Book - Contributions to Embryology Carnegie Institution No.20 part 7
| Embryology - 2 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. The histogenesis and growth of the otic capsule and its contained periotic tissue-spaces in the human embryo. (1918) Contrib. Embryol., Carnegie Inst. Wash. 8: 5-54.
Magnification Note
The magnifications stated in the figure and plate legends refer to the original published images, not those available online.
Explanation of Figures
Text Figures
Plate 1
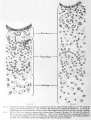
The figures on Plates I and II represent a series of photographs of the ear region in human embryos varying from 4 mm to 130 mm long. The photographs were taken at a magnification of 100 diameters and as far as possible at similar positions, so that a comparison of them would indicate the actual increase in size and the relative amount and form of the individual tissue-masses. In the reproduction tliey were reduced to about 90 diameters. The different figures include the principal stages in the development of the cartilaginous
capsule of the ear and show the gross features of the histogenesis of the periotic reticulum. Figures 5 to 7 cover the period during which the mesenchyme becomes condensed around the otic vesicle. Figures 8 to 10 show the otic capsule in its precartilage stage and the manner in which the precartilage becomes differentiated into relatively permanent and temporary zones. The latter encircle the epithelial ducts and correspond to the future cartilaginous canals. In figures 11 to 13 the main capsular mass has become true cartilage, whereius the temporary zone of precartilage surrounding the canal is on the point of dedifferentiating into periotic reticulum. A focal area of vascularized reticulum is already established at the inner margin of the epithelial duct.
Fig. 5
Frontal section through the region of the ear in a human embryo 4 mm. long (Carnegie Collection, No. 588, slide 6, row 6, section 6). The section is 15 um thick and is enlarged 90 diameters. It shows part of the brain-wall and the otic vesicle with the surrounding mesenchyme. The nuclei of the latter are more numerous in the neighborhood of the vesicle, indicating the beginning of the capsular condensation.
Fig. 6
Horizontal section through the region of the ear in a human embryo 9 mm. long (Carnegie Collection, No. 721, slide 5, row 2, section 1). The section is 15 um thick and is enlarged 90 diameters. It shows a distinct condensation of the mesenchyme around the otic vesicle, particularly on its lateral surface (above) where it extends frotn the surface of the vesicle to about half the distance from the vesicle to the ectoderm.
Fig. 7
Frontal section through the labyrinth in a human embryo 11 mm. long (Carnegie Collection, No. 353, slide 16, row 3, section 4). The section is 10ft thick and is enlarged 90 diameters. It shows the vestibular part of the labyrinth with the appendage opening out of it and passes transversely through the pouches whose margins are to form the superior and lateral semicircular ducts. There is now a very complete capsule of condensed mesenchyme surrounding every part of the labyrinth, with the exception of the appendage and the regions of the interna! auditory meatus and the fenestra cochleae.
Fig. 8
Horizontal section through the otic capsule in a human embryo 15 mm. long (Carnegie Collection, No. 719, slide 3, row 2, section 3). The section is 40 um thick and is enlarged 90 diameters. It shows a portion of the utricle below and the superior semicircular duct above. Surrounding these is a definite capsule of precartilage tissue.
Fig. 9
Sagittal section through the otic capsule in a human embryo 18 mm. long (Carnegie Collection, No. 144, slide 4, row 1, section 3). The section is 40 um thick and is enlarged 90 diameters. Above is the posterior semicircular duct, and just below the center is the lateral semicircular duct. The otic capsule is now differentiated into relatively permanent are:is of prccarlilago and other are;is that are more temporary. The latter surround the epithelial ducts and indicate the future cartilaginous canals.
Fig. 10
Frontal section through the otic capsule in a human embryo 27 mm. crown-rump length (Carnegie Collection, No. 756a, slide 47, section 2). The section is 50 um thick and is enlarged 90 diameters. It passes transversely through the lateral semicircular canal. The epithelial duct is surrounded by a zone of temporary precartilage corresponding to the future cartilaginous canal. Just median to the duct (below it in the photograph) is a group of nuclei that forms the focus of the future growth of reticulum.
Fig. 11
Section through the lateral semicircular canal in a human fetus 30 mm. crown-rump (Carnegie Collection, No. 86, slide 46, section 2). The section is 50 um thick and is enlargetl 90 diameters. The main capsular mass is now differentiated into true cartilage. The zone of temporary precartilage is beginning to recede from the epithelial duct, leaving a reticular area in the interval, which is more pronounced on the median side of the duct (below it in the photograph).
Fig. 12
Section through the lateral semicircular canal in a human fetus 37 mm. crown-rump length (Carnegie Collection, No. 972, slide 20, section 1). The section is 50 um thick and is enlarged 90 diameters. The nuclei of the zone of temporary precartilage form a dark field that corresponds to the future cartiliiginous canal. Along the inner margin of this zone are seen large blood-vessels that belong to the periotic reticulum.
Fig. 13
Section through the lateral semicircular canal in a human fetus 35 mm. crown-rump length (Carnegie Collection, No. 199, slide 58, section 2). The section is 50 um thick and is enlarged 90 diameters. It is stained deeply with hematoxylin, showing the matrix of the cartilage but not the zone of precartilage that is to become the cartilaginous canal.
Plate 2
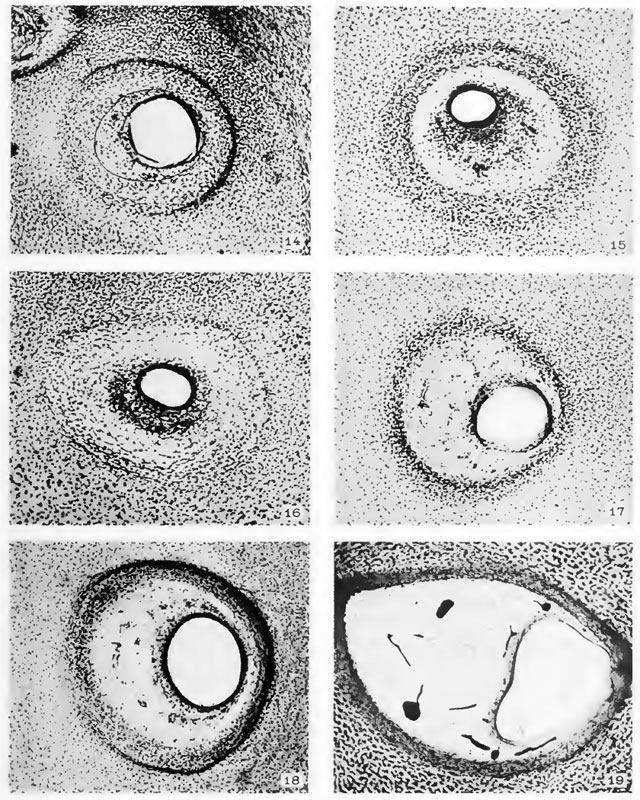
The figures on Plate II are in continuation of those on Plate I and show the final establishment of the periotic reticular tissue. They also show, on being compared with younger stages, the manner in which the cartilage becomes excavated in order lo yield room for the enlarging duct and also to allow for its changing position. The excavation is brought about by the dedifferentiation of cartilage into reticulum tissue. Throughout this period the margin of the cartilaginous canal continues in an unstable condition and is gradually either receding or advancing, through the processes of dedifferentiation, into precartilage or differentiation from precartilage respectively. The periotic reticulum in its later stages develops fibrous membranes at its inner and outer borders. The one at the inner border forms the membrana propria for the epithelial duct, and the one at the outer border becomes the perichondrium.
Fig. 14
Section through the lateral semicircular canal in a human fetus 43 mm. crown-rump length (Carnegie Collection, No. 886, slide 42, section 3). The section is 100 um thick and is enlarged 90 diameters. The zone of precartilage is expanding around its peripheral margin by dedifferentiation of the surrounding cartilage and on its central margin the precartilage is giving way before the advancing reticulum. A crescentic area of periotic reticulum is established on the median side (to the left) of the epithelial duct, about 8 mm. deep in the photograph.
Fig. 15
Section through the lateral semicircular canal in a human fetus 46 mm. crown-rump length (Carnegie Collection, No. 9.5, slide 72, section 1). The section is 100 um thick and is enlarged 90 diameters. The original area of precartilage is now all dedifferentiated into reticulum, and a new area of precartilage has formed outside of this at the expense of the smioundiiig cartilage. The new area of precartilage is about OS cm. deep in the photograph. Everything between this and the epithelium is reticulum, the peripheral part of which is not yet completely vascularized.
Fig. 16
Section thiough the posterior semicircular canal in a human fetus 50 mm. crown-rump length (Carnegie Collection, No. 184, shde 23). The section is 50 um thick and is enlarged 90 diameters. The dedifferentiation of precartilage into reticulum is nearly complete, there being left ordy a narrow hne of it along the margin of the cartilage. The vascularization of the reticulum is not yet completed. The small diameter and the thick wall of the epithelial duct in this figure and in figure 15 result from contraction. If they were distended in the process of fixation they would doubtless be as large as those in figures 14 and 17.
Fig. 17
Section through the posterior semicircular canal in a human fetus 52 mm crown-rump length (Carnegie Collection, No. 96, shde 12, section 2). The section is 100 um thick and is enlarged 90 diameters. It differs from figure 16 in having a more mature periotic reticulum.
Fig. 18
Section through the posterior semicircular canal in a human fetus, 85 mm. crown-rump length (Carnegie Collection, No. 1400-30, slide 43, section 2). The section is 100m thick and is enlarged 90 diameters. At the inner margin of the reticulum can now be seen the membrana propria supporting the semicircular duct and at the outer margin is the thick perichondrium, between which and the cartilage there is a narrow open space that is better seen on the left part of the photograph. The sharp dark line along the margin of the cartilage on the right is an appearance due to the excavation of cartilage at that point. It consists of an intermediate zone in which the cartilage is being dedifferentiated into precartilage and that in turn into reticular tissue.
Fig. 19
Section through the superior semicircular canal in a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018, slide 30, section 1). The section is 50 um thick and is enlarged 90 diameters. It shows a rather mature perichondrium closely attached to the cartilage, separated from it, however, by a narrow intermediate zone that is not seen in the photograph. This zone is connected with the further enlargement of the cartilaginous canal, the growth of which is not yet completed. In the outer part of the canal the perichondrium fuses with the membrana propria of the semicircular duct. The periotic reticulum is beginning to break up in the formation of larger spaces, which it does by the retraction of its trabecute, thereby allowing adjacent spaces to coalesce. The blood-vessels in this specimen were injected with India ink.
Plate 3
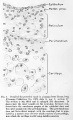
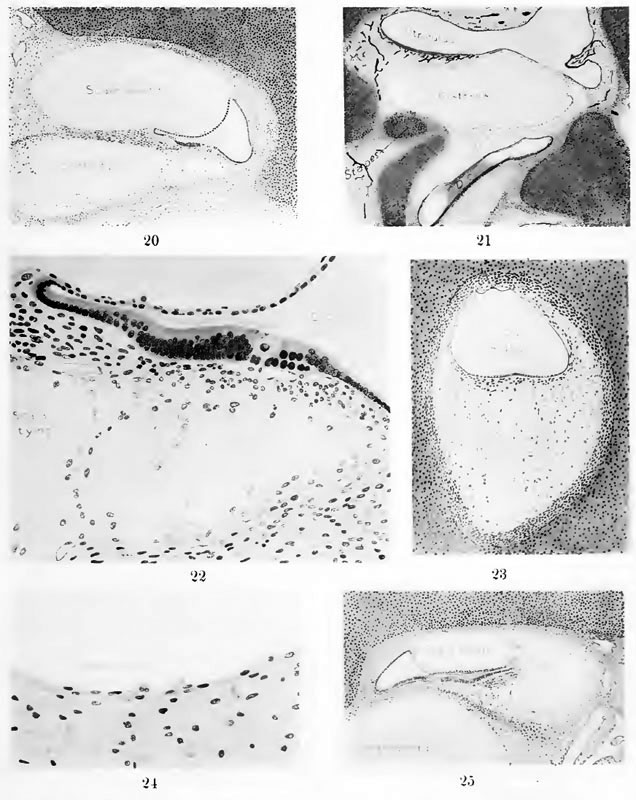
The figures on Plate III show the histological appearance of the periotic tissue-spaces and the manner in which they are formed from the periotic reticulum. This is accomplished by the disappearance of the trabeculae and the consequent repeated coalescence of adjoining spaces.
Fig. 20
Section through the second turn of the cochlea in a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018, slide 32, section 2), enlarged 57 diameters. This section shows the topography of the cochlear duct and the general character of the periotic spaces that are developing along its inner margins. Details of this same section as seen under higher magnification are shown in figures 22 and 24.
Fig. 22
Detail of the section shown in figure 20, enlarged 278 diameters. This figure shows the part of the cochlear duct that is to form the organ of Corti with the adjacent tissue that becomes incorporated in the basilar membrane. Below is the periotic reticulum, whose spaces are in the process of enlarging. By repeated coalescence these spaces finally unite with the large space which constitutes the scala tympani. This figure shows the histological appearance of the reticulum where the formation of ti.ssue-spaces is in active operation.
Fig. 24
Detail of the section shown in figure 20, enlarged 300 diameters. It shows the character of the margin of the scala vestibuli in a fairly mature condition. The scala vestibuli is inclosed by a membrane consisting of the cells that had previously constituted the reticulum occupying this area and which have been modified in form in adaptation to the formation of this large tissue-space, closing it off from the surrounding tissue.
Fig. 21
Section through the vestibular portion of the labyrinth in a human fetus 52 mm. crown-iump length (Carnegie Collection, No. 448, slide 154, section 2), enlarged 31 diameters. This section shows the general character of the periotic spaces and their relation to the different parts of the membranous labyrinth and the surrounding cartilaginous capsule. The first space to develop and the largest shown in this figure is the vestibular cistern, situated between the utricle and the cartilaginous stapes. The smaller spaces, belowthe cistern and extending downward along the cochlear duct, represent the scala vestibuli in an early form. The arteries in this specimen were injected with Imlia ink and are shown in black.
Fig. 23
Section through the superior semicircular canal in a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018, slide 29, section 2), enlarged 90 diameters. The periotic reticulum is undergoing the alterations characteristic of the early stages of the formation of tissue-spaces. Along the margins of the cartilage the reticular tissue is condensed and constitutes the fibrous perichondrium. Around the epithelial canal there is developed a layer of supporting tissue which forms the membrana propria. This layer fuses with the perichondrium along the peripheral margin of the canal and thereby constitutes a ligament that attaches each membranous duct throughout its whole length to the cartilaginous space in which it is suspended.
Fig. 25
Section through the apex of the cochlea of a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018, slide 32, section 2), enlarged 57 diameters. This section shows the tip of the cochlear duct and the character of the communication that develops between the two scate forming the helicotrenia. It will be seen that the margins of the periotic spaces are not so mature here as in the proximal parts of the cochlea of the same fetus, on comparing tliis figure with figure 20.
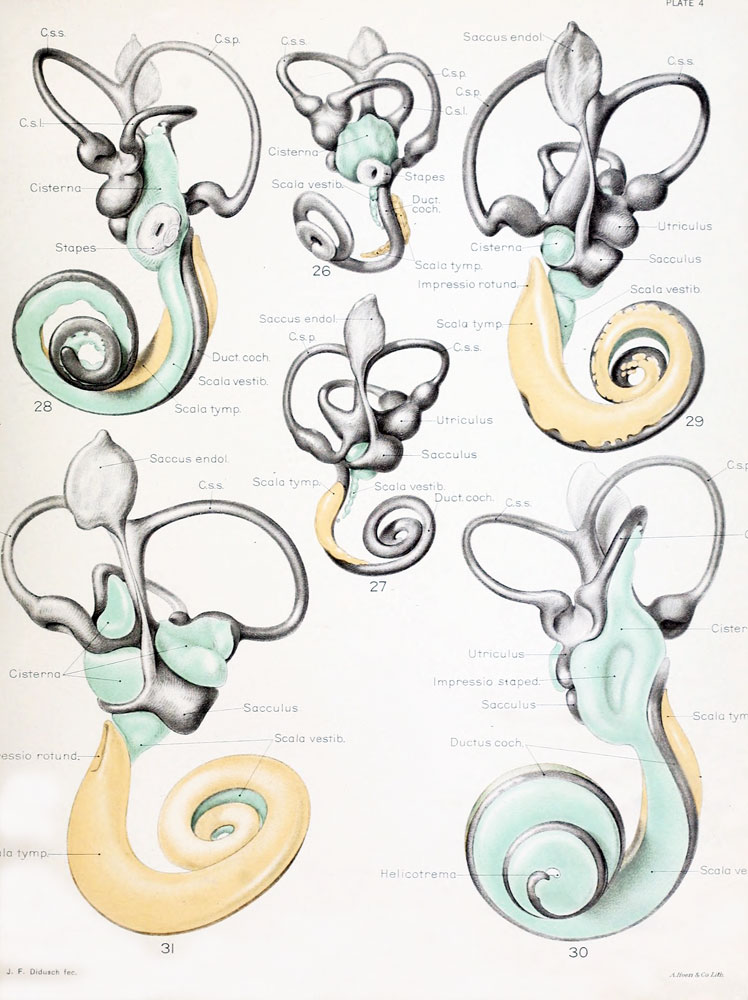
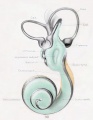
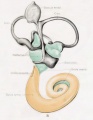
Plate 4
The figures shown on this plate represent a series of median and lateral views of wax-plate reconstructions of the membranous labyrinth and the surrounding periotic tissue-spaces. They illustrate under the same scale of enlargement three typical stages in the development of these spaces. Abbreviations: C. s. 1., ductus semicircuiaris lateralis; C. s. p., ductus semicircularis posterior; C. s. s., ductus semicircularis superior; Duct, coch., ductus cochlearis; Impressio rotund., area opposite the fenestra cochleae; Impressio staped., area in contact with base of stapes; Saccus endol., saccus endolymphaticus; Scala tymp., scala tynipani; Scala vestib., scala vestibule.
Fig. 26
Lateral view of a model reconstructed from a human fetus 50 mm. crown-rump length (Carnegie Collection, No. 84). The cistern and the scala vestibuli are shown in green and the scala tympani is shown in orange. The scala vestibule is in the first stage of its development and consists of a row of large reticular spaces which extend from the ventral margin of the cistern downward along the apical surface of the cochlear duct. The scala tympani is more advanced and shows more complete coalescence of its constituent spaces. Enlarged 11.4 diameters.
Fig. 27
Median view of the same model shown in figure 26. This view shows the topography of the scala tympani. Its large proximal end lies opposite the fenestra cochleae (rotunda) and corresponds to the focus at which its development originates. Distally it tapers off rapidly where the spaces are smaller and their coalescence less complete. Enlarged 11.4 diameters.
Fig. 28
Lateral view of wax-plate reconstruction of the left membranous labyrinth and the periotic spaces in a human fetus 85 mm. crown-rump length (Carnegie Collection, No. 1400-30), enlarged 11.4 diameters. The cistern and the connecting scala vestibuli are shown in green. Although the greater part of the cistern abuts against the stapes, it will be noted that it is also begiiming to spread over the liorsal surface of the utricle and along the inner border of the lateral semicircular duct. The scala vestibule communicates freely with the cistern and extends downward alotig the apical surface of the cochlear duct, throughout nearly two turns, showing the characteristic sacculated appearance near its tip, where the coalescence of the spaces is less complete.
Fig. 29
Median view of same model shown in figure 28, enlarged 11.4 diameters. The scala tympani is shown in orange. The oval indentation in its proximal end corresponds to the fenestra cochlea (rotunda). This space extends along the cochlear duct about the same distance as the scala vestibuli, but the two do not commuinicate yet at any place. The peripheral border of the scala tympani is characterized by sacculations corresponding to spaces that are coalescing with the main space. The grow th of the scala is due to a coalescence of new spaces along its peripheral border rather than along its central border.
Fig. 30
Lateral view of a wax-plate reconstruction of the left membnmoiis labyrinth and the periotic spaces in a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018), enlarged 11.4 diameters. The cistern and scala vestibuli are shown in green and the scala tympani is shown in orange, as in the previous figures. The cartilaginous stapes was removed from this model and the oval impression that it makes on the cistern can be plaiidy seen. The cistern has spread over the top of the utricle and part way along the lateral semicircular duct. The scala vestibuli extends to the ti|) of the cochlear duct, where it communicates with the scala tympani, thas forming the helicotrema.
Fig. 31
Median view of same model shown in figure 30, enlarged 11.4 diameters. The oval impression on the proximal end of the scala tympani corresponds to the fenestra cochleae (rotunda). As yet there is no conmiunication at this point between the scala tympani and subarachnoid spaces, such as is found in the adult and known as the aquaductus cochleae. The spaces making up the cistern cover almost the whole of the utricle and saccule except the places at which the nerves enter and a small part of the medial surface near the attachment of the appendage.
Carnegie Institution No.20 Otic Capsule: Introduction | Terminology | Historical | Material and Methods | Development of cartilaginous capsule of ear | Condensation of periotic mesenchyme | Differentiation of precartilage | Differentiation of cartilage | Growth and alteration of form of cartilaginous canals | Development of the periotic reticular connective tissue | Development of the perichondrium | Development of the periotic tissue-spaces | Development of the periotic cistern of the vestibule | Development of the periotic spaces of the semicircular ducts | Development of the scala tympani and scala vestibuli | Communication with subarachnoid spaces | Summary | Bibliography | Explanation of plates | List of Carnegie Monographs
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 2) Embryology Book - Contributions to Embryology Carnegie Institution No.20 part 7. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_7
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G