1987 Developmental Stages In Human Embryos - Stage 14
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
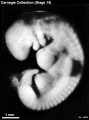
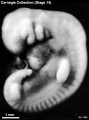
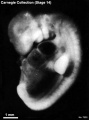
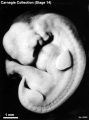
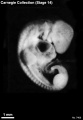
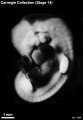
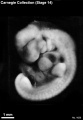
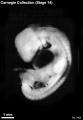
Stage 14
- Approximately 5–7 mm
- Approximately 32 postovulatory days
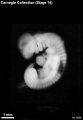
Stage 14 (6830)
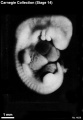
Stage 14 (1380)
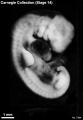
Stage 14 (7333)
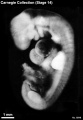
Stage 14 (6502)
Stage 14 (5654)
Stage 14 (4154)
Stage 14 (4629)
Stage 14 (7394)
Stage 14 (6848)
Stage 14 (7400)
Stage 14 (7394)
Stage 14 (1620)
Stage 14 (7400)
Summary
External: invagination of the lens disc but with an open lens pit; a well-defined endolymphatic appendage; elongated and tapering upper limb buds.
Internal: the ventral pancreas (if not detectable earlier) is distinguishable at stages 14 and 15; right and left lung sacs grow dorsad; the ureteric bud acquires a metanephrogenic cap; future cerebral hemispheres and cerebellar plates begin to be visible.
Size and Age
Approximately 70 percent of the embryos after fixation range from 5.5 to 7 mm in length, and most of these are 6 - 7 mm. A few, however, are only 5 mm and some are 7.2 - 8.2 mm. The longer specimens can be accounted for in part by the fact that they were fixed in an exceptionally straight posture. Their organs (eye, ear, and lung) have the degree of development characteristic of the group. It happens that the least advanced specimen of the stage is found among the exceptionally small ones, and the most advanced is among the largest ones. When the embryos are arranged in order of their size, however, their sequence departs considerably from one based on the advancement of their organogenesis.
There is a greater range of variation in the size of the chorion for a given stage than in the size of the embryo. That the growth of the chorion is much influenced by environmental nourishment is indicated by its poor performance in tubal pregnancies, where it is likely to become isolated in stagnant blood. A small chorion is to be expected in a tubal specimen. Omitting three such cases in the present group, most specimens for which records are available fall into three groups: small, average (about 70 percent), and large. These have overall largest diameters (1) between 20 and 25 mm, (2) between 30 and 38 mm, and (3) between 40 and 47 mm, respectively.
The age of the embryos of stage 14 is believed to be approximately 32 postovulatory days.
External Form
(figs. 14-1 to 14-3) The contour of the embryo appears still to be determined largely by that of the central nervous system. Here also there are long specimens and short curved ones but, as they acquire more mass, the embryos have become more uniform in shape. In most of them there is a depression in the dorsal contour at the level of sclerotomes 5 and 6. This is the Nackengrube of His. This cervical bend began to appear in the more advanced members of the preceding stage, but from now on itis a characteristic feature of embryos up to 30 mm. It is a ventral offset to the dorsal convexity of the precocious hindbrain above and the dorsal curve of the thoracic levels of the spinal cord below. It is apparently the forerunner of the flexure sequence of the primate vertebral column, although it so far antedates the latter that one hesitates to attribute to the central nervous system the entire determination of the flexures of the vertebral column.
The upper limb buds are no longer the simple ridges that were found in 4- and 5-mm embryos. Instead they are rounded, projecting appendages curving ventrally. They taper toward the tip, the terminal rim of which will form the hand plate, although the latter is not yet demarcated. In some of the specimens, however, a beginning marginal blood vessel can be recognized. The lower limb buds do not repeat the form of the upper buds. Extending from the upper bud to the lower bud are muscular plates that apparently give origin to the musculature of the ventrolateral body wall, and in front of them can be seen the slender mesonephric duct. Under suitable lighting the caudal cardinal vein appears as a dark band intervening between them. The segmental tributaries to the cardinal vein may give an appearance of segmentation of the overlying mammary crest (fig. 14-2A, B) (O'Rahilly and Müller, 1985). In most cases the atria are distended, and their thin walls stand out in contrast with the thick trabeculated walls of the ventricles.
In the head region the mandibular and hyoid arches are large and conspicuous (fig. 15-3), whereas the third arch in the more advanced members of the group is relatively small and partly concealed along with the depression of the cervical sinus. The hyoid arch, which in the preceding stage was already subdivided into a dorsal and a ventral segment, now tends to show a subdivision of its ventral segment. This produces a “ventralmost” segment which later will play a part in the subdivision of the sixth auricular hillock. In translucent specimens one can see much of the anatomy of the head, for example the outlines of the optic cup, the trigeminal ganglion with cells streaming into the maxillary and mandibular processes, the vestibulofacial mass extending into the dorsal segment of the hyoid arch, the otic vesicle with an elongated and well-marked endolymphatic appendage, the glossopharyngeal and vagus nerves. Winding through these various structures in a characteristic way is the primary head vein. In less-advanced members of the group the lens is indistinct. In more-advanced specimens, however, its opening stands out as a sharply outlined pore. The opening of the lens vesicle to the surface, being an especially definite characteristic, is taken as one of the criteria that determine the inclusion of a given specimen in this stage. When it is closed the embryo is moved to the next group.
The nasal plate (Bossy, 1980a) is usually flat, but it may be concave, although it has not yet acquired a distinct lip. One can usually recognize the nasal area by its thickened opaque ectoderm. If illuminated in a special way, the rim of the disc stands out in an exaggerated manner, which is the case in the photograph shown in figure 14-1D. In the same photograph the pore-like opening of the lens vesicle can be seen.
The term nasal disc (or plate) is considered useful for the peripheral feature distinct from the central components, such as the olfactory bulb etc. The term placode is avoided because of its long-standing association with supposedly “branchial sense organs” and because of its frequent usage for structures as disparate as the nasal plate and the lens disc.
Some difficulties in staging embryos, particularly stages 14 and 15, from only external criteria are mentioned in an abstract by Pearson et al. (1968).
Cardiovascular System
(fig. 16-7) The four major subdivisions of the primary cardiac tube that were seen in the previous stage are clearly defined. The atrioventricular canal is more-evenly apportioned between the ventricles (i.e., shifted relatively to the right), so that it has been claimed that the inlet of the right ventricle is probably derived from the “primitive” (left?) ventricle (Anderson, Wilkinson, and Becker, 1978). Whether the rostroventral and caudodorsal atrioventricular cushions may be beginning to be approximated is disputed, but they are not fused. The outflow tract still retains a single lumen, although functional septation has been in progress since the previous stage. Conotruncal ridges or, more probably, cushions (Pexieder, 1982) are generally described in the wall of the conus and truncus at this time, appearing at stages 12–15 (de Vries and Saunders, 1962) or 14–16 (McBride, Moore, and Hutchins, 1981). The ridges are said to be simply local remnants of disappearing cardiac jelly rather than new formations (Los, 1965). The role of the various “cushions” in the developing heart is not entirely clear; they may serve merely as a temporary adhesive. Normal cellular necrosis is a characteristic feature of the cushions (Pexieder, 1975). The sinu-atrial node is said to be identifiable (Yamauchi, 1965).
The spiral course of the aortic and pulmonary streams has been accounted for in a number of different ways: e.g., by hemodynamic factors, by differential growth, or by local absorption (Anderson, 1973; Goor and Lillehei, 1975). Pexieder (1982) has proposed that three pairs of cushions (conal, truncal, and aorticopulmonary) are simply arranged in different planes, and that their coming together inevitably leads to a spiral, without any supposed torsions and counter-torsions. Los (1978) believes that the spiral arrangement of the great vessels results from asymmetric growth of the main stem of the originally symmetric fourth and sixth pairs of arch arteries, without any twisting of the truncal septum.
The pulmonary (sixth aortic) arch is formed by a ventral sprout from the aortic sac and a dorsal sprout from the aorta (Congdon, 1922, fig. 34). At least in some instances a fifth aortic arch arises from the aortic sac or from the fourth arch and ends in the pulmonary arch (ibid., fig. 22).
Digestive System
(fig. 14-4) The adenohypophysial (craniopharyngeal) pouch is a prominent feature of this stage. The notochord ends near its dorsal wall.
The pharyngeal pouches, which previously have been relatively simple lateral expansions of pharyngeal epithelium intervening between the aortic arches, are now more pocket-like structures. The future thymus and the “parathyrogenic zones” (Politzer and Hann, 1935) can be recognized. The thyroid pedicle (fig. 14- 7D) shows further elongation but is still connected to the epithelium of the pharynx. In addition, right and left lobes and an isthmus may perhaps be presaged (Weller, 1933).
The composition of the tubotympanic recess remains unclear. Goedbloed (1960) has expressed “reservations concerning the concept that the middle ear derives from the first pharyngeal pouch” because it is claimed that the developing middle ear and pharyngeal pouch 1 do not agree topographically and belong to different periods of development: “the period in which the first pharyngeal pouch disappears is exactly the period when the new extension in the oral cavity occurs which is to become the middle ear,” namely 5–10 mm (approximately stages 13–16). In another study it was concluded that, between 10 and 20 mm (approximately stages 16–20), “there is a gradual reduction in the contributions from the second arch and second pouch to the tubotympanic recess so that the tympanum and tube are formed solely from the first pouch” (Kanagasuntheram, 1967). Clearly, further work is required.
From the outset these derivatives of the lateral and ventral parts of the pharyngeal outpouchings are characterized by diverse individualities. They soon lose the uniform serial appearance that marked the pouches in their earlier and simpler form, when they were clearly secondary to the pattern of the aortic arches and to the mesenchymal masses of the pharyngeal arches. In contrast with the development of the lateral and ventral parts, the pharyngeal roof remains mostly thin and inactive. A conspicuous feature is the contact in each interaortic interval with the skin ectoderm. These contact areas, although becoming progressively smaller, are still present in stage 14. It is to be noted, however, that the telopharyngeal body is devoid of a contact area with the skin ectoderm, whereas pharyngeal pouch 3 has one. It is to be noted further that the median thyroid primordium, which is to form the main body of the thyroid gland, has no contact with skin ectoderm at any time. This structure is shown in figure 14-7D. It still retains its stalk of origin from the floor of the pharynx. It is already a bilobed structure, surrounded by a fluid-filled reticular mesenchymal space. It comes into close relation with the aortic sac, a part of which is shown as the space at the bottom of the photograph in figure 14-7D.
A typical specimen belonging to the less advanced half of the group is illustrated in figure 14-4. When this figure is compared with figure 13-9 of the preceding stage, it is seen that the epithelial alimentary canal has become relatively more slender and the different parts are more definitely marked off from one another. The small intestine, because of its increase in length, is slightly deflected from the median plane and is deflected even more ventrally and dorsally. Thus we have the initiation of the intestinal coils. The junction of the small intestine with the colon is marked by the circumstances that the latter still lies in the median plane and is also slightly larger in diameter than the ileum.
The epithelium of the alimentary canal provides the form of the tube, which reflects the growth of its different parts. Surrounding coats appear secondarily.
The ventral pancreas (which may perhaps be distinguishable as early as stage 13) appears as an evagination from the bile duct at stages 14 (Blechschmidt, 1973) and 15. It is generally described as unpaired but, at least in some cases, may perhaps be bilobed (Odgers, 1930)[1] or even multiple (Delmas, 1939).
Respiratory System
(figs. 14-5 and 15-6) The hypopharyngeal eminence and arytenoid swellings appear in the floor of the pharynx, and the epithelial lamina of the larynx develops in the median plane between the arytenoid swellings (O'Rahilly and Tucker, 1973).[2]
The trachea is recognizable. The separation point (between it and the esophagus) remains at a constant level during at least the remainder of the embryonic period proper, whereas the bifurcation point (of the trachea) descends (O'Rahilly and Müller, 1984c).
The right and left lung sacs are shown in geometric projection in figure 14-5. The eight specimens demonstrate the range of development covered from the less advanced to the more advanced members of the group. These frontal projections do not reveal the fact that the sacs, as they become larger, curve dorsally to a position lateral to the esophagus, embracing it on each side. Thus the esophagus comes to lie within the two prongs of a fork. The right main bronchus soon shows a tendency to be longer and directed caudally, the left bronchus being shorter and more transverse.
In the preceding paragraph attention has been restricted to the epithelium, the seemingly dominant tissue of the lung. There now clusters about it a zone of condensed mesenchymal tissue, some of the cells of which are undergoing active angiogenesis. An extensive capillary network is taking the form of a basketlike envelope enclosing the mesenchymal tissue of the lung. It is fed from above, as is shown in figure 14-4, by irregular bilateral channels from the plexiform pulmonary aortic arch on each side. These channels become the pulmonary arteries. The pulmonary plexus is drained below by a short stem anastomosing with the dorsal surface of the left atrium. This anastomosis appears to be a joint product participated in by a thin fold of the atrial wall and the developing capillaries of the pulmonary mesenchyme, an example of affinity of one endothelial outgrowth for another. It is later that subsequent adjustments transform this primary anastomosis into the paired right and left pulmonary veins. This development is facilitated by the fact that the mesenchymal zone in which it occurs is continuous between the cardiac wall and the bifurcation of the lung. The mesenchymal cells that invest the epithelial lung are derived from the overlying coelomic wall. In stage 12 the active proliferation of these coelomic cells and their detachment from the surface (fig. 12-1QA) have already been noted. They can be traced as they move in toward the lung and alimentary epithelium, leaving behind them a layer of cells that will finally constitute the mesothelium. This is the condition in the present stage, as shown in figure 14-7E. The lung is thus composed of an innermost epithelial tubular system surrounded by a richly vascularized mesenchymal zone, which in turn is partially enclosed by a mesothelium (pleura) facing the coelom.
At the level shown in figure 14-7E, the mesenchymal layer of the lung is still blended with that of the esophagus. The latter can be seen in the center of the section, and the circular contours of its mesenchymal layer are marked by their deeply staining capillaries.
Urinary System
(figs. 14-6 and 14-7) In embryos of this stage the mesonephros is well along in its organogenesis. The steps in this process are made easier to follow by the fact that the development occurs progressively in a rostrocaudal direction. Thus in a single specimen, as shown in figure 14-6, one can retrace the gradation, from the units just starting at the caudal end as simple unattached vesicles, to the more advanced units at the rostral end, which have partially vascularized glomerular capsules, each connected by an S-shaped tubule with the mesonephric duct.
Here again is an organ in which the epithelial elements constitute its primary tissue and seem largely to determine its form. The non-epithelial mesonephric elements, though necessary complements for epithelial-mesenchymal interaction, give the appearance of being subsidiary. It has already been seen that the coelomic surface cells possess various inherent potentialities. The surface of the coelom can be mapped in definite areas in accordance with the distribution of these various kinds of surface cells. Depending on the area, there are produced such diverse tissues as cardiac muscle, the framework of the liver, and the connective tissue and muscular coats of the esophagus, stomach, and intestine. In the present case the coelomic cells give rise to epithelium. Running along each side of the median plane is a narrow strip of coelom where, by the proliferation and delamination of its surface cells, there is produced a longitudinal series of epithelial tubules that constitute the units of the mesonephros. This follows the manner in which nephric elements were formed in previous stages, and it is now about to be repeated, with certain modifications, in the development of the metanephros, which is still in the primordial state of a budding ureter with its nephrogenic capsule (fig. 14-6).
One can go a step further in regard to the inherent constitution of these coelomic epithelial tubules. Not only do they become tubules, but from the beginning they show regional differentiation. The proximal end promptly blends with and opens into the mesonephric duct, and this part of the tubule persists as a collecting duct. The distal free end at the same time begins its expansion into a highly specialized part of the tubule, namely the mesonephric corpuscle. The intervening central segment of the tubule becomes the convoluted secretory portion. Embryos in this stage are especially favorable for the study of the process of formation of the mesonephric corpuscle. As can be seen in figure 14-6, the proliferation of the tubular epithelium at the free end results in its maximum expansion. This occurs in such a way as to produce an indented flattened vesicle, known as a glomerular capsule. As seen in section, it has an arched floor-plate several cells thick and a thin, single-layered roof membrane. The two are continuous with each other but are very different in their potentialities. The roof membrane becomes attenuated as an impermeable membrane. The floor plate continues active proliferation and many of its cells were believed by Streeter to delaminate and apparently become angioblasts, participating in the formation of the vascular glomerulus and its supporting tissues. It is now maintained, however, that the glomerular capillaries (in the metanephros) come from adjacent vessels and never develop in situ from epithelial cells (Potter, 1965). Further details of renal development have been provided by several authors (e.g., Potter, 1972).
The residual cells facing the capsular lumen in the mesonephros at stage 14 are reduced in the more advanced phases to a single layer, covering and conforming everywhere to the tabulations of the underlying capillary tufts.
Angiogenesis around the secretory part of the tubule is not far advanced. Angiogenic strands connect with the caudal cardinal vein, and throughout the mesonephros there are isolated clumps of angioblasts, particularly around the capsules. In figure 14-7F, G is shown a continuous row of six mesonephric tubules, covered above by coelomic mesothelium and closely adjacent to the caudal cardinal vein below. These show the typical difference in complexity of the three parts of the tubule: (1) collecting duct, (2) secretory segment, and (3) glomerular capsule. In figure 14-7H the same structures are shown in the same plane at a more advanced phase, at the transition into stage 15.
Reproductive System
The primordial germ cells migrate from the mesentery to the gonadal ridges (Witschi, 1948). Each gonadal ridge appears as a mesodermal proliferation along the medial surface of the mesonephros (Witschi, 1948; Jirásek, 1971).
Nervous System
Median-plane views of three brains typical of stage 14 are shown in figure 14-8. The constancy in the detailed form of these brains is a striking illustration of the undeviating character of the process of organogenesis. All of them are definitely larger than the brains found in the preceding stage. When the representatives of the two groups are measured, it is found that the dimensions of the brain at stage 14 are about 50 percent larger than those at stage 13.
In the spinal region, the neural tube presents little variation in form from level to level, which is what one would expect from its uniformity in the mature individual. In the present stage the wall of the spinal cord is composed of three distinct zones: (1) the ventricular or ependymal zone, which contains the germinal cells from which neurons, glial cells, and ependymal cells will be derived, (2) the mantle or intermediate zone, in which characteristic clusters of precocious neurons give origin to the ventral rootlets, which thread their way ventrolaterally, and (3) the marginal zone, an expanding cell-free territory. This differentiation is more advanced in the motor or ventral half of the cord than in the dorsal half, and it is more advanced in the cervical region than in the more recently laid down caudal end. The dorsal funiculus develops in the cervical area and reaches C2.
The roots of the hypoglossal nerve have united. The cerebellar primordium is present now as a thickening of the alar plate in rhombomere 1. It consists of ventricular and intermediate layers, but does not possess a marginal layer. The sulcus limitans proceeds from the rhombencephalon throughout the mesencephalon. In the midbrain two parts can still be distinguished; the marginal layer covers three-quarters of the surface, the tectal area being the least developed. Cranial nerve 3 is present in all embryos. In the diencephalon the distinction between Dl and D2 is no longer possible. Between dorsal and ventral thalami a sulcus begins to form; it seems to be the beginning of the sulcus medius. The chiasmatic plate thickens and hence is distinct in the median plane. The preoptic sulcus (optic groove of Streeter) rostral to the optic stalk runs from the floor of one optic evagination to that of the other. The future medial striatal ridge, a part of the subthalamus, begins to form. The primordia of the corpora striata of the two sides are connected by the commissural plate. The olfactory areas also merge in this striatal field.
In the telencephalon the future hemispheres begin to be marked off by an internal crest: the velum transversum. In the median plane the telencephalon medium can be distinguished from the diencephalic roof by its thinner wall. The terminal-vomeronasal crest begins to make contact with the brain and clearly indicates the olfactory area.
Sections selected at intervals through the midbrain and forebrain are shown in figure 14-8, sections A–E. The levels at which they are taken are indicated by dotted lines on the drawing of the third brain. In these sections it can be seen how the various areas at this time are marked off from one another by ridges or grooves. The thickness of the wall may be misleading if the section is partially tangential. Thus one can identify functional areas by the contours of the wall long before the component cells have acquired individualities in form. Section C is a particularly good example of well-defined territories, such as can be seen in carefully preserved specimens.
The three brains of embryos belonging to this stage, illustrated in figure 14-8, show a convincing uniformity in the outlines of their respective functional areas. Each area has its own individual site, size, and form.
To summarize the main subdivisions of the brain, it has been seen that (l) the prosencephalon, mesencephalon, and rhombencephalon can be distinguished as regions in the completely open neural folds at stage 9, (2) the diencephalon and the telencephalon medium can be detected in stage 10, and (3) the future hemispheres and the cerebellar plates appear by stage 14.
The pons of the postnatal brain, in the sense of the apparent bridge shown by Eustachius and Varolius in the 16th century, appears early in the fetal period but does not possess a counterpart during the embryonic period proper. The pontine region, however, can be distinguished very early by the attachment of the appropriate cranial nerves (7/8 at stage 10, 5 at stage 11, and 6 at stage 15) and in relation to the summit of the pontine flexure (by about stage 16). The pontine flexure appears gradually during stages 14–16 but its initial appearance is not readily visible in lateral views.
The spinal nerves are now attached to the muscular primordia, and rami communicantes are present (Bossy, 1980c).
The innervation of the upper limb buds begins at stage 14, that of the lower limb buds at stage 15. The cutaneous innervation follows the external form but is one or two stages behind (Bossy, 1982). Diagrams purporting to show the dermatomes during the embryonic period are, at least in the present stage of knowledge, figments of the imagination.
Eye
(fig. 14-9) In stage 14 the lens disc shows various degrees of indentation, and in the more advanced members of the group it is cup-shaped, communicating with the surface by a narrowing pore. There may be a slight variation between the right and left vesicles, but as long as one of them is definitely open to the surface of the embryo, the specimen is included in this stage. The final closure and pinching off occur subsequently.
Sections of the eye, typical of stage 14 and arranged in order of the indentation of the lens ectoderm, are shown in figure 14-9. It will be seen that the lens closely follows the retina in the rate of invagination, and as it does this it becomes partly enclosed by the latter. A significant and constant accompaniment of lens development is the accumulation of a clump of disintegrating cell remnants in the lumen of the lens pit. Photographs of this material are shown in figure 14- 7A–C. From an examination of these and the serial sections themselves one concludes that they are nuclear remnants extruded by the lens ectoderm. The latter is in a state of active cell division, and many of the daughter nuclei can be seen migrating or perhaps being crowded to the surface. Different stages in the emergence of these “unwanted nuclei” and their aggregation in free clumps in the lens depression can be recognized.
A uveocapillary lamina becomes defined. As the retinal disc is invaginated to form the optic cup, the retinal (“choroid”) fissure is delineated. The inverted layer of the optic cup comprises a terminal bar net (the future external limiting membrane), proliferative zone (the mitotic phase), primitive zone (the intermitotic phase), marginal zone, and an internal limiting membrane (O'Rahilly, 1966). The developing cerebral stratum of the retina is closely comparable to the developing cerebral wall (ibid, fig. 8).
Ear
Aside from becoming about one-fourth larger and showing an increased delineation and tapering of its endolymphatic appendage, there are no striking characteristics in the otic vesicle at this stage that are not already present in the more advanced members of the preceding group. If, however, three-dimensional reconstructions are made of them, it is seen that the oval vesicle of stage 13 has become elongated by the ventral growth of the primordium of the cochlear duct. One can also recognize the beginning differential thickening of the walls of the vesicle, the thicker areas marking the location of what are to be the definitive parts of the labyrinth. A section typical of this stage is shown in figure 14-10. It can be compared directly with those illustrated in figure 13-12.
Proliferation of mesodermal cells indicates the beginning of the condensation that precedes the cartilaginous otic capsule, which will enclose the otic vesicle.
Specimens of Stage 14 Already Described
- H. Braus, 6-mm embryo, Heidelberg. Enlarged views of this well-preserved curettage specimen are included in the Hochstetter (1907) portfolio of pictures of the outer form of a series of human embryos. The nasal plate is more advanced than in others of this stage, but the limb buds are like those of the older members of the group.
- E. Gasser, 6.5 mm. Leyding embryo, Marburg Anatomisches Institut. Description of this advanced embryo is in the Keibel and Elze Normentafeln (1908).
- J. A. Hammar, 5 mm Vestberg embryo, Anatomisches Institut, Uppsala. Embryo described by Hammar in the Keibel and Elze Normentafeln. Typical sections and models of the pharyngeal region and gut are illustrated. This well-preserved embryo has been studied by several investigators, including Hammar on the development of the foregut, salivary glands, and tongue, and Broman on the development of the diaphragm and omental bursa.
- Wilhelm His (1831-1904), Embryo R, 5.5 mm. Illustrated by His (1885). It was concluded by His that this embryo approximated the Fol embryo (stage 13). The lens pit indicates stage 14. This assignment is supported by other features: e.g., the ductus venosus.
- Wilhelm His (1831-1904), embryo B, 7 mm, and embryo A, 7.5 mm. These two embryos are described jointly, in great detail, by Wilhelm His (1831-1904) (1880). Embryo A is evidently a little more advanced than embryo B and perhaps should be placed in stage 15.
- Keibel, 6.5 mm embryo “forensis.” Embryo described in the Keibel and Elze Normentafeln. This embryo was used by Keibel in a series of studies on the development of the urogenital system.
- Keibel, 6.8 mm embryo, No. 501. Embryo included in the Keibel and Elze Normentafeln. It is described in monographic form by Piper (1900).
- Strabl, 6.75 mm embryo (Walther), Giessen. Described in the Keibel and Elze Normentafeln. The external form is pictured by Hirschland (1898).
- 6.5 mm embryo, University of Minnesota. Drawings of external views of this and of a 2.9-mm (stage 11) embryo were published by Wells and Kaiser (1959).
- Free Hospital for Women, Brookline, Massachusetts, No. 33, 6 mm, No. 29, 7 mm, and No. 31, 7 mm. The histochemistry of these embryos of stage 14 was studied by McKay et al. (1956).
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ Odgers PN. Some observations on the development of the ventral pancreas in man. (1930) J. Anat., 65(1): 1-7. PMID 17104298
- ↑ O'Rahilly R. and Tucker JA. The early development of the larynx in staged human embryos. (1973) Ann. Otol Rhinol Laryngol, 82, Suppl. 7: 1-27.
See also Müller F & O'Rahilly R. (1988). The first appearance of the future cerebral hemispheres in the human embryo at stage 14. Anat. Embryol. , 177, 495-511. PMID: 3377191
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 26) Embryology 1987 Developmental Stages In Human Embryos - Stage 14. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_14
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G