prominences (figs. 5, 6). Seen from without, the plane of the intercondyloid incisure looks downward. The region lying between the prominences faces almost directly backward and also a little downward. Viewed from the side it is seen to present a distinct dorsal concavity, corresponding to the posterior
condylar notches. At these points the foramen is widest (fig. 1). The superior occipital incisure, whose plane is directed backward and slightly upward, is filled with the spino-occipital membrane. It is much wider than in la. The superior occipital incisure may persist as a wellmarked notch in adult skulls of certain dogs. Dr. A. H. Schultz has shown me three very striking examples in skulls of bull dogs and pugs. These are Nos. 71, 381, and 382 of the Schultz collection.
Cervical Vertebrae
The cervical vertebrae (figs. 3, 4, 5, 6) are well developed. The distance between the tips of the hemiarches of the atlas is the same as that between the dorsal foraminal prominences, but below that the hemiarch tips gradually become more closely approximated, those of the seventh vertebra being separated by a comparatively short interval. A comparison with LeM-is's figures of the same region gives a graphic demonstration of the closure of this part of the spinal canal and foramen magnum.
The atlas presents a distinct anterior arch or hj'pochordal bar, which is separated from the dens epistrophei by a thin sheet of connective tissue (fig. 11). The late al ma'=ses are stout and show concavities upon the upper surface for the condyle? of the occiput. The costo-transverse foramen on the left side is closed, but that on the right side lacks a very short piece of the costal process which, however, appears to be forming.
The epistropheus presents a stout dens, from whose tip emerges the notochord, as shown in figure 11. The bodies of the vertebrae form a line which is almost straight and which makes with the basal plate an angle of 125°. The corresponding angle in the Lewis 21-mm. embryo was 110°, as measured by me from his figiires. This angle is probably variable.
Type of Cartilage
With the exception of the ossification centers, which have already been described, the occipital region is made up almost entirely of a mature type of cartilage. The character of this varies somewhat in the different regions. The paracondyloid process is tipped with young cartilage. The condyle, too, reveals a younger type of cartilage at the region of the future articular surface.
Otic Region
Parietal Plate
The parietal plate (figs. 1, 5, 6, 14) is thin and slightly concave entally. Its junction with the squama occipitalis has been mentioned. Anteriorly it is connected with the otic capsule by the capsulo-parietal commissure which is interrupted by the large and elongated capsuloparietal foramen. The plate, above the commissure, bends outward over a groove which is bounded below by the otic capsule.
The dorsalmost, u'regularly rounded part of the plate is the highest part of the chondrocranium and is partially cut off from the main portion by distinct notches, most closely approximated on the right side. Its anterior edge does not overlap the developing parietal bone, as in la, but is separated from the posterior edge of this bone by a very narrow interval (fig. 5). There are no small cartilaginous remnants above it, as in Ia\ indeed this portion of the plate shows a relatively greater development than in la, or (even more pronounced) than in the Hertwig model, in both of which it is evidently undergoing reduction.
The anterior extremity of the main portion of the plate is rounded and does not project far beyond the capsulo-parietal commissure. Posteriorly the plate curves inward, gradually narrows, and ends in a point, separated from the supraoccipital cartilage by the occipitoparietal notch. The upper edge of the entire plate is rather rough.
Dorsal Tecta
The idea that there are two tecta represented in the dorsal part of the occipital region has recently been given attention by several authors. Kernan (1916, p. 620) regards the tectum synoticum, which joins the otic capsules through the parietal plates, as a primitive structure which is "formed early, and is absorbed as the tectum posterium reaches its development." The tectum posterius joins the occipital wings and thus completes the foramen occipitale magnum. This view Kernan arrives at from a comparison of the findings in his 20-mm. human embryo with those of my 40-mm. human embryo, together with a consideration of the earlier evidence brought forward hy Levi. In his specimen Kernan describes the parietal plates (which are marked off from the underlying occipital wings by the occipito-parieial grooves) as being joined behind to form a true cartilaginous tectum synoticum. In this way the primitive foramen magnum is
completed, for the occipital squamae are not yet in union. In la the parietal plates, although stretching inward toward one another, do not unite, while below them the occipital wings are united in the tectum posterius. Levi, according to Kernan, "showed that the dorsal union between the two sides occurred cranially and advanced caudally and ventrally, the more cranial union between the parietal plates being absorbed as the ventral union is formed." Thus Kernan remarks : "The conditions in Macklin's 40-mm. and this 20mm. embryo would appear to bear out this statement of Levi." This idea has been enunciated by Rice (1920, p. 137).
Fawcett (1918a, p. 227) writes: "In man there are two tecta (Bolk, Fawcett) : one a very wide one, the more posterior, therefore called the tectum cranii posterius, from the middle of whose anterior border a processus ascendens arises (Fawcett; in pig, Mead) ; the other, the tectum cranii anteriorly, is very slender and quite isolated, not reaching the parietal plate on either side, nor being in any way connected with the tectum cranii posterius. Recently I have observed two tecta in the cat : one certainly the ordinary tectum posterius, the other small, median, and anterior to this, which may be an isolated processus ascendens or may be looked upon as a tectum anterius." In Weddell's seal Fawcett (19186) reports two cartilages, one on either side of the midline, situated very far forward, and belonging to the anterior tectum. They showed slight signs of fusion with one another.
The condition in No. 886 does not add anything to the information given by la, the parietal plates ending as free points projecting into the dorsal membrane. It is possible that the most anterior cartilage of Bollc (1904) is to be looked upon as a rudiment of the link which once joined the parietal plates and, if this be so, then it is possible that the posterior cranial cartilages of la belong to this band.
Otic Capsule
In addition to the connections of the otic capsule already noted, there is a small cartilaginous union with the processus alaris, one with the incus, and one with the styloid process.
As Lewis remarks for his 21 -mm. specimen, the capsule is placed in about the same position, with regard to the basal plate, as is the petrous portion of the temporal bone. Its shape, roughly that of half a pear (with the large end situated dorsolaterally and the cut surface toward the cranial cavity), is best appreciated from a study of the figures. Its walls, except at certain regions to be described, are thin.
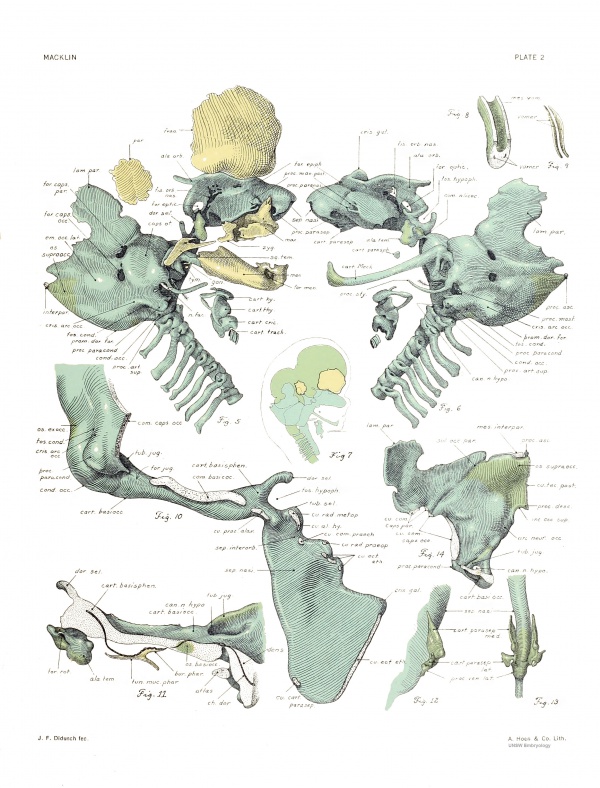
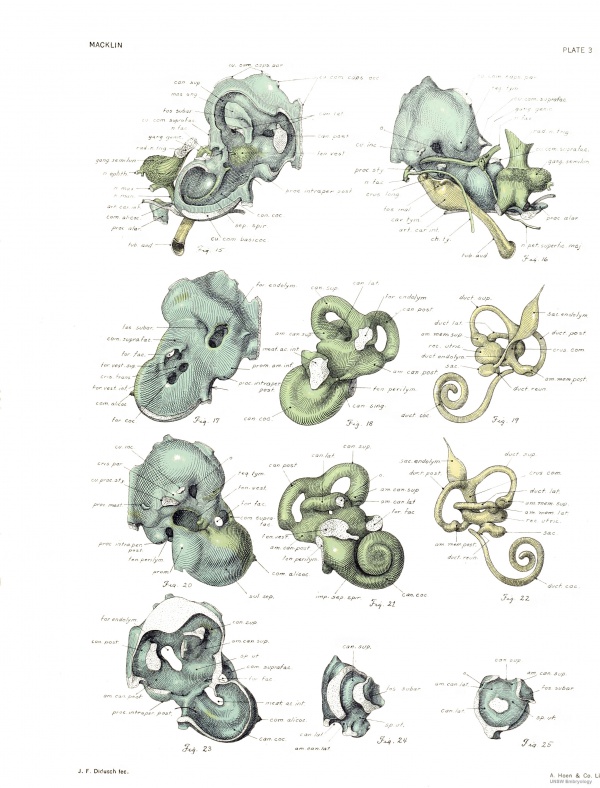
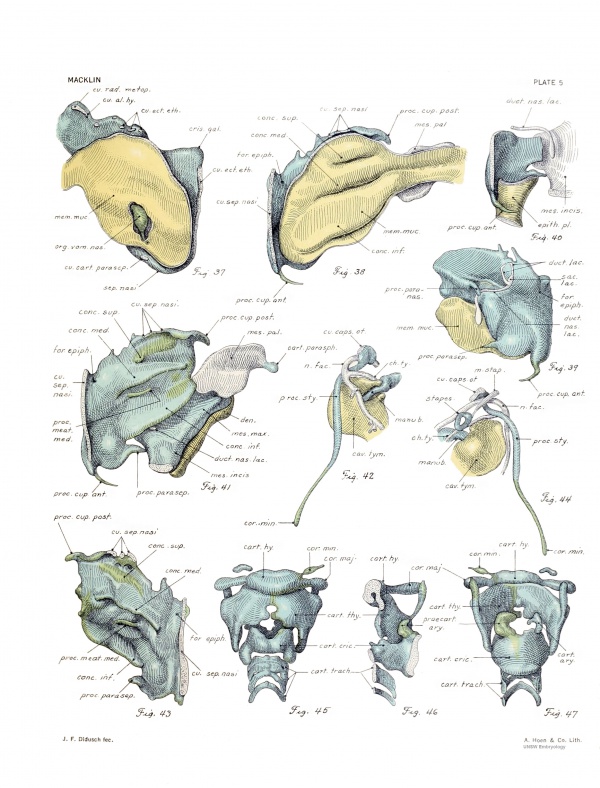
Cranial surface. — The entire cranial sm-face of the capsule is seen in frank view in figure 17. The pars cochlearis is here quite smooth and flattened, and presents the wide internal acoustic meatus. The more irregular pars canalicularis shows distinct rounded eminences for the superior and posterior canal spaces, above which is the groove for the transverse sinus. The relation of this surface to the inner cavity is ascertained by a comparison of figui-es 17 and 15. The capsule was drawn from the same viewpoint in the two cases, but in figure 15 most of the medial wall has been removed. Above the space for the superior canal is the cut edge of the capsulo-parietal commissure, interrupted by the long capsulo-parietal foramen. The posterior slender limb of this commissure is joined to the angular mass of the capsule. Proceeding downward, we note the connection with the squama occipitaUs, broken by the large capsulooccipital foramen. The cut edges of these unions are seen in figure 14. Their line of attachment is along a rounded border separating the medial and lateral surfaces of the pars canalicularis. The subarcuate fossa, overarched by the .superior semicncular canal, is very deep. It contains only loose connective tissue and small vessels. Just behind it, in the prominence for the crus commune, is the endolymphatic foramen. It is wide and elongated and has very thin borders of young cartilage. The ductus endolymphaticus, traversing the foramen very obliquely, fills but a small fraction of it, Ihe remainder being completed by membrane. The relationship of the transverse sinus to the endolymphatic duct and sac of this embryo may be seen in plate 5 of an article by Streeter (1918). In la the endolymphatic foramen was narrower and had thicker walls; there was a short process projecting dorsaUy from the upper lip, which is not present in No. 886.
Behind the endolymphatic foramen is a sharp spur of cartilage which projects inward and backward medial to the transverse sinus. It is a short distance below and behind the endolymphatic sac. Underlying it the waU is quite thick, so that the posterior canal is removed some distance from the surface here. The inferior ampuUary prominence is conspicuous below. It terminates in the posterior intraperilymphatic process, which projects downward into the perilymphatic foramen.
Lateral surface. — The lateral surface can not be satisfactorily seen from one standpoint. Figure 20 presents the most comprehensive view. From below upward may be distinguished the cochlear vestibular, and canalicular areas.
The cochlear area, looking principally downward, is very convex and bulging. It shows a very shallow furrow, the septal sulcus, which has the form of a helix and is very indefinite towards the pole (fig. 20). It corresponds to the Kne of attachment of the spiral septum upon the interior of the capsule (fig. 15). The internal carotid artery lies in a short length of this groove, as seen in figure 2. Turning upward around the cranial pole of the cochlea, it traverses the lateral side of the carotid foramen, keeping close to the alicochlear commissure, and thus gains the cranial cavity. Just lateral to the ventral basicochlear groove is a long prominent rounded ridge, formed by the space for the first turn of the cochlear duct. This terminates posteriorly in the promontory; anteriorly it turns upward to end at the rounded ventral pole of the cochlea. Somewhat below the apex of the pole (figs. 1, 6, 20, 23) is the slender alicochlear commissure.
There is, at this stage, no evidence of the small supracochlear cartilage, which I described in la just above the cranial pole of the cochlea and below the semilunar ganghon. Its position in la is somewhat above that of the union of the alicochlear commissure with the cochlea, found in No. 886.
The vestibular area is sharply marked off medially by the rounded contour of the cochlea and is laterally blended with the inferior canalicular surface. It shows, adjoining the promontory, the large vestibular window. Above is seen the facial foramen, surmounted by the suprafacial commissure. Across this area the facial nerve com'ses (fig. 16), lying close to the cartilage. The great superficial petrosal nerve is seen leaving the geniculate ganghon.
The canalicular area is crossed by a ridge which, beginning above at the capsulo-parietal commissure, runs downward over the tegmen tympani and then backward and downward over the parotic crest to end at the dorsahnost extremity of the jugular foramen. Medial to this ridge the siu'face looks principally downward and extends inward as far as the inferior ampullary prominence. The major portion of the canalicular surface, lying behind and above the ridge just outlined, looks directly outward (figs. 5, 6). It is somewhat convex, especially from before backward. All of the canals make prominences, but these are all very low and rather indefinite. The central and superior, almost plane, portion of the surface belongs to the angular mass, a large lump of cartilage (figs. 24, 25) which is inclosed by the semicircular canals. A small area in front of the anterior limb of the lateral semicircular canal has failed to chondrify fig. 20, o). It is not seen in later stages.
The fenestra vestibuli (fig. 20) is roughly oval in contour, with a narrow anterior extremity. Its ventral border, which is formed by the upper free edge of the promontory, is almost straight. The edges are thin, and of a young type of cartilage, which is indefinitely merged with the membrane which fills it. This membrane, which represents t he annular ligament, bulges into the cavity of the capsule, as shown in figure 15. It resembles precartilage and its inner surface is cellular and heavily staining. The stapes occupies only a small fraction of its area. A slender isthmus of cartilage separates the fenestra vestibuli from the fenestra perilymphatica, and from this a low ridge runs directly backward to the laterahnost end of the jugular foramen.
The fenestra perilymphatica, at the caudal extremity of the cochlea, looks directly backward, and hence can not be shown in the ordinary views, but in figures 5, 6, 17, and 23 arrows are used to indicate its position. Its margin has the form cf an irregular ring, sharply bent upon itself, the bent parts being situated (laterally) at the commissure separating the two fenestrae, and (medially) at the inner corner of the foramen, which will form the cochlear aqueduct. The parts of the ring which approach one another are the posterior intraperilymphatic process, situated above and posteriorly (in my former article referred to as the interperilymphatic process) and the anterior intraperilymphatic process situated below and infcriorly. The latter is a small ridge of cartilage which appears upon the inferior border of the fenestra. It seems to correspond to that described by Terry in the cat, and marked with an asterisk in his figures 2 and 12. These two points are apparently growing together, as shown by later stages. In la the posterior process was somewhat longer. Thus is accomphshcd the partitioning of the perilymphatic fenestra into the cochlear fenestra laterally and the cochlear aqueduct medially. The medial half of the border is -seen in figure 23.
Jugular foramen. — The posterior margin of the jugular foramen (fig. 10) has already been described with the occipital region. The anterior margin, lying at a higher level, is seen in figures 15 and 17 between the basicochlcar and capsulooccipital commissures. This border is bent downward by the inferior ampullary prominence and posterior intraperilymphatic process. Between this process and the anterior intraperilymphatic process is a gap in this border which will be completed by the union of these processes.
The intracranial ganglia of the glossopharyngeal, vagus, and accessory nerves are continuous and form a conspicuous object just above the level of the jugular foramen. This chain of nerve cells lies almost directly antero-posteriorly, with a slight medial inclination of the caudal end, and crosses the jugular tubercle, which it almost touches. The jugular ganglion of the vagus is the largest element of the chain; it has the form of a ring, the central part being composed almost entirely of fibers. The outer edge of this ring, situated at a higher level than the inner, lies just medial to the inferior ampullary prominence. In front of this ganglion, and a little above the site of the cochlear aqueduct, is the jugular ganghon of the glossopharyngeal. The accessory portion of the chain is long and thin. The petrous ganglion of the glossopharyngeal is situated close behind the lower border of the perilymphatic foramen, its upper hmit reaching as high as the anterior intraperilymphatic process. It is larger and thicker than the jugular gangUon of the same nerve. Between its ganglia the trunk of the glossopharyngeal nerve runs downward and slightly outward just medial to the line joining the intraperilymphatic processes. The ganglion nodosum of the vagus is a much larger structure. It is very much elongated, with attenuated upper end, which reaches to the lowermost limit of the petrous ganglion but lies slightly medial to this. Its main du-ection is downward, with an appreciable inchnation backward.
The suprafacial commissure is seen in figures 17 and 20, and the cut surfaces, after the removal of the conmiissure, appear in figures 15 and 16. It connects the pars canalicularis just above the region of the recessus ellipticus with the superior border of the cochlea. It is flattened from above downward and is a little narrower below than above. The edges are quite thin. The relation to the facial nerve is seen in figures 15 and 16. In Kernan's specimen the commissure was incomplete, but it was complete in Lewis's No. 460.
The legmen tympani (fig. 20), which overlies part of the developing ossicles, is a well-marked ridge of cartilage projecting forward from the superior ampullary prominence to form the most anterior part of the pars canalicularis.
The crista parotica, though less prominent than in la, is quite con.spicuous and forms a ridge lying between the hole (fig, 16, o) in the capsule and the mastoid process. Just above its anterior end there is a small area for union with the crus breve of the incus. Its posterior portion is concerned with a union with the styloid process (figs. 5, 6, 16). The facial nerve courses under the shelter of the ridge (fig. 16) in the site of the future facial canal.
The styloid process (figs. 4, 5, 6, 42, 44) is attached to the otic capsule, thus differing from the condition in 7a, where it was separate. In figures 42 and 44 of the process and figure 20 of the capsule the division has been made higher up than one would think should be the case, judging from figure 16, and it would seem that a little of the crista parotica has been excised and appears as the upper expanded end of the process in figures 42 and 44. There is along this line of section, however, a layer of lighter-staining cartilage which was used as a guide in making the division. At the 20 mm. stage Kernan states that the styloid process is "received in a depression of the cartilage" at the dorsal extremity of the crista parotica.
The first part of the process passes almost directly inward, with a slight inclination downward, and approaches quite close to the capsular wall, which here rises slightly as a very low narrow ridge. Thus we have almost a complete primitive stjdo-mastoid foramen for the exit of the facial nerve, the relation of which to the styloid process appears in figures 42 and 44. The stjdoid next sweeps downward around the posterior edge of the developing tympanic cavity, forming a curve with a strong backward convexity. It then passes into the terminal long, tapering, and almost straight part which is directed downward, inward, and forward. It shows a membranous connection with the lesser cornu of the hyoid (figs. 42 and 44).
Tho facial nerve, after passing around the styloid process, turns downward and then forward, and breaks up into the usual branches (fig. 42). From the lateral aspect of the concavit}' thus made, and but a short distance below the root of the styloid, the chorda tj'mpani is given off (fig. 16), which passes almost directly forward, just above the superior extremity of the tympanic cavity and between the crus longum of the incus and the manubrium of the malleus, after which it turns downward and runs also a little forward and inward, crossing the line of the tympanic cavity and tuba auditiva, but diverging from these to reach the lingual, as shown in figures 31 and .34.
The mastoid process of the right side is a small nodule of young cartilage separated from the capsule by perichondrium. Its shape is that of a short rod, about 300 micra long antero-posteriorly. The nodule on the left side is similar. In la the process was connected with the otic capsule by cartilage. In No. 460 it was somewhat longer,
relatively, than m No. 886, but there it consisted of blastema. Lewis found, in other embrj'os of about the same stage as No. 460, that a separate cartilage in the blastema of the mastoid process was present. He notes the attachment of the digastric and stapedius muscles to the process. Kernan notes the process in his specimen.
Lewis describes the "mastoid cartilage" to which the mastoid process is attached. In his models it is a fiange of cartilage which is attached to the caudal and dorsal border of the pars canahcularis. In No. 886 the mastoid process is attached to the pars canahcularis itself, a short distance in advance of the posterior end of the posterior semicircular canal. It would seem that part, at least, of this mastoid cartilage of Lewis is later taken up into the caudal end of the pars canahcularis. In No. 886 the region corresponding to the mastoid cartilage of No. 460 is very much less prominent.
Spiral septum.— Looking into the interior of the cochlea (fig. 15) the spiral septum is seen. In my former article it was referred to as the spiral lamina. It springs above from the posterior edge of the roof of the cochlea (which also forms the floor of the facial canal, as in fig. 15), and shows here a slender connection posteriorly with the adjacent capsular wall, just medial to the impression for the recessus eUipticus. In this way is formed the transverse crest (fig. 17). Terry describes a similar formation in the cat. Just below this union is a short spur of young cartilage (fig. 15) which projects backward under the anterior extremity of the sacculus.
This medial part of the septum is much the highest; it diminishes rapidly in height as it passes downward and forward (fig. 15). It makes a conspicuous impression upon the cast of the cochlear cavity, as seen in figure 21. As yet the entiie septum shows scarcely one turn, and the second (outer) portion of this (rather less than onehalf) is not at all prominent; it gradually becomes obliterated in the depths of the cave which is thus cut off. The edge is of a young type of cartilage, which is evidently growing rapidly.
The first portion of the cochlear canal space lies medial to the high first portion of the spiral septum, and upon the corresponding region of the medial wall of the cochlea there is a very low ridge (fig. 23), also edged with young cartilage. This is seen in the model of the space (fig. 18) as a shallow groove which, beginning caudad above this perilymphatic foramen, is continued forward and finally forward and upward around the medial acoustic meatus to lose itself in front of this opening. This ridge is less developed than in la, where the posterior portion of it was referred to as the "pyramidal mass." The spiral septum and this ridge seem to be approaching one another over the cochlear duct and spiral ganglion and it is probable that in this way the first portion of the cochlear canal space is cut off — indeed it would seem that the canal coils are gradually separated in this way. As yet, however, there is a wide interval separating these ridges.
Internal acoustic meatus. — The upper border of the meatus (fig. 17) is formed by the inner edge of the suprafacial commissure and a posterior continuation of this saUence, while the lower border, lying considerably nearer the median plane, represents the upper edge of the medial wall of the cochlea. Both of these borders are sharply marked, while the anterior and posterior, joining them, are more rounded. From the anterior border is continued backward and downward the beginning of the spiral septum; the free edge of this septum may be followed forward and inward as it curves sharply to meet the inner border of the meatus, thus outhning the anterior end of the inferior acoustic foramen. The intermediate portion of the posterior meatal border is formed by the wall of the elliptic recess, which, as we have noted, narrows and descends into the depths of the meatus to join the upper part of the spiral septum and thus to form the transverse crest.
The superior acoustic foramen, opening into the elliptic recess, is situated lateral to the upper part of the crest, and looks directly forward, so that it can not well be seen in figure 17. The position is indicated by an arrow in figure 15. It represents the future superior vestibular area. Lying medial to the crest, and delimited medially by the sharp inferior border of the meatus, is the elongated inferior acoustic foramen. This is not shown well in figure 17, since it looks directly upward, but figure 15 discloses its medial wall. When viewed directly from above it is seen that the spnal .septum approaches the ridge upon the medial cochlear wall (already mentioned) and thus the foramen is incompletely divided. In 7a there was a narrow cartilaginous union here. The anterior portion, slightly the larger, is the site of the future central canal and spiral foraminous tract; the posterior portion represents the inferior vestil)ular area and the foramen singulare. The latter opens into the cartilaginous canalis singularis (fig. 18) carrying the branch of the vestibular nerve to the ampulla of the posterior canal, and in this respect resembles the condition in la and also in the rabbit (Voit).
Thus we have, as in the mature bone, a space above the transverse crest, representing the fossula superior, containing the passageways for
the facial nerve and the superior division of the vestibular nerve, and a space below the crest, representing the fossula inferior, for the inferior division of the vestibular nerve.
The facial nerve is shown in the internal acoustic meatus in figures 15 and 16. It passes almost directly outward, and a little downward, and lies in front of the vestibular ganglion. The genicular ganglion is placed a little beyond the lateral edge of the suprafacial commissure.
The vestibular ganglion is crescent-like in form. Its outer end — a thin point projecting laterally and forward — underlies the commissure, but does not reach as far as its outer border. It lies in front of the superior acoustic foramen. The upper extremity is tucked under the inner margin of the meatus. The ganglion narrows as it curves downward and backward, presenting a medial convexity, and terminates deep in the caudal end of the inferior acoustic meatus.
Massa angularis. — The angular mass, already referred to, is seen from the front in figure 24 and from below in figure 25. In figure 15 it appears from within, in its relation to the canal spaces and to the remaining cartilage of the capsule. Its lateral surface, quite smooth, has been noted in figures 5, 6, and 20. Thus it extends from the medial to the lateral surface of the pars canalicularis.
In direct antero-posterior views it presents a triangular outhne, the lateral surface forming the base, and the region of the fossa subarcuata representing the apex. The mass of cartilage projecting from the lower border, in figure 24, is the trabecula inclosed by the lateral semicircular canal. In figure 25 it is seen trimmed more closely to the angular mass proper. A similar, though somewhat less obvious, triangular outline appears when the mass is viewed from above, or from below, as in figure 25, all the borders being more rounded and the apex, still in the same locahty, being less marked. Seen from within or from without, the mass is roughly quadrilateral. The relation of the semicircular canals to its anterior, medial, and lower aspects may be seen from the figures. The fossa subarcuata penetrates deeply into its interior and ends in a very .slight dilatation. Underlying the space for the crus commune (fig. 15), there is a ridge which becomes sharpened posteriorly, where it joins the trabecula inclosed by the posterior canal space. The cartilage is lightly staining, with scattered nuclei.
The space within the otic capsule is very large. Figures 15 and 23, of the lateral and medial walls, and figures 18 and 21, which show the external form of the cavity, modeled as a solid, will be helpful in gaining a true concept of it.
Cochlear space. — The cochlear space begins at the foramen perilj-mphaticum and proceeds forward in the trough-like inclosure for the commencement of the first turn of the cochlear duct and spiral ganglion. Here it is partially overhimg by the low ridge already described, which projects outward from the cranial wall of the cochlea and is wailed in laterally by the medial part of the spiral septum and outer wall of the capsule behind this. Continued forward, upward, and finally backward and downward, the space is seen to suggest strongh' the spiral character of the adult bone. The spiral cochlear duct contained in this space makes one and three-quarter turns, as seen in figures 19 and 22.
Vestibular space. — The vestibular portion of the space is verj' capacious. There is but little evidence of the sacculation which is present in the osseous condition. Just above the superior opening of the medial acoustic meatus (representing the future superior cribriform area) there is a faintly marked depression for the recessus utriculi of the utriculus, the representative of the future recessus ellipticus. Of the recessus sphericus, there is little evidence. The foramina tvhich pierce the wall have been mentioned.
The semicircular canal spaces are all large and all show dilatations for the ampullge. They bear the usual relationships to each other and to the vestibular cavity. The plane of the lateral canal is very nearly parallel with that of the basal plate. In its course it ciixles around a short trabecula (cut in fig. 25), which joins the angular mass with the floor of the capsule just behind and lateral to the fenestra vestibuli. The caudal non-ampullated end opens into the vestibule immediately in front of the trabecula inclosed by the posterior canal.
Membranous labyrinth. — But little need be said as to the membranous labjTinth (figs. 19 and 22), since its features are familiar. It approximates in form the mature condition. AU the parts are represented. As in the adult, it occupies but a comparatively small volume of the space contained within the otic capsule. The cochlear duct does not show such a distinct projection of the apex of the coil from the base as in the adult condition. Lying within the coil is the spu-al ganglion, which ends in a slight enlargement, just medial to the smallest coil of the duct; this enlargement shows a short spur, directed toward the medial portion of the middle meatus. The ganglion ends dorsally at the angle between the dorsalmost straight portion of the duct and its basal limb, and thus does not reach the vestibular caecum.
The ductus endolymphaticus is long and slender and crosses the crus commune in a direction from above downward and forward. Its saccus is broad, flattened latero-medially, and of oval outline, and it Ues medial to the transverse sinus. Its dorsal extremity is a very slender filamentous process containing an almost imperceptible lumen.
Stapes. — The stapes (figs. 31, 34, 44) is a ring of cartilage with a thick cellular perichondrium. A section of the ring, representing the future footplate, is in tbe membrane filling the vestibular window. The ends of this primitive foot-plate are indefinitely marked off from the fenestra! margins; indeed, anteriorty there is a union by j-oung cartilage with the anterior edge of the fenestra, shown as a blunt spur in figure 20. This primitive foot-plate presents a marked bowing inward and upward, and pushes in the membrane filling the oval window so that it bulges into the vestibular cavity (fig. 15).
The section of the ring opposite to the footplate is bent a little to form a rounded angle which articulates laterally with the crus longum of the incus. Here the tendon of the stapedius muscle is inserted (fig. 44). The stapes makes with the crus longum of the incus an angle of about 90°.
Incus. — In form the incus approximates the mature bone, presenting a bodj' and two crura. The body is separated from the adjoining head of the malleus by membrane, but as yet there is no joint cavity. There is a well-marked notch on the cranial aspect of the body for the cog-tooth of the malleus. A distinct spur of cartilage, tipped with young cartilage, marks the cranio-lateral limit of the body and projects forward to the upper extremity of the malleus. The crus breve points backward, with a cmrve of its tip downward (fig. 6) to apply the medial surface of its extremity (fig. 31) to the wall of the otic capsule just lateral to and a little below the anterior limb of the lateral semicircular canal — the site of the future fossa incudis (figs. 16, 20). This connection is accomplished by young cartilage. In la it was membranous. This represents the sole connection of the otic capsule with the first visceral arch. The crus longimi, underlying the facial nerve, points inward, downward, and a little backward. It is parallel with the handle of the malleus, but is considerably shorter than this. The representative of its future processus lenticularis is edged with young cartilage.
Malleus. — The head of the malleus (fig. 6) is directly continuous with Meckel's cartilage, showing no evidence of the future separation. The neck is relatively longer than in the adult condition, and the manubrium is shorter. The lateral process (fig. 42) is a distinct tubercle; here the neck and manubrium join to form a gentle curvature, with concavity upward and inward. The chorda tympani hes just medial to this between the processes of the malleus and incus. The manubrium, from the processus lateralis to the tip, is just medial to the stratum cutaneum of the tympanic membrane. Medially it is inserted into a distinct bay upon the lateral aspect of the developing tympanic cavity (figs. 16 and 42), the representative of the stratum mucosum of the future tympanic membrane. The head shows a ridge, edged with young cartilage, directed upward and backward, which represents its spur or cog-tooth. This is loosely fitted into the notch upon the body of the incus. A small tubercle is directed forward from the anterior aspect of the head.
The anterior process, or processus gi-acilis of Folianus, is a slender spicule of very young bone ; indeed, most of the substance is composed of osteoblasts with but little ground substance. It arises in the perichondrium of Meckel's cartilage, some distance below the head of the malleus and a little below the chorda tympani nerve (figs. 31 and 34). It is attached to this perichondrium on the caudo-medial aspect of the malleus, and the spicule projects from this upward and a little backward and outward, slightly approaching the chorda, to end freely a little in front of the neck of the malleus. A connection, however, is made through connective tissue and the malleus shows at this point a small amount of young cartilage which indicates the future fusion of the process to the neck. This bone, homologous with the goniale of lower forms (Gaupp), was somewhat farther developed in la.
Auditory Tube and Tympanic Cavity
The auditory tube comes off from the pharynx just behind the level of the developing medial
pterygoid plate and follows a course outward, upward, and slightly backward. It is much flattened from above downward and outward and there is a very gentle curvature with concavity downward and outward. At a point about half way between the pharyngeal opening and the caudalmost extremity of the tympanic cavity the expansion for the latter begins to appear, and the outer end of this epithelial evagination is very broad and flat. When the entire structure is looked at in frank view the medial border shows a much stronger inward concavity than the lateral. The tympanic cavity presents practically no lumen, the walls being approximated. It lies quite close to the promontory of the cochlea. The medial aspect presents a shallow concavity and the lateral aspect a corresponding convexity, which, however, is even less marked. The upper area is specially modeled, presenting a depression (figs. 16 and 42)- — representing the stratum mucosum of the future membrana tympani — for the manubrium of the malleus. From the superior border of this depression there are tongue-like prolongations which partially embrace the manubrium, as shown in figure 42. A little medial to this upper edge is found the end of the crus longum of the incus (fig. 16). There is a small convexity upon the medial aspect corresponding to the depression for the malleus (fig. 44).
Type of Cartilage
The type of cartilage of the otic region is almost all mature. In the angular mass it is palestaining with sparse nuclei. The upper portion of the cochlea shows a younger type of cartilage than the remainder of the capsule. Edges of young cartilage are found bordering the endolymphatic and vestibular foramina, the spiral septum and the small ridge upon the opposite wall, the outer edge of the suprafacial commissure, and the joint surfaces of malleus and incus; and, upon the malleus, the lateral process, the tip of the manubrium, and the site of future union with the goniale. There are no ossification centers.
Orbito-Temporal Region
The orbito-temporal or sphenoidal region contains a central unpaired ma.ss, representing the body of the sphenoid, and three lateral paired masses representing the two wings and the medial pterygoid plates.
The basisphenoid has been described with the basal plate. The anterior end of its connection with the cochlea juts out toward the side (fig. 10) and here forms part of the posterior boundary of the carotid foramen. This projection represents the posterior petrosal process of the mature bone. The dorsum sellse, less developed than that of la, is directly continuous with the basisphenoid and is slightly concave anteriorly. It terminates in two lateral, somewhat divergent and blunt tips, the developing posterior clinoid processes. The upper edge is of a somewhat younger tj-pe of cartilage than that of the lower portion of the plate.
In front of the dorsum sellse the body of the sphenoid, which here forms the floor of the hypophyseal fossa, is wide and thin and slightlj' hollowed, but unperforated. Low upon the lateral border appears the thin uninterrupted cartilaginous union with the alar process (fig. 10). Joining the root of this process with the posterior petrosal process is a narrow ridge, edged with young cartilage, forming the lateral border of the carotid foramen.
In front of this thiu floor the cartilage narrows transversely and broadens dorso-ventrally as it passes forward. The upper surface presents four distinct elevations in front of the dorsum sellse and, including the hypophyseal fossa, four depressions or notches between these. The anterior wall of the hj'pophyseal fossa is shghtly hollowed and is surmounted by the tuberculum seDse. To its extremities are attached the stout metoptic roots of the lesser sphenoidal wing (figs. 1, 10). The tuberculum seUse is not so wide as in la, nor is there a middle clinoid process present, as there was on the right side in 7a.
In front of the tuberculum sellse the cartilage, now known as the lamina hypochiasmatica, narrows rapidly from side to side and descends into the sulcus chiasmatis. The upper surface is slightly convex. To the lateral margins are attached, just in front of the caudal roots of the lesser wing (with which their bases are continuous), the alse hypochiasmaticse.
ALA HTPOCHIASMATICA.
These projections seem to be homologous with the orbital processes of Terry, in the cat. They are thin, wing-like structures of young cartilage with rounded borders of precartilage which project outward and a little downward into the optic foramina. Thus each forms with the anterior margin of the posterior root, a notch opening outward into the optic foramen. Anteriorly each wing is separated from the posterior end of the prechiasmatic commissure by another notch — also an extension of the optic foramen.
The ala hypochiasmatica of No. 886 is less developed than that of la. In relation to the orbital wing it is a very insignificant object in both stages and it is therefore of interest that Kernan (1916) has reported this structure in his 20 mm. human embryo as relatively very large, when compared with the lateral part of the orbital wing. He describes it as a free cjdinder of cartilage in the position, apparently, of the future posterior root of
the orbital wing, to which he considers it to belong. Lewis, in the 21-mm. stage, describes the same structure as the "basal part" of the orbital wing. Here, too, it is relatively very large when compared with the lateral part of the wing. Lewis thinks that the basal part "must ultimately become incorporated into the body of the sphenoid." From a consideration of these different stages it would seem that the ala hypochiasmatica is very precocious in its development, early attaining a prominence which is not maintained in later life. It is possible that the ala hypochiasmatica, as described by me in No. 886 and la, does not represent all of the structure described by Kernan and Lewis. It would be of interest to ascertain how far the development of this structure is concerned with the attachment of the muscles of the orbit. Fawcett (1919) finds, in the chondrocranium of the bat, all the ocular muscles except the two obliques attached to the hj-pochiasmatic wing, and Lewis (1920) reports a similar finding in his human embryo. Voit finds an independent ossification center in the ala in the rabbit chondrocranium.
PRECHIASMITIC COMMISSURES AXD FORAMINA.
Connected with the edges of the hj'pochiasmatic plate (figs. 1, 10), lateral to the sulcus chiasmatis and a little in front of the hjqjochiasmatic wings, arethecaudalmost extremities of the prechiasmatic commissures, slender strands of precartilage with extremities of young cartilage, which extend forward to the attenuated anterior roots of the lesser wings of the sphenoid. They are almost negligible when compared with those of la and are evidently just developing.
These commissures cut off the small prechiasmatic foramina from the optic foramina. Fawcett (1918, p. 425), on the basis of his researches with the chondrocranium of Weddell's seal, ferret, and cat, prefers to think of the prechiasmatic foramen as an isolated part of the orbito-nasal fissure rather than as a cut-off part of the optic foramen. These foramina are oval in shape, with long axes directed caudocranially, with a slight deflection of the caudal extremities laterally. Underlying them are the upper extremities of the ectethmoids. They were present in la, being a little farther apart and relatively smaller. Thej' are also present in the Hertwig model.
In my former article I stated (p. 390), that the prechiasmatic foramina are not to be found in the osseous condition. This statement should be revised, for, although in some skulls they are not to be formed, they may persist in others. I have found fhem present in the sphenoid of a young adult in the osteological collection (No. 101) of the Johns Hopkins Medical School. The skulls in which they were absent were apparently from old individuals.
INTERORBITAL SEPTUM.
The part of the central stem forming the narrow medial delimitation of the orbital cavities is known as the interorbital septum. Its anterior end lies above the level of the posterior extremity of the ectcthmoid. It is much reduced in area in the human subject as compared with lower forms. It extends forward slightly beyond the limbus sphenoidalis, coming to an end at the point where the attachments of the ectethmoid reach to the ujjper margin of the septum.
Anterior to the sulcus chiasmatis the upper border of the interorbital septum passes forward and slightly upward to reach a distinct eminence, the limbus sphenoidalis. Its upper narrow extremity is prolonged laterally into thin ridges, to which, at bilaterally opposite points, are attached the small preoptic roots of young cartilage. It is to the posterior aspect of these roots that the anterior extremities of the prechiasmatic commissures are attached.
In front of the limbus sphenoidalis is a much smaller notch, followed by a low but distinct eminence, capped by young cartilage. Somewhere in this region is the position of the future sphenoidal spine, although there is nothing here in the condition of the cartilage indicative of the future separation of the sphenoidal and ethmoidal elements of the septum. This ridge presents, on either side, very slender connections with the roof of the ectethmoid, and directly behind this are two other junctions with the same structure. These attachments, of which there are thus three on either side, are separated by minute foramina and He in almost a straight line (fig. 10). The caudalmost junction is below the limbus. They spring from a ridge of cartilage which projects laterally from the septum. The anterior end of this is level with the upper edge of the septum, but the posterior end is some distance below the limbus, as figure 10 shows.
In front of the eminence just described the upper border passes forward upon the nasal septum, descending into a distinct notch and rising upon the very prominent crista galli, to fall away finally upon the anterior border of the mesethmoid.
The lower border of the body of the sphenoid, or more accurately here, the presphenoid, after
leaving the distinct notch beneath the floor of the hypophyseal fossa, follows an almost direct com-se downward and forward, as figure 10 shows. Somewhere in the vicinity of the dorsal extremity of the vomer we have to place the ventral limit of the presphenoid, though, as with the upper margin of the plate, there is no trace of any dehmitation.
PROCESSUS ALARIS.
The temporal wing proper is supported by the processus alaris (figs. 1, 2, 15), a short, rather plate-like rod of cartilage, flattened from above downward, which projects laterally at right angles to the body of the sphenoid, with but a very slight inclination downward. Its medial extremity, of which the cut edge appears in figure 15, is thin, and is attached to the lower surface of the body of the sphenoid, as shown in figure 10. The lower surface shows a slight concavity from before backward, and the upper surface a corresponding convexity. There is upon the upper surface, too, a slight side-to-side concavity, and in this the internal carotid artery lies (fig. 15). It is the representative of the future sulcus caroticus. The sulcus is bounded laterally by a raised, knobhke eminence, which marks the latero-dorsocaudal extremity of the alar process, and from this is directed, backward and slightly upward and outward, the alicochlear commissure (fig. 6), a well-marked spur of cartilage. This, it will be remembered, is confluent by its slender caudal tip with the cochlea just below the cranial pole. From its relation to the internal carotid artery and to the sphenoidal fundament, it is indoubtedly the primitive lingula which, in the mature skull, is formed of bone and projects backward into the foramen lacerum, often being quite elongated. Although the slender junction of this commissure with the cochlea is of young cartilage, the knob-like eminence from which it springs shows sHght changes in the cartilage premonitory of ossification. This region represents the sphenotic center. Fawcett (1910) describes this independent center in a 110-mm. human embryo and remarks:
"Its large size is somewhat surprising, if it form only that part commonly in our text-books called lingula. From its posterior end a pointed cartilaginous process is seen to pass backwards towards the auditory capsule."
The alar process, thus, is for the most part included in the body of the mature bone rather than in the greater wing. Its outer extremity, with the continuation into the alicochlear commissure, forms the lingula. Lewis (1920) thinks it probable that the alar process is largely incorporated into the body of the matm-e bone and brings forward several lines of evidence favoring this view, including the relations of the carotid artery and the nerve of the pterygoid canal to the process, and also some interesting craniometric findings. He agrees in the general view that the alar process also forms the lingula, but does not mention the alicochlear commissure or alicochlear process.
ALICOCHLEAR COMMISSURE.
The ahcochlear commissure is 20 micra in length and conical in form. The lateral diameter of its base is slightly greater than the dorso-ventral. With the projecting cochlear pole it forms the lateral border of the carotid foramen. Jacob}' found it in the 30 mm. stage, but Levi did not describe it. Fawcett (1910a, p. 216) found that in a 30-mm. embrj-o "the processus alaris sends backwards a pointed process which comes into contact with the auditory capsule." He added that "this condition is even visible in the 110 mm. stage." It is not clear whether there were actual cartilaginous unions with the otic capsule. Kernan found, in the 20 mm. stage, an alicochlear process which came into contact with the cochlea but did not unite with it. He considers it to be only temporary in man. In the corresponding region of la there was a projection backward toward the cochlea, but no actual cartilaginous
CAROTID FORAMEN.
The carotid foramen is ii-regularly rounded in outline (figs. 1, 2) with a narrow lateral diverticulum between the alicochlear commissure and the cranial border of the cochlea. Views of its medial and lateral walls, which have been described, are seen in figures 10 and 15 respectively. The internal carotid artery occupies only this outer angle. It passes in an oblique direction from below upward, forward, and slightly inward, close to the alicochlear commissm-e, to overlie the alar process in the region of the future cavernous sinus. Its course beneath the otic capsule has already been noted. There is no indication of the canal through which the artery later passes in the osseus skull and it is evident that the future development of the region at the cranial end of the cochlea must be characterized by considerable extension, there being developed between the carotid foramen and the membranous cochlea a lengthy piece of bone. In the mature skull, too, the carotid foramen is merely the inner end of the foramen lacerum, since the backwardly projecting lingula does not reach to the petrous portion of the temporal bone, as a rule.
TEMPOR.U. WING.
The connection of the temporal wing with the alar process does not take place dii-ectly from the outer extremity of the latter, but rather from an area at the outer end of its ventral surface. This is somewhat triangular (fig. 16) with the apex directed forward and inward, and is gently convex. It is directed downward, forward, and shghtly outward. The reverse side, upon the dorsal surface of the wing, appears in figures 26 and 27, its inner boundary being separated from the adjoining surface by a low ridge. In the sections where this division has been made, a very thin strip of young cartilage may be seen, which separates the two portions of the wing.
If the alar process and wing be looked at from below, there is seen a sharp notch where they join, but when the separation of the parts is effected as outHned above it is evident that this notch belongs to the alar process. In it is the nerve of the pterygoid canal, lying just above and a Uttle medial to the caudo-medial angle of the -ndng (fig. 26). It is in this region that the future pterygoid canal will be formed. This notch is continued around upon the caudal aspect of the vrmg, and may be seen from the side, as in figure 6, between the lateral e.xtremity of the processus alaris (above), and the caudo-medial angle of the ala (below).
The temporal wing, resembling that of la quite closely, is a rhomboidal block of cartilage which hes with its long axis dii-ected forward, outward, and upward. It hangs altogether below the level of the alar process. Perforating the block from above, downward, and forward, is the conspicuous foramen rotundum (figs. 2, 26, 27), situated nearer the medial than the lateral border and nearer the anterior than the posterior end. The antero-lateral salient portion may be recognized as the ascending lamina, while the pterygoid process projects farthest ventrally and is just lateral to the medial pterygoid plate (fig. 29). The two surfaces may be described as dorsal and ventral, though they look a little posteriorly and anteriorly respectively. The dorsal surface (fig. 1) is somewhat convex from side to side and from before backward, and presents a sharp upward edge of young cartilage which borders the foramen rotundum laterally. In front of the foramen there is a narrow and shallow transverse concavity, while medial to the foramen is a narrow isthmus of young cartilage which joins the thicker anterior and posterior parts (figs. 11, 26, 27). This isthmus is by far the most slender part of the wing and seems to be the last part of the foraminal border to chondrify; for in earlier stages the foramen is incomplete here, the greater wing appearing as a hook with its tip stretching medially, forming the notch which represents the foramen (Lewis et al.). In la it was stouter, though still of comparatively young cartilage. Behind the foramen is the stoutest portion of the wing. Its upper surface slopes downward and backward, its medial region, underlying the alicochlear commissure, presenting a low ridge, directed principally backward, which is edged with young cartilage. This ridge terminates caudally in the caudo-medial angle of the wing, some distance above the level of the pterygoid process.
The ventral surface, which also looks forward and inward (figs. 2, 3), shows the rounded lateral and superior portions of the foraminal border. The slender medial border is also seen. Just below the foramen is a rounded forward projection from the pterygoid process, which passes backward to be lost at the caudo-lateral angle of the mass. A shallow groove is medial to this ridge. It extends from the foramen to the caudal edge of the wing. Medial to this is a rounded area which curves upward to the mesial border.
The anterior boundary is quite sharply marked ; the lateral is distinct in front, becomes blunt and rounded behind, and shows a shallow outward concavity. The posterior is best marked medially, where is terminates in the blunt point already mentioned. The medial border is thin in front (figs. 11, 27); from the slender foraminal portion of this the boundary is continuous, medially, with the thin cranial edge of the processus aiaris (fig. 11), there being here a narrow edging of young cartilage. The caudal portion of the medial boundary of the wing is lost in the connection witli the alar process, except posteriorly, where it runs over upon the caudo-medial angle.
Evidences oj heginning ossification. — Particularly in the region of the pterygoid process, and lateral to and below the foramen rotundum (see Fawcett, 1910a, fig. 13), but extending out from this, especially forward, the cartilage shows evidence of preparation for o.ssification (figs. 11, 26,27, and 29). It involves practically the entire outer half of the wing and extends inward to about the middle of the mass lying in front of the foramen rotundum. The cranial half, however, is not so strongly marked as the caudal and, as has been noted, the upper edge of the lateral margin of the foramen is edged with precartilage. Below this ridge the lateral wall of the foramen shows preossification changes.
The semilunar ganglion lies above the greater wing, though separated therefrom by an interval equal to its own thickness. Its posterior third is applied quite closely to the antero-lateral aspect of the cochlea (figs. 15, 16). Its root and branches are all well seen in these figures. The root pierces the dura just above the level of the ganglion. The ophthalmic division, much the smallest, passes forward through the wide superior orbital fissure, seen in figure 1. The maxillary division traverses the foramen rotundum (figs. 26, 27, 29) and gives off its sphenopalatine branches and twigs to the sphenopalatine ganglion (fig. 27), while the remainder of the fibers pass downward and forward as the infraorbital nerve. The mandibular division courses downward and a little outward and backward, as seen in figm'e 16. As yet there is no foramen ovale.
In figure 16 the course of the great superficial petrosal nerve may be followed as it passes forward close to the outer surface of the cochlea just behind the mass of the semilunar ganglion. At the cranial end of the cochlea it comes to underlie the alicochlear commissure and the internal carotid artery. It then turns more directly forward, diverging from the artery, which is now separated from it by the alar process. In making this turn it passes above the caudomedial angle of the temporal wing (figs. 26 and 27) and comes, as the nerve of the pterygoid canal, to occupy the notch between the process and the wing proper. Traced forward from this, it is seen entering the sphenopalatine ganglion, as the figures show.
The medial pterygoid plate (figs. 26, 29) is a thin strip of membrane bone which is dii'ectly continuous below and behind with the rounded parasphenoidal cartilage, representing the hamulus. The bone and cartilage form a right angle (figs. 26, 28). The hamulus alone is seen in figures 2, 3, and 6. The cartilage and bone seem to be quite intimately joined together, and there is no definite boundary between them — in fact, the appearance of the cartilage suggests that it is in process of calcification preparatory to ossification. The lower end of the anterior border of the plate resembles cartilage. In the description of la it was noted that this cartilage differs in histological appearance from that of the chondrocranium proper. The pointed upper end of the bony plate lies but a short distance below the caudomedial angle of the greater wing. It is inclosed in condensed mesenchyme, which is continuous with that enveloping the palate bone, as seen in figures 28, 30, and 41.
ORBITAL WING.
The orbital or lesser wings of the sphenoid are seen from above, below, in front, and from the side in figures 1, 2, 3, and 5, respectively. The two wings make with one another an angle of 131°, measuring from the uptm-ned tips of the dorsolateral processes and hmbus sphenoidalis. The corresponding angle was measured in four adult skulls and found to range from 147.5° to 154.5°, the average being 150°.
The wings have been described by many authors as sickle-shaped plates of cartilage joined medially to the presphenoid and anteriorly continued as the spheno-ethmoidal cartilage. It is convenient to divide each wing into four parts: the body, the dorso-lateral process, and the two roots. The body is thin and almost plane anteriorly, while posteriorly it is thicker and narrower. It is this latter portion of the wing which is the first to develop, according to the accounts of earlier stages, as that of Lewis (1920), where the thape is also that of a sickle, but with the concavity of the blade dii'ected inward to form the boundary of the optic foramen. The anterior root, in No. 460, has not joined with the main stem and in No. 886 it has evidently just joined, for it is very slender and of young cartilage. Lateral to the optic foramina, the upper sm-face of the body shows a gentle concavity upward and inward (fig. 1) and below this region there is a corresponding convexity downward. When seen from the side (figs. 5, 6) the combined lesser wings and sphenoethmoidal cartilages present a distinct concavity downward, this plate of cartilage forming part of the roof of the orbit. The anterior border of the body forms the caudal boundary of the orbitonasal fissure — a triangular cleft which partially separates the wing from the spheno-ethmoidal cartilage. The lateral border of the two plates, beginning in front at the spheno-ethmoidal commissure, describes a compound curve, the edge of the spheno-ethmoidal cartilage showing a strong outward convexity, which is followed by a gentle outward concavity as the line passes over upon the dorsolateral process. Just beyond this border is the posteromedial edge of the frontal bone.
The posterior border, beginning at the inner extremity of the superior orbital fissure, also describes a compound curve, the first portion of which is very sharply convex backward, where it passes around the pointed anterior clinoid process; there is next a long sweeping curve with concavity backward, the first portion of which shows a thickened margin.
The dorsolateral process is an almost straight cylindrical rod pointed backward, outward, and upward, and is somewhat thicker than the portion of the wing from which it springs.
The posterior root of the lesser wing is a .short, stout rod of cartilage which springs laterally from the tuberculum sellae (fig. 10). Each root shows a rather wide, shallow groove upon the dorsal surface, that on the right side being slightly better marked. A narrower groove is found upon the ventral surface of each root, which is continued forward as the groove separating the ala hypochiasmatica from the medial stem. From the anterior aspect of the junction of root and presphenoid, the ala hypochiasmatica is continued forward. The anterior clinoid processes are rather blunt and tipped with young cartilage.
Lewis (1920, p. 309) .surmises that there are separate centers of chondrification for the two parts of the orbital wing, the basal and the lateral — a point which was advanced by Kernan (1916), who identified the "basal part" as the ala hypochiasmatica. Lewis thinks that the basal part is later incorporated into the sphenoidal body.
The anterior roots of the ala orbitalis, formed of young cartilage, are very slender. In la they were very much wider and stouter.
Optic foramen. — But little remains to be said as to the optic foramen. The lateral border, which may be considered to be formed by the margins of the body and the two roots, is semicircular in outline and lies at a higher level than the medial, which is made anteriorly by the prechiasmatic commissure and posteriorly by the outer margin of the hjTDOchiasmatic lamina, bearing the salient hypochiasmatic wings.
The triangular spheno-ethmoidal cartilage (figs. 1, 5, 6) is confluent caudally with the lesser wing of the sphenoid. Its cranio-ventral angle is connected with the projecting upper edge of the ectethmoid by a narrow bridge, the sphenoethmoidal commissm-e, and behind this the connection with the ectethmoid along the inner border of the cartilage is accomplished by several cartilaginous strands. This connection is very imperfect caudally. Most of the caudal borderthat medial to the connection with the orbitosphenoid— is free, forming the cranial limit of the orbitonasal fissure. The medial part of this border is irregular, and marked by projections of young cartilage into the fissure. The part of the plate in front of this border is perforated by several small holes. The spheno-ethmoidal cartilage shares with the orbitosphenoid in the formation of the cartilaginous roof of the orbit and the floor of the anterior cranial fossa. It is almost plane, showing but a suggestion of a downward concavity.
The orbitonasal fissure (figs. 1, 5, 6) lies between the inner portions of the orbitosphenoid and the spheno-ethmoidal cartilage. The cranial border, irregular and broken by projections of young cartilage from the spheno-ethmoidal cartilage, lies at a lower level than the caudal, which it meets at a sharp angle, marking the lateral limit of the fissure. The caudal border, rather blunter, shows a gentle forward bend near the point where it passes over upon the slender anterior root of the orbital wing. The medial border may be followed forward along the laterally projecting edge of the sphenoidal limbus; from the cranial extremity of this it crosses over the surface of the tectum nasi to join the cranial boundary. The medial portion of the fissure will doubtless become somewhat cut off by reason of the enlargement of the connection of the lesser wing with the main stem. Laterally the fissure overlies the orbital cavity, and medially its plane is above an inward extension of this cavity overlying the tectum nasi.
Type Of Cartilage
The material of the orbitotemporal region is for the most part mature cartilage. In the alar process and greater wing, changes in the cartilage have been noted which indicate the position of the future centers of ossification here. There is rather more precartilage and young cartilage than in the posterior regions of the chondrocranium. The details have already been mentioned.
Ethmoidal Region
The ethmoidal region contains the mesethmoid and paraseptal cartilages, the ectethmoids and related cartilages. It is noticeably less developed than that of la.
Mesethmoid
The shape of the mesethmoid can be best ascertained from figme 10. Three slender connections with the ectethmoid are noted upon its upper margin, while anteriorly there is a more elongated union. The slightly thickened crista galli is conspicuous above, and the upper border of the septum for some distance behind this separates the fcnestrae crlbrosse (fig. 1). There is a small union on either side with the paraseptal cartilage. This was present on the right side only in la. The mesethmoid is thinner than that of la and does not possess such a thick lower border. There is no indication of a superior paraseptal cartilage, as in la.
The ventrolateral processes arc rudimentary, being low ridges of very young cartilage extending out slightly from the lower thickened border of the septum (figs. 12, 13) just in front of the paraseptal cartilages. For some distance behind these processes the septal margin between the paraseptal cartilages is of a very young type of cartilage, covered with precartilage.
Mucous membrane of septum. — Models have been made of the mucous membrane covering the medial (fig. 37) and lateral wall (fig. 38) of the right nasal cavity. The mucous membrane of the septum, shown in figure 37 (which should be compared with figure 10 to get the relation of the membrane to the septum) shows a surface almost plane. There is an exceedingly low, indistinct, and wide elevation near its middle, which runs almost parallel with the upper border of the septum. Very low hollows border this ridge.
The vomeronasal or Jacobson's organ appears upon either side of the nasal septum. In figure 37 the mucous membrane of the right side has been cut away to expose the organ. It consists of a dorsal enlarged portion, of somewhat fusiform shape, which terminates dorsocaudally in a fine point. From the lower end a fine tube leads downward and outward to empty into the lower portion of the nasal cavity. The main mass of the organ is a congregation of more or less uniform cells which suggests a coiled tube, but the lumen is very indistinct and the walls are made up of several layers of cells. The septum does not show a distinct concavity for the organ, as in la.
The paraseptal or Jacobson's cartilages (figs. 12 and 13) show a much 3'ounger type of material and are more rudimentary in form than those of la, but the two main masses, medial and lateral, may be recognized.
The lateral mass is relatively small. It is roughly of triangular shape, the apex being directed forward, and being separated from the fundament of the ventro-lateral process by a narrow cleft. This end of the plate is of precartilage. The base, of young cartilage, is continuous medially with the medial mass, near the anterior end. The lateral edge is situated a little higher than the medial, making the lower surface look somewhat outward as well as downward.
The medial mass is a vertical plate of very young cartilage, edged with precartilage, which shows but little of the differentiation that characterized its condition in 7a. In shape, instead of being roughly quadralateral, as in la, it is like a rightangled triangle, the projecting right angle being situated caudoventrally. The horizontal base is notched. The posterior side, longer than the base, is vertical in position and is bowed outward a little about its middle. It terminates below in a very shght enlargement. Its outlines are indefinite, merging into the surrounding mesenchme, and it is of precartilage. The vomer is some distance behind it and is situated closer to the midline. The hypotenuse runs practically parallel with the lower edge of the septum and is but Uttle above this, so that the portion of the septum lying between these plates is very small. The anterior angle Ls continuous with the septum (fig. 10) and with the fundament of the ventrolateral process. The latter connection was not present in la. This end probably represents the cranioventral process of la. A connection with the lateral mass, just behind this region, seems to represent the position of the ventral process of la. The surfaces of the plates look ahnost directly outward and inward. They are separated by an interval but little wider than the lower edge of the septum and lie practically parallel with one another.
These cartilages, being of such young material, are evidently developing rapidly and would doubtless soon have become differentiated as in la. They are somewhat difficult to outline, for they lie in a mesenchyme of which the parts, as modeled, are often but the most condensed portions. No connection could be made out between the lateral mass and the paraseptal process of the ectethmoid, which project toward one another and which are linked by a zone of loose tissue in la.
Ectethmoid
The ectethmoid is a thin crumpled plate of cartilage which presents on the outer surface a number of eminences and depressions, which appear on the inner surface in reverse. In addition, the inner surface shows the developing conchse. In the ectethmoid there are represented a roof, a lateral wall, and a floor, though the first and last are not extensive.
The roof occupies little more than half of the entire length of the ectethmoid and is bounded laterally by the attachment of the sphenoethmoidal cartilage. The border is completed anteriorly by the upper edge of the inturned ectethmoid which meets the crista galh; the boundary of the posterior part of the roof is indefinite, being marked by a low medially curving ridge, which ends somewhat behind the paraethmoidal process in the region of the limbus sphenoidalis.
Most of the roof is incomplete, showing anteriorly the wide fenestra cribrosa (fig. 1). Its margin presents no cribro-ethmoidal process, as in la. Posteriorly the roof is joined to the nasal septum, and is of young cartilage. Some four or five small holes appear in this evidently newly formed part. Behind this area the roof can hardly be said to exist, being represented only by the sharply rounded upper edge of the antorbital plane. The posterior part of it underhes the preoptic root of the lesser wing.
The side wall is ii-regular in formation and anteriorly turns inward to meet the nasal septum. Underij-ing the lesser wing and spheno-ethmoidal cartilage the upper border flares outward and thus gives rise to a shallow and wide groove below.
The posterior or orbital portion is flat and shows near its upper exiremity the small para-ethmoidal process of young cartilage. This has a blunt, free posterior end. In la there was here a free nodule of cartilage, which lay very close to the wall.
The upper border is connected anteriorly with the nasal septum through the roof; posterior to this it turns sharply downward to terminate in a free and very thin edge which, however, is continued downward beside the nasal septum as a membrane. The wedge-shaped presphenoid lies between these downturned parts of the border, and so they are widely separated behind. They are separated from the presphenoid by the very narrow cupulo-septal fissure.
The posterior end of the antorbital plane is somewhat indented, but does not show such a sharp dorsal palatine notch as did la. The upper corner, though tm-ned downward as a sharp point of young cartUage, does not turn forward to form a distinct posterior cupular process, as in la. The ventrocaudal corner shows a backwardly projecting point of young cartilage.
The lower part of the plate turns in sharply to form the inferior concha. At the anterior end of the angle thus formed is the posterior nasal prominence, bearing the posterior baaxillarj' process, formed of young cartilage.
The posterior maxillary process is considerably smaller than in la, and between it and the posterior prominence there is a distinct cleft. Projecting forward from it is a hardly distinguishable osseous fragment — the beginning lacrimal bone. Immediatelj" lateral is the hook-like paranasal process which shows a rather knobbed extremity, l3'ing somewhat below the level of the lacrimal bone. The process projects downward and forward and its pedicle is slightly constricted. It is of a young type of cartilage. In la, it was disconnected from the wall. Medial to the process is the lacrimal duct (fig. 39).
Paranasal process. — The wall to which the paranasal process is attached is the widest part of the lower border of the antorbital plane. This ridge continues anteriorly, with an inward, upward, and forward curve, upon the posterior prominence. It corresponds to the deepest part of the middle meatus.
It is convenient here to examine the inferior concha, which forms the greatest part of the floor. It is inclined a little downward and shows a downward-looking, wide, shallow groove. This is of thin young cartilage. It is represented in the floor of the middle meatus by a low, rounded anteroposterior ridge. The free border of young cartilage is thickened and turned downward and is somewhat irregular. The caudoventral angle is rounded and the caudal border shows a backwardly projecting, short point, surmounted by a low vertical ridge of precartilage. Between this point and the ventrolateral angle of the plane there is a notch, the representative of the ventral palatine notch of la, which, however, is small when compared with the older stage. In it is found the anterior extremity of the palate bone (fig. 41). The concha gradually projects more steeply downward, as it is followed forward, and comes to an end some distance below the posterior prominence at the post-transverse incisure.
The anterior or nasal portion of the wall is more irregular than the posterior. Above appears the small superior prominence, which is not represented upon the internal .surface by a concavity. A little below and jjehind it is the foramen epiphaniale. The middle prominence (called by Voit the supraconchal prominence or Sakterwulst) in less conspicuous than in la. Overlying it is a band of mesenchyme continuous with the frontal process of the maxilla. A corresponding but relatively less marked concavity appears upon the inner surface. Although there is a slight depression here in the mucous mem])rane, which is continuous caudodt3r.sally with the cleft for the middle meatus, yet it is not so prominent as the eminence on the outer surface would lead us to expect. The inferior prominence, in front of the paraseptal process, is quite evident.
Cupular process. — The lower margin, at the front of the anterior naris, presents several projections. Anteriorly is the curious, slender, hookhke cupular process of precartilage (figs. 38, 39, 40, 41), which is an extension of what I termed the cupular cartilage. It extends for 700 micra anteroposteriorly. It at first curves sharply inward, coming to underlie the septum and also to lie quite close to its neighbor of the opposite side. It terminates in a sharp tip turned outward and backward. Only its stump was described in la. It shows a distinct outward concavity which embraces the epithelial plug of the anterior naris, as shown in figure 40. Rehmke (1913), according to Peter (1913), showed that this process later becomes cartilaginous and forms the medial crus of the greater alar cartilage, and that in doing so it fuses with what seems to correspond to the ventrolateral process. Rehmke 's latest stage was 275 mm. sitting height (30-32 weeks).
Behind this process is a notch and the edge here is of very young cartilage. We then come upon the superior alar process, which projects downward from the lower- edge of the inferior prominence. Behind this is the distinct paraseptal process, terminating in a point of precartilage which projects backward and upward. It forms the lower border of the post-transverse incisure. This incisure is made a foramen by the lower edge of the maxilla. Both the superior alar process and the paraseptal process are found in Rehmke's figures in Peter's Atlas. The former is included in the lateral crus of the greater alar cartilage, while the latter breaks up to form the lesser alar cartilages, as shown in Peter's figure 56d, from the 275 mm. stage.
The interior of the ectethnoid (figs. 41 and 43) presents all the young conchee. There are three slender connections above with the nasal septum and anteriorly an elongated union with the septum appears. These all fit to the cut edges shown upon the septum in figure 10. Behind the posterior connection is seen the down-turned edge of young cartilage already mentioned. This was more advanced in la, where the appearance suggested a concha. Just below it, and above the posterior end of the superior concha, is a space which represents the spheno-ethmoidal recess. The superior concha is short, broad centrally, and low. It is of very young cartilage, with an envelopment of precartilage. The anterior extremity is rather narrow and turned downward. Underlying this concha is the shallow superior meatus.
The middle concha is well marked and widest in its central region. It overhangs the middle meatus and does not show a perforation, as it did in la. Posteriorly it does not pass over upon such a distinct ridge joining the posterior border of the inferior concha as in la, but there is a low ridge of young cartilage joining the two and development is evidently proceeding rapidly here. The middle meatus is very capacious. Its floor, as already observed, is steeper in front than behind and it continues forward into the cavity formed by the middle prominence; beyond this the concavity leads downward into the anterior naris. The deepest part of the meatus is the posterior. About its middle is the process of the middle meatus, ahogether of precartilage, which represents the isolated cartilage of the middle meatus of 7a, together with its pedicle of precartilage. It projects almost straight backward for 600 micra to end freely. It may represent the uncinate process. The connection with the wall is at the medial side of the posterior prominence. The cartilage of the wall is quite thick here and the part joined to this process is partially separated from that lying laterally by an upwardly-dii-ected cleft.
The inferior concha needs no further description. The inferior meatus, below the inferior concha, is seen in figure 41. The floor, in front, is formed by the paraseptal process, while behind this it is made by the developing premaxilla and inaxilla. The paraseptal process comes ahnost into contact with the cranial end of the condensed mesenchjine of the premaxilla and the post-transverse incisure above it leads backward into the meatus. The end of the nasolacrimal duct is shown in figure 41. The inferior meatus is very narrow as compared with the middle.
Just in front of the paraseptal process is the concavity formed by the inferior prominence. This is quite evidently the wall of the future vestibule. Above the indentation for the middle prominence is a low ridge which probably represents the agger nasi. It is continued backward to the anterior end of the middle concha.
The mucous memhrane of the outer nasal wall is shown in figure 38, which should be compared with figure 41 drawn from the same point of view. We note all the turbinate folds, covering thenrespective cartilaginous conchse. The superior membranous concha is the shortest and least distinct and the two lower are very well marked. The superior meatus is short and shallow and the middle one is deeper and forms a very distinct cleft. The inferior meatus, longest of all,
is quite shallow but projects into the meatus as a wide double layer of epithehal cells. This is shown from without in figure 39, where it is seen that the epithelium underlies practically the inner half of the inferior concha. In figure 41 the tip of the nasolacrimal duct, which is here somewhat expanded, is seen projecting into the space of the inferior meatus; its relation to the epithelium is seen in figure 39.
The caudal extremities of the meatuses show the famihar approximation where the nasal capsule narrows into the elongated nasopharyngeal canal, as shown in figure 38. This canal is supported laterally by the palate and internal pterygoid bones, in their common investment of mesenchyme (fig. 41).
Nasolacrimal Duct System
The nasolacrimal duct system was modeled and a view of it is seen in figure 39. Commencing laterally, we note the two lacrknal ducts, terminating in the punctae lacrimalise and opening into the somewhat dilated nasolacrhnal sac. From this point the nasolacrimal duct runs directly backward for some distance, crossing above the maxilla and coming to he between the lacrimal bone and the tip of the paranasal process. The duct does not lie in the depths of the notch which is made by this process and the posterior maxillary process. It now ciu-ves downward, inward, and backward, under the posterior maxillary process, terminating in an expansion (fig. 40) which is contiguous with the epithelial plate of the future inferior meatus (fig. 39). It has not yet broken through the epitheUum. Throughout its extent no definite lumen can be recognized in this system, though the epithelial cells are arranged in ring-like formation with a potential lumen within.
The lower portion of the nasolacrimal duct in the human fetal skull is situated some distance posterior to that in the lower forms, as (to cite one of numerous examples) the chondrocranium of the cat (Terry), where the duct passes forward lateral to the anterior transverse lamina to empty into the nasal cavity in front of this lamina. The anterior transverse lamina is represented, in the human chondrocranium, by the paraseptal process and, if the position of this part of the duct were the same in homo as in the lower forms, it would pass forward around the paraseptal process to connect with the mucous membrane of the nose in front of this process. It would then empty into an extension of the anterior nai-is, as it does in the lower forms. Instead, it is situated far behind the paraseptal process and its ostium is, accordingly, shifted backward from its earlier position. It would appear that the present position of this portion of the duct in man, far behind the paraseptal process, is consequent upon the disappearance of the cartilaginous connection between the iiaraseptal process and the nasal septum, which completes the anterior transverse lamina in the lower forms.
Basal Fissure
The basal fissure (fig. 2) ,which is represented by a fenestra in most of the lower mammals, extends throughout the entii'e length of the ectethmoid, being continued forward as the incisura narina. Its lateral border has recently been outhned in the discussion of the lower border of the ectethmoid and its medial border follows the lower margin of the septum, being indented by the paranasal cartilages. The lateral and medial borders meet in front of the confluence of the ectethmoid with the septum. There is no such confluence behind, as we have seen, the caudal extremity of the ectethmoid being separated from the medially lying septum by the very narrow cupulo-septal fissui'e. This fissure runs forward to the caudalmost junction of the ectethmoid with the main stem of the chondrocranium. The basal fissure is interrupted by the inward projection of the alveolar process of the maxilla.
Type of Cartilage
Summarizing the type of cartilage in the ethmoidal region, we note that it is younger than that of the other regions of the chondrocranium. It has long been known that this region of the skull develops later than the others and accordingly it shows a rapid growth at this stage. In the region of the connections with the paraseptal cartilage, and with the upper portion of the ectethmoid and at the crista galli, the septum shows a young type of cartilage. In the ectethmoid, the roof and edges are, for the most part, of young cartilage, while the two upper conchffi and the posterior part of the inferior concha are lined with precartilage. Precartilage, too, forms the process of the middle meatus and tips the paraseptal and posterior maxillary processes.
Cartilaginous Branchial-Arch Skeleton
Meckel's Cartilage
Meckel's cartilage (figs. 1, 3, 5, 6, 31, 32, 34) is a long rod of mature cartilage continuous above with the head of the malleus and terminating below in a tip, turned forward and inward, which approaches very close to, but does not meet, its neiglibor of the opposite side. The cartilage is of practically uniform thickness throughout, excepting at the angle near the tip, where it is flattened from above downward and forward, and widened. In front of this the cartilage rapidly narrows to a point. There is a constriction just below the head of the malleus. The two rods converge to form a wide angle (figs. 3, 4), in which lies the floor of the mouth. The shaft, when seen from the side, as in figure 6, has a practically straight course with a very faintly marked anterior concavitj'. When seen directly from the front there is, just above the angle, an inward bending, which makes a gentle concavity outward and downward here. Above this point the shaft appears straight from this point of view. Throughout almost its entire extent the cartilage (as shown in figures 5, 31, and 34), is covered by the mandible. At the lateral surface in the region of the ventral bend (fig. 32), there is a very close approximation of the cartilage to the mandible, and here the cartilage is undergoing change preliminary to ossification.
The perichondrium here has become ossified. The modified cartilage cells extend far into the interior. The corresponding area, on the mandible, is seen in figs. 33 and 35. The relation of Meckel's cartilage to the slender goniale has been referred to.
Stylo-Hyoid Arch
The cartilage of Reichert, representing the future styloid process, has been described with the otic capsule.
Hyoid Cartilage
The hyoid cartilage (figs. 5, 6, 45 46, 47) is roughly semicircular in form, resembling closely the mature bone. It consists of a body and paired lesser and greater cornua.
The body, cartilaginous, with an edging of young cartilage in regions adjoining the cornua, is oval in form when seen from the front, with a rounded cranioventral face. The caudal face presents a distinct transverse notch, as seen in the mid-sagittal section (fig. 46). Just above this the upper edge of the thyroid cartilage is confluent with the hyoid. At the site of connection with the lesser cornu the cartilage is somewhat pointed, but there is no direct cartilaginous connection here, a fine strand of membrane joining the two.
The greater wing, on the contrarj', shows a direct continuity of its cartilage with that of the body.
The lesser cornua are two thin, fusiform bodies of young cartilage covered with precartilage, which are connected by fine strands of membrane with the body of the hyoid below and with the styloid process above.
The greater cornua, directly continuous with the body, spring backward to meet the superior cornua of the thyroid cartilage, there being a thin strip of membrane intervening. The anterior surface near the body, and the caudal tips, show precartilage.
Thyroid Cartilage
The thyroid cartilage, too, has acquired most of its adult form, but there are some outstanding differences. We note at once, from the figures, that its upper edge is closely associated with the body of the hyoid and histological examination reveals a continuity of the cartilage. Thus the hyo-thyroid membrane has not developed. The laminsB approach one another so as to form an even, rounded convexity ventrally and a corresponding concavity dorsally. Thus there is no evidence of the laryngeal prominence, the hyoid body even projecting farther forward than the thyroid cartilage, as figures 5, 6, and 46 show. There is no thjToid notch as in the adult; on the contrary, if the young cartilage is removed, there is a distinct notch on the lower border of the thyroid cartilage. This, however, is closed by a bridge of young cartilage, and above it there is a small foramen with precartilaginous borders, where chondrification is incomplete. The line of junction of the two laminae, although cartilaginous, is edged with young cartilage. It shows, upon the inner or dorsal surface, just above the foramen mentioned, a low ridge (fig. 47). There is no evidence of an oblique line. An inferior thyroid tubercle is distinctly seen upon the lower border and also a low superior thyroid tubercle, projecting laterally from just below the upper border — rather closer to it than in the adult. The lateral surface presents a shallow concavity, running craniocaudally. The superior border makes a rather sharper angle with the superior cornu than is the case in the adult. The superior cornu is directly connected by cartilage to the lamina, the portion immediately above this connection being more slender than the terminal portion. The extremities lie caudomedial to the extremities of the greater cornua of the hyoid, but, although they are closely approximated (figs. 46, 47), they show no continuity, being separated by a thin sheet of membrane.
It is to be noted that the superior cornu is continuous throughout, not interrupted by the lateral hyo-thjToid hgament, as in the adult. The triticeal cartilages are doubtless vestiges of this cornu. The upper extremity is covered with young cartilage.
The inferior cornu is much shorter than the superior, and rests upon the shoulders of the cricoid, as shown in figures 45 and 47, being separated by a thin sheet of membrane. The lower extremity is edged with young cartilage.
Cricoid Cartilage
The cricoid cartilage displays its signet-ring form, the lamina, bearing the young arytenoids, lying between the inferior cornua of the thyroid cartilage. The lamina presents posteriorly a ridge in the midline, and from this the sides slope down to the vertical ridges, which join the positions of the future facets for the arytenoid and inferior cornu and divide the lamina from the arches. At the upper end of the posterior ridge is a small notch, almost completely filled with precartilage, which extends the superior opening of the cricoid here. To either side of this notch are the areas bearing the arytenoids. As figure 47 shows, there is a direct continuity of the precartilage investing the arytenoid with that covering the upper portion of the lamina. When, however, this investment is removed, as was done upon the right side in the models (fig. 47), it is seen that the arytenoid is separated from the lamina of the cricoid by a thin strip of membrane, representing the position of the future articular cavity. The part of the lamina bearing the arytenoid is raised into a tubercle, best seen from the side.
The upper border, passing forward upon the arch, shows in front of this tubercle a notch and (in front of the region for articulation with the inferior cornu) the lower border also shows a notch , the two serving to constrict the region between the lamina and the arch. In front of both arches there are tubercles, seen in side view upon the upper and lower borders. In front of the upper of these the border descends rapidly, the two lateral borders meeting to form a distinct notch in the midline, flanked by precartilaginous tubercles (fig. 45). In front of the notches upon the lower border the contom* descends a little, but there is no notch upon the lower border, as seen from the front. The anterior section of the arch is by far its most slender part. Like the mature cartilage, the inferior opening is rounded, while the superior is oval, showing flattened sides.
Arytenoid Cartilages
The arytenoid cartilages are in a very undeveloped condition, being represented by paii-ed masses of precartilage of rather indefinite outline, investing cores of cartilage of young type, which outwardly grade off into the surrounding tissue. The precartilaginous arytenoid, as I have modeled it, shows the surfaces and borders of the adult condition. The dorsal surface is slightly concave from before backwards, and even less so from side to side. In its ventral portion the cartilaginous core comes to the surface (fig. 47). This surface shows caudally a continuation of the precartilage over the surface of the cricoid lamina. The medial surface, smallest in area, presents a roughly triangular shape, with the apex craniad (fig. 46). The wider ventrolateral surface is somewhat hollowed out, as in the adult condition. The ventral border is convex downward, presenting a blunt processus vocalis; here the core of cartilage shows a corresponding projection (fig. 47). The dorsal border is sharply concave,
while the lateral border shows an outward convexity with a distinct muscular process as the posterior extremity. The entire mass is, as already noted, fused with the upper portion of the cricoid lamina. The cartilage proper, though separated from the cricoid by membrane, is closely applied to it and is to be described as a cylinder having a general caudocranial direction, with a slight medial inclination. Upon its ventromedian aspect, rather nearer its cranial than its caudal end, is a spur of cartilage, the core of the vocal process, projecting downward and inward (fig. 47). The cavum laryngis is very narrow. There are no corniculate and cuneiform cartilages.
Tracheal Rings
The tracheal rings lie parallel with the basal plate and are of a very young type of cartilage. The upper three show fusions with one another. Each describes about half a circle. Only the upper four were modeled.
Membrane Bones
Interparietal
The interparietal part of the future occipital bone is represented in No. 886 by two slender osseous spicules, lying one on either side of the processus ascendens (figs. 1, 4, 5), just above the upper border of the tectum posterius. The outer ends are somewhat medial to and below the projecting extremities of the parietal plates. Each half is slightly flattened antero-posteriorly and is invested by condensed mesenchyme, as seen in figure 14. This bone could not be modeled in la and is not included in the Hertwig model, so that comparisons with these specimens could not be made.
Parietal
The parietal bone is a very fragile reticulum of osseous substance lying in a plane just lateral to that of the parietal plate. It is very small in comparison with the frontal bone. In outline it is roughly oval with the long axis dorsoventral. The bone is very slightly concave inwardly and lies in the membranous calvarium. Its caudal edge does not as yet overlap the cranial edge of the parietal plate, as it does in la and the later Hertwig model. The peripheral part is but little more delicate than the central. The texture of the bone was too tenuous for modeling, so that it had to be represented by profile reconstruction. The dorsal edge does not extend above the line joining the dorsal extremities of the parietal plate and frontal bone. In la the bone has grown enormously upward and in the Hertwig model this growth is even more marked, while the parietal plate has undergone reduction.
As in the case of the parietal, the outline of the right half of the frontal bone was worked out in plaster of paris and the details of structure were obtained by profile reconstructions in the sagittal and transverse planes. From the side (fig. 5) the bone appears as a roughly rounded plate bent into an outward convexity. The margins are serrated. Arching across this surface is a low but distinct ridge which separates the orbital from the frontal portions. This ridge is recognized as the representative of the supraorbital margin and probably the supercihary arch as well. It slants much more sharply backward than in the adult and extends from a projection of the bone in front, representing the medial angular process, to a point some distance behind the dorsocaudal angle of the bone. The caudal lunit doubtless represents the position of the future zygomatic process. The zygomatic bone is separated from this point by a wide interval. This ridge marks the densest part of the bone and appears to be in the position of the original center of ossification, as I have pointed out (Macklin 1914, p. 416). The densest bone (surrounded by a dotted line in fig. l) forms a crescentic plate of homogeneous osseous tissue, from which gradually narrowing spicules radiate to the periphery. The relation of the bone to the cerebral hemisphere is seen in figure 7.
The orbital portion of the bone forms an anterolateral extension of the surface of the orbitosphenoid and spheno-ethmoidal cartilages. Its lower surface is very slightly concave, being molded for the orbital cavity, and its upper surface shows a corresponding gentle convexity, where it forms the peripheral portion of the floor of the anterior cranial fossa. Its tissue, although reticular throughout, is rather denser than that of the frontal part above the region of the supraorbital ridge. The inner margin of the plate hes but a short distance from the edge of the ala orbitalis and spheno-ethmoidal cartilage (fig. 1), though never in actual contact. In 7a this edge had grown under the cartilages mentioned.
It is apparent from a comparison of the mature bone with the present specimen that the sphenoethmoidal cartilage must undergo resorption, the orbital plate growing backward and inward, to join the ethmoid and the lesser wing of the sphenoid. The result of this process is to form practically a right angle by the approximation of the margins articulating with the sphenoid and with the ethmoid. Already there is an indication of the beginning of such an angle in a spm* of bone jutting inward and backward from the margin in question toward the spheno-ethmoidal cartilage.
The frontal portion forms by far the largest part of the bone and makes with the orbital portion a very obtuse angle. It presents a concavity looking inward and upward ; in antero-posterior planes, however, this concavity does not appear, the bone in such sections being almost straight. Its margin describes rather more than half a circle. Upon the exterior no frontal tuberosities are yet apparent.
Maxilla
The maxilla is cancellous in structure. In it the main features of the bone as at term can be made out. The facial surface looks ventrolaterally — much more laterally than in the adult. It shows a gentle outward convexity and, although presenting roughenings and small holes, is nevertheless smooth in comparison with the medial or palatine surface, which is very rough on account of numerous bony excrescences, and is very slightly concave. The palatine surface is seen from within in figure 26, and froir the front and a Uttle above
in figure 27, but neither of these figures gives a frank view of this surface. The facial surface is shown from below in figure 3 and from the side in figure 5, but here, again, its full area does not appear. If the bony processes are removed, these surfaces become very small. It is hardly possible to speak of the orbital and infratemporal surfaces, for the area which represents them embraces merely the groove for the infraorbital nerve and a very insignificant region behind this, which comes to an end at the caudal extremity of the alveolar process.
The palatine surface presents a very interesting condition. There is a distinct wide groove containing mesenchjTne, which begins above just in front of the apex of the frontal process and traverses the bone to end below 0.2 mm. from the medial extremitj'. The lower end is wider than the upper and is straight, while the upper turns forward a little, as shown in figure 26. This groove represents the incisive suture, marking the line of division between the maxillary and premaxillary elements, and agrees in position with the figures of Felber (1919), who has recently investigated the development of the maxilla (see abstract by Schultz, 1920). The borders of the groove are sharply marked and conspicuous, the upper being the longer and reaching to the apex of the frontal process. These borders are somewhat nodular and at the lower end they project medially as processes, the posterior being the longer. Behind and lateral to this is the alveolus for the canine tooth, while medial to the lower end of the anterior border of the groove, in the territory of the premaxilla, are found the alveoli of the lateral and medial incisors (fig. 29). The floor of the groove is fairly smooth. Above, it is incomplete, there being a cleft, 0.1 mm. long, which extends through to the facial surface just behind the anterior border of the groove. A little behind this there is a much .shorter fissure.
Felber has shown that during growth of the maxilla the premaxillary and maxillary elements preserve a certain independence. They arise from separate centers. The frontal process grows upward as two spicules, a maxillary and a premaxillary, separated by a cleft, and the union of the two elements occurs from the alveolar process upward. Thus it is that the elements unite last at the apex of the frontal process. The fusion, too, is completed earlier on the facial than on the palatal aspect.
The body of the maxilla may be defined as all that is left after the processes are removed, and since the processes are the most prominent parts they will be described first.
The frontal process is thin and triangular in shape and lies in a hollow on the lateral aspect of the ectethmoid, between the region of the lacrimal bono above and the paraseptal process and adjoining cartilage l)elow. With the basal plate horizontally jilaced, it projects almost directly forward and lies in a sheath of condensed mesenchyme which reaches forward around the developing nose to join with its neighbor of the opposite side in the formation of a band or chaplet in which the nasal bone will later be developed. It is quite evident that the frontal process is extending upward in this band, the inner surface of which is applied medially, near its upper extremity, against a portion of the ectethmoid which presents, upon the inner surface, the representative of the agger nasi. It is clear, therefore, that the frontal process later takes to itself the modeling of the cartilage here.
The outer surface of the process shows a few small foramina and at the apex the aforementioned unclosed end of the incisive sutm-e appears. Its inner surface is largely occupied by the groove already described, together with its borders. There is a rough, spiculated area above and behind the groove. Just above the upper border of the process the nasolacrimal duct courses backward for some distance before turning downward to its termination. The lower border cui'ves inward close to the paraseptal process of the ectethmoid, making of the post-transverse incisure a foramen.
The zygomatic process is represented by a thin plate of bone which ends in a dorsolateral spur (fig. 29) and which is separated from the nearest point of the zygomatic bone by a wide interval. It projects much more directly backward than in the adult. Its lateral edge is the duct extension of a ridge which forms the lateral side of the groove for the infraorbital nerve. Its anterior surface looks also somewhat upward. It narrows as it descends into the depths of the infraorbital groove. The posterior surface, as it passes downward, turns outward, widens, and goes over upon the lateral surface of the body. The medial border of the plate is continuous with the lateral edge of the tooth gutter (fig. 29). The process shows several irregular spicules of bone.
The groove for the infraorbital nerve is formed laterally by a forward extension of the lateral border of the zygomatic process and medially by a continuation backward of the upper border of the frontal process. It is narrower in front than behind and its floor is pierced by foramina carrying the superior alveolar nerves.
The alveolar process is as yet represented only by the crescentic, irregular, roughened edges of the tooth gutter (fig. 29). Beginning caudally Ijut a little in front of the extremity of the zygomatic process, it sweeps forward and inward practically to the midhne. The gutter is filled with developing teeth (fig. 30). The outer border, which separates the alveolar process from the facial surface, commences caudally as the medial margin of the zygomatic process. It is marked near this point by a small, backwardly projecting spur. Opposite this point there is a much larger spur of the same character upon the inner border. In front of this the two borders proceed to their craniomedial end with m,any slight but no great irregularities. The tooth gutter lies in almost a perfect plane. Its two borders are crossed near the middle, by an imperfect bridge (the interalveolar septum between the canine and the first milk molar) and in its depths are seen the superior alveolar nerves. The crescentic bar of tissue representing the developing teeth (figs. 30, 41) extends craniomedially almost as far as the midline and caudally a little beyond the bone, There are enlargements representing imperfectly the individual teeth.
The palatine process is rudimentary. It is seen best in figure 29 as a shelf of irregular bone fitted into the concavity formed by the inner border of the tooth gutter. Caudally, it comes close to but does not touch, the palate bone. The medial edge, of serrated appearance, is widely separated from its fellow of the opposite side and forms an obtuse angle with the caudal border. The lower surface, although irregular, contrives to form with the laterally lying alveolar process a surface which is, in general, plane (figs. 26, 29). The superior surface, on the contrary, slopes upward, especially in its cranial extremity, upon the body of the bone and is honeycombed by holes. It thus displays maxillary and premaxillary parts, the latter being very small. Upon the os incisivum there is, as yet, but slight trace of a palatine process.
Among other features of the mature bone which are represented in this maxilla may be briefly mentioned the nasal notch, which embraces the anterior end of the ectethmoid and appears as a sharp crescentic border running down from the lower border of the frontal process to the region of the future anterior nasal spine; also the infraorbital margin, passing backward and outward from the upper edge of the frontal process, crossing the infraorbital groove, and continuing upon the outer border of the zygomatic process.
The condensed mesenchyme enveloping the maxiEa was modeled and is shown in figures 30 and 41. Its outlines are smooth and conform closely to those of the bone within. Its boundaries are somewhat indefinite in places. There are continuations with the mesenchj-me of the maxilla of the opposite side in two places, viz, through an extension of the frontal processes over the bridge of the nose and through a connection between the anterior extremities of the alveolar processes of the two bones, which occurs just below the paraseptal cartilages. There is, too, a caudolateral connection from an extension of the zj'gomatic process to the mesenchjTne of the zj-gomatic bone. The interior of the mass shows a lessening in density, where the fibers for the superior alveolar nerves traverse it. Seen from within in figiu-e 41, we note that the anterior end of the alveolar process and (behind this) the palatine process ai-e forming a floor for the inferior meatus, which, however, is complete only in front. Caudally, it will be observed, the mesench3Tne of the maxilla comes into close contact with that of the palate bone, which, as yet, shows a very poorly developed palatine process.
A comparison of the maxilla of No. 8C6 with that of la shows at once that in the latter there have been noteworthy developments. Not only has the body become larger but the processes all show progress, the zygomatic reaching out to join that of the zygomatic bone, the alveolar projecting fartner downward, and the palatine, with extension much farther toward the midline, being the most noteworth}% In the Hertwig model these growth features are still more evident, there being here almost a complete closiue of the infraorbital foramen and an upward extension of the bone forming the medial boundary of the gi'oove for the infraorbital nerve around the adjoining ectetnmoidal cartilage.
The palate bone of No. 886 is represented by a thin plate of irregular outline bent into an inward concavity situated between the maxilla and medial pterygoid plate (fig. 26). Although showing slight roughenings, the plate is remarkably smooth in comparison with the adjoining inner surface of the maxilla. In its medial concavity rests the mucous membrane of the lateral wall of the nose, as seen in figure 38. Though there is none of the elongation and little of the speciaUzation of the adult condition, yet already many of the parts of the mature bone may be recognized. The main portion of the bone — a plate lying almost vertically — represents the pars perpendicularis. The inward concavity — seen in dorsoventral, but not in caudocranial planes — is found only in its caudal two-thirds and is due to the incurling of the upper and lower borders. The plate is pierced by two small foramina, carrying twigs from the palatine nerves, as shown in figure 26. It is continuous below with the horizontal part, behind with the pyramidal process, above with the sphenoidal and orbital processes, and in front with the maxillary process, all of which are as yet very rudimentary. The lateral surface (fig. 28), affording a less favorable view of these extensions, presents a shallow fiu-row, the pterygopalatine sulcus, deepening above to form the sphenopalatine notch. In it appear the orifices for the exit of the aforementioned two nerve-twigs. Though the middle of this surface shows an outward convexity in the coronal plane, corresponding to the concavity upon the medial surface, it also shows a marked outward concavity in the horizontal plane, due to the lateral projection of the caudal extremity — the representative of the pjTamidal process.
The representative of the horizontal part, or palatine process, forms no angle with the perpendicular plate; on the contrary, there is simply a bending in toward the midline of the lower edge of the bone, especially marked in its caudal portion, shown by figiu-e 29, giving a view of the bone from below. The cranial end of this part of the bone is separated from the palatine process of the maxilla by a distinct space (fig. 29).
The representative of the pjTamidal process is the most lateral part as well as the most posterior part of the bone. It is separated from the horizontal part by an indentation and projects backward to the level of the most anterior edge of the medial pterygoid plate, but Hes some distance lateral to this (fig. 29). It includes three spicules, of which the middle, terminating in a flattened point, is much the longer and is separated from those above and below by marked notches.
The upper border of the bone shows a sharp inbending opposite the nerve trunks descending from the sphenopalatine ganghon. This, the representative of the future sphenopalatine notch, marks the upper end of the pterygopalatine sulcus and separates the rudimentary orbital and sphenoidal parts. The former, overlaid by a small branch from the sphenopalatine ganghon, is seen in figure 27 from above and figure 26 from within. It is, as j-et, only a roughened projecting edge, curving outward and forward from the sphenopalatine notch. The sphenoidal process, equally rudimentary, rises upward and inward from this notch and is surmounted by the caudal end of the sphenopalatine ganglion. Its upper edge descends upon the uppermost spicule of the pyramidal process.
The maxiilarj' process, representing the cranialmost part of the bone, is seen from within in figure 26, and from above in figure 27. It is flattened, its anterior edge is turned a little inward, and its ventral border projects downward toward the adjoining maxilla, giving to the process a hook-like appearance. Its upper portion fits into a notch upon the caudal aspect of the ectethmoid, between the caudal extremities of the maxillo-turbinate below and the planum antorbitale above, as figure 41 shows. There is a wide interval separating it from the maxilla. Seen from within (fig. 26), the caudal edge of the palatine process of the maxilla seems to fit into the notch which the lower border of the process makes with the perpendicular plate, but when seen from below (fig. 29) we note that there is a wide space separating the two bones. In la the bone was somewhat more advanced.
Views of the condensed mesenchyme enveloping the palate bone are seen in figures 30 and 41, and from these it will be noted that it is continuous with that of the medial pterygoid plate. This mesenchyme is shown, partially cut away, in figure 28. Its relation to the nasal cavity appears in figure 38. Its surfaces are somewhat more smooth than those of the bone, but the outlines of the two are everywhere in general agreement.
The spheno-palatine ganglion is, roughly, a three-sided pyramid of nerve cells with the base directed downward and the apex dorsocraniad. It lies in what will be the sphenopalatine fossa, with the sphenoidal process of the palate bone below, the processus alaris of the sphenoid dorsocaudal, and the ala temporalis lateral. A medial projection overlaps slightly the upper margin of the palate and thus encroaches somewhat upon the space from which it will later be excluded by the upward growth of the horizontal plate. Communicating with the caudal extreniity of tlie base is the nerve of the pterygoid canal, while the medial side shows the two sphenopalatine branches from the maxillary nerve. The palatine nerves emerge from the base and lie in the pterygopalatine sulcus.
Medial Pterygoid Plate
The medial pterygoid plate has been described with the orbito-tcmporal region and its mesenchymal investment has also been referred to with the palate bone.
Zygomatic
The zygomatic bone is a rather thin plate of osseous tissue stretching between the zygomatic processes of the temporal and maxilla, but separated from both (figs. 3 and 5). Its lower edge is seen in figure 29. It is not divided into separate parts. It possesses four sharply defined angles, the dorsal, caudal, ventral, and cranial. Wide notches cut into the upper and lower borders of the plate, giving to it an S-shaped appearance. The bone lies with the lateral surface looking a little downward and forward, as well as outward. Many of the characters of the adult condition will be recognized. The dorsal angle, rounded and projecting upward, but separated from the frontal bone by a very wide space, represents the frontosphenoidal process. The temporal, the longest of the processes, which forms the caudal angle, is more slender and is directed upward, backward, and slightly outward. It does not overlap the zygomatic process of the temporal, as in la, but rather is separated by a short space from that process, as shown in figure 5. Thus the zygomatic arch is incomplete. Between the temporal and frontosphenoidal processes is the representative of the temporal border of the adult bone, a thin edge with a deep upward concavity. This curvature is more sharply marked than in the adult.
The malar tubercle forms the ventral angle and is the bluntest of all the processes. The edge of the bone joining it with the temporal process — representative of the masseteric border of the adult — pm-sues a more or less direct course, although marked by minor irregularities. Theremaining angle, representing the infraorbital process, points cranially and a little medially and is separated from the malar tubercle by a wellmarked incisure, du-ected downward, the representative of the portion of the bone which will later articulate with the maxilla. Between the infraorbital and frontosphenoidal processes the edge, representing the infraorbital border, is straight, except for slight roughenings. The lateral surface is fairly smooth and, in general, plane, with a slight outward concavity near the cranial end. The medial surface is similar and, in a position corresponding to the outward concavity, there is an inward convexity, which may be the representative of the orbital process. The thickest portion of the bone is in the region of the malar tubercle.
Squama Temporalis
The squamous portion of the temporal bone consists of a thin narrow plate, placed just lateral to the upper portions of the malleus and incus. It is prolonged in front into a slender point, and below into the long, spiu--like zygomatic process. The squama proper is seen from the side in figure 5. Its lateral surface, quite smooth, shows a very slight convexitj' in the coronal plane and in the horizontal plane a more marked one, owing to the fact that the cranial end turns medially in such a marked manner. The rounded caudal end is only a short distance in front of the extremity of the short cms of the incus. The upper border, lying but a Uttle below the upper border of the incus, pursues a faii-ly direct course forward to the level of the root of the zygomatic process, after which it curves rapidly downward. It is slightly serrated behind and markedl}' so in front (figs. 31, 34). The lower border, showing small irregularities, describes a gentle curve with concavity downward and passes over upon the zygomatic process. Just above the root of the zj-gomatic process the squama displays a small foramen, and from this point forward the plate becomes very narrow and thin and terminates in a slender process, with irregular saw-like edges, which projects downward, forward, and inward. It deviates widely from the zygomatic process, forming Mdth this a deep notch. The medial sm-face of the squama, fairly smooth, and displajing concavities in the frontal and horizontal planes, is separated from the upper parts of the incus and malleus by but a narrow space.
The zygomatic process is very long and almost straight. It projects downward and forward, tapering gradually to a point which lies close to the tip of the temporal process of the zygomatic bone. This process is not so heavj' as that of la, nor is the squama so wide. These differences are even more exaggerated in the Hertwig model.
The goniale, seen in figures 31 and 34 as a separate bony element, has already been described in connection with the description of the malleus.
Tympanic Bone
The tympanic bone (fig. 5) is so shghtly developed as to be found only after careful search. It is a very small rounded nodule, consisting mainly of cells with but little ossified matrix. It appears from the side in the angle between the handle of the malleus and Meckel's cartilage, but is just outside the plane joining these structures. Its ventral tip is below and a little lateral to the goniale, while its posterior and larger end is below and extends a little medial to that bone, with which its mass of condensed mesenchyme is continuous. It is situated quite close to the lateral surface of the tympanic cavity and just in front of the developing external acoustic meatus. It is not nearly so far on in development as the tjTiipanic of la, and even in the latter there was none of the ring-like form which is so characteristic of the new-born condition and which is well seen in the Hertwig model.
The lacrimal bone is a very thin, narrow slip of osseous tissue which projects forward from the outer and upper edge of the posterior maxillary process (fig. 39), inclosed by a shell of mesenchyme. It measures scarcely 200 micra in length and is rather less than half as wide. The nasolacrimal duct hes just below it, as the figure shows. As we have noted in the description of the ethmoidal region, there is a long and clubshaped projection of cartilage, the paranasal cartilage, which points downward and forward from the ectethmoidal cartilage and thus forms a sharp notch between itself and the posterior maxillary process within, opening cranioventrally. The paranasal process occupies the position of the future hamulus lacrimalis, the lower salient end of the posterior lacrimal crest, and the notch within it would then become the sulcus lacrimalis. The lacrimal bone undoubtedly comes to occupy the position of the paranasal cartilage and the groove medial to it. As to the exact manner of its growth we do not have much information. It is usually described as arising from a single center, though Thompson (1907, Morris's Human Anatomj') states that not infrequently the hamulus is a separate element. In such a case it seems probable that this center would occur in or upon the paranasal process and would later fuse with the medial center around the lacrimal sulcus. Thompson also states (p. 75): "The hamular process is regarded as representing the remains of the facial part of the lacrimal seen in lower animals."
The vomer consists of two separate, very slender strips of bone which he side by side along the lower border of the nasal cartilage, as shown in figure 10. They are widest in the middle portion (fig. 9), the ends tapering off to pomts. Only at the widest point do the bones reach the level of the lower edge of the nasal septum. The anterior ends curve a little upward. The thicker lower borders are closer together than the upper, so that the two form a V-shaped trough which, however, is as yet open below. The upper edges are thin and suggest the edge of a knife. In la a narrow fusion between the lower borders has taken place near the anterior end, antl it is well known that such a fusion soon occurs along the entire length of the bone, as the Hertwig model shows, the plates growing upward and hedging in the nasal septum. The two strips of bone are enveloped by a common sheath of mesenchjTne, which is shown in part in figiu-e 8. In this figure the anterior tips of the bones appear in their correct relation to the septum (which is shown in negative manner as a groove) and to the mesenchyme. The anterior tip comes into close relationship with the posterior end of the paraseptal cartilage, but lies closer to the midsagittal plane than this.
Mandible
The mandible is represented at this stage by paired plates of bone which lie just lateral to Meckel's cartilages. The right half alone has been modeled and is shown from without in figure 5. The extremities of the plate are wider than the intermediate portion, which shows somewhat below the middle a constriction. Here the lower end of the bone gives the appearance of having been bent forward upon the upper, forming a wide angle open cranially. The caudal border, in contrast to the cranial, shows scarcely any change in direction. Just below this constriction the large mental foramen appears and from this region the bone turns inward, its ventral extremity coming to lie very close to its partner of the opposite side, without, however, actually touching it. This inturning is represented in figure 3.
The upper end of the bone is shaped somewhat like a dagger, the point, which is notched, projecting toward the root of the zygomatic process of the squama temporalis — the region of the future mandibular fossa — from which, however, it is separated by a wide interval. This region, obviously, represents the future condyle. From it the boundaries rapidly diverge to projections of bone upon the caudal and cranial borders, which are almost opposite each other and which mark the extremities of the widest part of the mandible. The caudal projection, which also points medially, is the upper extremity of the inturned caudal edge or base of the mandible and represents the angle and the Umit of the ramus here. The ventral delimitation of the ramus is not definite. The ventral projection, which shows a thin spur of bone directed upward and forward, represents the future coronoid process. Upon the border of the bone joining the coronoid and condyloid processes, there is a gentle upward concavity which is crossed, a little above, by the nerve to the masseter muscle. It represents the mandibular notch.
The lateral surface is fairly smooth, somewhat more so above than below. Due to the turning inward of the base, there appears on the lateral surface a rounded vertical ridge, which is best marked above and which, as it descends, flattens out and approaches the caudal border, which it meets about the level of the upper border of the mental foramen. Upon the medial surface there is a corresponding groove, seen in figures 33 and 35, as the upward continuation of the inferior alveolar groove. In it, as seen in figures 31 and 34, a portion of Meckel's cartilage lies, though the cartilage and bone are separated by a substantial interval, most marked above, where the groove is deepest; indeed, in the space between the condyle and Meckel's cartilage the auriculo-temporal nerve is found and it occupies only a small part of the available space. In front of the ridge upon the lateral surface there is a vertical groove, best marked above, whose cranial side passes forward upon the cranial border of the bone. A corresponding ridge occurs upon the inner surface. "This bending of the plate gives to cross-sections of it an s-shaped appearance; indeed, such a shape is perceived by looking directly at the upper end of the bone.
The mental foramen is much larger relatively than that of the adult bone. It is roughly quadrilateral with the long axis dorsoventral. Its anterior, posterior, and upper boundaries are free and made of narrow, bony plates; its ventral border, on the contrary, is formed by the angle made by the junction of the medial alveolar wall with the lateral sm-face. It is a low ridge showing a sharp spike of bone at its cranial end. When the foramen is looked at du-ectly from the side the medial alveolar wall obstructs the view completely. The plate of bone forming the dorsocranial boundary of this foramen is thin and narrow. In front and behind, the lower part of the foramen opens into the V-shaped lower 'art of the inferior alveolar groove.
The surface of the bone immediately below the foramen is somewhat depressed, and there is a distinct groove passing downward from the lowest part of the opening to the ventral margin of the bone. This groove serves as the anterior delimitation of a small eminence of bone whose position justifies the conclusion that it represents the mental tubercle. It is situated some distance below the mental foramen and forms a projection upon the lower border of the bone. Upon the corresponding inner surface there is a small depression. The lateral sm'face, in the region of the mental foramen and below it, is rather rough.
Turning now to a consideration of the medial wall, we note as its most prominent characteristic the plate of bone forming the medial wall of the inferior alveolar gi'oove. This, the so-called splenial element, is highest in the region of the mental foramen. In front of this it becomes much thicker, and progressivelj' lower, passing forward and inward to terminate as a thin spur of bone, with an extremity turned a little upward. Behind the foramen, too, the ridge decreases graduallj' in height and extends backwards rather more than two-thirds of the distance from the anterior tip of the bone to the angle, as shown in figures 33 and 35. The upper margin is much serrated and lies about on the same level as the lateral wall of the inferior alveolar groove, which also shows a very jagged edge. A little in front of the mental foramen there is a narrow bridge of bone joining the medial and lateral alveolar walls, which covers the incisive nerve, as shown in figm-e 34. The upper border of the medial alveolar wall, barring the irregularities mentioned, has a diiection in general straight, with the exception of its caudal extremity, which turns upward a little. On the other hand, the attachment of the plate to the main portion of the mandible follows a curved hne, with concavity upward and forward. This line crosses almost the entire bone, beginning in front not far from the anterior extremity of the mandible, and continuing backward with an increasingly strong inchnation upward, almost to the posterior border of the bone. This accounts for the varying depth of the inferior alveolar sulcus— deep in the region of the mental foramen, and decreasing in front and behind — following the variation in height of the inner wall. WTiere the wall ends dorsocaudallj' the alveolar groove is continued as the groove formed by the inturning of the base of the bone, as far as the condyloid process (figs. 33 and 35).
Below the medial alveolar wall there is a region presenting a marked inward concavity This is open behind, due to the fact that the lower border does not tm-n inward here, but rather projects outward to form a sahent upon the lateral aspect. In this concavity rests the lower end of Meckel's cartilage (figs. 31 and 34). In the depths of this concavity there is an area, shown in figures 33 and 35, where the bone and Meckel's
cartilage are in contact; indeed, the appearance is as though the perichondrium were becoming ossified here. The cartilage, too, in this region (fig. 32) shows evidence of calcification and is evidently undergoing the change preliminary to ossification. Similar findings were described by Low and were also reported in mj' former paper. The area marked involves the region of confluence of the medial alveolar wall with the main plate and extends a short distance downward from this.
In connection with the medial surface, the relation of Meckel's cartilage should be described . We have noted that there is a short portion of the rod which hes between the uppermost extremity of the mandible and the head of the malleus. Opposite the mandible it hes, as figures 31 to 35 show, in the groove which has been described. As it descends it comes to he medial to the upper end of the medial alveolar wall and in close contact with this. It crosses the upper border of this wall at an angle, its cranial border faUing somewhat behind and below the edge of the bone, as figures 31 and 34 show. From the angle of the mandible to its lower extremity the posterior edge is turned inward partially to envelop the cartilage, except in the region alreadj- described.
The position of the inferior alveolar nerve, as shown in figure 34, is interesting, as marking the position of the future canal. A short distance behind the coronoid process and below the mandibular notch, as shown in the figure, the nerve comes to he quite close to the bone. At this point it hes just behind the ridge which has been mentioned and which occurs as a groove upon the outer surface. This region doubtless represents the site of the future mandibular foramen. The nerve, from this point, courses fairly close to the bone, crossing the ridge obhquely from behind forward, to divide, a little before the mental foramen is reached, into the mental and incisive branches. These nerves appear in figure 34, the former passing through the upper extremity of the mental foramen, the latter continuing downward, in the depths of the alveolar groove, to the tip of Meckel's cartilage.
The remainder of the mandibular nerve, in so far as it relates to the mandible, may be briefly described. The stout trunk seen in figures 31 and 34 shows, springing from almost the same region, the auriculo-temporal, masseter, lingual, and inferior alveolar branches. The auriculo-temporal, showing two strands, winds backward and outward, around Meckel's cartilage, and between it and the condyloid process, to emerge upon the face. The masseter has been referred to as passing over the representative of the mandibular notch, some distance below and medial to the zygomatic process of the squama temporalis. The lingual is shown in the figures descending to the medial side of Meckel's cartilage, and being joined by the chorda tympani. The borders of the mandible may be briefly suimnarized. Beginning above at the sharply marked condjdoid process, and passing forward and downward, we traverse the mandibular notch shallow when compared with that of the adult condition. From this the thin cranial edge of the bone is passed over. It is fairly smooth. Reaching the region of the mental foramen, the border becomes much more irregular, showing deviations from side to side and from above downward. There is a large notch just in front of the lower part of the foramen. This border terminates upon a sharp point projecting medially. Beginning again at the condyloid process, but proceeding backward, we pass downward over the dorsal border of the ramus. Between the condyloid process ahd the angle there is a strong outward convexity. From the angle downward the border becomes thicker and is somewhat roughened. As has been mentioned, it is tui'ned inward, so as to present a sahent edge when viewed from within (figs. 33 and 35). In la there was a very thin fissure visible upon the lateral surface which partially cut off this edge from the main plate. This fissure is only suggested in this specimen, as a very faint incutting of the bone, parallel to the caudal border and visible from without.
The turning inward of the base of the mandible does not occur below the level of the upper border of the mental foramen; on the contrary, when the bone is regarded from behind, it is seen that there is a sweeping outward of the border at and below this level, to form an outward projection. The curvature thus described marks the caudal lunit of the concave area below the medial alveolar wall, already described. The border, continued downward and forward, passes over upon the symphysial portion, which shows fine serrations and gradually approaches its partner of the opposite side. This border ends, like the cranial, upon the ventrocranial tip of the bone.
The condensed mesenchyme of the mandible follows, in general, the outlines of the bone. It is confluent with its partner of the opposite side at the symphysis by a wide area, as shown in figure 36. The groove occupied by Meckel's cartilage is rather more conspicuous and, of course, there is a general increase in thickness of the mass.
The developing teeth, seen in figure 36, occupy the tooth gutter but do not extend as far back as the medial alveolar wall. As the figm-e shows, there are two main masses, connected by a narrow isthmus. This narrow point is just in front of the upper extremity of the mental foramen, at a point where we have noted the occurrence of a rod of bone joining the medial and lateral alveolar walls. The mesenchyme here is, of course, of greater volume than the inclosed bridge.
The nasal bone has not yet formed.
Summary
I. Chondrocranium
General development. — The chondrocranium of No. 886 is well developed and is practically a homogeneous mass of cartilage, with but little trace of the regions of earlier separation of the parts. Almost all of it is represented.
Type of cartilage. — The cartilage is almost all of mature type. Young cartilage and precartilage are found principally at the anterior end, which is undergoing the most rapid development.
Evidences of preossification change.~ln all, ten centers were found where the cartilage showed the change which ushers in ossification. Of these, four were paired and two unpaired. In none was actual bone formed. At the peripheries of these centers there was a gradual transition of the modified cartilage into the surrounding normal cartilage. The areas of these centers have been carefully indicated in the figures and have been described. They may be briefly recapitulated:
In the occipital region there are: (1) a single center for the basi-occipital; (2) paired centers for the exoccipitals; (3) a single large center for the supraoccipital.
In the orbito-temporal region there are: (1) paired centers in the temporal wings; (2) paired centers in the alar processes, these being very slightly developed.
The remaining paired centers are found one in each lower end of JNIeckel's cartilage.
Central stem.— The angle made by the chordal and prechordal parts of the central stem is 115°, as in la. It has become narrower since the 21 mm. stage of Lewis, where it was 125.
Orbital wings. — That the outer ends of the orbital wings become depressed, thus widening the angle made by the hnes joining the Umbus sphenoidaUs with the lateral extremities of the wings, following the stage of No. 886, is evident from a comparison of this angle in No. 886 with the corresponding angle in the adult skull. This flattening of the floor of the anterior cranial fossa is probably associated with the growth of the brain.
Comparison of Chondrocranium of No. 886 With That of 1a
The chondrocranium of No. 886 is considerably less developed than that of la and much of the cartilage is of a younger type. The points of contrast may be summarized as follows :
(a) Parts absent from No. 886 and -present in la.
- Supracochlear cartilages.
- Superior paraseptal cartilages.
- Lateral cranial cartilages.
- Posterior cranial cartilages.
(b) Parts -present in No. 886 and absent from 1a.
- Alicochlear commissure. It was represented in 1a by a process. The carotid foramen was thus closed in No. 886 and open in la. From the alicochlear commissure is developed the lingula.
(c) Parts showing a difference in No. 886 as compared with la.
- Styloid process. It is attached by cartilage to the otic capsule in No. 886, and is separate in la.
- Mastoid process. It is a free nodule in No. 886 and was attached to the otic capsule by cartilage in la.
- Paraethmoidal cartilage. It is attached to the ectethmoid by cartilage in No. 886 and is separated by membrane in la. It is very rudimentary on the right side.
- Process of the middle meatus. This may represent the uncinate process. It is altogether precartilaginous in No. 886 and is long and slender. In la it is a nodule of very young cartilage with a pedicle of precartilage attaching it to the ectethmoid.
- Paranasal process. It was a separate cartilaginous nodule in la.
- Ala hypochiasmatica. Less developed in No. 886 than in la.
- Prechiasmatic commissures. In No. 886 they are of precartilage and are very slender; in la they are cartilaginous and much stouter.
- Prechiasmatic foramina. They are relatively larger in No. 886 than in 7a. They were found in the skull of a young human adult.
(d) Parts found in No. 886 and not described in la.
- Cupular process. In No. 886 it is long, slender, and of precartilage. It partially encircles the epithelial plug in the anterior naris. Only a short projection of cartilage was described in la. This process develops into the medial crus of the greater alar cartilage (Peter, 1913). It is of interest that the process is so fully developed in precartilage at this stage.
- Processus ascendens is represented by a spheroidal nodule attached to the upper border of the supraoccipital cartilage in No. 886. The corresponding region of la is missing.
- Processus descendens is plainly marked in No. 886 and is not distinct in la.
- Posterior petrosal process is represented in both skulls.
- Anterior intraperilymphatic process. It is a very small projection.
II. Additional Cartilages
- Parasphenoidal cartilage, or hamular process, is present and intimately associated with the medial, pterygoid plate.
- Branchial-arch skeleton. The following cartilages have been described: Meckel's and Reichert's, with ossicle fundaments; hyoid; thyroid; arytenoid; cricoid; upper tracheal.
- Cervical vertebrae. They show arch tips widely separated; those of the atlas are the same distance apart as those of the occipital vertebra.
- Paraseptal cartilages and ventrolateral processes are rudimentary in comparison with those of la.
III. Membrane Bones
All of the membrane bones, with the exception of the nasal, are represented. Some of them are very small, as the medial pterygoid plate, the parietal, and particularly the interparietal, lacrimal, tympanic, and goniale. The palatine surface of the maxilla shows a groove indicating the line of earlier complete separation of the maxillary and premaxillary elements.
IV. Non-Skeletal Parts
The following structures which are intimately related to the developing skull have been figured and described :
- Notochord. It follows a typical course and perforates the anterior end of the preossification center for the basioccipital,
- The outlines of the external form, brain, frontal, and parietal bones, and the chondrocranium, as seen from the right s'"de, have been drawn by profile reconstruction, to show their common relationships.
- Mucous membrane of the nose, showing relation to the nasal septum and vomeronasal organ and to the developing conchae.
- The vomeronasal organ is plainly indicated and is situated one upon either side of the septum. It is considerably above the paraseptal cartilages.
- Nasolacrimal duct system. The lower end, not yet connected with the nasal cavity, is situated some distance behind the position which the corresponding structure occupies in the lower forms and its migration backward may be connected with the solution of continuity of the cartilaginous anterior transverse lamina.
- The following gangUa have been mentioned, and some of them have been figured: semilunar, geniculate, sphenopalatine, cochlear, vestibular, jugular, and petrous of glossopharyngeal, jugular and nodosum of vagus, accessory.
- The following nerves have been noticed and most of them have been figured: trigeminal and branches, facial, chorda tympani, great superficial petrosal, nerve of the pterygoid canal, glossopharyngeal, vagus, accessory, hypoglossal.
- Auditory tube and tympanic cavity are shown in their relation to the otic capsule and ossicle primordia.
- Internal carotid artery is shown in relation to the otic capsule, carotid foramen, and alar process of the developing sphenoid. Upon the alar process it lies in the representative of the carotid sulcus.
- Membranous labyrinth and endolymphatic duct and sac. The slender extension from the last mentioned lies medial to the anterior end of the occipitoparietal groove.
- Stapedius muscle and tendon are shown in relation to the styloid process, facial nerve, and surrounding structures.
In conclusion, I wish to express my thanks to Dr. G. L. Streeter, Director of the Carnegie Laboratory of Embryology, for the faciUties of that Department, which were freely placed at my disposal.
Explanation of Plates
All drawings were made by Mr. James F. Didusch according to geometric projection. With the exception of figure 7, which was made from a profile reconstruction, all figures were drawn from the original plaster-of-paris models made from human fetus No. 886 of the collection of the Carnegie Laboratory of Embryology. The number of the model from which each figure was drawn is given, together with the magnification.
Note - the magnifications refer to the original print versions, not the online images.
Color Scheme Of Plates
In general, blue is used to indicate cartilage and precartilage, yellow to indicate bone, and green for beginning ossification centers. Cut edges are white. The chief departures from this scheme are as follows:
Plate 2. — Figure 7, brain green; figure 8, mesenchyme green; figure 11, mucous membrane of pharynx yellow; figures 12 and 13, paraseptal cartilage and precartilage green.
Plate 3.— Figures 15 and 16, mucous membrane of auditory tube yellow, nerves and ganglia green. Figures 17, 20, and 23, young cartilage green; figures 18 and 21, cavity of otic capsule green; figures 19 and 22, membranous labyrinth yellow.
Plate 4- — Figures 28, mesenchyme green; figures 30 and 36, developing teeth yellow, mesenchyme green; figures 33 and 3.5, ossifying perichondrium green.
Plate 5. — Figures 37, 38, 40, 42, and 44, mucous membrane yellow, precartilage green; in figure 37, epithehum of Jacobson's organ green; in figures 38 and 40, mesenchyme white; in figure 41, teeth yellow, precartilage green, mesenchyme and epithelium of nasolacrimal duct white. Figures 43, 45, 46, and 47, precartilage green.
Virtual Slides
|
|
| |||||||||
|
|
Plate 1
Note - the magnifications refer to the original print versions, not the online images.
Fig. 1. Chondrocranium from above with frontal and parietal bones on right side. The densest part of the frontal bone is inclosed by a dotted line. The basal plate is not quite horizontal, the cranial end being a little the closer to the eye of the observer. Model 1. X6.25.
Fig, 2. Chrondrocranium from below with cartilaginous branchial arch skeleton extirpated. Frontal and parietal bones are shown on right side. The basal plate is not quite horizontal, the caudal end being a little the closer to the eye of the observer. The view is directly into the anterior nares. Model 1. X6.25.
Fig. 3. Skull from front, showing membrane bones on right side. Face is seen in frank view. The cervical vertebrae and cartilaginous branchial arch skeleton are also seen. Model 1. X6.25.
Fig. 4. Skull from back, giving a frank view of the foramen oocipitale magnum. The cervical vertebrtae are seen, their arches beiag as yet unclosed dorsally. Note the alignment of the hemiarch tips with the dorsal foraminal prominences, representing the extremities of the hemiarches of the occipital vertebra. The right half of the interparietal bone is seen. Model 1. X6.25.
Plate 2
Fig. 5. Skull from right side, showing membrane bones. The cervical vertebrae and cartilaginous branchial arch skeleton are included. Only the right half of the skull is shown. Model 1. X6.25.
Fig. 6. Left half of chondrocranium, cervical vertebrae, and cartilaginous branchial arch skeleton as seen from left side. Model 1. X6.25.
Fig. 7. Profiles of external form of head, brain and upper end of spinal cord, and skull, in their normal relation to one another, as seen from the right side. Drawn from a profile reconstruction. Xl-9.
Fig. 8. Condensed mesenchyme enveloping the vomer, seen from front, side, and above. The anterior extremities of the vomer are indicated. The gutter in the center is for the lower edge of the nasal septum. There is a slight amount of lateral curvature. The cut edges of the mesenchyme are indicated. Model 22. X12.5.
Fig. 9. Two halves of the vomer from the same point of view as that of figure 8. They are very slender spicules of bone lying along the lower border of the nasal septum. Model 21. X12.5.
Fig. 10. Median stem of skull as seen from right side. It consists of the basal plate behind and the interorbital and nasal septa in front, forming ai- "btuse angle at the body of the sphenoid. The adjoining exoccipital cartilage is shown in part. Junctions with cartilage lying laterally are shown. Model 4. X12.5.
Fig. 11. Right half of basal plate and parts of upper two cervical vertebrae, sectioned in the mid-sagittal plane. The cut surface is seen in frank view. Shows the preossification center for the basioccipital, the notochord, the pharyngeal bursa with a little of the epithelium of the roof of the pharynx, the temporal wing, dorsum seUae and a portion of the exoccipital. Model 8. X12.5.
Fig. 12. Left cartilage of Jacobson from left side with neighboring septum. Models 2 and 25. Xl2,5.
Fig. 13. CartUages of Jacobson from below in relation to nasal septum. Models 2 and 25. X12.5.
Fig. 14. Right half of occipital cartilage and parietal plate from in front and within, with the ascending process and right half of inter-parietal bone in its mesenchyme. Connections with adjoining cartilages are shown. Model 24. X6.25.
Plate 3
Fig. 15. Interior of right otic capsule from within and above. The inner wall of the capsule has been cut away to show the lateral wall of the cavity. Figure 21 shows this cavity modeled as a solid. The superior and posterior semicircular canals are shown, with the entrances to the lateral canal. A good view is afforded of the spiral septum in the wall of the cochlear space. The membrane fiUing in the vestibular window is indicated. Other features are the
medial end of the tuba auditiva with its entrance into the pharynx, the alar process of the temporal wing of the sphenoid, the aUcochlear commissure, the internal carotid artery, and views of the fifth and seventh cranial nerves. Model 14. XlO.
Fig. 16. Right otic capsule and associated structures seen from right side, front, and a little below. The suprafacial commissure has been removed. The facial nerve is shown in its relation to the otic capsule and styloid process, with its off-shoots, the chorda tympani and the great superficial petrosal nerves, the latter arising from the geniculate gangUon. The tip of the long crus of the incus appears in relation to the chorda tympani. A prominent object is the tympanic cavity fundament, of which the lateral surface is shown, presenting an impression at the site of the future tympanic membrane. The auditory tube is shown in its full extent. The immense semilunar ganglion with its root and branches, the internal carotidartery, and the processus alaris of the temporal wing of the sphenoid are seen. Model 14. XlO.
Fig. 17. Right otic capsule, medial surface, frank view, showing connections with adjoining cartilages and openings toward the cranial cavity. Model 5. XlO.
Fig. I8. The space within the right otic capsule seen from within, modeled as a solid; the surface presented fits into the cavity shown in figure 23. Openings toward the cranial cavity are shown. Note the large volume of this cavity in comparison with that of the membranous labyrinth wliich fills it (fig. 19). Compare also figures 21 and 22 in this respect. Model 6. XlO.
Fig. 19. Membranous labyrinth of right otic capsule contained within the space shown in figure 18. Figures 17, 18, and 19 were all drawn from approximately the same point of view, so that an accurate idea may be gained of the space contained within the otic capsule and the membranous labyrinth within that. Model 7. XlO.
Fig. 20. Right otic capsule, lateral surface, frank view, with openings looking outward. The attachments of the cartilage of Reichert and of the short process of the incus are seen. Model 5. XlO.
Fig. 21. The space within the right otic capsule, seen from without, modeled as a sohd; the openings are indicated. Model 6. XlO.
Fig. 22. Membranous labyrinth of right otic capsule contained within the space shown in figure 21. Figures 20, 21, and 22 were all drawn from approximately the same point of view. Model 7. XlO.
Fig. 23. Medial wall of right otic capsule, seen from without, the lateral wall having been cut away. The cutting of the capsular wall was not done in quite the same way as in the model shown in figure 15, so that the cut edges do not fit together exactly. The inner wall of the space is seen with the endolymphatic and internal acoustic foramina. Model 26. XlO.
Fig. 24. View from front of mass of cartilage (massa angularis) partially inclosed by the semi- circular canals of the right otic capsule. Above is seen the superior canal leading into the space for its ampulla and, farther downward and to the right, into that for the utriculus. To the left is the space for the ampulla of the lateral canal and, farther back, the lateral semicircular canal appears. Model 15. XlO.
Fig 25. View of same mass of cartilage shown in figure 24, but seen from below. The lateral semicircular canal is conspicuous to the left, passing above into an enlargement for the ampulla of this canal and for the utriculus, with the beginning of the superior canal above. Below, the lateral canal passes medially into an erdargement for the inferior extremity of the posterior canal and for the crus commune. Model 15. XlO. Views of the angular mass from other aspects are seen in other figures, as from without in figures 20, 5, 6, and from within infigure 15.
Plate 4
Fig. 26. Right maxilla, palate, medial pterygoid plate with hamular process, temporal wing, sphenopalatine ganglion, and associated nerves, seen directly from within. Cut surface showing junction of temporal wing with alar process is seen. Note the incisive suture partially separating the maxilla and premaxilla. Model 11. X12.5.
Fig. 27. The same structures as those seen in figure 26 with the exception of the medial pterygoid plate, seen from above. The relations of the maxillary division of the trigeminal nerve and its branches to the different structures are shown. Model 11. X12.5.
Fig. 28. Lateral aspect of the right palate bone and medial pterygoid plate, with their investment of condensed mesenchyme. Model 18. X12.5.
Fig. 29. The same structures seen in figures 26 and 27, but viewed from below. The tooth gutter of the maxilla and premaxilla is conspicuous and the lower end of the incisive suture appears. The zygomatic bone also is shown. Compare with figure 30. Model 11. X12.5.
Fig. 30. Condensed mesenchyme enveloping the right maxilla, palate, and medial pterygoid plate. The developing teeth are seen in their gutter in the maxilla. (Compare with figure 29 drawn from approximately the same point of view.) The cartilaginous hamular process is seen projecting from the medial pterygoid lamina. Compare also with figure 36, forbdeveloping teeth of the right lower jaw. Model 25. X12.5.
Fig. 31. Frank view of the right mandible, Meckel's cartilage, and associated structures, seen from within. The cartilaginous precursors of the auditory ossicles are seen above, in relation to the facial and chorda tympani nerves. A ghmpse of the squama temporalis is given and also of the goniale. Note the relations of the mandibular division of the trigeminal nerve. Model 9. X12.5.
Fig. 32. Lateral aspect of lower end of the right Meckel's cartilage, showing especially the area applied closely to the mandible, where the cartilage is showing the changes preliminary to ossification. (Compare with figures 33 and 35.) Model 16. X12.5.
Fig. 33. Right mandible from same viewpoint as in figure 31, showing the tooth gutter and area of close apposition to the lower end of Meckel's cartilage. Model 10. X12.5.
Fig. 34. The same structures as those shown in figure 31, but the model was rotated somewhat medially around its long axis. While presenting all the structures from a new angle, it shows especially the relation of the nerves to the mandible. Model 9. X12.5.
Fig. 35. View of right mandible with the model in the same position as that shown in figure 34; it is rotated so as to afford a good view of the tooth gutter. Model 10. X12.5.
Fig. 36. Condensed mesenchyme around right mandible viewed from approximately the same point as that of figure 35. It shows the developing teeth of the right lower jaw. The mesenchyme is connected across the midhne with its partner of the opposite side, the cut edge being shown. Model 23. X12.5.
Plate 5
Fig. 37. Mucous membrane of inner wall of right nasal cavity, overlying the septum; it is cut away to show the right organ of Jacobson. (Compare with figure 10.) Model 20. X12.5.
Fig 38. Mucous membrane of lateral wall of right nasal cavity, overljing the right ectethmoid, showing folds for the developing conchce. The mucous membrane fits over the structures seen in figure 41. The elongated nasopharyngeal canal, flanked by the developing palate and medial pterygoid plate, is well seen. Model IS. X12.5.
Fig. 39. Lateral aspect of right ectethmoid from the front, side, and a little below, showing especially the nasolacrimal duct, with the nasolacrimal sac and the lacrimal ducts above, and, below,the expanded end, applied to but not perforating the external aspect of the mucous membrane of the inferior meatus. The tip of the paranasal cartilage hes just lateral to the duct and the tiny shred of osseous tissue representing the lacrimal bone is seen lying along the posterior maxillary process. The cupular process of precartilage is conspicuous in the lower part of the figure. The broad plate of epitheUum, which represents the future inferior meatus, but which has not yet undergone cleavage except posteriorly, is plainly shown. Model 18. X12.5.
Fig. 40. Anterior end of right ectethmoid, with the epithelial plug in the anterior naris, embraced medially by the cupular process of precartilage. The terminal portion of the nasolacrimal duct is shown, entering the space for the inferior meatus, with a small portion of the mesenchyme of the maxilla. Model 19. X12.5.
Fig. 41. Medial aspect of right ectethmoid, showing the developing concha. Precartilage is especially evident in the superior concha, the small process of the middle meatus, and the cupular process. The other conchce are edged with it. Themesenchyme envelopes of the maxilla, the palate, and the medial pterygoid plate are seen. The cartilaginous hamular process is conspicuous, as are also the developing teeth of the right side of the upper jaw. (Compare figure 30.)
Note also the tip of the nasolacrimal duct in the space for the inferior meatus. Model 25. X12.5.
Fig. 42. View of right hyoid arch from without, below, and behind. The connection with the otic capsule is seen above and below appears the lesser cornu of the hyoid cartilage. The relations of the facial nerve, chorda tympani, and tympanic cavity are well seen, and the handle of the malleus is plainly shown in a concavity representing the stratum mucosum of the future tympanic membrane. Model 17. X12.5.
Fig. 43 Medial aspect of left ectethmoid (compare with figure 41), showing developing conchse. The anterior portion of the tectum nasi has been trimmed a little farther laterally than on the right side, and hence the cut surface is not quite the same in the two figures. The cupular process is omitted. Model 3. X12.5.
Fig. 44. View of right hyoid arch from within and slightly above, with its membranous connection with the lesser cornu of the hyoid below (as in fig. 42) and the cut edge of its connection with otic capsule above. Fitting into the curvature of its upper portion is the epithelium of the developing tympanic cavity, which from this point of view is almost parallel with the plane of the paper. The ring-like stapes is seen and to it is attached the tendon of the stapedius muscle, with the muscle itself passing medial to the facial nerve and to the upper end of the styloid process. The handle of the malleus is also seen, with the chorda tympani lying just medial to it. Model 17. X12.5.
Fig. 45. Cartilages of the hyoid, thyroid, cricoid, and upper end of the trachea, seen from the front. On the left side of the model the precartilaginous edging of the upper three cartilages is shown. Model 12. X12.5.
Fig. 46. Right half of the same structures seen in figures 45 and 47, viewed from within. The precartilage of the arj'tenoid is seen and should be compared with the young cartilage of the same structure shown in figure 47. Model 13. X12.5.
Fig. 47. Same structures seen in figure 45, but viewed from behind. The asymmetry evident in the arytenoids and in other places is due to the fact that on the left side of the model the precartilage was shown, whereas on the right side only the cartilage and young cartilage appear. Model 12. xl2.5.
Bibliography
Bolk, L. 1904. Entwioklungavorgange in der occipitalen Region des Primordial-Craniums beim Menschen. Petrus Camper, Bd. 2, S. 315-327.
Fawcett, E. 1910a. Notes on the development of the human sphenoid. Jour. Anat. and Phys., vol. 44, p. 207-222.
. 19106. Description of a reconstruction of the head
of a thirtv-miUimetre embryo. Jour. Anat. and Phys.. voi. 44, p. 303-311.
. 191Sa. The primordial cranium of Brinofeus europa-US. Jour. Anat. and Phys., vol. 52, 2d part, p. 211-250.
. 19185. The primordial cranium of Pacilophoca
uedddli (Weddell's seal) at the 27 mm. CR. length. Jour. Anat. and Phys., vol. 52, p. 412441.
. 1919. The primordial cranium of Miniopterus schreibcTsi at the 17 millimetre total length stage. Jour. Anat., vol. 53, part 4, p. 315-348.
Felber, Pavl. 1919. Anlageund Entwicklungdes Maxillare und Praemaxillare beim Men.schen. Gegenbaurs Morphologisches Jahrbuch, Bd. 50, S. 451— 499.
Gaupp, E. 1900. Das Chondrocranium von Lacerla agilis. Ein Beitrag zum Verstandnis des Amniotenschadels. Anat. Hefte, Bd. 15, S. 433-595.
HvBER, G. C. 1912. On the relation of the chorda dorsalis to the anlage of the pharyngeal bursa or median pharj-ngeal recess. Anat. Record, vol. 6, p. 373-404.
Jacobt, M. 1895. Ein Beitrag zur Kenntniss dea menschlichen Primordialcraniums. Arch. f. mikr. Anat., Bd. 44. S. 61-86.
Kernan, J. D., Jr. 1916. The chondrocranium of a 20 mm. human embryo. Jour. Morph., vol. 27, p. 605-645.
Levi, G. 1900. Beitrag zum Studium der Entwickelung dea knorpeligen Primordialcraniuma des Menschen. Arch. f. mikr. Anat, Bd. 55, S.341-414.
Lewis, W. H. 1915. The use of guide planes and plaster of Paris for reconstructions from Serial sections; some points on reconstruction. Anat. Record, vol. 9, p. 719-729.
. 1920. The cartilaginous skull of a human embryo, twenty-one millimeters in length. Contributions to Embryology, vol. 9, Carnegie Inst. Wash. Pub. No. 272, p. 301-324.
Macklin, C. C. 1914. The skull of a human fetua of 40 mm. Amer. Jour. Anat., vol. 16. p. 317-426.
Mead, C. S. 1909. The chondrocranium of an embryo pig, Sus scTofa. A contribution to the morphology of the mammalian skull. Amer. Jour. Anat., vol. 9, p. 167-210.
Peter, K. 1913. Atlas der Entwicklung der Nase und des Gaumens beim Menschen, mit Einschluss der Entwicklungsstorungen. Jena, Gustav Fischer.
Rehmkb, Gr. 1913. Die Entwicklung der Knorpel der ausseren Nase. Noeh unveroffentUcht. Referred to by Peter (1913).
Rice, E. L. 1920. The Development of the skull in the skink, Eutneces quinquelineatus L. I. The Chondrocranium. Jour. Morph., vol. 34, p. 119-243.
Schultz. A. H. 1920. Abstract in American Journal of Physical Anthropology, vol. III. No. 2, AprilJune, 1920, upon an article by P. Felber. (See Felber 1919).
Streeter, G. L. 1918. The developmental alterations in the vascular system of the brain of the human embryo. Carnegie Inst. Wash. Pub. No. 271, Contribution to Embryology No. 24, vol. 8, p. 5-38.
Terry, R. J. 1917. The primordial cranium of the cat. Jour. Morph., vol. 29, p. 281-433.
Thompson, P. 1907. Osteology, Morris's Human Anatomy, 4th ed., Philadelphia. P. Blakiston's Son &Co.
Voit, M. 1909. Das Primordialcranium des Kaninchens unter Berilcksichtigung der Deckknochen. Ein Beitrag zur Morphologie des Saugetierschadels. Anat. Hefte, vol. 38, p. 425-616.
Abbreviations
ala hypoch.
ala orb. ala tem. am. can. lat. am. cau. post, am. can. sup. am. mem. lat. am. mem. post. am. mem. sup. ang.
arc. neur. occ. art. car. int.
bur. phar.
can. coc.
can. lat.
can. n. hypo.
can. post.
can. sing.
can. sup.
cips ot.
cart. ary.
cart, basiocc.
cart, basisphen.
cart. eric.
cart. hy.
cart. Meek.
cart, parasep.
cart, parasep. lat.
cart, parasep. med.
cart, parasph.
cart. sph. eth.
cart, supraocc.
cart. thy.
cart, trach.
cav. tym.
ch. dor.
ch. ty.
com. alicoc.
com. basicoc.
com. caps. occ.
com. prsech.
com. sph. eth.
com. sph. par.
com. suprafac.
cone. inf.
cone. med.
cone. sup.
cond. occ.
cor. maj.
cor. min.
cris. arc. occ.
cris. gal.
cris. par.
cris. trans.
crua com.
crus long.
cu. al. hy.
cu. caps. ot.
cu. cart, parasep.
cu. com. caps. occ.
cu. com. caps. par.
cu. com. praech.
cu. com. suprafac.
cu. ect. eth.
cu. inc.
cu. proc. alar.
ala hypochiasmatica ala orbitalis ala temporalis
ampulla canalis semicircularis lateralis ampulla canalis semicircularis posterior ampulla canalis semicircularis superior ampulla membranacea lateralis ampulla membranacea posterior ampulla membranacea superior angulus
area in close apposition to the beginning ossification center in Meckel's cartilage arcus neuralis vertebrae occipitalis arteria carotis interna
bursa pharj'ngea
canalis spiralis cochlese
canalis semicircularis lateralis
canalis nervi hypoglossi
canalis semicircularis posterior
canalis singularis
canalis semicircularis superior
capsula otica
cartilago arytsenoidea
cartilage basioccipitalis
cartilago basisphenoidalis
cartilago cricoidea
cartilago hyoidea
cartilago Meckeli
cartilago paraseptalis
cartilago paraseptalis lateralis
cartilago paraseptalis medialis
cartilago parasphenoidalis
cartilago sphenoethmoidalis
cartilago supraoccipitalis
cartilago thyreoidea
cartilagines tracheales
cavum tympani
chorda dorsalis
chorda tympani
commissura alicocWearis
commissura basicochlearis
commissura capsulo-occipitalis
commissura prschiasmatica
commissura sphenoethmoidalis
commissura sphenoparietalis
commissura suprafacialis
concha nasalis inferior
concha nasalis media
concha nasalis superior
condylus occipitalis
cornu m.ijus
cornu minus
crista arcuata occipitalis
crista galU
crista parotica
crista transversa
crus commune
crus longum
cut edge of ala hypochiasmatica
cut edge of connection with otic capsule
cut edge of connection with paraseptal
cartilage cut edge of capsulooccipital commissure cut edge of capsuloparietal commissure cut edge of prechiasmatic commissure cut edge of suprafacial commissure cut edge of connection with ectethmoid cut edge of connection with incus cut edge of connection with alar process
cu. proc. cup. ant. cut edge of root of anterior cupular process
cu. proc. sty. cut edge of connection with processus styloideus
cu. rad. metop. cut edge of radix metopticus of ala orbitalis
cu. rad. prseop. cut edge of radix prajopticus of ala orbitalis
cu. sep. nasi. cut edge of connection with nasal septum
cu. tec. post. cut edge of tectum posteriua
den. dentes
dor. sel. dorsum sellse
duct. coc. ductus cochlearis
duct, endolym. ductus endolymphaticus
duct. lac. ductus lacrimale
duct. lat. ductus semicircularis lateralis
duct. nas. lac. ductus nasolacrimalis
duct. post. ductus semicircularis posterior
duct. reun. ductus reuniens
duct. sup. ductus semicircularis superior
em. occ. lat. eminentia occipitalis lateralis
epis. epistropheus
epith. pi. epithelial plug in anterior naris
fen. crib. fenestra oribrosa
fen. perilym. fenestra perilymphatica
fen. vest. fenestra vestibuli
fis. orb. nas. fissura orbitonasalis
fis. sph. par. fissura sphenoparietalis
for. caps. occ. foramen capsulooccipitale
for. caps. par. foramen capsuloparietale
for. car. foramen caroticum
for. coc. foramen cochleare
for. endolym. foramen endolymphaticum
for. epiph. foramen epiphaniale
for. fac. foramen faciale
for. jug. foramen jugulare
for. men. foramen mentals
for. occ. mag. foramen occipitale magnum
for. optic. foramen opticum
for. paracond. foramen paracondyloideum
for. prsech. foramen pr.nechiasmaticum
for. rot. foramen rotundum
for. vest. inf. foramen vestibulare inferior
for. vest. sup. foramen vestibulare superior
fos. cond. fossa condyloidea
fos. hypoph. fossa hypophyseos
fos. mal. fossa manubrii mallei
fos. subar. fossa subarcuata
fron. OS frontale
gang, genie. ganglion geniculi
gang, semilun. ganglion serailunare
gang. sph. pal. ganglion sphenopalatinum
gon. goniale
ham. ptery. hamulus pterygoidcus
imp. sep. spir. impression for the spiral septum
inc. incus
inc. cond. post. incisura condyloidea posterior
inc. intercond. incisura intercondyloidea
nc. occ. par. incisura occipitoparietalis
inc. occ. sup. incisura occipitalis superior
incis. OS incisivum
nterpar. os interparietale
ac. 03 lacrimale
am. med. pteryg. lamina medialis processus pterygoidei
am. par. lamina parietalis
tn. stap.
mat.
man.
manub.
mas. ang.
max.
meat. ac. int.
mem. muc.
me9. incis.
mea. interpar.
mes. man. mes. max. mes. pal. mea. vom.
n. alv. inf.
n. aur. tem.
n. fac.
n. infraorb.
n. ling.
n. man.
n. max.
n. men.
n. ophth.
n. pet. euperfio.
orb.
org. vom. nas.
OS. basiocc.
OS. exocc.
OS. Meek.
08. supraocc.
pal. par.
praecart. ary. proc. alar, proc. alar. sup. proc. art. sup. proc. asc. proc. cond. proc. coron.
musculua stapedius
malleus
mandibula
manubrium mallei
massa angularis
maxilla
meatus acousticus internus
membrana mucosa
condensed mesenchyme of incisive bone
condensed mesenchjTne of interparietal
bone condensed mesenchiTne of mandible condensed mesenchjTne of maxilla condensed mesenchyme of palate bone condensed mesenchyme of vomer
nervus alveolaris inferior
nerv-us auriculotemporalis
ner\'us facialis
nervus infraorbitalis
ner\-U3 lingualis
nervus mandibularis
ner\-u3 maxillaris
Der\'U3 mentalis
nervus ophthalmicus maj. ner%'us petrosus superficialis major
orificium, in the otic capsule, the result of incomplete chondrification
orbita
organon vomeronasale
preossification center of the basioccipital cartilage
preossification center of the exoccipital cartilage
preossification center of Meck'el's cartilage
preossification center for the supraoccipital cartilage
OS palatinum
03 parietale
prsecartilago arytsenoidea
processus alaris
processus alaris superior
processus articularis atlantis superior
processus ascendens
processus condyloideus
processus coronoideus
proc. cup. ant. processus cupuiaris anterior
proc. cup. post. processus cupuiaris posterior
proc. desc. processus descendens
proc. intraper. ant. processus intraperilj-mphaticus anterior
proc. intraper. post, processus intraperilymphaticus posterior
proc. mast. processus mastoideus
proc. max. post. processus maxillaris posterior
proc. meat. med. processus meatus medii
proc. paracond. processus paracondyloideus
proc. paranas. processus paranasalis
proc. parasep. processus paraseptalis
proc. sty. processus styloideus
proc. ven. lat. processus ventrolateralis
proc. zyg. processus zygomaticus
prom. promontorium
prom. am. post. prominentia ampullaris posterior
prom. dor. for. prominentia dorsalis foraminis magni
prom. nas. sup. prominentia nasi superior
radix nervi trigemini
rad. n. trig, ram. incis. rec. utric.
sac.
sac. endoljTn. sac. lac. Sep. interorb. Sep. nasi Sep. spir. sp. ut. sq. tem.
St.
sul. occ. par.
sul. Sep.
sul. suprasep.
sut. inc.
teg. tym.
tub. aud.
tub. jug.
tub. sel.
tun. muc. phar.
tym.
vom.
sacculus
saccus endoljTuphaticus
saccus lacrimalis
septum interorbitale
septum nasi
septum spirale
spatium utriculi
squama temporalis
stapes
sulcus occipitoparietalis
sulcus septalis
sulcus supraseptalis
sutura incisiva
tegmen tympani
tuba auditiva (Eustachii)
tuberculum jugulare
tuberculum sellje
tunica mucosa pharj'ngea
OS t>Tnpanicum
vomer
zyg. OS zygomaticum
3, 4, 5, 6, 7, vertebrae cervicales
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Contributions to Embryology Carnegie Institution No.48. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.48
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G