Book - Contributions to Embryology Carnegie Institution No.21
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
van der Stricht O. The genesis and structure of the membrana tectoria and the crista spiralis of the cochlea. (1918) Contrib. Embryol., Carnegie Inst. Wash., 21: 55-86.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Genesis and Structure of the Membrana Tectoria and the Crista Spiralis of the Cochlea
By O. Van der Stricht with 4 plates (1918)
Introduction
The membrana tectoria belongs to a group of organs produced at the surface of the epithelium and termed superficial cuticles or superficial cuticular formations. Once developed, the generating epithelium persists in its entirety beneath the cuticle or exceptionally may disappear, as in the case of the ameloblasts, which atrophy after forming the enamel at their bases.
One may subdivide these structures into three groups: In the first the process of development can not be doubted or denied. It occurs just within the superficial layer of the cytoplasm and the cuticle produced remains in close contact, even continuity, with the generating cells. Examples are the striated borders of the columnar epithelium of the intestine, of the crypts of Lieberklihn, of the convoluted tubules of the kidney, of the syncytial layer of chorionic villi in the human placenta, and of osteoclasts.
The second is represented by the series of reticulares or fenestrated membranes covering the surface of sensorial epithelia for example, the reticular membrane of the cristae and macula acustica and the organ of Corti, the membrana limitans externa of the retina, the membrana limitans olfactoria. The openings of the membrane are traversed by the apices of sensorial cells, the hairs of the acoustic cells, the rods and cones of the retina, the ciliated vesicles of the olfactory cells. These membranes in adult sensorial organs are in close contact wdth the surface of the epitheUum, but are completely separated from the generating substratum. Hence their origin must be studied -during the embryonic period of their development. Many authors regard them as a real cuticle derived from the free surfaces of the subjacent, that is to say, sustentacular cells. N. Van der Stricht (1908) has demonstrated that the reticular membrane of the acoustic epithelium is formed by a system of terminal bars closing the intercellular spaces between the embryonic epithelial cells. G. Leboucq (1909) proved that the membrana limitans externa of the retina is not formed at all by the Muller cells, but by the closing bars separating the apices of the rods and cones and Muller cells. The present writer (1909) found the membrana limitans olfactoria to have a similar origin. The zona pellucida surrounding the ovarian ovum in mammals and traversed by the prolongations of follicular cells which reach the surface of the egg must be considered as a fenestrated membrane of the same nature. According to the investigations of Dubreuil and Regaud (1908), it is derived from exoplasmic fibers produced within the intercellular spaces of the follicular cells. My own preparations of ovaries of bats and dogs show that it is formed by the terminal bars, and AHcc Thing (1917) considers that the very thick zona pellucid of the ovum of the turtle is produced by the terminal bars of the surrounding epithelium. They extend over the free surfaces of these cells, where appears a delicate network of the same nature as that of the bars. This network, together with the bars, gives rise to the cuticular fundamental substance of the zona pellucida.
Enamel and the membrana Corti or membrana tectoria are included in a third group. In both cases the adult organ becomes completely detached from its generating substratum, the first from the bases of the ameloblasts, each of which produces a kind of cuticular prism (the enamel prism). These elements are separated by the calcified cement substance which is considered to be a kind of intercellular product, although its origin has not been clearly described. The second, the membrana tectoria, becomes detached from the surface of the greater and the lesser epithelial ridges in the cochlear duct and remains fixed to only the least active portion of its generating substratum, the crista spirahs. Held (1909), discussing the nature of the membrana Corti, thinks that the membrane should not be considered cuticular, not because its layer first formed is not homogeneous, but because its constituent elements, its fibrils, as they become more and more elongated, proceed from the cytoplasm as different plasmic products and not as cell prolongations. A cuticle, he states, is not represented by flagella, by cilia of a ciliated epithelium, or by sensorial hairs. In addition a cuticle always remains attached to the surface of the cell. Hence Held regards the membrana tectoria as a specific product of the free surface of the greater and lesser epithelial ridges, the sensorial cells of which do not take part in its development. Therefore the fibrils of the membrana Corti can not be termed cuticular. Held seems to forget the recognized fact that enamel prisms are real cuticular elements, although they become completely detached from their anatomical substratum.
The object of my research is the study of the development and structure of the membrana tectoria. Although this problem has received the attention of many investigators, it seems to me that the results obtained have been rather contradictory and give for the most part no satisfactory interpretation because of differences between the morphological substratum and the real structure of the membrane derived from it. Recent investigators have more or less neglected the structure of the crista spiralis. I intend to devote to it special attention.
Methods
I have investigated the following material: Pig embryos of 00.0, 93.5, 95.0, 127.0, 137.0, 150.0, and 190.0 mm.; a new-born dog; young kittens; the following adult animals: bat, dog, rat, and mouse. The isolated cochlea was fixed by one of the following agents: Trichloracetic acid, 5 per cent in water; this decalcifies bone very well after one, two, or three days, according to the size of the cochlea; Bouin's fluid; Zenker's fluid.
After fixation by the first agent, the pieces were transmitted directly to absolute alcohol, to which some drops of iodine had been added. After remaining one or two days in the second or third fluid, the pieces were washed in running water and hardened for many weeks in 70 per cent alcohol with some drops of iodine. The iodine effectively acts as a mordant. Where necessary, decalcification was completed by 2 per cent nitric acid in 70 per cent alcohol.
Before embedding in paraffin the pieces were stained by borax carmine. The series was then stained by iron hematoxylin, Congo red, and light green. In advanced stages of development the best results were usually obtained by the following treatment :
- Immerse one day in 2.5 gm. ferric alum in 100 c.c. distilled water.
- Wash one second in distilled water.
- Immerse for some minutes in a solution of Congo red, 1 gm. in distilled water 200 c.c.
- Wash in distilled water.
- Immerse one day in a 0.5 per cent aqueous solution of crystallized hematoxylin.
- Decolorize by a 1 per cent aqueous solution of ferric alum.
- Wash for one hour in running water.
- Stain for some seconds in a solution of 0.5 gm. light green in 200 c.c. of 95 percent alcohol.
- Treat in succession with absolute alcohol, xylol, and Canada balsam.
By this method the nuclei, the central corpuscles, and the terminal bars are stained very dark blue, the cytoplasm and its prolongations red, the ground substance of the connective tissue and the membrana tectoria green. Mallory's method is also very useful for staining blue the ground substance of the connective tissue and the membrane of Corti. The membrana tectoria possesses very delicate structures in which shrinkage and agglutination are provoked by the best fixing agents, although some of my series give results which are good and are confirmed by a new and better method tried during the past few weeks. Before treatment by one of the three fixing fluids mentioned above, I made one or two small openings in the bony wall of the cochlea and exposed the piece for 15 minutes to vapors from an aqueous solution of osmic acid or submerged it in a 1 per cent acfueous solution of the same for one hour. Afterwards fixation was completed by immersion in trichloracetic acid, Bouin's fluid, or Zenker's fluid and the series of sections was stained by iron hematoxjdin, Congo red, and light green. By this method some of the turns of the cochlea give very good prei)arations of the structure of the membrana tectoria. The mitochondria also are visible within osteoblasts, osteoclasts, connective-tissue cells, all epithelial cells, and the sensorial elements.
Anatomical Substratum of the Membrana Tectoria
In spite of numerous investigations many features remain obscure in the histogenesis and structure of the membrana tectoria, in its connections with the adult organ of Corti, and in its extension beyond the sensorial epithelium. Embryologists almost all agree that the membrana tectoria appears in the earliest stages of development of the membranous cochlea before the appearance of the greater and the lesser epithelial ridges and the crista spiralis, as a kind of very thin membrane on the surface of the somewhat thick epithelial layer covering the interior wall of the ductus cochlearis next to the scala tympani. While the two epithelial ridges and the crista spiralis are developing, the membrane thickens and is in close contact with the surface of their superficial epithelium, which I consider as the generating substratum, the matrix of the membrane of Corti, according to the investigations of most authors (Boettcher 1869, Nuel 1878, Pritchard 1876, Retzius 1884, Denis 1901, Rickenbacher 1901, Held 1909). Others believe that the process of formation extends farther over the surface of Hensen cells and Claudius cells (Hensen 1871, Tafani 1882, Dupuis 1894, Coyne and Cannieu 1895, Czinner and Hammerslag 1898, Vasticar 1909, Prentiss 1913). Previous authors asserted that the membrana tectoria reaches and is attached to the ligamentum spirale (Corti 1851, Claudius 1855, Boettcher 1859, Henle 1866, Loewenberg 1868, Barth 1889). Everyone who has studied this cjuestion has recognized that the greater ridge is the most active segment of this substratum; the crista spiralis, at the surface of which the membrane remains fixed in the adult cochlea, is of less significance; indeed, its activity ceases before the stage of complete development of the organ of Corti. A few authors, with Koelliker (1859), assert that the lesser ridge does not take part in the formation of the membrane, and Vernieuwe (1905) and Hardesty (1908, 1915) attribute very little importance to it.
Most investigators concur in regarding the membrana tectoria as a cuticular product of the cytoplasmic apices of the surface epithelial cells (Koelliker 1861, Hensen 1863, Middendorp 1867, Rosenberg 1868, Winiwarter 1870, Gottstein 1870, Pritchard 1876, Nuel 1878, Retzius 1884, Coyne and Cannieu 1895, Denis 1901, Rickenbacher 1901, Vernieuwe 1905, Hardesty 1908, Vasticar 1909, Prentiss 1913); whereas some (Boettcher 1869, Ayers 1891, Czinner and Hammerslag 1898) consider it to be produced by hairs, ciha, or filaments. Held (1909) practically confirms this opinion. I must, therefore, consider the apices of the superficial epithelium which enter into this process of genesis.
The Greater and Lesser Epithelial Ridges
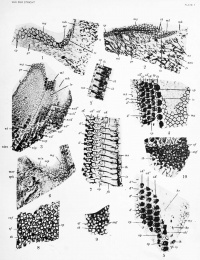
On a transverse section of the tympanic wall of the cochlear duct, in the earliest stages before the appearance of the crista spiralis (pig embryo 60 mm.), and later, when the crista spiralis and the two ridges are visible but before any trace of differentiation has taken place in the sensorial elements, the wall of these regions is lined by a rather thick (epithelium which was regarded by certain earlier authors (Koelliker 1859, Middendori' 18()7, etc.) as formed by superposition of many rows of celLs. But Hensen (1863), Boettcher (1869), Baginsky (1886), and other more recent investigators describe it as a simple columnar epithelium. The elongated prismatic cells reach the inferior and superficial part of the layer and their nuclei arc situated at various heights. Figure 1, from a new-born dog, shows such a section near the toj) of the cochlea, with the first indication of the greater ridge (gr) and with the future le.sser ridge (Ir) not yet iJiomincnt. A superficial mo.saic is visil)le at my, ml, on the two segments, but without any dilTcnMitiation in the sensorial fields. Many rows of nuclei (//) arc a|»p:ir('nt in the scguicnt of the future lesser epithelial ridge.
In figure 2 are displayed the same details in a transverse, slightly oblique section of the second turn of the cochlea in a pig embryo (93.5 mm.). The lesser epithelial ridge is barely indicated (Ir) by a superficial mosaic (ml), of which all the polygons belong to indifferent epithelial cells, although below them are seen three special nuclei (ns) separated by a considerable distance from others more deeply situated (nsn). This superficial location of the nucleus is the first sign of sensorial differentiation in an epithelial cell. The deep nuclei belong to future supporting cells. The superficial mosaic of the greater ridge is visible (wf/), and just at its lateral or outer side is a row of five fields, three larger (ih) separated by two smaller (is), three apices of future inner hair-cells separated by two apices of future inner supporting cells. The outer hair-cells appear later. Toward the axial part of the greater ridge exist three mitotic figures (mi) located below the superficial mosaic ; they are the last existing traces of the proliferation zone of Baginsky (1886), which is very well marked in earlier stages.
Figure 3 represents a transverse, slightly oblique section of the greater (gr) and lesser (Ir) ridges on the second turn of the cochlea in a new-born dog. N. Van der Stricht (1908) describes many similar figures in his photos 42, 42', 44, 45. I will emphasize only the details reproduced on a greater scale in figure 4, a section tangential to the surface of the two epithelial ridges. These confirm for the dog the description given by X. Yan der Stricht of embryonic bats, as shown in his photo 52 among others.
From the axial towards the outer region of the two ridges in figure 4 are the following:
- The superficial mosaic of the greater ridge (mg), the most lateral polygons of which are differentiated into one row of circular inner sensorial fields, the apices of the inner hair-cells (?70 regularly separated by compressed elongated narrow fields, the apices of the inner supporting cells (is).
- A row of small poh'gons, the apices of the inner pillars (ip).
- A first row of apices of outer hair-cells (oh') separated by the phalanges of the outer pillars (op).
- A second row of apices of outer hair-cells (oh") separated by the phalanges of the first row of Deiters cells (d').
- A third row of apices of outer hair-cells (oh'") separated by the phalanges of the second row of Deiters cells (d").
- The apices of the third row of Deiters cells in the form of small polygons (d'") similar to those of the inner pillars.
Figure 5 is from a segment between the second and third turns of the same cochlea and shows identical structures. But there exists a fourth row of outer hair-cells (oh'^') and a fourth row of Deiters cells (d") along a very small portion of the cochlea. Retzius (1884) states that the organ of Corti in the rabbit and the dog exhibits a fourth row of outer hair-cells in the superior part of the middle turn and along the largest part of the apical turn. Waldeyer (1872) mentions a fourth row in man and Retains (1884) confirms this, but adds that the fourth row and even a fifth belong to the upper part of the cochlea and are largely interrupted. I must point out that the outer sensorial fields of the recently differentiated part of tilt' organ of Corti are much smaller than the older inner fields (, fig. 4). In figure 5 all the ai)ice.s of the hair-cells are of the same size and the superficial horseshoes c^Jt from their subjacent cuticular dark plate from which the hairs proceed, are more clearly visible.
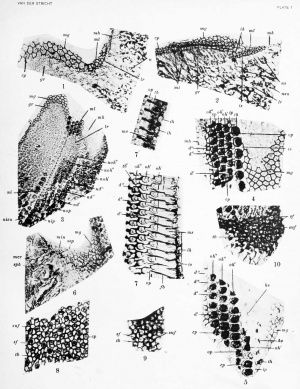
If figures 1, 2, 3, 4, and 5 be carefully compared with one another and with other similar epithelial areas in the cochlea duct, it will be observed that the polygons vary in size. In the first stages of development (figs. 1 and 2) they are largest on the surface of the greater epithelial ridge and much smaller on the future lesser thickening, where their size does not exceed that of the apices of the Hensen cells {mh). But in more advanced stages the fields of the crista spiralis {tncr, fig. 6) and of the Hensen cells become the largest. They do not alter very much on the surface of the greater thickening, although when the organ of Corti is differentiated they extend a Uttle and retain this size more or less until the membrana tectoria becomes detached from its anatomical substratum (fig. 6, ssp). But now they (mg) manifestly decrease along an inner segment [rnin) of the greater ridge near the vestibular lip (fig. 6). Figure 24 (N. Van der Stricht) shows this detail much better than my figure 6.
Figure 4 shows the alterations already mentioned as undergone by the primitive small polygons (ml) in figures 1 and 2 when the sensorial (fig. 4) and supporting fields appear. During the develoj^ment of the membrana tectoria they remain more or less unchanged, as may be seen in figure 5; but before reaching the stages of the adult organ of Corti, gradual transformations occur at the apices of the inner and outer pillars and in the terminal bars which form the superficial membrana reticulatis, as described by N. Van der Stricht. To recall details discussed by this author I will give figure 7, which shows the apices of all the constituents of an adult organ of Corti in the bat, in order to demonstrate in connection with the fields of the inner supporting cells a fact of some importance, upon which I shall dwell later, when I speak of the terminal bars.
Within each area of the indifferent mosaic of the greater ridge exists a central corpuscle (cp), a constituent of the attraction sphere (figs. 1, 2, 4, 6). In reality, on transverse section one sees a diplosome, two granules superposed, of which only one is visible in a tangential section. Generally central, they may become eccentric and even reach the i)eriphery of the field. The corpuscle is surrcnmded by a small, clear area, the medullary zone of the attraction s]ihere of E. Xan Beneden, which is itself encircled by a darker cortical zone (fig. 4).
The diplosome also exists in the small fields of the indifferent mosaic covering the future lesser ridge (figs. 1 and 2). When differentiation occurs and is comjilcted the dijilosome persists within the sensorial fields, where it becomes peripheric, occupying the outer or lateral i)art of the round apex of the hair-cell. It is always surrounded by a small, clear medullary area [rp, fig. 5) l)eyond the dense, intensively stained circular central plate from which proceed the hairs. This superficial plate is considered by N. Van der Stricht (190?) and Held (1909) as a cuticular product of the cytoplasm. Series of preparations of the cochlea of young cats, fixed by osmic vapors or 1 per cent osmic acid followed by treatment by other agents, show the presence of innumerable mitochondria and chonch-iomites throughout the cytoplasm of the sensorial cells. Near the free surface of these elements the mitochondria increase in number and are in very close contact; on the surface they form a plate which is more or less homogeneous, as if the granules were fused together. In successful thin preparations the mitochondrial nature and the granular structure of the superficial plate may be observed. This proves that cuticular formations belonging to the first series mentioned above may be of mitochondrial origin, but in addition it is a striking proof of the mitochondrial nature of the acoustic hairs formed by this plate. F. Spee (1901), Held (1902), and N. Van der Stricht (1908) have described the central corpuscles of the hair-cells, and the last two authors are agreed that these diplosomes do not take part in th6 formation either of the cuticular plate or the hairs. According to N. Van der Stricht, the superficial central corpuscle of the hair-cells (crista and macula acustica) forms a flagellum; Held (1909) observes two flagella for each diplosome within all the epithelial cells lining the cochlear duct. The superficial central corpuscle shows a flagellum prominent on the surface and on the deep face a flagellum directed into the protoplasm towards the nucleus.
The diplosomes within the irregular polygons of the sustentacular fields of the organ of Corti are repelled into the enlarged axial or inner portion of the inner supporting cells (fig. 4), into the enlarged lateral portion of the phalanges of the outer pillars as observed by X. ^'an der Stricht and Held. In the phalanges of the first and second rows of Deiters cells (and of the third in case of an additional fourth row) in the new-born dog they are divided into two central corpuscles, one of which reaches the axial segment and the other the lateral segment of the field. At this stage of development (figs. 4 and 5) the central corpuscles are not displaced in the small polygons of the inner pillars, in the third row of Deiters cells, and in the fourth when it exists.
What is more important in elucidation of the anatomical substratum of the membrana tectoria is the appearance of the terminal bars - the system of lines which separates all the polygons, the apices of the cells, of the superficial mosaic, and closes the intercellular spaces. These bars, described for all endothelia and columnar epithelia, represent a denser and superficial portion of the intercellular substance, the chemical composition of which is altered, for it takes up intensively various stains (such as iron hematoxylin) in the same manner as do the central corpuscles and the cuticular superficial plates of the acoustic cells. The size of the terminal bars varies according to the stage of development and the region. Originally thin, they remain thus in the region of the cells of Hensen and of Claudius. They enlarge slightly at the surface of the crista spiralis, but become much thicker on the greater epithelial ridge and between the constituents of the organ of Corti.
My prejmrations from pig, bat, dog, cat, and mouse enable me fully to confirm the results obtained upon bat (Vespertilw noctula) by N. Van der Stricht, who considers the membrana reticularis of the crista and macula acustica and of the organ of Corti as formed exclusively by a gradual enlargement of the terminal bars. In 1876, after fixation of material by silver nitrate which stained the intercellular ceniciit (Kittsubstanz) in black, Lavdowsky expressed the oi)inion that the membrana reticularis is formed by this metamorphosed substance.
Figure 8, a section tangential to the surface of the crista acustica in a newborn dog, shows a system of thick terminal bars {(b) between the smaller polygonal supporting fields (suf), each of which presents a central corpuscle and the larger more circular sensorial fields (sf), within which a dark central plate and an eccentric central corpuscle (cp) are visible.
Figure 9 represents a similar appearance of the crista acustica in an adult bat {Vespertilio fuscus). Here the bars (tb) are very much enlarged and extend over the greater part of the clear sustentacular fields, leaving uncovered only their central area (suf). The more or less circular openings (suf) of the membrana reticularis become smaller in figure 10, the crista acustica of an adult white rat, and are narrowest in figure 11, the macula acustica of an adult mouse. The much larger sensorial fields {sf) of these last three figures show the central dark cuticular plate from which proceed the hairs traversing these large openings of the membrana reticularis. The power of enlargement and extension of the originally thin terminal bars is fully demonstrated by these four figures, as also is the real origin of the fenestrated membrane derived from them.
As regards the origin of the membrana reticularis of the organ of Corti, figures 4, 5, and 7 are noteworthy. During the earliest stages in the process of development of the membrana tectoria the bars separating sensorial and su])i)orting fields are rather thin, although much thicker than those visible in my preparations at the surface of the cells of Hensen and of Claudius, but they gradually enlarge, chiefly after the membrana tectoria is formed. In the adult organ of Corti one sees (fig. 7) well how the bars have become thicker everywhere and are enlarged most between the first and second rows of outer hair-cells and in such a way that between these two rows, and again between the second and third row, there is to be seen a system of lines alternately thin and thick Ub"), but relatively thicker in the latter situation. Finally, along the row of inner hair-cells between two neighboring sensorial fields (fig. 7', ih), there is a small, dark veil hiding the apices of the inner supporting cells. This originates as an extension of the terminal bars, and I was able to sec similar figures and superficial veils in the organ of Corti of adult rats and dogs. The development of this veil is another striking proof of capacity for extension over neighboring cells possessed by the terminal bars. It may be recalled here that the existence of a small plate or a prolongation of the head of the inner pillar has been mentioned previously by Retzius (1(SS4) and Held (1902). According to Retzius, who did not recognize the inner supporting fields, it extends between the apices of two inner hair-cells. Held, who terms this jjrolongation a rostrum (Schnabel) or a bill, describes it as spreading over a small outer zone of the inner supporting cells. I presume that this rostrum is a part of the superficial veil which I have described a.s derived from the terminal bars, but which these two investigators (lid not recognize.
During the development of the nicinhraiia tectoria the terminal bars, as already stated, possess the power to grow and thicken ui)on the surface of the greater epithelial ridge and even of the crista spiralis. This property may result in further alterations. At certain places the bars show a tendency to spUt longitudinally into two parallel lines, the clear space between which is bridged across, as in the case of so many intercellular spaces between epithelial cells. According to N. Van der Stricht (1908), this process of longitudinal splitting of the bars occurs regularly in the course of development of the membrana reticularis covering the crista and macula acustica. It also takes place during the formation of the membrana limitans olfactoria (1909). The power of extension of the bars over the apices of neighboring cells I will discuss in a later chapter.
The superficial epithelial mosaic of the cochlear duct has been described by Lavdowsky (1876) and Retzius (1884). Both mvestigators used silver nitrate as a fixing agent and thus stained the intercellular cement black. Vernieuwe (1905), after staining the terminal bars intensively blue by iron hematoxyUn, first recognized the true nature of these elements and their special chemical composition. He observed the indifferent mosaic of the greater ridge, and since the number of fields and the number of nuclei deeply situated are approximately the same, he concluded that all the cells reach the surface, and consequently that this thick epithelium ought to be considered as a simple columnar epithelium. In 1902 the terminal bars on the surface of the adult organ of Corti had already been described by Held; in 1908 X. Van der Stricht and in 1909 Held studied them in the embryonic and adult cochlea on the surface of the indifferent and sensorial epithelium.
Crista Spiralis, Limbus Spiralis, Habenula Dentata, Habenula Sulcata
Former authors, including Huschke (1832) and Corti (1851), mention the existence of two regions in the surface of the crista spiralis. One, lateral — the zona dentata or sulcata, near the vestibular lip of the sulcus spiralis displays a series of elongated protuberances more or less parallel the teeth of Huschke, separated by furrows within which Corti had previously noted vestiges of nuclei. Another region, axial, near the attachment of Reissner's membrane, exhibits prominent "warts," "swellings," or papillae, and may be termed the zona papillaris.
The crista is formed by connective tissue which was regarded by Hensen (1871) as cartilaginous, or as intermediary between cartilage and connective tissue. Gottstein (1870) and Waldeyer (1872) considered it, likewise the teeth, as osteoid substance and calcified. Hensen (1863) and Kolliker (1867) described the teeth as a product between and derived from the superficial epithelial cells, but Boettcher (1869), Waldeyer (1872), Denis (1901), and Vernieuwe (1905) among others, demonstrated that they are formed by a proliferation of the subjacent connective tissue between these elements. What becomes of these epithelial cells during and after this proliferation? Boettcher (1869) and V. Winiwarter (1870) noticed rows of nuclei without cytoplasm within the furrows separating the teeth, and Winiwarter described a kind of superficial mosaic without nuclei. He stated it in the following terms: "Sehr eigenthiimhch ist die auf der oberen Flache des Gehohrwulstes mit starkeren Vergrosserungen wahrnehmbare Epithel-Zeichnung, hervorgebracht durch feine, scharf ausgedriickte Contouren ohne Spur von Kernen." It is, of course, the real mosaic figiiird and described later by Lavdowsky (1876), Hetzius (1884), N. Van der Stricht (1908), and Held (1909). After treatment by silver nitrate, Lavdowsky notices on the limbus spiiralis a layer of small endothelial cells devoid of nuclei, more exactly endothelial i)lates which are quite distinct from the subjacent cells located within the interdental furrows.
On the limbus spiralis of rabbit, cat, and man, Retzius, also using silver nitrate, states that the interdental cells situated within the furrows reach the surface and line by their superficial flat ajiices the prominences, the teeth, and the warts (Warzen). Hence in adult individuals is formed a comjilete cell mosaic, continuous with the cell-layer of the Reissner's membrane and of the sulcus spiralis. This statement is confirmed by N. Van der Stricht and Held, who used other fixing agents and stained the terminal bars between the apices of the epithelial cells and found a diplosome within each polygon of the mosaic.
It is to be pointed out that the last three authors, who accurately describe the superficial mosaic and its connections with the teeth and the primitive epithelial cells, do not give exact details of the location of the cell bodies and of the intermediary connective tissue, because they did not examine transverse sections of the crista spiralis at different stages in its development. There is no wonder that in many text-books of histology the description of the superficial elements of the crista is partly erroneous. I will content myself with referring to the Histology of Stohr translated by Billstein, 1898. On page 380 one reads: "the surface of the Hmbus is covered by a simjile layer of flattenedep ithelial cells. " R. Krause, in the Handbuch der vergleichenden und experimentellen Entwickelungslehre der AVirbelthiere, O. Hertwig, 1900, Bd. 1, p. 118, expresses himself in the following terms: Die anfangs kubischen Zellen werden ganz platt und bilden eine feine, endothelartige Membran, welche hier den Ductus cochlcaris begrenzt. " In the text-book of Microscopic Anatomy, by E. A. Schafer, 1912, one reads on page 28o that the cells "arc continued as a pavement-epithelium over the limbus." In 1909 A'asticar described very flattened polygonal cells at the surface of the teeth of Iluschke.
In order to get a true picture of the structure of the crista spiralis at various stages in its development, it is necessary to compare tangential sections with the vertical, and to follow, step by step, the formation of the teeth of Huschke and the alterations in the epithelial cells. I will distinguish four stages in succession.
1. The first is represented in the second turn of the cochlea from a pig embryo of 9.'5.5 mm. The colunuiar epithelium is separated from the subjacent connective tissue by a basement-membrane stained blue by Mallory's method and green by light green, and is in continuity with the much thicker epithelium which outlines both ridges. On vertical and somewhat obli(}ue sections this basement-membrane is fenestrated and provided with small openings; small nuclei of connective tissue, cells are incorporated in its thickness, their axis parallel to the surface of the epithelium. Beneath the membrane exists an embryonic areolar connective-tissue consisting of cells the nuclei of which are stained reil by .Mallory's method or blue by iron hematoxylin, and surrounded t)y a very small (•yt()|)lasnuc zone stained faintly red by fuclisin or rosy by Congo red; this zone is in continuity with prolongations taking up the wime stain. But around and between the cells one sees an alveolar system, on optical sections a network of blue (if stained by Mallory's method) or green (if impregnated by light green) collagenous sheets or filaments in continuity with the basement-membrane. Within the spaces of the reticulum sections of the protoplasmic prolongations of the cells are visible. The alveoli, large in the deep layers, become gradually smaller in the neighborhood of the columnar epithelium. The superficial epithelium is represented by a row of prismatic cells, the nuclei of which may be situated at various heights. Each cell contains a single nucleus and the intercellular spaces are closed by the terminal bars already mentioned.
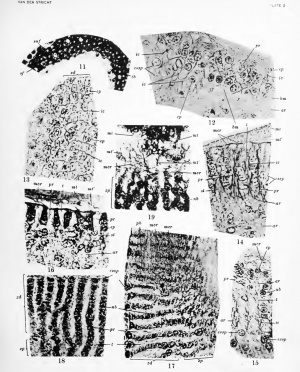
2. The second stage is that of the beginning proliferation of the connective tissue between the epithelial cells. Figure 12, from a photograph of the second turn of the cochlea in a 127.0 mm. pig embryo, shows this process in vertical section. The basement membrane (bm), more or less visible on the right toward the future zona capillaris. has disapjjeared toward the left near the sulcus spiralis (essp), where the proUferation first begins and is always most advanced. There exist below the epithelium larger alveolar spaces, and the constituents of the membrane with the collagenous elements of the connective tissue extend between the bases of the epithelial cells in the form of dark intercellular filaments. At first sight this intraepithelial connective substance seems to be homogeneous and no transverse sections of bundles are iJercejjtible. Upon careful observation, however, it shows very small spaces within which thin prolongations (pr) of connective-tissue cells are detected. Hence it must be recognized that from the first stage of proliferation cellprolongations and collagenous w^alls or sheets penetrate between the epithelial elements. Tangential sections of the crista spiralis demonstrate that this proliferation is performed in such a way that the epithelial cells are pressed together in more or less parallel rows along the future zona dentata (fig. 13, zd). The axis of the cellular rows (ep) is also parallel to the surface of the limbus and is directed from the future vestibular lip toward the zona papillaris. Xo collagenous substance exists between cells of the same row. Figure 12 proves that neighboring epithelial cells of the greater ridge, which later will cover the sulcus spiralis (essp), participate in this alinement and special arrangement of the epithelial elements. These very small parallel intraepithelial connective sheets [ic, fig. 13) represent, of course, the future teeth of Huschke, which in oblique tangential sections (/, fig. 14) are clearly in continuity with thicker subepithelial parallel septa of the same nature {st, fig. 14). Consequently, it is obvious that this intraepithelial arrangement in the form of teeth of Huschke is in the first place induced by a special and similar disposition of the subepithelial substratum.
3. The distinctive feature of the third stage is that the connective-tissue teeth are more or less as large as the interdental epithelial sheets, ^'ertical sections of the crista sinralis in a pig embryo of 127 mm. stained by Mallory's method show this fact (fig. 16). Each superficial epithelial cell (ep) seems to contain two or three nuclei (owing tn the oblique direction of the section) and to be cj'lindrical ; but the red-stained cytoplasm at its free surface is in continuity with a similar small superficial layer (mcr), dark in the photograph, covering the intermediary teeth (/) and representing; the superficial mosaic, which is never invaded or traversed by the proliferating connective-tissue. The transverse diameter of some cell-bodies is the same at various heights, but in others it is slightly reduced and constricted near the surface.
Between the dark epithelial elements exists a clearer collagenous mass (0, deeply stained by aniline blue, within which are noticed darker filaments stained red. These are the prolongations of subjacent connective-tissue cells {pr). These connective intereinthelial teeth of Huschke extend bodily into the depth (si) and are largely in continuity with the subepithelial substratum. Many preparations show this detail more distinctly.
Tangential sections at this stage are very interesting (figs. 17, 18, 19). Figure 17 shows a portion of the third turn of the cochlea in a new-born dog. Above one sees the superficial epithelial mosaic (incr) like a veil formed by elongated polygons, the apices of the epithelial cells, which are separated by darker lines, thin terminal bars stained blue by iron hematoxylin. The long axis of these fields is perpendicular to the axis of the rather dense subjacent bands (pb) which represent cytoplasmic epithelial zones deprived of their nuclei because the razor has taken only the superficial segments of the cells. In the lower j^art of the figure the veil disappears and one notices nuclear bands (nb), the section of the deeper segment of the epithelial elements. These bands are more or less parallel in the zona dentata (zd), ramified and anastomosed in the form of a network in the zona papillaris (zp). Figure 19 shows much better the reticulum of nuclear bands {71b) in a pig embryo of 127 mm. The zona dentata is cut more superficially and one sees its mosaic hncr) and a part of the membrana tectoria (mi'). Figures 17, 15, and 18 demonstrate also that the neighboring epithelial cells of the greater ridge (essp), conspicuous by their larger and darker nucleus, join the epithelial elements of the crista spiralis in the formation of the cell-bands. The connective tissue between the nuclear bands represents the teeth of Huschke and consists of a clear collagenous mass and darker granules (pr) , the transverse section of the prolongations of subjacent connective cells.
In areas of figure 17 and more clearly in figure 18 one sees other details. A system of regular parallel filaments crosses the teeth (/) transversely like intercellular bridges and connects two neigliboring nuclear rows (iib). There seem to exist as many bridges as there are nuclei in one column. Preparations of pig embryos of 127 and 137 mm. display similar bridges and their regularity proves that they represent real structures. I think that they are thin cytoplasmic membranes persisting between the cells of two neighboring rows after their separation by the proliferating connective tissue. At this third stage of its development the subepithelial parts of the teeth of Huschke are marked on tangential sections by large, thick, den.se, parallel, collagenous l)uii(li('s, within which many cell prolongations are embedded.
4. The final or adult stage is characterized by the fact that the cytoplasmic epithelial sheets are very thin and constricted in their siijierficial non-nuclear segment, which is in continuity with the persistently intact superficial mosaic; they are much larger at the level of their deep nuclear segment.
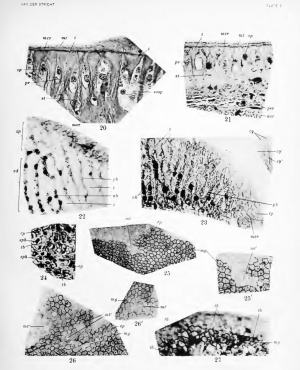
The two vertical sections represented in figures 20 and 21 give a true picture of these conditions. The first is from a pig embryo of 190 mm., the second from a young dog of about 4 months. Compared with figure 16, thej' show that the teeth (t) and the interdental epithelial sheets (ep) become much longer, as seen in figure 20. The teeth enlarge superficially, and by compression the intermediary cytoplasmic sheets are mechanically reduced to a kind of membrane which remains in direct continuity with the superficial epithelial mosaic (mcr). This latter is stained a little darker than the thin cytoplasmic sheets. In the depth the teeth keep their previous size or enlarge very little between the nuclear portions of the sheets, but from there their transverse diameter rapidly increases toward the surface and toward the depth. On the contrary, the epithehal bands become thinner in both these two directions. At their base the teeth with their cell prolongations merge with the subjacent connective-tissue.
Figure 21 shows the different layers of the adult crista spiralis:
- Superficially the membrana tectoria (mt).
- The cytoplasmic mosaic (mcr) beneath the former.
- The epithelio-connective layer formed by the teeth (t) and the interdental epithelial sheets (ep).
- A subepithelial connective laj^er formed by a collagenous substance, including occasional spaces with stellate cells and a system of canals with cell prolongations (pr). These latter are visible in length, in great number reaching the surface of the teeth. The teeth of Husehke are real extensions of this subepithelial layer.
- A deep layer where the fundamental substance is less abundant and the cells with their prolongations more numerous.
- A periosteal membrane (per), which later will undergo ossification and form a bone lamella separating the crista spiralis from the subjacent nerve-fibers (ner) .
My description of the connective tissue is for the most part similar to that of Vernieuwe (1905). Tangential sections of the adult stage of the crista spiralis are represented by figures 22 and 23. The first is from a pig embryo of 190 mm., the second from an adult bat {Vespertilio fuscus) . They show three different planes in succession. The first and most superficial is the mosaic (^mcr), consisting of clear polygonal fields separated by rather thin terminal bars (fig. 23), which in figure 22 are spHt longitudinally and exhibit intercellular bridges. Figure 24 displays a similar veil from the cochlea of a young dog, but within the polygonal fields one sees a dark circular mass, a kind of plate which represents the attraction sphere (sph) formed by a central corpuscle {cp), a small, clear medullary zone, and a larger dark cortical zone. In the cochlea of the adult mouse I could notice the successive stages of division of this dark layer into two smaller dark plates. It is an unexampled and surprising process in the evolution of the sphere, the function of which in the cell completely ceases after the last mitosis during the earUest stages of development. A second plane, cut by the razor a little more deeply, shows a system of dark bands, granular and non-nuclear (pb), which become nuclear in the still deeper third i)lano [nb). They are more or less parallel in the zona dentata and anastomosed in the zona papillaris {zp, fig. 22). In jjreparations from the pig embryo they are .stained rosy by Congo red, and in those from the bat they are faintly blue from iron hematoxylin, the nuclei being dark blue. The nuclear bands are larger than the cytoplasmic.
The teeth of Huschke (<) between these epithelial sheets are clear and homogeneous in figure 22, like the ))a])ilUe of the zona ])ai)illaris (zp). But in figure 23 the teeth are striated from the presence of long granular filaments stained faintly blue, the prolongations of subjacent connective cells. The fundamental substance of the teeth is stained rosy by Congo red.
Transverse compared with tangential sections i^ermit one to conclude that in the adult crista spirahs the interdental epithelial sheets (lamellae) are much longer than in previous stages and reach a deeper level in the subjacent connective-tissue, but that they remain in direct continuity with the suj^erficial cyto]ilasmic mosaic, covering entirely the surface of the teeth and the jjapillje. Beneath this mosaic their transverse diameter is the smallest, while in their deeper nuclear jiortion it remains practically the same as in the preceding stage or decreases a little. This elongation and thinning of the sheets is due to a broadening and mechanical pressure of the intermediary teeth.
The reduction in size and in luuiil^er of nuclei of the epithelial lamellae is shown much better by the tangential sections than by the transverse. This change, more apparent than real, may be imputed to their elongation in a direction parallel with the axis of the primitive cell and undoubtedly to the wide extension of the sheets during the increase in size of the crista spiralis. But comparing figures 22 and 23 (where long cytoplasmic bands separate two rather small nuclei with figures 18 and 19 (where the neighboring nuclei are in close contact it seems to me that during the development of the limbus spiralis an increase of the cytoplasmic mass occurs and can not be denied.
This consideration brings me to the important (juestion of study of the epithelial lamella; of the crista spiralis; all authors describing vertical sections of this admit the existence of separated cells, the i)rimitive eijithelial cells. Indeed, figures 12 and 13 prove that during the first and second stages this view is correct, and figures 10, 20, and 21 .seem to confirm it for the third and fourth stages. All sections tangential to the surface of the crista, however, show diflerent figures. At the third stage (figs. 17, 18, 19) no boundaries between cell-areas can be detected either in the cytoplasmic or in the nuclear bands where the nuclei are very closely i)ressed together. This statement is also true for the fourth stage (figs. 22 and 23). C'onse(juently these ei)ithelial sheets must be considered as r(>al .syncytial masses formed by fused epithelial cells, which are seiiarated only during the first and .second stages of their development. This fusion is brought about by a mechanical factor, the compression from the broadening t(>eth. This multinuclear syncytium is unexampled in other organs, while here the primitive apices and central corpuscles of the epithelial cells persist intact, the boundaries being marked by the terminal bars. Ketzius (1884) and \. Van der Stricht (1908) refer to similar figures, but the former author, though his illustrations show undivided nuclear cytoplasmic masses, speaks of "interdental cells;" the second mentions "nuclear bands." Neither describes the syncytial nature of these formations.
As a matter of fact, in many preparations some exceptions to this rule may be found with sjjaces between the neighboring cells. I do not refer, of course, to figure 20, where the razor cut the teeth vertically just at the edge of the vestibular lip and struck the epithelial cells of the subjacent sulcus spiralis, an unchanged columnar epithelium (essp) ; I refer to figures which may be observed in the middle of the limbus. Their existence proves that when the mechanical pressure is weak fusion does not occur, or when it diminishes in adult stages boundaries may reappear.
Do the numerous nuclei of this special kind of syncytium represent elements of the primitive isolated cells? Or are new nuclei formed at the time of fusion of the cell-bodies or afterward? As a matter of fact, after the first stage described above, no mitoses occur within the epithelial cells. On the other hand, some preparations seem to confirm the idea that occasionally nuclei undergo nuclear amitosis, increasing in size and elongation, and exhibiting direct division into two smaller daughter nuclei by a process of constriction.
Retzius was able to investigate this question. His figures represent the superficial mosaic with terminal bars stained black by silver nitrate; below each polygon he notices a single nucleus, hence he asserts that there exist as many apices of cells as nuclei, although near the vestibular lip some fields contain two nuclei which he explains as due to the fact that the supplementary elements belong to the sulcus spiralis. But by a careful examination of the figures of Retzius I can sometimes count three nuclei beneath two superficial fields at a short distance from the vestibular lij) (his figure 1, plate 24) and also in the zona papillaris.
If, in preparations similar to that displaj-ed in figure 17, I compute the number of superficial fields along one row of polygons of the zona dentata and the number of nuclei of one nuclear band, I find that I obtain as a rule the same number (about 12) ; but there are some exceptions where there are one or two nuclei more than fields. I am inclined, consequentlj', to believe that some occasional nuclei of the primitive epithelial cells undergo the jirocess of amitosis during the second or third stage of development of the crista sj)iralis.
Finally, I think that it is worth while to emjihasize the fact that furrows mentioned bj^ many authors between the prominences of the teeth, within which (according to V. Winiwarter and others) nuclei or remains of epithelial cells are located, do not exist at all. There are large interdental spaces completely filled by the epithelial syncytial lamellae. In the adult organ the deep portion of the.se spaces is broad, but the superficial gradually becomes very narrow at the level where the cytoplasm is in continuity with the superficial mosaic. Superficial iiits between the teeth are either exceedingly small or are lacking; if they do exist they are covered by only a part of the mosaic. The fused epithelial cell-bodies are embedded between the teeth in such manner that I can not agree with Retzius (1884, p. 345) when (in describing these conditions of the adult man) he states : " Diese Epithelzelleh welche sich beim Embryo als eine cvlinderzellen Schicht anlegten sind also noch beim Erwachsenden als Cylinderzollon orhalten, nur sind sic reihenweise von einander durch die von unten her emporwachsenden Vorsi)runge fj;etrennt worden. "
Retzius adds that these cells are actually so adherent as to remain attached to the membrana Corti when it is torn away from the underlying tissues and that even after maceration they still remain connected with the membrane! Such incidents may occur only when the first or second embryonic stage persists. In this respect I am able to state that large areas of the zona jjapillaris from cats 1 to 11 days old are covered by a cubical epithelium free from connective-tissue, while other parts of the same region and the zona dentata show the structure of the adult stage. According to Gottstein (1870) and Waldeyer (1872) half the surface of the crista spiralis, next to the attachment of Reissner's membrane, remains covered by a continuous layer of epithelial cells. Of course, such elements may become detached by maceration or by stripping off the membrana tectoria.
Development of the Membrana Tectoria on the Surface of the Greater Epithelial Ridge
The process of genesis of the membrana tectoria is conspicuous mainly at the most active portion of its anatomical substratum — that is to say, at the surface of the greater epithelial thickening. Tangential sections must be obtained, since they exhibit the best figures, as demonstrated by figures 25 and 26. One observes the superficial mosaic (mg) removed by the razor from small areas {mt'), where the recently formed part of the membrana tectoria is visible. At mt' exists a kind of pale mosaic of another nature, reproducing that of the subjacent anatomical substratum. The terminal bars are replaced by larger dense lines and the apices of the cells by paler, more circular areas. The lines exactly overlie the system of terminal bars which is cut off, and their substance is not only in close contact with the bars, but is in continuity with them at the periphery of the small islands. The pale, more fiuid substances in the mazes of this network overUe the polygons of the mosaic, the cytoplasmic apices of the epithelial cells, and are also in continuity with them. Hence the compact lines must be considered as produced by the bars and the content of the mazes by the superficial cytoplasm. By Mallory's method the lin(\s arc; stained blue, the bars red; by the use of iron hematoxylin and Congo red the former are rosy, the latter dark blue; after iron hematoxylin and light green the first are intensely green and the second dark bluish. Consequently, the chemical composition of the line must be regarded as different from that of their generating sul)stratum.
The cochlea of a pig embryo of 93.5 mm. fixed by the uranium nitrate method of Ramon y Cajal shows in some places (fig. 27) the terminal bars (//>) and the dense jjart (tb') of the superficial membrana tectoria stained black. The comi)act lines {lb') are thinner than the bars and situated in a different i)lane; hence they are not quite in focus. The result of this treatment affords another striking proof of the real origin of the lines and of a chemical composition more or less similar to that of the Ijars. The lines {nW, figs. 25 and 2(5) are larger than the bars and at first sight seem to be homogenous and structureless; })ut on careful examination (mi', figs. 25', 26') they look double, as if spht longitudinally into two parallel thin lines severed bj^ a clear space which is at times bridged across, the bridges being immersed in a kind of intercellular substance.
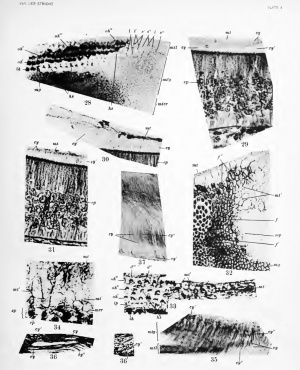
A tangential section through all the layers of the membrana tectoria (mtg) over a large extent (fig. 28) proves that the same structures are visible everywhere, the mazes of this kind of network becoming a little smaller toward the surface of the membrane (on the right side of the figure) than in the vicinity of its inferior side, next to the mosaic of the greater ridge. Nowhere can there be seen transverse sections of filaments or of fibrils. Hence the reticulum in figure 28, and ?«<', figures 25 and 26, must be considered as the optical section of a sj-^stem of walls, of membranes surrounding cylindrical or prismatic tubules filled with a pale fluid. In other words, the membrana tectoria is formed by a system of cylinders or prisms consisting of a dense outer wall derived from the terminal bars and of a contained portion, the more fluid part, derived from the C3'toplasmic apices of the epithelial cells.
This view is confirmed by vertical sections through the greater epithelial ridge, as represented by figures 29, 30, and 31, taken from the cochlea of a pig embryo of 95 mm. These show the cylinders or prisms lengthwise as double lines (cy) and cut across as circular or polygonal fields (cy'). Their transverse sections in figures 30 and 31 are quite similar to those in figures 28, but the longitudinal sections (cy) demonstrate better that between the cyhnders exists an interprismatic clear substance with delicate structures, within which elements like bridges can be noticed. Figure 29 shows most clearly that the young membrana tectoria consists of a basal clear layer and one more superficial, darker and more compact, the walls of the cylinders being denser near the surface and their diameters being a little smaller, with an intermediary' substance less abundant. Finally, the base of each prism is obviously in continuity with one of the slightly prominent apices of the epithelial cells; but these vertical sections can give no sure knowledge concerning the origin of the constituents of the membrana tectoria. Therefore, tangential sections are needed.
We shall see that the interprismatic substance undoubtedly exists in the membrane of adults; hence it is not an artificial product, the result of shrinkage. It is derived from the terminal bars which may split longitudinally into two thinner parts connected together by short bridges. The primitive bars close the subjacent intercellular spaces and separate the intercellular cement from the interprismatic substance, which must therefore be considered as derived from the bars themselves.
Some authors, Coyne and Cannieu (1895), Hardesty (1908), Held (1909), and Prentiss (1913), have drawn and described figures similar to those in my figures 25, 26, 7nt', and also regard them as representing the first stage of the developing membrana tectoria. Hardesty and Held consider these figures as a network of filaments derived from the superficial cytoplasm of the epithelial cells and forming the fibers of the adult membrane. Hardesty (1915, p. 60) states:
"In the production of the tectorial membrane each cell of the greater epithelial ridge may contribute an average of 25 fibrils to the membrane. Each fibril seems to show a slightly elongated onlargpniput at its junction with its cell. Tn the region of the immediate surface of the ridge the interfihriUar matrix does not appear as abundant or so completely jiroduoed as in the older body of the membrane."
Ill many fifj;iir('s Hardcsty and Prentiss represent the bars, but do not describe them.
Held computed 33 to 38 fibrils per each lOO/i of the surface of the greater ridge. They are stained red and visible as red granules on sections tangential to the superficial mosaic, each polygon displaying some of them (his figure 3, guinea-jiig). Nowhere else does this author mention transverse sections of these elements in the developed membrane, although occasional figures seem to show them. He describes the terminal bars everywhere, but does not attribute to them any importance in the genesis of the membrane. However, investigating the origin of the cupula of the surface of the macula acustiea in the rabbit, he states that the reticulum, representing the first stage of develoi)ment of the cupula, remains attached to the apices of the sustentacular cells by delicate filaments, "wobei sie oft nicht in mitten der ZelWache, sondern mehr einer Schlussleiste zu sich anheften" (p. 265). He mentions further, beneath the cupula, special filaments in connection with the surface of the sensorial epithelium, and adds: " merkwiirdigerweise sind diese besonderen Fiiserchen mit den Schlussleisten seitlich verbunden, oft in reicher Zahl von diesen abgehend, welche eine Haarzelle umgreifen." Finally, referring to the filaments of the tectorial membrane in the hen, the chicken, and the pigeon he states that they "an den freien Fliichen der Stiitzzellen und oft dicht an den Schlussleisten sich anheften" (p. 275). In spite of these statements, Held accords no importance to the terminal bars in the development of the membrana tectoria.
Held gives further details of the anatomical substratum of the membrane. In the rabbit he observes a special homogeneous border on the surface of the epithelial celLs, within which the dii)losomes and the terminal bars are inclosed. It is his "Randsaum" and undoubtedly my superficial mosaic. But above it appears another very thin striated border, his "Decksaum, " and on page 203 he states: "Die Vorstellungen die ich nur auf Grund dieser Beobachtungen gebildet habe, ist, dass der durch den Rand- und Decksaum ausgezeichnete Epithelbezirk die erste Bildungszone der Cortischen Membran ist."
Figure 32 represents a portion of the membrana tectoria and the superficial mosaic of the subjacent greater ridge in the vicinity of the future sulcus spiralis (ssp) partially free and detached from the membrane. It displays some structures very interesting in relation to the fibrillar origin of the membrana tectoria as described by Hardesty and Held. While some fields of the mosaic are clear (/), others are covered by a dark veil (/') or a granular ^'eil stained like the bars. Indeed, many i)reparations jirove that these latter are able to enlarge and extend over the neighboring polygons. But some structures, real filaments (/") derived from the bars, incline and join together over the polygons more superficially where they (the said structures) generate the dense i)art of the cylinders. If these fibers persist in the walls of the i^risms one would have to recognize the fibrillar structure of the walls, but my j)rei)arations do not allow me to ascertain witli certainty if this be so.
The same figure shows also the extent of shrinkage often produced b^- fixing agents through artificial distention of the more fluid part of the recently formed layer {mt') of the membrana tectoria.
Co3me and Cannieu describe the membrana Corti as by no means formed by a homogeneous clear substance with dense fil)rils, but by memliranes or sheets ("cloisons") of a special nature circumscribing polygonal cavities which form a network in perpendicular sections. "The surfaces of junction of these membranes thickened at the angles of the reticulum" (p. 280) represent the alleged fibers; and on page 285 they add: "these sheets circumscribe polj-gonal cavities which gradually become narrower from the organ of Corti toward the prominence of Huschke."
My results upon the development of the membrana tectoria are chiefly comparable with those of Prentiss. On page 442 this author states:
- "To sum up the development of the membrana previous to fetuses of 15.0 cm., we may say that it is a cuticular organ with a definite though irregularly chambered structure which is secreted between, and at the ends of the cells composing the basal epithelium of the cochlea."
In his conclusion Prentiss adds:
- " In sections through the axis of the cochlea the membrana has a striated or lamellated appearance. ... In sections perpendicular to the lamellae the structure of the membrana is that of a reticulum with thickenings at the angles of the meshes. It is therefore neither lamellar nor reticular but a chambered structure or ' honeycomb ' of hollow tapering cuticular tubes or chambers normally filled with a fluid resembfing the endolymph. The bases of chambers during development rest between the ends of the epithelial cells."
The main difference between the results of Prentiss and my own is that according to my investigations there may exist an interprismatic substance among the chambers, and that the walls of the cylinders are produced b}' the terminal bars which were not recognized by Prentiss, while their content alone is formed by the cytoplasmic apices of the cells.
On the Surface of the Lesser Epithelial Ridge
Figure 28 displays a section tangential to the surface of the organ of Corti, the neighboring greater ridge and a segment of the crista spiralis between the second and third turn of the cochlear duct in a new-born dog. Ih and oh', oh", oh'" show respectively the row of inner hair-cells and the three rows of outer hair-cells. Two hair-cells, oh", belong to an interrupted fourth row of outer sensorial elements. This segment of the section and another (mg, the superficial mosaic of the greater ridge) are not in focus; therefore they are blurred, but the structures of the membrana tectoria are plainly visible on the right of the figure. In continuity with the rows of outer hair-cells one sees three different, more superficial planes s', s", s'". The first (s') displays horseshoe-like elements, the bases of the acoustic hairs; they occupy more or less the center of clear areas separated by a system of darker, thick, longitudinal lines (l) and of thinner transverse lines (V). Some of these lines are double and short bridges connect the two halves. The clear areas undoubtedly correspond to the sensorial round fields of the outer hair-cells and overlie their apices and the pale fluid must be considered as derived from this cytoplasmic substratum. The longitudinal, thick, irregular lines overlie the apices of the suj)porting fields better visible in figure 5, op, d' , d" , d'", where the terminal bars between two neighboring hair-cells of the same row seem to form a single thick line; while the thinner transverse lines overlie those bars, also irregular, visible between the different rows. Hence the longitudinal and transverse lines of the first superfif'ial plane {s') should be considered as derived from the subjacent terminal bars— that is to say, from the real membrana reticularis of the organ of Corti.
I believe, consequently, that I am justified in drawing the conclusion that the membrana tectoria is formed at the surface of the organ of Corti by the same process as that observed on the surface of the greater ridge. The differences in appearance of the figures are induced by corresponding differences in the anatomical substratum.
The second more superficial plane is also very instructive. It consists of two fields (s") identical in size with those of the preceding (,s')- Their longitudinal lines (/) are undoubtedh' double and the two neighboring halves are connected by short bridges. The transverse lines can not be recognized and a delicate pale network is visible within the clear areas. The structures of this plane resemble those of the third superficial plane {s'"), where the longitudinal lines have also disappeared, and the constituents are the same as those of the neighboring membrana tectoria belonging to the greater ridge (jnty).
What is the significance of the pale network appearing within the clear spaces of the second plane {s")? Two different explanations may be given. The network represents the most superficial portion of the membrane formed in the early stages of its development, before the appearance of the hair-cells, when the subjacent mosaic is formed b}' undifferentiated polygons (figs. 1, 2, ml). Figure 33, from a pig embryo of 150 mm., shows the membrana tectoria recently produced {lut') on the surface of the second and third rows of Deiters cells {d", d'") and the third row of outer hair-cells {oh'") . One sees clearly {mt') a series of pale round fields surrounded by very thick bands which, with similar lines around smaller areas, rei)roduce more or less the subjacent membrana reticularis of the lateral part of the organ of Corti. This figure jiroves that in the course of development the structures of the tectorial membrane (change here greatly, as do those of the subjacent membrana reticularis, and that the differences between its first (nd) and its laterformed layers {mt') become gradually more pronounced. I am thus induced to accei)t this first explanation.
A second explanation would biijig me to consider this delicate reticulum as derived from the bars in such way that the dense part of the membrana tectoria directly formed by them and covering them may grow and extend over the ai)ices of neighboring cells and there give rise to structures like those on the crista sjMralis. I will refer to this question later.
Held (1909) describes a system of thick fibers derived from the apices of the sustentacular cells of the organ of C*orti and chiefly from the first and second rows of Deiters cells; these filaments he terms "Haftfasern dcr Lamina reticularis externa," adhering or attached fibers. They take origin from the phalanges by two Umbs :
- "Fin innere Schenkel ist von einem aiisseren bei diesem zweispaltigen Ursprung der Haftfasern der I und II D. Zellen zii iinterscheiden, von denen sich jeder aus einer Sumnje von Fibrillen sammelt, welche auf die beiden entgegengesetzten und breiteren Ecken der Phalangen-platten hauptsachhch verteilt sind, wenn auch eine geringe Anzahl auf dem schmaleren Mitteil stehen kann" (p. 221).
The double lines or double filaments of Held's figure 15 and the coarse fibers in his figure 16 are undoubtedly the same elements as my lines, figure 29, which I regard not as fibrillar, but as bands or walls, homogeneous Hke the subjacent membrana reticularis.
It may be pointed out that, with N. Van der Stricht, I consider the membrana reticularis of the organ of Corti as fenestrated and constituted by a system of enlarged terminal bars separating openings within which the apices of the sensorial and of the supporting cells are located. Held (1904) and others describe the apices of the Deiters cells as a constituent of this membrane.
The membrana tectoria of the kitten deserves special mention. Fixed bj^ osmic acid and Bouin's fluid or by trichloracetic acid it shows on its lateral edge a regular series of coarse filaments, the thickness of which equals the diameter of the apices of the third row of Deiters cells. Upon each of these apices is adherent a thick, solid, long cylinder, a real cramp (un cramjion), even where the entirely free part of the membrana tectoria is detached from the organ of Corti. I am not able to determine if these attachments persist in adult animals. The cramps mark clearly the lateral boundaries of the membrane and of its anatomical substratum. I have not, so far, had the opportunity of investigating the earliest stages of their development, since I have at present no embrj'onic cat material available. It seems to me that Retzius (1884) observed these elements in the cochlea of the cat, since he described, in the Randfasernetz, "glanzenden parallel neben einander von innen unten nach aussen oben verlaufende Fasern, welche sich am Ausserrande zur einem Randstrang sammeln. "
On the Surface of the Crista Spiralis
All authors agree that the crista spiralis takes only a slight part in the development of the membrana tectoria. According to my investigations, the alterations of its superficial mosaic in the earliest stages of its activity are essentially the same as those described for the greater epithelial ridge, although thinner terminal bars and larger cytoplasmic fields result in some differences. Figure 28 shows that the most superficial layer of the tectorial membrane, first developed and visible on the right of the figure (mtcr), is formed by a system of larger fields and thinner intermediary membranes than those belonging to the neighboring segment (tntg) of the greater ridge. But in a layer a little deeper and more to the left the wide areas disappear, because a kind of delicate network covers them. Finally, still more to the left, the membrana tectoria of the crista spirahs exhibits the same structures as those of the greater epithelial thickening.
Figures 19 and 34, from a pig (embryo of 127 mm., illustrate better the a])pearance of these formations. The two tangential sections are a little oblique and involve four successive planes. The first belongs to the nuclear and cytoplasmic layer of the zona capillaris (zp); the second and more superficial shows the superficial mosaic {trier) of the zona dentata; the third exhibits wider areas {mt') enlarged by the fixing agents. Some of these areas contain only homogeneous clear fluid, obvioush' a product of the superficial cytoplasm; they are separated by thick, coarse lines as dark in the figures as the much thinner lines belonging to the subjacent mosaic (mcr), the real terminal bars, altliough in my preparations they are but faintly stained by Congo red. This dense i)art of the tectorial membrane more recently formed (ml') is derived from the bars and is in direct continuity with a delicate secondary network of the same nature (and therefore of the same origin) covering some of these clear areas. Finally, a fourth quite superficial plane (mt) represents the older jjart of the membrana tectoria and its constituent structures, but is more comjiact and reminds one of the structures in the membrane produced by the greater ridge.
The vertical sections, figures 14, 20, and 21, illustrate also the fact that the tectoria membrane (mi) on the surface of the crista spiralis is formed by a dense part, the uninterrupted walls between small cavities or chambers filled by a clear fluid. On the right of figure 20 the dense part is marked by long lines which are in continuity with similar constituents or cj'linders belonging to the free membrane overlying the sulcus spiralis.
The features just described force me to conclude that at this stage of its develoj)ment the membrana tectoria of the crista spiralis is formed by a system of chambers or cjdinders with a fluid content derived from the superficial cytoplasm of the epithelial cells, and by denser walls ])roduced at least in part by the terminal bars. Indeed, as already stated above, during the development of the membrana olfact oria limitans, of the membrana reticularis of the crista and macula acustica;, and of the membrane covering the ai)ices of the inner supporting cells in the organ of Corti, the substance of the bars extends over the neighboring fields and may form a kind of delicate network which gives rise to secondary structures of the same nature and of the same chemical composition as those of the bars.
In the case of the develoi)ing tectorial membrane the primitive bars generate on their surface a coar.se primary network of a different chemical composition, the large meshes of which contain a secondary, more delicate reticulum of the same nature and of the same chemical composition. This secondary network is derived at least in part from the primary perhaps in part also from similar cytoplasmic structures. To what extent the apices of the epithelial cells take jnirt in the genesis of this delicate reticulum 1 am no more able to determine than Alice Thing has been (1917) in regard to a similar si'condary network generating the fundamental substance of the zona pellucida in the turtle.
Many series of preparations of adult and embryonic stages showing features similar to those of figures 14, 19, 34, 20, and 21, prove that a great number of cylinders of the free membrana tectoria reach and are connected with one cytoplasmic polygon of the superficial mosaic covering the crista spiralis. Each of them is for the most part derived from one polygon of the mosaic of the greater ridge, but also somewhat from a mesh of the secondary network covering one polygon of the crista spiralis, which (according to all investigators represents the least active segment of the anatomical substratum. But it is impossible to give even an approximate estimate of the exact parts played by these two substrata.
I have been able to describe the genesis of an interprismatic substance between the prisms produced by the greater ridge, but the genesis of similar spaces between the portions of cylinders formed by the crista spiralis and by the membrana reticularis of the organ of Corti is still obscure. If further investigation should confirm the existence of this kind of cement for the first segment and the lack of it in the other two segments, this distinctive feature might enable other investigators to determine accurately the parts of the prisms derived from each of the three segments of the anatomical substratum.
Structure of the Adult Membrana Tectoria
Most authors distinguish three segments in the adult tectorial membrane: a thin, innermost axial segment coveruig the crista sjMralis; an outermost lateral, derived from the lesser ridge; a thick middle segment produced by the greater ridge according to the investigations of: Henle (1866), Loweuberg (1868), Boettcher (1869), V.Winiwarter (1870), Gottstein (1870), Hensen (1871), Lavdowsky (1876), Nuel (1878), Retzius (1884), Barth (1889), Dupuis (1894), Coyne and Cannieu (1895), Held (1909), and Prentiss (1913).
The middle segment is striated and the striations incline from the vestibular lip toward the organ of Corti, the inclination being due to the existence of fibers or of fibrils as stated by Hensen (1863), Henle (1866), Boettcher (1869), v. Winiwarter (1870), Lavdowsky (1876), Nuel (1878), Tafani (1882), Retzius (1884), Ferre (1885), Ranvier (1889), Barth (1889), Ayers (1891), Dupuis (1894), Hardcsty (1908), Held (1909), and Vasticar (1909). According to Coyne and Cannieu (1895), Shambaugh (1907), and Prentiss (1913), they are due to the jiresence of lamella^, Most authors agree upon the presence of a homogeneous fluid between these constituents, though Retzius denies it.
The inner segment is described by Henle (1866), Boettcher (1869), v. Winiwarter (1870), Hensen (1871), and Nuel (1878) as a more or less homogeneous but fenestrated membrane provided with openings. Gottstein (1870) and Lavdowsky (1876) regard it as structureless and without openings. Retzius (1884), Barth (1889), and Dupuis (1894) consider it as formed by thin radiating fibrils which according to Held are collected in bundles separating openings. The outer segment, as stated by Henle (1866), Boettcher (1869), v. Winiwarter (1870), Gottstein (1870), Nuel (1878), Retzius (1884), Dupuis (1894), and Held (1909), consists of a network of anast'^mosed fibrils, filaments, or hyalin bands. Hardesty (1908) describes an accessory tectorial membrane as formed by two sets of fibers crossing at an acute angle.
Prentiss (1913) subdivides the membrana tectoria into the following zones:
- A thin, structureless zone of the inner portion of the labium vestibulare.
- A second thicker zone of flattened horizontal chambers over the outer portion of the labium vestibulare.
- A still thicker zone of chambers curving downward and outward, unattached, over the sulcus spiralis.
- An outer zone thickest in the upper turn, with chambers trending downward, outward then inward, largely attached to the cells of the spiral organ and probably normally and wholly attached in this manner.
Many investigators describe a superficial layer in some regions of the membrane, the superficial network of filaments, Fadennetz of Lowenberg, who mentions its existence throughout the two-thirds of the outer segment. Its existence is confirmed by Hensen (1871), Boettcher (1872), Retzius (1884), Dupuis (1894), Spee (1901), and Held (1909) as extending farther towards the inner segment. Spee and Held recognize that it reaches the attachment of Reissner's membrane and forms the inner zone of the tectorial membrane.
According to the process of histogenesis, the adult membrane is formed throughout its entire thickness and breadth by a system of cylinders or prisms separated by a pale and homogeneous fluid which seems to be lacking in the most superficial and densest layer. Figure 35, from an adult rat, represents a tangential section of the outer segment (mtl) and the greater part of the middle segment (mtg). At first sight the figure shows a system of double lines, the longitudinal axial section of the cylinders (cy) . The intraprismatic liquid seems to be a little darker than the more abundant interprismatic fluid. One sees, moreover, other single, often darker lines, tangential sections of cylinders {cy"). At the ends of some obliquely cut cylinders the double lines join together and are in continuity with a single line. On the left of the outer segment {mtl) and in the middle segment (cy') the prisms are pressed together and their transverse section is visible in the form of small jjolygons, between which the" interprismatic substance seems to be lacking.
In my preparation the segment of the membrana tectoria photographed (fig. 35) is in continuity with its inner segment on the surface of the crista spiralis. This axial portion exhibits the same structures, but the prisms are in closer contact, while the interprismatic fluid is loss abundant.
The superficial network of Lihvenberg and similar structures of the outer and inner segments arc represented, according to my preparations, by more comixict jwrts of the membrane, the interjirismatic substance of which is less abundant or lacking, so that a real chambered structure (Coyne and Cannieu, Prentiss), or, in tangential sections, a kind of network appears; but the walls of the chambers or the filaments of the reticulum represent the transverse section of the membranes of neigld)()riiig cylinders. Figure 35 displays these longitudinal prisms on the right side of the outer segment, while their apices are cut transversely, pressed together, on the left.
Figure 23, from an adult bat, exhibits a small portion of the middle segment of the tectorial membrane within which at least three sinxilar longitudinal sections (cy) of the cylinders and some obliquely tangential (cy") can be noticed.
In addition to these two figures, 23 and 35, of adult stages, I give another, similar, figure 36 and 36', from a young dog, and one, 37, from a pig embryo 150 mm., with similar structures, longitudinal (cy), obliquel}' tangential {cy"), and transverse sections (cy') of prisms. They are larger and the intraprismatic and inter23rismatic fluid is more abundant. As a matter of fact, in some instances in these figures, instead of prisms with fluid content, there seem to be present transverse sections of solid filaments, and in transverse sections {cy') darker granules like sections of fibers within the walls of the cylinders. Further investigation is wanted to decide if these elements correspond either to real structures or to products of the shrinkage induced by fixing agents, and if some delicate fibrils or bridges may persist within the pale intraprismatic or interprismatic fluid.
From this description I must conclude that the adult membrana tectoria is formed throughout its extent by prisms or cj'linders consisting of an outer dense membrane or wall and of an axial clear fluid. They are separated by a more fluid intermediary substance, which may be lacking in some places upon the surface of the middle segment, but chiefly of the other segments, while a wall common to two neighboring prisms separates their pale content . This superficial layer is the densest and formed earliest in the course of development. Fixing agents, by reason of the consequent shrinkage, seem to have a great influence on these structures and induce many alterations.
The results of these investigations permit me to emphasize the analogy of structure and origin of the membrana tectoria and enamel of teeth. Both organs are cuticular formations derived from a columnar epithelium, and their immediate anatomical substratum is represented in the case of the former by the superficial mosaic of the cells, and in the latter instance by a basal mosaic, the cytoplasmic polygons of which are also separated by terminal bars according to the investigations of Cohn (1897). Both are formed by a system of prisms, each produced from one cell. The axial, more fluid part of the prisms of the tectorial membrane is derived from the cytoplasm and the outer denser wall from the terminal bars, which at the same time give rise to an interprismatic fluid. The soUd calcified prisms of the enamel are produced by the cj'toplasmic base of the ameloblasts, while the part taken by the terminal bars in the origin of the calcified cement has not thus far been demonstrated. It should be pointed out that one epithehal cell of the crista spiralis, and perhaps of the organ of Corti, may produce or be in connection with many cylinders.
After development the enamel becomes completely detached from its embrj^ological substratum. The membrana tectoria, on the other hand, while becoming free in greater part, keeps its primitive connections with the crista spiraUs, the least active part of its anatomical substratum.
These results concerning the genesis of the tectorial membrane are of general biological interest, for they prove the importance of the intercellular substance, the terminal bars, in ihe origin of special constituents of organs considered as of cuticular nature, now estabhshed for the membrana reticulares of sensorial organs. In this respect the intimate structure, fibrillar or reticular, of the prisms or of some segments of the membrana tectoria must be regarded as of secondary interest.
The present investigation has been carried out in the anatomical laboratory of the medical school of the Western Reserve University, Cleveland, Ohio. It is an agreeable duty to me to express my very sincere thanks to this university and to the staff of the anatomical department, in which I received gracious hospitality for nearly two years and was allowed to continue my scientific researches. My best thanks go mainly out to Professor Dr. T. Wingate Todd, who put at my disposal all the material, the reagents, and the instruments needed for my investigation. Owing to his kindness, to his abihty and assistance, I was able to perform this work. I am very happy to pay to him the tribute of my deepest and most heartfelt gratitude. I also thank very much Mr. G. G. Marshall, who supplied me with a very interesting collection of bats.
Bibliography
Aybrs. H., 1891. Die Membrana tectpria — was sie ist, und die Membrana basilaris — was sie verrichtet. Anat. Anz., vi, 219. , 1898. On the membrana basilaris, the membrana teetoria and the nerve endings in the human ear. Zool. Bull., i, 27.5-278.
Baginsky, B., 1886. Ziir Entwicklung der Gehor .schnecke. Arch. f. mikr. Anat., xxviii, 14-37. Earth, A., 1889. Beitrag zur Anatomie der Schnecke. Anat. Anz., iv, 620-624.
BoETTCHER, A., 1859. Weitere Beitrage zur Anatomie der Schnecke. Virchow Arch. f. path. Anat., XVII, 243-281. , 1869. Ueber Entwickelung und Bau des GehorlabjTinths nach Untersuchungen an Sau gethieren. Dresden. , 1872. Kritische Bemerkungcn und neue Beitrage zur Literatur des Gehorlabyrinths. Dorpat.
Cannieu, a., 1904. L'oreille interne. In Traite d' anatomie humaine de Poirier et Charpy. T. 5, f. 2. Paris. Claudius, M., 1855. Bemerkungen liber den Bau der hautigen Spiralleiste der Schnecke. Ztschr. f. wiss. Zool., VII, 1.54-161. CoHN, Th., 1894. Ueber Intcrcellularluckcn und Kittsubstanz. Wiesbaden. (Inaug.-Diss. V.'iirzburg.) , 1895. Ueber Intercellularlucken und Kittsubstanz. Anat. Hefte, 1. Abt., v, Heft 15, 295-333. , 1897. Ueber epitheliale Schlussloisten an embrjonalen und ausgebildeten Geweben. Verb. d. physik.-med. Gesellsch. zu VVurzburg, n. F., XXXI, 171-200. CoRTi, Alphonse, 1851. Recherches sur Torgaue de I'ouie des mammiferes. Premiere partie. Ztschr. f. wiss. Zool., Ill, 109-169. Coyne et Cannieu, 1895. Contribution a I'etude de la membrane de Corti. Journ. de I'Anat. et de la Physiol., XXXI, 261-287. Czinner, H. I., und V. Hammerschlag, 1898. Beitrag zur Entwieklungsgeschichte der Corti'schen Membran. Arch. f. Ohrenh., XLiv, 50-88.
Deiters, O., 1860. Untersuchung tiber die Lamina spiralis membranacea. Bonn. Denis, P., 1901. Recherches sur le developpement de I'oreiUe interne chez les mammiferes. (Vesper tiUo murinus.) Arch, de Biol., xviil, 377-49.3. Dubreuil, G., et Cl. Regaud, 1908. Sur les productions e.xoplastiques des cellules foUiculeuses de I'ovaire chez la lapine. Verb. d. Anat. GeseUsch., Berlin, 152-156.
Dupuis, A., 1894. Die Coiti'sche Menibran. Anat. Hefte 1, Abt. in. Heft 10, 447-508. Duval, M., 1897. Traite d'histologie. Cours de Physiologic. Ferr6, 1882. Etude de la crfete auditive. Ann. des sciences nat. Bordeaux. , 1885. Contribution a I'etude du nerf auditif. Soc. Zool. de France, x. GELLfi, 1879. Gaz. hebdom de mdd. et de chir., 2. s. xvi, 512. GoTTSTEiN, J., 1870. Beitrage zum fcineren Bau der Gehorschnecke. Centralbl. f. d. med. Wiss., No. 40, 625-«28.
Hardesty, I., 1908. The nature of the tectorial membrane and its probable role in the anatomy of hearing. Amer. Journ. Anat., viii, 109-179. , 1915. On the porportions, development, and attachment of the tectorial membrane. Amer. Journ. Anat., .xviii, 1-73.
Held, H., 1902. Untersuchungen tiber den feineren Bau des OhrlabjTinths der Wirbeltiere. I. Zur Kenntnis des Corti'schen Organs u. der ubrigen Sinnesapparate des Labyrinths bei Saugetieren. Abhandl. d. k. Sachs. Ges. d. Wiss., Math.-phys. Kl., xx\iii, 1-74. , 1909. Untersuchungen uber den feineren Baudes Ohrlabyrinths der AVirbeltiere. II. Zur Entwieklungsgeschichte des Corti'schen Organs und der Macula acustica bei Saugetieren und Vogeln. Abhandl. d. k. Sachs. Ges. d. Wiss., Math.-phys. KL, xxxi. No. 5, 19.5-294.
Henle, J., 1866. Handbuch der systcmatischen Anatomie des Mcnschen. Braunschweig.
Hensen, v., 1863. Zur Morphologic der Schnecke des Menschen und der Saugethiere. Ztschr. f. wiss. Zool., xiii, 481-512. -, 1871. Ueber Boettcher's Entwicklung und Bau des Gehorlabyrintlis nach eigenen Untersuchungen. .\reh. f. Ohrenh., vi, 1-34., 1873. Besprechungen. Arch. f. Olirenh., n. F., I, 64; II, 163. Huschke, E. Froriep's Notizen, 1832, xxxiii, 33-36. Isis von Oken, 1831, 950; 1833, 675-679; 1834, 107. Katz, L., 1890. Histologisches tiber den Sclineckenkanal speciell die Stria vascularis. Arch. f. Ohrenh., XXXI, 6(j-72. KisHi, J., 1902. Ueber den Verlauf und die periphere Endigung des Nervus CochleiB. Arch. f. mikr. Anat., Lix, 144-179. KiSHi, K., 1907. Corti'sche Membran und Tonempfindungstheorie. Pfliiger's. Arch. f. d. ges. Physiol., cxvi, 112-123. KoELLiKER, A., 1854. Ueber die letzten Endigungen des Nervus Cochlea; und die Function der Schnecke. Festschr. beim 50-jahi'igen Doktor Jubililum Fr. Tiedemann's. Wiirzburg. , 1 859 . Handbuch der Gewebelehre des M enschen 3. Aufl. Leipzig. , 1861. Der Einbryonale Schneckenkanal und seine Beziehungen zu den Theilen der fertigen Cochlea. Wtirzburger naturwissensch. Ztschr., II, 1-9. ,1867. Handbuch der Gewebelehre. 5. Aufl.
Lavdowsky, M., 1876. Untersuchungen uber den akustischen Endapparat der Saugethiere. Arch. f. mikr. Anat., xiii, 497-.557. Leboucq, G., 1909. Contribution a I'etude de I'histogenese de la retine chez les mammiferes. Arch. d'anat. micr., x, 555-605. Leydig, Franz, 1857. Lehrbueh der Histologic des Menschen und der Thiere. Frankfurt, a. M.
Loew'ENBERG, B., 1868. La lame spirale du Umagon de l'oreille de I'homme et des mammiferes. Journ. de I'Anat. et de la Physiol., v, 626-657. . Meyer, P., 1876. Etudes histologiques sur le labyrinthe membraneux et plus specialement sur le limaQOn chez les reptiles et les oiseaux. Strassburg (Th^se) .
MiDDENDORP, H. W., 1867. Hpt vlipzig slakkciihuis in zijne wording en in den ontwikkelden toestand. Groningen. NCEL, J. P., 1872. Beitrag ziir Krnntniss der SauROthiprschneokp. Arrh. f . mikr. Anat., viii, 2(H)-215. , 1878. Rpchprchps miproscopiqups sur ranatomie du limagon dps mammiferps. M6m. coiironn^s et m^m. dps savants dtrangprs publids par I'Acad. roy. dp Bplgiqup, XLii, No. 1. Prenant, a., p., BotTiN, and L., Maillard, 1911. Traits d' Histologip, ii, 569. Prentiss, C. W., 1913. On thp dpvplopment of the membrana tpctoria with rpferencp to its structure and attachments. Amer. Journ. Anat., xiv, 425-458. Pritchard, U., 1876. The development of the organ of Corti. Journ. Anat. and Physiol., xiii, 99-103, and Quart. Journ. Micr. Sc, n. s., xvi, No. 64, 398-404. Ranvier, 1889. Traits technique d'histologie. Paris. RETZirs, G., 1872. Anatomische Untersuchungen. 1. Stockholm. , 1882. Biologische Untersuchungen. No. 6. , 1884. Das Gehororgan dpr Wirbelthiere. II. Das Gehororgan der Rpptilien, der Vogei und der Saugethiere. Stockholm. RicKENBACHER, O., 1901. Uutersuchungen iiber die embrjonale Membrana tectoria der Meerschwpin chens. Anat. Hefte, I. Abt., xvi. (Inaug. Dissert., Basel.) Rosenberg, E., 1868. Untersuchungen iiber die En twickelung des Canalis cochlearis der Saugethiere. Dissert., Dorpat. Shambaugh, G. E., 1907. A restudy of a minute anatomy of the structures in the cochlea. Amer. Journ. Anat., vii, 245-257.
Spee, 1'., 1901. Mitteilungpn zur Histologic des Corti'schpii Organps in dpr (iphor.schnpcke dps erwachspnpn Mpnschen. Vcrh. d. anat. Gespllsch. Bonn, 13-23.
Schwalbe, G., 1887. Anatomic dpr Sinnpsorgane. Erlangpn.
Tafani, A., 1882. Gh ppitchi acustici. Lo sperimentale, I, 587-607. , 1884. L'organc de Corti chez les singes. Arch. ital. de Biol., vi, 207-247.
Thing, A., 1917. The formation and structure of the zona pellucida in the eggs of the turtle. Cleveland Med. Journ., xvi, 210.
Van der Stricht, N., 1908. L'histogendse des parties constituantes du neuro-^pithdlium acoustique, des taches et des cretes acoustiqups pt de I'organe de Corti. Arch, dp Biol., xxiii, 541-693.
Van der Stricht, O., 1909. Le neuro-i^pith^liura olfactif pt sa mpmbrane hmitantp interne. M^m. couronn^s et autres ni^m. public's par I'Acad. roy. dp Med. de Belgique, xx, fasc. 2._
Vasticar, E., 1909. Notes d'histologie. Etude sur la tectoria. Journ. de I'Anat. et de la Physiol., XLV, 459, 473. , 1911. Notes d'Hi.stologie. Les sangles des. cellules de soutenement de I'organe de CortiJourn. dp I'Anat. pt de la Physiol., xlvii, 60-99.
Veknieuwe, 1905. Contribution k I'dtude du ddveloppement embryonnaire et post-embryonnaire du limagon des mammifferes et de I'homme. La Presse oto-laryngol. beige, iv, 241, 289.
Waldeyeh, W., 1872. Hornerv. und Schnecke. In S. Strieker, Handbuch der Lehre von den Geweben der Menschen und der Thiere, Leipsig, ii, 91.5-963.
VON WiNrwARTER, A., 1870. Untprsuchungen iiber die Gehorschnecke der Saugethiere. Sitzungsb. d. k. Akad. d. Wiss., Wien, Math.-naturw. Cl., lxi, 1. Abt., 683-714.
Explanation of Figures
Photograph taken at a magniflcation of 750 diameters, except figure 'i, which is 400 diameters, and figure 25', which is 800 diameters. By the reproduction they have been reduced one-tenth.
Abbreviations
1'..
It.,
mcr., mg.,
mh.,
ml., ms.,
mt., mt'..
areolar tissue. basement membrane. central corpuscle. cylinders or prisms of membrana tectoria cut lengthwise. cylinders or prisms of membrana tectoria cut transversely. cylinders or prisms cut tangen" tially. apices of the four rows of Deiters cells. epithelial ceUs. epithelial cells of the future sulcus spiralis. clear cytoplasmic fields. dark cytoplasmic fields. filamentous fields. fibrillar bundles or superficial segments of the bodies oi outer pillars. greater epithelial ridge. superficial hor.seshoe-like elements, the bases of the acoustic hairs. intraepithelial connective-tissue. apices of the inner hair-cells. apices of the inner pillars. apices of the inner supporting cells. longitudinal lines of the recently formed parts of membrana tectoria. transverse lines of the recently formed parts of membrana tectoria. lesser epithelial ridge. superficial mosaic. superficial mosaic of crista spiralis. superficial mo.saic of greater epithelial ridge. superficial mosaic of Hensen cells. mitotic figures. superficial ino.saic of axial segment of the greater ridge. superficial mosaic of lesser ridge. striated membrane or apices of inner pillars. membrana tectoria. recently formed part of membrana tectoria.
mtcr.,
mtg.,
mtl..
n.,
nb.,
nd'., nd"., nd'"
ner.,
ni.,
nip.,
nisu.,
noh'., noh".. no
nop., ns., nsu., oh., oh'., oh"
per., pr.,
sf.,
sph.,
ssp.,
St.,
suf.,
t..
tb., tb'.,
tb".,
zd., zp.,
- membrana tectoria formed on the surface of the crista spiralis,
- membrana tectoria formed on the surface of the greater ridge,
- membrana tectoria formed on the surface of the lesser ridge, nuclei.
- nuclear bands of epithelial cells, nuclei of the three rows of cells of Deiters. nerve-fibers.
- nuclei of the inner hair-cells, nuclei of the inner pillars.
- nuclei of the inner supporting cells,
- nuclei of the three rows of outer hair-cells, nuclei of the outer pillars,
- nuclei of the sensorial cells,
- nuclei of the supporting cells, outer hair-cells, oh"'., oh".,
- apices of the four rows of outer hair-cells, apices of the outer pillars.
- protoplasmic bands of epithelial cells, periosteal membrane, prolongations of connective-tissue cells. '.,
- three different successive planes of recently formed part of the membrana tectoria at surface of the lesser epithelial ridge, sensorial fields, attraction sphere, future sulcus spiralis,
- subepithelial part of teeth of Buschke, fields of the supporting cells,
- tb. - teeth of Buschke,
- tb'. - terminal bars, dense part of the membrana tectoria.
- tb". - terminal bars alternately thicker and thinner,
- zd. - zona dentata of the crista spiralis
- zp. - zona papillaris of the crista
spiralis.
Description of Plates
Plate 1
|
1. Photograph of transverse section through tympanic wall of cochlea near apex. New-born dog. Fixation, trichloracetic acid; stain, iron hematoxylin, Congo red. 2. Photograph of transverse section through second turn of spiral duct. Pig embryo 93. mm. Trichloracetic acid; iron hematoxylin, Congo red. 3. Photograph of transverse, slightly oblique section through the greater and lesser ridges of second turn of cochlea, New-born dog. Trichloracetic acid; iron hematoxylin, Congo red. 4. Photograph of section tangential to surface of the two epithelial ridges in second turn of cochlea. New-born dog. Trichloracetic acid; iron hematoxyUn, Congo red. 5. Photograph of section tangential to surface of the two epithelial ridges between .second and third turn of cochlea. New-born dog. Trichloracetic acid; iron hematoxylin, Congo red. 6. Photograph of section tangential to surface of crista spiralis and the greater epithelial ridge. Pig embryo 137 mm. Bouin's fluid; iron heraato.xylin, Congo red. 7. 7'. Photographs of a section tangential to the surface of the organ of Corti in the adult bat (Vesperlilio fuscus) Bouin's fluid; iron hematoxylin, Congo red. 8. Photograph of section tangential to surface of crista acustica. New-born dog. Trichloracetic acid; iron hematoxyUn, Congo red. 9. Photograph of section tangential to surface of crista acustica in adult bat (Vesperlilio fuscus). Zenker's fluid ; iron hematoxylin, Congo red. 10. Photograph of section tangential to the surface of crista acustica in adult white rat. Trichloracetic acid; iron hematoxylin, Congo red. |
Plate 2
|
11. Photograph of section tangential to surface of macula acustica. Adult mouse. Trichloracetic acid; iron hematoxylin, Congo red. 12. Photograph of section vertical to surface of the crista spiralis of second turn of cochlea. Pig embryo 127 mm. Bouin's fluid; Mallory's stain. 13. Photograph of section tangential to the surfaceof the crista spiralis of the third turn. Pig embryo 9.5 mm. Zenker's fluid; iron hematoxylin, Congo red, light green. 14. Photograph of section tangential and slightly oblique to surface of crista spiralis. Pig embryo 127 mm. Trichloracetic acid; Mallory's stain. 15. Photograph of section tangential to surface of crista spiralis. Pig embryo 137 mm. Bouin's fluid; Mallory's stain. I6. Photograph of section vertical to surface of crista spiralis. Pig embryo 127 mm. Trichloracetic acid; Mallory's stain. 17, 18. Photographs of section tangential to surface of crista spiralis of the third turn. New-born dog. Trichloracetic acid; iron hematoxylin, Congo red. 19. Photograph of section tangential to the crista spiralis. Pig embryo 127 mm. Trichloracetic acid; iron hematoxylin, Congo red. |
Plate 3
|
20. Photograph of section vertical to surface of crista spiralis. Pig embryo 190 mm. Bouin's fluid; Mallory's stain. 21. Photograph of section vertical to surface of crista spiralis. Young dog, age 4 months. Trichloracetic acid: iron hematoxylin, Congo red, light green. 22. Photograph of section tangential to surface of crista spiralis. Pig embryo 190 mm. Trichloracetic acid; iron hematoxylin, Congo red. 23. Photograph of section tangential to surface of crista spiralis in adult bat (Vesperlilw fuscus). Zenker's fluid, iron hematoxylin, Congo red. 24. Photograph of section tangential to surface of crista spiralis. Young dog. Bouin's fluid; iron hematoxylin; Congo red. 25, 25', 26, 26'. Photographs of sections tangential to surface of greater ridge. New-born dog. Trichloracetic acid; iron hematoxylin, Congo red. 27. Photograph of section tangential to surface of greater ridge. Pig embryo 93.5 mm. Fixation by uranium nitrate method of Ramon y Cajal. |
Plate 4
|
28. Photograph of section tangential to surface of the two epithelial ridges and the crista spiralis and their membrana tectoria. between the second and third turns of cochlear duct. New-born dog. Trichloracetic acid; iron hematoxylin, Congo red. 29. 30. Photographs of sections vertical to surface of the greater epithelial ridge. Pig embryo 9.5 mm. Zenker's fluid; iron hematoxyUn, Congo red, light green. 31. Photograph of section vertical to the surface of the greater epithelial ridge. Pig embryo 95 mm. Zenker's fluid; Mallory's stain. 32. Photograph of section tangential to surface of the greater epithelial ridge. Pig embryo 127 mm. Trichloracetic acid; iron hematoxyUn, Congo reil. 33. Photograph of section tangential to surface of organ of Corti and its superficial membrana tectoria. Pig embryo 1.50 mm. Zenker's fluid; iron hematoxylin, Congo red, light green. 34. Photograph of section tangential to surface of crista spiralis. Pig embryo 127 mm. Trichloracetic acid; iron hematoxylin, Congo red. 35. Photograph of section tangential to surface of membrana tectoria. Adult rat (Mus decumanns). Bouin's fluid; iron hematoxylin, Congo red, light green. 30. Photograph of section tangential to surface of membrana tectoria. Pig embryo 150 mm. Bouin's fluid; Mallory's stain. |
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Contributions to Embryology Carnegie Institution No.21. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.21
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G