Book - Contributions to Embryology Carnegie Institution No.20 part 5
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. The histogenesis and growth of the otic capsule and its contained periotic tissue-spaces in the human embryo. (1918) Contrib. Embryol., Carnegie Inst. Wash. 8: 5-54.
Development of the Perichondrium
In the description of the development of the periotic reticulum we have seen how it begins as a small focus along the central border of the epithelial semicircular duct and spreads at the expense of the temporary precartilage, forming as it does so a crescentic-shaped area of reticulum inclosing the duct. We have also seen how the im-asion or spread of the reticulum into the surrounding area of precartilage is brought about, at least in the later stages, by a dedifferentiation of the latter into the former.
Furthermore, along with this latter process, the inner margin of cartilage surrounding the duct is dedifferentiated into precartilage, so that a new area of precartilage becomes established as the old area disappears. The conversion of precartilage into reticulum in the later stages, however, is more rapid than the conversion
of cartilage into precartilage, and consefjuently there comes a time when the precartilage has nearly all disappeared. In such specimens the reticuhnn extends
practically from the epithelial duct to the margin of the cartilaginous canal. The
(|ualifying term "practically" is used because the inner and outer margins of the
reticulum are modified in a special manner. The inner margin becomes condensed
into a membrane-like coat of fibrous tissue that constitutes the membrana ])ropria
of the membranous canal. The outer margin at about this time undergoes changes
that result in the formation of the jjerichondrium.
In discussing the lu'richondrium it is important to kcej) in imnd the active
alterations in the tissue along the margin of the cartilage that accompany the
growth of the labyrinth. It has been seen how the enlargement of the cartilaginous canals and their alterations in form and position is obtained partly by excavation of cartilage and partly by the laying down of new cartilage, the excavation
being accomplished by its dedifferentiation into precartilage and reticulum, and the
new cartilage being built up through a precartilage stage from the periotic reticular tissue. Throughout the entire period of growth of the cartilaginous canals
the elements of this continual transformation exist along their margin. The margin
during this period is in a state of temporary eciuilibrium and is capable of advancing or receding as the conditions determine.
The first and relatively the major part of the hollowing-out of the cartilaginous
canals is complete before the perichondrium makes its appearance. This is illustrated, for instance, by the fetus of 52 mm. crown-rump length, in figure 17, where
there is as yet no indication of it shown. In fetuses between 40 and 50 mm. long
the zone of precartilage surrounding the margins of the canals, as seen in figures
14 and 15. might be mistaken for perichondrium. This area, however, in fetuses
sUghtly older is converted almost entirely into reticulum. Kolliker (1879), in the
second edition of his text-book on embryologj', pictures a transverse section
through the lateral canal of a rabbit embryo (fig. 457, page 735), in which this
zone of precartilage is labeled as periosteum of the future bone.
The real perichondrium does not make its appearance until the fetus reaches a
a length of about 70 mm. A specimen of this age is represented in te.xt-figure 4,
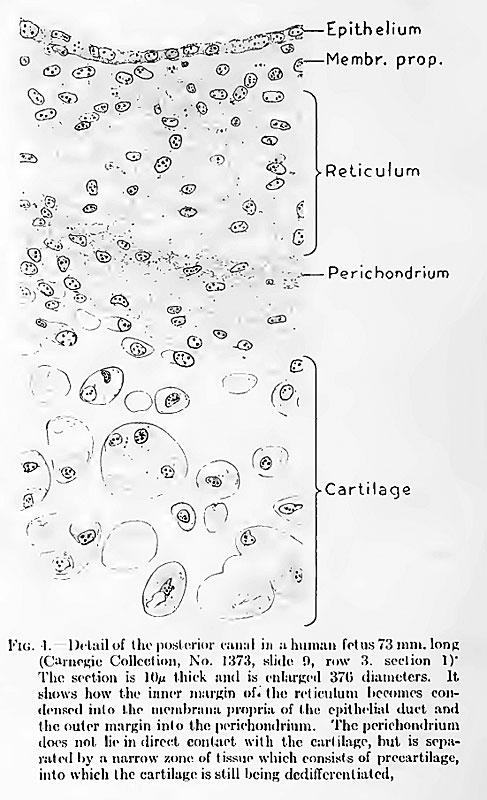
which shows a segment of the posterior semicircular canal in a fetus 73 mm. crownrump length (Carnegie Collection, No. 1373). On examination of this specimen it is
found that there is a distinct condensation of the reticulum along its inner margin, so
that it forms a membrana propria for the epithehal duct with which it is in contact.
This area has largely lost its reticular character and now resembles embryonic
fibrous connective tissue. Along the outer margin of the reticulum a similar condensation of its trabeculse has taken place, forming a thin fibrous lamina or membrane near the margin of the cartilage. This is the perichondrium in its early
form. It does not abut directly against the cartilage, but is separated from it by
a thin layer of transition tissue that is in process of dedifferentiation from precartilage into reticulum.
Passing inward from the cartilage, the transitions are rapid from cartilage to
precartilage, from precartilage to the tissue that is in transition to the reticulum and
then to the perichondrium. These are found as narrow zones that merge quickly
from one into the other. One should remember that the cartilaginous canal has not
reached its full size yet, and that the margin of the canal is still in an unstable condition. However, as the canal becomes larger and the tissues more mature, it is
found that the transitions between the different zones become more abrupt and
in this process the precartilage zone becomes relatively much narrower. This can
be seen by comparing text-figures 3 and 4. The width of the reticulum in these
two figures can not be compared, because the.v represent diflferent canals, lateral
and posterior, and no attempt was made to take them from the same relative positions. The fact that the reticulum is narrower in figure 4 has no significance in
the question of growth. The wide precartilage zone in figure 3 as compared with
that in figure 4, on the contrary, has a direct bearing on the relative age of the two
specimens. A relatively wide zone of precartilage is characteristic of younger
stages. After fetuses become 70 mm. long the precartilage zone becomes quite
narrow, so that the transition from cartilage to perichondrium is relatively abrupt.
In older si^ecimens one might easily obtain the impression that the perichondrium
rested directly against the cartilage, as doubtless it does in the adult. In the oldest
fetus examined, 130 mm. crown-rump length, there is still found a distinct though narrow precartilage-reticular transitional zone between the cartilage and the perichondrium. Presumably this indicates that the margin is still in an unstable
condition.
After the perichondrium has
made its first appearance it rapidly becomes thicker and more
conspicuous. In a fetus 80 mm crown-rump length (Carnegie Collection, No. 172) it is found as
quite a dense fibrous coat, more
than twice as thick as that shown
in the 73 mm. embryo in figure 4.
It is clearly separated from the
cartilage and precartilage by a
narrow zone of reticular tissue.
The character of the perichondrium as existing in slightly
older fetuses is shown in figure 18,
which represents a section through
the posterior semicircular canal of
a fetus 85 mm. crown-rump length
(Carnegie Collection, No. 140030). Here the perichondrium
consists of a relatively broad
zone of enibrj'onic fibrous connective tissue, which in the photograph is about 5 mm. wide,
encircling the whole canal. It
can be seen on the median side
(to the left) that it is sejxirated
from the cartilage and adjacent
transforming precartilage zone by
a narrow, lighter area, which under higher magnification is found
to consist of reticular tissue. The membrana propria at the inner margin of the
reticulum is fairly well developed and it can be seen how it forms a supporting
coat to tho epithelial duct.
When one examines the cartilaginous semicircular canals in fetuses 130 mm. long there can no longer be any ([uestion as to the identity of the perichondrium.
A specimen showing the superior semicircular canal at this stage is represented in figure 19, which is taken from a fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018). The blood-vessels are injected with India ink. The main cartilaginous mass in this specimen is quite mature; the capsules are well defined and the cartilage cells now possess a considerable amount of granular body-protoplasm.
Fig. 4. Detail of the posterior canal in a human fetus 73 mm. long
- (Carnegie Collection, No. 1373, slide 9, row 3. section 1) The section is 10 microns thick and is enlarged 370 diameters (in original printed version). It shows how the inner margin of the reticulum becomes condensed into the membrane propria of the epithelial duct and the outer margin into the perichondrium. The perichondrium does not lie in direct contact with the cartilage, but is separated by a narrow zone of tissue which consists of precartilage, into which the cartilage is still being dedifferentiated.
In many instances capsules are found containing more than one cartilage cell, showing the tendency to cell columns.
A casual glance at a section under lower powers might indicate that the inner margin of the cartilage is in direct contact with the perichondrium. Examination under higher magnification, however, shows that between the thick perichondrium and the cartilage there is a narrow zone of dedifferentiated cartilage. In it the matrix has largely disappeared and the capsules have collapsed and are flattened out, allowing the elongated endoplasm of adjacent cartilage cells to come in contact, separated only by the remnants of the capsular margins. Dyes that stain endoplasm red cause this zone to appear as a deep-red line. This zone represents a state of transition between cartilage and precartilage and its presence doubtless indicates that the margin of the cartilage is still in an unstable condition. The narrowness of the zone and the abruptness of the transition are characteristic of later stages, where the process is more gradual and relatively small in amount.
The transition from this zone to the perichondrium is likewise abrupt. The perichondrium consists of a dense protoplasmic stratum thickly studded with nuclei, and has all the appearance of late embryonic fibrous connective tissue. It is of about the same tliickness around the whole margin of the canal. At the outer margin (toward the right) it fuses wdth the membrana propria of the epithelial duct, therebyforming an attachment which is regarded as a suspensory ligament for the support of the membranous labyrmth. The trabeculae of the reticulum
extending between the membrana propria and the perichondrium are just beginning to break apart, allowing the adjacent spaces of the reticulum, as they are seen in section, to coalesce in the formation of larger spaces.
Having completed the review of the early history of the reticulum and its formative relations to the adjacent tissues, we are now in a position to consider the development and the fate of these larger spaces in the reticulum, which have hitherto been generally known by the misleading term "perilymphatic spaces."
Development of Periotic Tissue Spaces
In the preceding pages of this article the main features of the development of the cartilaginous capsule that incloses the membranous labyrinth have been described. We have traced the process step by step from the first condensation of the mesenchjone around the otic vesicle, through its differentiation into a precartilaginous mass and the maturation of the latter into true cartilage, with the formation through dedifferentiation of cartilaginous chambers in wliich the membranous labyrinth is suspended. It has been shown how these spaces within the cartilaginous capsule are modified in adaptation to the continued growth of the membranous labyrinth and how they finally come to be fiUed with an open-meshed reticulum which everjT\ here bridges the space existing between the membranous labyrinth and the surrounding cartilage. It has further been shown that the membrana propria supporting the epithehal part of the labyrinth on the one hand and the perichondrium on the other are derived from and serve as the hmiting membranes of this reticulum. It is a modification in the meshes of this same reticulum that results in the formation of the so-called perilymphatic si)aces, or periotic spaces as they will be referred to in this paper, the development of which will now he outlined.
Thus far attention has been directed primarily to regions included in typical
transverse sections through the semicircular canals. This was done for the purpose
of uniformity and simplicity and because of the- ease with which successive stages
could be compared with one another. For studying the periotic spaces, however,
the region of the canals is not so favorable, because the spaces are late in developing
there, and even in their completed form they are not so well defined and highly
differentiated as those in the region of the vestibule and cochlea.
The earliest evidence of a periotic space makes its appearance opposite the
stapes. It is developed in the reticulum that fills the interval situated between
the saccule, utricle, and the cartilaginous stapes. Even before the general periotic
reticulum becomes very extensive, in embryos between 30 and 40 mm. long, it can
be seen that its meshes are more irregular and more open in this region than elsewhere. This is the rudimentary form of the periotic vestibular cistern, which is
the first space to become established.
Development of the Periotic Cistern of the Vestibule
Aside from the scala vestibuli and the scala tympani, the largest of the periotic spaces is the large reservoir situated between the tympanic wall of the bony vestibule with its articulated stapes and the vestibular chambers of the membranous labyrinth. This is the spatium perilymphaticum vestibuli (BNA) or the cisterna perilymphatica (Retzius). In order to eliminate the word lymphatic from the terminology it will be designated here as the cisterna periotica vestibuli, or less formally the periotic cistern. In this manner the descriptive term introduced by Retzius is retained.
Before there is any trace of the scalse the initial steps in the formation of the
cistern can be seen. This is well illustrated in an embryo 35 mm. long (Carnegie
Collection, No. 199). This particular embryo is cut in a sagittal series and the
sections on slides 53 and 54 show the periotic cistern in its most rudimentary form.
It consists of an area of reticulum bounded by the utricle, saccule, ductus reuniens,
the proximal end of the cochlear duct, and the ampulla of the jiosterior semicircular
duct. The greater part of the periotic reticulum at this time (35-mm embryo) is
characterized by a narrow and uniform mesh that is interrupted only by numerous
cajjillaries branching through it; in the area mentioned, however, the spaces are
larger and are more irregular both in shape and in size. They i)resent the appearance seen along the semicircular ducts in considerably older embryos, for instance,
in the 52-mm. embryo, as is shown in figure 17. From the very first the increase in
the size of the mesh seems to be attained by the detachment and retraction of its
constituent i)rotoplasmic bridges, thereby allowing adjacent spaces to unite in the
formation of composite large spaces. Thus in the above section a few irregular
protopla.smic free-ends are seen still projecting into the newly enlarged spaces.
This interesting histogenetic process will be taken up again later in connection with the development of the two scalae. The area of this rudimentary periotic cistern
is as yet very small and merges indefiniteh' into the adjoining reticulum. It is
not until we come to fetuses about 40 mm. long that it develops spaces of any considerable size, and it is not until we come to fetuses about 50 mm. long that we find
a single large space with walls that are definitely outlined, so that it can be satisfactorily modeled.
In a fetus 43 mm. long (Carnegie Collection, No. 886), which is cut in a coronal
series, the spaces forming the rudimentary cistern stand out much more definitely
than is the case in the 3o-mm. embryo that has just been referred to. There is
now just opposite the stapes one space which is much larger than the adjoining
spaces. On part of its margin the protoplasmic bridges are stretched along so as to
form a smoothly curved continuous boundary, which is defective in some portions,
and at such places the space merges with the adjoining secondary spaces. Within
the space are some fainth' refractive branching threads of coagulated plasma. The
scala vestibuli is not yet laid down and the scala tympani is only represented b}'
a moderate widening of the meshes of the reticulum in the neighborliood of the
fenestra cochleae (rotunda), along the basal border of the first turn of the cochlear
duct.
In fetuses 50 mm. long the outlines of the cistern become very distinct, due to
the marked increase in the size of its main cavity and to the more definite membrane
at its junction with the rest of the reticulum. Its form and relations are shown in
figures 26 and 27. They represent a median and a lateral view of a wax-plate
reconstruction of this region in a human fetus 50 mm. long (Carnegie Collection,
Xo. 84). Only the main cavity is shown in the model. At certain places around
its borders the meshes of the reticulum are uniting into larger spaces and these in
turn are taken up by the main cavity as it advances into the new territory. These
smaller incomplete spaces were omitted in constructing the plates of the model.
The rule was adopted that only the spaces that were outlined by a membrane-like
border should be traced on the plates and included in the model. This rule was
adhered to in all the models of this series.
Figures 26 and 27 show that the periotic cistern in 50-mm. embryos consists
of a flattened, rounded, bursa-like cavity intervening between the stapes and the
lateral surface of the saccule and adjoining utricle. It extends forward to the
ijmpuUa of the lateral canal and upward to the beginning of the crus commune.
Posteriorly it crowds backward against the ductus reuniens, filling in the space
between the utricle, saccule, and the proximal end of the cochlear duct. Both on
its median and lateral surfaces there is no further opportunity for expansion except
as the vestibule itself enlarges. The deUcate membrane-like wall of the cistern
hugs closely against the parts of the membranous labyrinth on the one side and the
tympanic wall of the cartilaginous vestibule on the other, being separated from them
only by a thin layer of the original reticulum. Along the dorsal margin of the cistern, however, there is room for expansion, and the reticulum in this region shows
enlarging spaces in the process of uniting with the main cavity. On its ventral
margin, near the cochlea and extending along the apical surface of the latter, there is a definite row of reticular spaces actively coalescing and constituting the beginning of the scala vestibuli. These are shown in figure 21, which is a section of a
fetus of about the same age. The spaces of the scala vestibuli lie between the
cochlear duct and the cistern. This section also shows veiy well the relation of
the stapes to the cistern. The scala tymi)ani is already well started at this time,
but its development is quite independent of the cistern. Within the cistern can
be seen scattered clumps of faintly refractive granular threads of what seems to be
a coagulated constituent of the plasma.
The subsequent growth of the cistern is shown in figures 28 to 31. Figures 28
and 29 show respectively a median and lateral view of a wax-plate reconstruction
of the membranous labyrinth and its periotic spaces in a human fetus 85 mm.
long (Carnegie Collection, No. 1400-30). The growth of the cistern here has kept
pace with the increase in size of the lab3'rinth and maintains the same general
relations as regards the stapes and the parts of the membranous labyrinth. The
view of the cistern in figure 28 is an oblique one which would tend to mislead
one as to its width. In reality it is relatively a little wider. It has also extended
upward on the dorsal surface of the utricle and is beginning to creep along the
inner side of the jjostcrior end of the lateral semicircular duct. Ventrally it communicates freely with the scala vestibuli, which now extends well down along the
cochlear duct.
The oldest stage studied is shown in figures 30 and 31. These show two views
of a wax-plate reconstruction of these structures in a human fetus 130 mm. long
(Carnegie Collection, No. 1018). At this time the periotic cistern has spread over
the vestibular part of the membranous labyrinth, covering it nearly eveiywhere
excepting at the macular jiortions where the nerves terminate. In figure 31 it can
be seen that the mesial surface of the saccule is not covered ; this lies close against
the wall of the cartilaginous vestibule. The uppermost division of the cistern,
situated between the crus commune and the ampulla of the posterior semicircular
duct, does not yet open into the general cavity It has formed separately and owing
to the i)osition in which it lies its coalescence with the other parts of the cistern is
retarded ; otherwise, free communication exists between all divisions of the cistern.
Development of the Periotic Spaces of the Semicircular Ducts
From the descriptions given of the adult the reticulum along the ducts never develops a single continuous wide periotic space like that of the cistern and the two scala?. There always remain a few trabecular, such as are seen in the cistern and scala? in their earlier stages, and these constitute partitions which traverse the space and give it in sections the a])pearance of a series of separate spaces extending along the inner margins of the semicircular ducts. Although these spaces along the ducts are inc()mi)lete as compaicd with the cistern and scahc, they are, however, entirely analogous with them in their formation.
The space along the lateral semicircular duct is the largest. Its posterior end exists as a continuation of the cistern. This can be seen in the lateral view of the
model shown in figure 30, where the cistern extends for a considerable distance along the inner border of the lateral duct. Along the other two ducts of the same specimen (130 mm. crown-rump length) the reticulum has commenced the process of space-formation, but complete channels are not yet established. A typical section through one of the semicircular ducts in a fetus of this size, and this is the oldest fetus studied, is shown in figure 23. As compared with the scalse in the same fetus, as shown in figure 20, the space-formation along the ducts is very much retarded.
Development of Scala Tympani and Scala Vestibuli
The scala vestibuli may be regarded as an extension of the cistern downward into the region of the cochlea and as such its growth starts from a focus opposite the fenestra vestibuli (ovalis) . The scala tympani in a similar way makes its first appearance opposite the fenestra cochleae. From these two foci the scalae extend gradually downward along the cochlear duct as two separate spaces which do not communicate with each other until they reach the tip of the duct, where there is finally developed a free opening between them known as the helicotrema.
In their formation they go through a series of histogenetic changes essentially in the same manner that has been followed in the case of the formation of the cistern ; this (as we shall see) consists of the enlargement of the spaces of the periotic reticulum that originally occupied this region, the enlargement being a result of the disappearance of the protoplasmic bridges of the reticulum, whereby adjacent spaces unite in the formation of composite larger spaces. This process continues until there is a single continuous space extending down along the cochlear duct representing each scala and at the margins of each of them there is developed a membranous arrangement of the reticular cells which completely walls ofif the space from the surrounding tissue. In these alterations in the reticular mesh and in the formation of the surrounding membrane there is an active change in the form of the reticular cells, which repeatedly adapt themselves to the new conditions. There is no evidence to indicate that smy other cells take part in the formation of the scalae.
The first evidence of the formation, of scalae is found in fetuses about 40 mm. long, which stage is a little later than the first appearance of the cistern. In a fetus 43 mm. crown-rump length (Carnegie Collection, No. 886), along the proximal part of the cochlear duct on its basal surface there is a distinct widening of the meshes of the periotic reticulum. This is the beginning of the scala tympani. On the opposite side of the cochlear duct, where one would look for the scala vestibule, the periotic reticulum retains its primitive appearance characterized by a narrow and rather uniform mesh. Thus the scala tympani makes its appearance slightly in advance of the scala yestibuli that is, if we regard the latter as distinct
from the cistern.
In fetuses 50 mm. long both the scala tympani and the scala vestibuli can be plainly identified, although they are still very incomplete. A wax-plate reconstruction of them, representing their form and their relation to the membranous labyrinth in a human fetus 50 mm. crown-rump length (Carnegie Collection, No. 84), is shown in figures 26 and 27, being a median and a lateral view respectively.
In preparing this and tlie models shown in figures 28 to 31, it is to be remembered that only those periotic spaces are included that were outlined by a membranelike margin. In the adjacent reticulum there are spaces that are actively coalescing and gradually uniting with the main cavity. No attempt, however, was made to show such spaces in the models. From figures 26 and 27 it will be seen that the scala tympani is larger and more advanced in its development than the scala vestibuli. The hitter is in its earliest stage and consists of hardly more than a row of enlarged reticular spaces which extend downward from the cistern along the dorsal and apical surface of the cochlear duct. A section through the scala vestibuli in another fetus of about the same age (Carnegie Collection, No. 448) is roughly shown in figure 21, the spaces of the scala being situated between the cistern and the cochlear duct.
The scala tympani consists of an elongated oval space lying along the basal surface of the proximal part of the cochlear duct, about corresponding to the proximal half of the first turn of the duct. In the main part it is a single space with a distinct margin separating it from the general periotic reticulum. In the more apical portion it tapers off into multiple incompletely united smaller spaces which actively coalesce as the process advances into the new territory along the duct. It is of interest to note that the most mature and the largest part of this scala, representing the focus at which it first appeared, is opposite the fenestra cochleae (rotunda), just as the cistern forms opposite the stapes and the fenestra vestibuli. The scala tympani always begins at the same place and extends downward along the cochlear duct, at first a little in advance of the scala vestibuli, but subsequently the latter catches up with it and the two reach the tip of the duct at about the same time.
It is well known that the proximal portions of the cochlear duct mature sooner than the distal portions. One might expect that the accompanying periotic spaces would correspond in their development to the maturity of the duct and therefore the proximal parts of the scalse would differentiate first. In other words, the maturation of the cochlea proceeds as a wave from the proximal end to its tip, involving all of its constituent structures as it passes along, including mesenchymal parts as well as epithelial.
This conception might explain the direction of development of the scalae, but can hardly be applied to the cistern, the vestibular representative of the scala vestibuli. One can not say that those portions of the membranous labyrinth lying opposite the focus of development of the cistern (that is, the lateral walls of the saccule and utricle) mature in advance of the rest of the labyrinth. There is no indication
that a wave of differentiation passes through the epithelial elements of the labyrinth in the same direction and synchronously with the extension of the cistern as it advances from its primary focus upon the roof of the utricle and over on its median surface. In the case of the cistern it seems much more likely that the point at which it first apjiears is determined by the position of the stapes, which is doubtless an expression of the physical relation that subsequently exists between the two. By analogy this would yield additional significance to the relation existing between the fenestra cochlea; and the point of beginning development of the scala tympani.
In dealing with the cistern and also with the scalse one should not consider them as insignificant accessories that merely fill in the waste intervals between the membranous labyrinth and the surrounding cartilage. From studying their development it becomes apparent that they have a morphological individuality in many respects as definite as that of the ossicles themselves. They make their appearance at a definite time and at definite places, they spread in a definite manner, and eventually they attain a form and structure that are adapted to a definite function. This becomes more and more evident as we examine older stages.
The form and relations of the scalie in fetuses between 12 and 13 weeks old are shown in figures 28 and 29. These figures show median and lateral views of a waxplate reconstruction of the membranous labyrinth and the surrounding periotic spaces in a human fetus 85 mm. crown-rump length (Carnegie Collection, No. 1400-30). Attention has already been directed to these figures in the description previously given of the cistern. The scala vestibuli can be seen in figure 28. Above, it opens freely into the cistern and extends downward along the apical side of the duct as a single main space, possessing a rather uniform diameter. It extends along the first two turns of the duct, gradually tapering off and showing a less mature character in its distal portions. Along the second turn of the duct the spaces are incompletely fused and the contour becomes correspondingly irregular. As a rule the peripheral margin of the scala is less mature and more irregular than the central margin. The scala, vestibuli does not connect with the scala tympani at any point as yet. The two are separated in the first place by the cochlear duct and then more centrally by a framework of connective tissue in which are the radiating bundles of the cochlear nerve with the nodes of ganglion cells that form the spinal ganglion. These latter structures are not shown in the model; they occupy, however, the V-shaped groove seen between the two scalae.
The scala tympani, as can be seen in figure 29, extends downward on the basal side of the cochlear duct along its first two turns. This corresponds to about the same linear dimension as that of the scala vestibuli. In its proximal portion it shows a greater area in cross-section than the latter, but further toward the apical region it is of about the same size and in some places it is even smaller. The peripheral margin of the scala tympani is distinctly more irregular than the central margin. The irregularity is due to spaces along this margin that are actively coalescing with the main sjiace, but in which the fusion is not yet complete. The irregularity of this margin is thus an indication of the direction of the expansion of the scala. As the diameter of the whole cochlear mass increases, it is evident that the main growth of the scala must radiate outward in a peripheral direction. This is accomplished by the continual assimilation of new reticular spaces along this margin. At the proximal end of the scala tympani can be seen an oval depression which corresponds to the fenestra cochleie (rotunda) and with which it stands in intimate relation.
In fetuses about 16 weeks old the form and relations of the scalae have nearly attained the adult conditions, and this represents the oldest stage studied in connection with the present paper. The conditions found at this time are shown in figures 30 and 31, which present nuMhan and lateral views of a wax-plate model of a human fetus 130 mm. crown-rump length (Carnegie Collection, No. 1018). On comparing the scala tympani and scala vestibuli as seen in these figures with those in figures 28 and 29, it will be seen that they are larger in cross-section and more nearly cover the cochlear duct. Furthermore, they now extend to the extreme
tip of the duct and communicate with each other across its central margin, thus forming a helicotrema. A section through this point can be seen in figure 25, in which these structures are shown as seen under low magnification. It will be noted that now, even as far as the tip of the cochlea, each of the scalse consists of a continuous principal space, though both are more mature and larger in their proximal portions. Along the first turn of the cochlear duct they are walled off by a smooth membranous margin which separates them from the adjacent reticular tissue. The spaces of the latter do not seem to be taking any further part in the process of enlargement of the scala. Along the second turn of the cochlear duct, a section of which is shown in figure 20, the coalescence of reticular spaces with each other and with the scalse is still in active operation. This produces a greater irregularity of the scalar than is shown in the model. The subsidiary spaces are shown as a solid mass; the slender clefts separating them are not represented. The nearer one approaches the tip of the duct the more immature are the scalse, until the condition is reached that is shown in figure 25, where the membrane-like margin is quite incomplete and the spaces merge irregularly with the surrounding reticulum.
Thus a single specimen, if studied in its different parts, shows several stages in this interesting process of the formation and growth of the scalaj.
The figures grouped on plate 3 illustrate some of the histological features of this process. An early stage in space-formation is shown in figure 23. This is a section through the canal region where the changes in the reticulum are late in making their appearance. In fact, the periotic spaces never reach the same degree of differentiation here that occurs in the case of the cistern and scalse. The initial steps, however, are the same, and this figure presents very well the appearance of the periotic reticulum as it begins to open up into larger spaces. Unmodified reticulum is characterized by a rather uniform narrow mesh. The essential change in space-formation consists in the disappearance of some of the trabecule of the mesh, with the consequent coalescence of the corresponding adjacent spaces. The trabecuhe consist of the protoplasmic processes of the constituent cells of the reticulum and their disappearance is to be explained in either of two ways: It is possible that owing to some property of the fiuid element of the tissue the protoplasmic strands are dissolved or liquefied; this would account for their complete disappearance. On the other hand, the same result could be accomplished by an alteration in the form of the cell processes. A given trabecula could separate at either end, or at some jioint along its line, and the free ends of protoplasm could then retract and reshape themselves and become a part of the remaining framework. Whether we are dealing with a licjuefaction of tissue or with active motility of the cell, protoplasm involving detachment and retraction of the trabeculae can not be definitely determined by observations of fixed tissue; but the appearance of sections where the process is in active operation seems to the writer to indicate the latter.
In the above paragraph and elsewhere in this paper reference is made to trabeculae ser^dng as "partitions" between "spaces" and the disappearance of trabeculse resulting in the "coalescence of adjacent spaces." In making this use of the term "space" it should be explained that it is done in a descriptive sense, in application to the appearance of the tissue as seen in sections in which form human embryological material is mainly available. In thin sections of a reticular tissue one sees trabecule as partitions separating adjacent spaces. The same tissue in a mass would show that the spaces everywhere communicate freely with each other, like the spaces in a sponge, and that the trabeculse are thread-like strands which at the best are very incomplete partitions. Instead of a meshwork containing many small spaces, one could perhaps equally well describe reticular tissue as a single large space traversed by many trabeculse. If the latter practice were adopted, one would describe the development of the tissue-spaces with which we are concerned as a process of gradual decrease in the number of traversing trabeculae, with the result that the mesh thereby becomes coarser. For descriptive purposes, however, it is convenient to refer to the intervals between the strands of the mesh as spaces, at the same time not granting them the significance that is attached to such membrane-lined tissue-spaces as are represented by the vestibular cistern and the two scalse, though the latter are in reality derived from them.
In figure 23 the free detached ends of the trabeculse will be noted everywhere, as is characteristic of this stage of development. It is a necessary step in the coalescence of adjacent spaces. The detached trabeculse seem to be gradually retracting and adapting themselves to the formation of larger spaces. Their constituent protoplasm reshapes itself as a smooth border or as a part of other trabeculae. Larger spaces necessitate longer trabeculae, and as trabeculae become longer they also tend to become heavier. These phenomena are all in evidence in the spreading and enlargement of the scalse.
Figure 20 shows a characteristic view of the scalse as seen under low magnification. It will be noted that the scala vestibuli is relatively mature at this point; the scala tympani, however, is in the act of spreading peripherally, so as to
underlie, as it eventually will do, the future basilar membrane. The scala tympani
finally reaches the peripheral margin of the cochlear duct, and it does this by the
coalescence of the enlarging reticular spaces, which become incorporated with the
main cavity of the scala. This can be observed better in figure 22, which shows a
detail of the same section as seen under higher magnification. By comparing this
figure with figure 20 the exact location can be readily made out. A portion of the
main cavity of the scala is indicated and to the right of this are a few enlarged
reticular spaces that are uniting with each other and will in the end become part
of the main space. In addition to the enlarged reticular spaces there is a certain
amount of residual undifferentiated reticulum. It is this tissue that will play the
part of an adventitial coat to the completed scala. The trabecular that separate
the enlarged spaces seem to be under tension and about ready to snap apart. In
fact, in most sections one can see the fragmentary ends of trabeculse where this
interruption of continuity has apparently occurred.
The differentiation of the margin of the scalae constitutes the final feature in their maturation. During the period in which the enlargement of an individual scala is being brought about by the coalescence of enlarging reticular spaces, the margins of the main cavity can be seen to consist of smooth, delicate strands of nucleated protoplasm that resembles in all essentials that of the trabeculae between the large reticular spaces. These linear margins are interrupted here and there by openings into adjacent spaces, but they tend to form a continuous line that definitely marks off the space from the adjacent reticulum. An early stage in the formation of such a margin is shown in figure 25, where the margin is indicated at a few places, but for the most part the space abuts against the surrounding ragged reticulum. The margin of the space is more complete in the scala tympani shown in figure 22, but it is still thin and delicate and can be easily opened up to allow the taking in of new spaces. If we examine the borders of more mature spaces we find them inclosed by a firmer membrane, which finally reaches a state that will probably not admit of any further opening up for the coalesence of additional spaces. Any further growth must thereafter be limited to simple distention of the wall of the space with the consequent adjustment of its constituent cells. Such a condition is represented in figure 24. This shows a more mature section of the wall of the scala vestibuli, being a detail of the same section shown in figure 20. The only difference between such a membrane, as we must now call it, and the corresponding structure in younger stages is its density; it is wider and its protoplasm perhaps more opaque, or in other words, more protoplasm is accumulated there.
If figures 24, 22, 25, and 23 are compared and followed in that order, it will be seen that the lining membrane of the scala can be traced backward, step by step, to the ordinary trabeculie of the periotic reticulum. There is no histological evidence that any new cells enter into its formation. It seems to be simply a product of the proliferation and adaptive reshaping of the cells already there. In its final form the margin of the space resembles an endothelial membrane. One could describe, as immediately lining the space, a thin membrane with flattened nuclei, which is supported underneath by a thin coat of nucleated protoplasm thai,
has the form of fibrous connective tissue. The former, judging only from its final appearance, one might designate as endothelium and thus make a distinction between it and the underlying tissue. In its histogenesis, however, it differs in no way from the rest of the wall and the difference that exists later seems to be merely the result of its adaptation to the existing physical conditions. Its early behaviour is entirely different from that of vascular- endothcliuiu. Thus if its final appearance is stressed and the term endothelium is used for its designation, it must be done with a considerable amount of reservation. It is preeminently a place where the
term mesothelium could be used with great advantage.
Communication of Periotic Spaces with Arachnoid Spaces
The relation of the scala tympani and scala vestibuli to the subarachnoid spaces surrounding the hind-brain is of considerable interest, both on account of the possibiUty of their functional relationship and on account of the similarity that exists in their development. For a satisfactory investigation of the establishment and the character of the communications that are formed between these two allied systems of tissue-spaces, one should resort to other methods than those used in the present study, and, furthermore, one should examine older fetuses than those described here. In fact, a problem lies here that would be well worth careful study.
Certain observations, however, were made in the course of the above investigation that bear relation to these matters, and they will be briefly outlined here. In the first place, the histological picture of the periotic reticulum is essentially the same as that of the early stages of the pia-arachnoidal tissue investing the central nervous system. The enlargement of the meshes of the latter and the formation of the subarachnoid spaces and the arachnoid cistern, as has been recently described by Weed (1917), correspond exactly with the appearance seen in the histogenesis of the periotic spaces in the ear. The periotic spaces are not, however, extensions of the arachnoid spaces that have invaded the cavity of the cartilaginous labyrinth. If this were so we should find them first appearing among the rootlets of the vestibular and cochlear nerves, along which the subarachnoid space extends for some little distance. Instead, they begin at points where there can be no connection with the arachnoid tissue and their direction of growth is quite independent of it. The periotic spaces maj' be analogous to the arachnoid spaces, but they are not identical with them, nor are they an extension of them.
According to the descriptions of the adult anatomy of the ear, a communication becomes established between the scala tympani and the subarachnoid space near the fenestra cochleae, the so-called aquaeductus cochleae. Vague and conflicting statements are also made concerning a communication through the internal auditory meatus connecting the arachnoid spaces with the scalae. Such communication must be estabhshed quite late. In the oldest fetus examined, 130 mm. crown-rump length, they did not yet exist. As to the latter communication, it can be seen that the arachnoid spaces extend peripherally through the internal
auditory meatus along the trunk of the acoustic nerve-complex, and slender pockets and clefts from them extend along the larger bundles of the cochlear nerve; they terminate, however, before reaching the margins of the scalae, and there is no evidence at this stage that there is ever to be a conununication between them and the scalae. As to the aquaeductus cochleae, in the 130 mm. fetus it can be plainly seen that it is already forming as a derivative of the arachnoid spaces, although the communication with the scala tympani is not yet established. The arachnoid spaces invest the glossopharyngeal nerve and extend down along its trunk and pass directly by the region of the fenestra cochleae (rotunda). A thin-walled tubular pouch projects from these spaces, leaving the nerve trunk and extending obliquely toward the scala tympani in a direction that would meet it just distal to the fenestral impression on its basal surface. This fundament of the aqua?ductus cochleae is present in fetuses 85 mm. crown-rump length, but is longer in the 130 mm. fetus, where it nearly reaches the scala tympani. The communication must be established soon after this.
Carnegie Institution No.20 Otic Capsule: Introduction | Terminology | Historical | Material and Methods | Development of cartilaginous capsule of ear | Condensation of periotic mesenchyme | Differentiation of precartilage | Differentiation of cartilage | Growth and alteration of form of cartilaginous canals | Development of the periotic reticular connective tissue | Development of the perichondrium | Development of the periotic tissue-spaces | Development of the periotic cistern of the vestibule | Development of the periotic spaces of the semicircular ducts | Development of the scala tympani and scala vestibuli | Communication with subarachnoid spaces | Summary | Bibliography | Explanation of plates | List of Carnegie Monographs
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Book - Contributions to Embryology Carnegie Institution No.20 part 5. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.20_part_5
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G