User:Z3417843: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
*Lab 7 --[[User:Z3417843|Z3417843]] ([[User talk:Z3417843|talk]]) 11:06, 17 September 2014 (EST) | *Lab 7 --[[User:Z3417843|Z3417843]] ([[User talk:Z3417843|talk]]) 11:06, 17 September 2014 (EST) | ||
*Lab 8 --[[User:Z3417843|Z3417843]] ([[User talk:Z3417843|talk]]) 11:01, 24 September 2014 (EST) | *Lab 8 --[[User:Z3417843|Z3417843]] ([[User talk:Z3417843|talk]]) 11:01, 24 September 2014 (EST) | ||
*Lab 9 | *Lab 9 --[[User:Z3417843|Z3417843]] ([[User talk:Z3417843|talk]]) 12:00, 8 October 2014 (EST) | ||
*Lab 10 | *Lab 10 | ||
*Lab 11 | *Lab 11 | ||
Revision as of 11:00, 8 October 2014
Welcome to the 2014 Embryology Course!
- Links: Timetable | How to work online | One page Wiki Reference Card | Moodle
- Each week the individual assessment questions will be displayed in the practical class pages and also added here.
- Copy the assessment items to your own page and provide your answer.
- Note - Some guest assessments may require completion of a worksheet that will be handed in in class with your student name and ID.
| Individual Lab Assessment |
|---|
|
| Lab 12 - Stem Cell Presentation Assessment | More Info | |
|---|---|---|
| Group | Comment | Mark (10) |
| 1/8 |
|
7 |
| 2 |
|
7.5 |
| 3 |
|
7.5 |
| 4 |
|
8.5 |
| 5 |
|
8.5 |
| 6 |
|
8.5 |
| 7 |
|
7.5 |
Lab Attendance
- Lab 1 --Z3417843 (talk) 12:45, 6 August 2014 (EST)
- Lab 2 --Z3417843 (talk) 11:06, 13 August 2014 (EST)
- Lab 3 --Z3417843 (talk) 11:10, 20 August 2014 (EST)
- Lab 4 --Z3417843 (talk) 12:16, 27 August 2014 (EST)
- Lab 5 --Z3417843 (talk) 12:52, 3 September 2014 (EST)
- Lab 6 --Z3417843 (talk) 11:19, 10 September 2014 (EST)
- Lab 7 --Z3417843 (talk) 11:06, 17 September 2014 (EST)
- Lab 8 --Z3417843 (talk) 11:01, 24 September 2014 (EST)
- Lab 9 --Z3417843 (talk) 12:00, 8 October 2014 (EST)
- Lab 10
- Lab 11
- Lab 12
Individual Online Assessment
Assessment 1
Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization.
Vitamin D plays an essential role in the process of human reproduction. Women in their reproductive age are more prone to vitamin D deficiency. In recent studies, the levels of vitamin D in the body is somewhat correlated to the prevalence of vitamin deficiency in the population. For example, 79% of women undergoing IVF is suffering from vitamin deficiency. In a different study, female rats were tested for the link between vitamin D deficiency and infertility. Two groups of female rats were given different diets, one rich in vitamin D and the other low in vitamin D. Female rats fed with the vitamin D deficient diet had 75% reduced fertility and 30 % smaller litter sizes compared to the group fed with vitamin D rich diet.
The goal of this study is to investigate the correlation between vitamin D deficiency and lower rates of clinical pregnancy after IVF in women. Serum 25-hydroxy-vitamin D, 25(OH)D, is a marker for vitamin D levels. 173 women gave consent to participate in the experiment. The serum was used to determine the vitamin D level of each and were segregated in terms of their 25(OH)D levels, whether they are sufficient or insufficient.
It is unclear how vitamin D affects fertility in women. However, it is likely that vitamin D has an effect on the implantation process. Findings show that women in the sufficient group had higher rates of clinical pregnancy after IVF as well as implantation rates. It also suggested that vitamin D supplementation is an easy and cost-effective way of combatting vitamin D insufficiency. Furthermore, it suggested that vitamin D status should be taken into consideration when assessing for infertility in women.
Neurotensin Enhances Sperm Capacitation and Acrosome Reaction in Mice.
In order for a spermatozoa to be fertile, it has to undergo capacitation. Capacitation refers to the change in the physiology of spermatozoa when inside the uterus, which allows them to penetrate and fertilise the oocyte. While in the uterus, these cells are subject to influence by various factors that affect sperm function, Neurotensin (NT) is a hormone known to have multiple functions. It can function as a neurotransmitter and even participate in gastrointestinal motility and secretion. NT stimulation results to the production of secondary messengers such as cAMP and Ca2-, which are important for fertility in sperm. However, its function relating to reproduction is still unknown. This study is aimed to clarify the role and function of NT in the capacitation and acrosome reaction in spermatozoa.
Sperm were gathered from the epididymis of male mice, aged more than 12 weeks. To test for the effect of NT on acrosome reaction, the suspension of spermatozoa capacitated in HTF medium were divided into microtubules and given varying amounts of NT stock solution. Under a fluorescence microscope, the sperm that underwent acrosome reaction were identified and the acrosome reaction rate was calculated. To test for NT concentration, cumulus oocyte-complexes (COCs) were cultured in media with FSH and EGF.
The study’s results showed a rapid increase in the rate of acrosome reaction in sperm depending on the dosage of NT stock solution introduced. Acrosome reaction is triggered by the increase in levels of calcium ion (Ca2+), which occurs when NTR1 is stimulated. However, it is still unsure whether this is the direct cause for acrosome reaction in the spermatozoa. For the NT concentration test, it showed that the addition of FSH and EGF stimulated the production of NT in cumulus cells. All together, these evidences show that neurotensin play a role in the capacitation of spermatozoa and acrosome reaction.
--Mark Hill These are good summaries (5/5).
Assessment 2

--Mark Hill All the information is associated with the file, with some minor format issues, the reference link should also be on your own page. You should have also used a file name that describes the image not File:Heartfibertracttractography.png which is not identifiable. (4/5)
Assessment 3
--Mark Hill These are relevant articles, but you have not identified your project sub-section or explained in a sentence why you have selected these references (4/5).
Assessment 4
Migration and Differentiation of Human Umbilical Cord Stem Cells After Heart Injury in Chicken Embryos
With the increasing cases of myocardial infarction, or heart attack, the heart is a popular organ for regenerative cell therapy. Stem cells are known for their ability to differentiate and form into different organs, given the right signalling and stimulation, including myocardial structures. Because of this, stem cells are widely used in regenerative cell therapy. Cardiac injuries in animal models have been treated with the use of stem cells, including a few clinical trials of patients suffering myocardial infarction. Evidence shows that in a myocardial injury, stem cells are activated. Although, there is still little knowledge on the mechanisms and signalling pathways regarding the differentiation of stem cells. This study aims to analyse human umbilical cord blood stem cells (scHUCBs) and their behaviour when grafted onto the hearts of chicken embryos, whether normal (control) or damaged.
Stem cells where extracted by processing the whole blood from a human umbilical cord. The chicken eggs used were incubated and windowed to give access to the embryo. With a microinjector, scHUCBs were injected into the epicardium of the left or right ventricle of the chicken embryo’s heart. Some hearts were injured, specifically at the myocardial wall, using a needle. Experimental hearts, i.e. hearts with the microinjuries, had the stem cells introduced at the farthest site of the contralateral ventricle to test for the migration of scHUCBs. In addition to that, stem cells were grafted at different times (6hr, 12hr, 24hr later), to test for the effect of time on the result of this regenerative procedures.
The study’s results showed that the stem cells spread throughout the heart walls and it’s chamber’s walls. Stem cells have differentiated into myocardiocytes and were also seen within the lumen of blood vessels, suggesting that the stem cells have differentiated into endothelial cells as well. Majority (95%) of the grafted cells, introduced to the injured heart, migrated to the injury. This evidence proves that signals may promote the migration and differentiation of stem cells towards sites of injury and other parts of the organ. Grafted cells that were introduced 6hr after the injury showed a similar migration pattern; whereas grafted cells introduced 24hr later showed no signs of migration at all. This evidence shows that the gap between the ischaemic event and grafting could affect and predict the likely result of regenerative cell therapy.
- ↑ <pubmed>18393637</pubmed>
There are three vascular shunts in the vasculature of the fetus:
- foramen ovale - passage of blood from the right atrium to the left atrium, which bypasses blood flow to the right ventricle
- ductus arterioles - passage of blood from the pulmonary trunk to the aorta, which bypasses blood flow to the lungs
- ductus venous - passage of blood from the umbilical vein to the inferior vena cava, which bypasses blood flow to the liver
Assessment 5
Meconium Aspiration Syndrome (MAS) is a rare condition in neonates, that could lead to death if untreated or failed to be detected. It occurs when a foetus aspirates meconium-stained amniotic fluid (MSAF) during development, i.e. before or during labour. Meconium refers to the first stool of the infant, which consists of the contents of foetal gastrointestinal tract during development. This is excreted as a greenish-brown material in utero or post birth. In response to stress, e.g. hypoxia-ischaemia, stimulation of the GIT can occur, which results to peristalsis and relaxation of the anal sphincter. This will then result to the release of meconium by the foetus to the amniotic fluid, which contaminates it and is now meconium-stained. Foetal gasping is also a reflex in response to stress. This reflex leads to the aspiration of MSAF and result to MAS. This process allows the meconium to enter the foetal upper airway and eventually, the distal airways. A foetus may be hypoxic due to abnormal patterns in the heart rate.
In return, meconium aspiration can cause hypoxia in neonates via:
- airway obstruction
- surfactant dysfunction
- chemical pneumonitis
- pulmonary vasoconstriction
Signs of tachypnoea, cyanosis (when the neonate appears blue), and chest inflation are present in neonates with MAS. Further complications of this abnormality could lead to more serious diseases, such as atelectasis and pneumothorax, or respiratory failure. MAS can also result to chemical pneumonia and pneumonitis. There are currently a few methods of preventing or managing MAS, such as intratracheal intubation and suctioning, which was reported to have 100% survival. [1]
- ↑ <pubmed>19381312</pubmed>
- Jane Yizhen Lim, S. Arulkumaran Meconium aspiration syndrome Obstetrics, Gynaecology & Reproductive Medicine: 2009, 18(4);106-109
- Benjamin J. Stenson, Allan D. Jackson Management of meconium aspiration syndrome Pediatrics and Child Health: 2009, 19(4);174-177
Assessment 6
The pituitary gland is an endocrine gland responsible for the secretion of multiple hormones, including luteinizing-hormone (LH), follicle-stimulating hormone (FSH), and growth hormone (GH). Glucocorticoids are a class of steroids that have a role in the GH production in the pituitary gland. Adrenal glucocorticoid corticosterone (CORT) are essential for somatotrophic cell differentiation, which are the cells that produce GH. Another important factor for the differentiation of somatotrophic cells is cycloheximide (CHX), a protein synthesis inhibitor. In this research article, the authors study the effect of glucocorticoids on the gene expression of cells in the embryonic pituitary gland with the use of chicken embryos. With the use of a custom chicken embryo, the direct and indirect glucocorticoid-regulated genes were identified. This was conducted by determining the transcriptional profiles in pituitary cells influenced by CORT with or without CHX.
The presence of CHX blocks the induction of CORT to GH mRNA. This suggests that one or more protein is needed for the process to occur. Their findings show that the presence of CORT have diverse effects on the expression of 396 genes and that 11 of these were induced in the presence of CHX. The presence of CORT alone influenced the mRNA levels in these genes. DEXRASI and RASDVA, two of the identified genes, are believed to have a role in the regulation of CORT in GH mRNA. Another identified gene was FKBP5, which is involved in the stimulation of GH release in somatotrophic cells. The 11 genes that underwent GH induction, despite the presence of CHX, could be involved as a mediator on the effects of glucocorticoids on embryonic GH production.
- ↑ <pubmed>23572539</pubmed>
The embryonic layers and tissues concerning the development of teeth include the ectoderm of the first pharyngeal arch and neural crest, ectomesenchymal cells. Odontoblasts are neural crest-derived mesenchymal cells that secrete predentin, which calcifies later in development to form the dentin of the teeth. Ameloblasts are cells that arise from the inner enamel epithelium, which produce the enamel of the teeth.
Assessment 7
Assessment 8
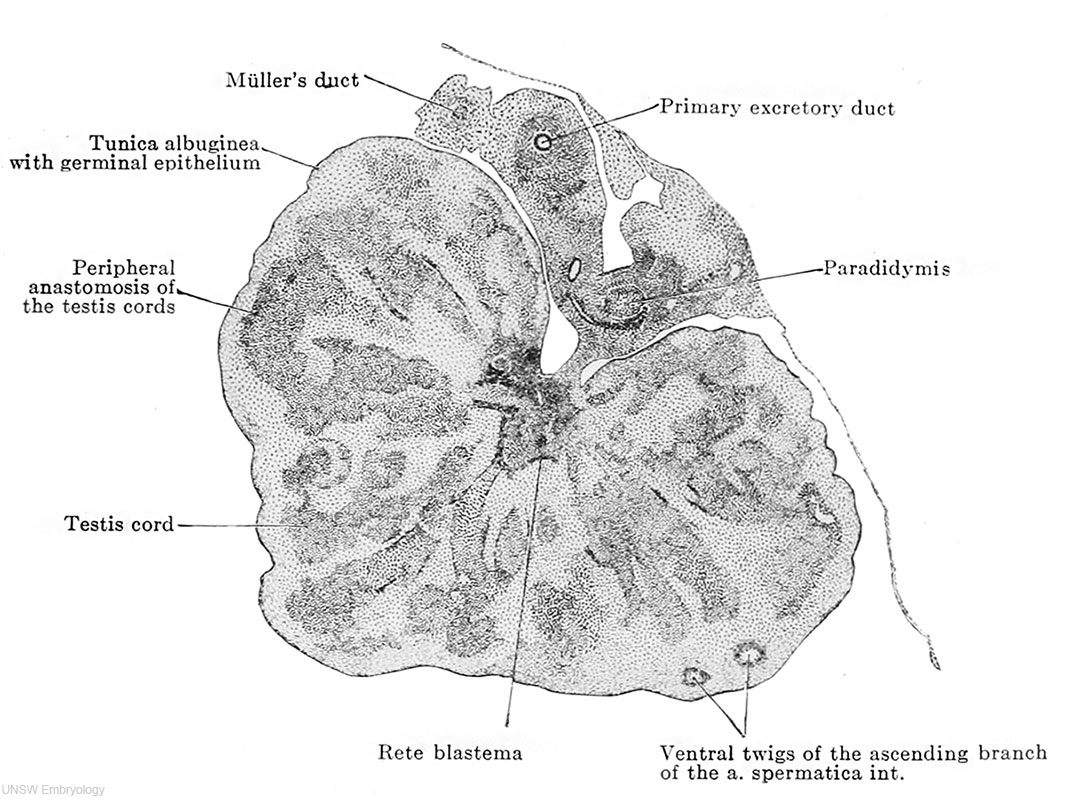
Embryonic Development of the Testis In the embryo, gonads come from three sources: the mesothelium, underlying mesenchyme, and primordial germ cells. Primordial germ cells (PGCs) are undifferentiated sex cells. By week 3, they migrate to the primitive streak at the stage of gastrulation. At week 5, the mesothelium and underlying mesenchyme proliferate and forms a bulge on the medial side of the mesonephros. This structure is called the gonadal ridge. In the same week, the gonadal cords grow into the underlying mesenchyme. Both structures form an indifferent gonad and in XY embryos, this becomes the testis. By week 6, PGCs go to the hindgut yolk sac junctional region and then migrate to the gonadal ridge at early embryonic development.
After that, gametogenesis occurs, where PGCs enter the gonads and differentiate. The Y chromosome contains a protein-coding gene called sex-determining region Y gene, better known as SRY gene. This gene transforms supporting cells into Sertoli cells. Sertoli cells are responsible for signalling cells to undergo male gonadal differentiation. They secrete anti-Mullerian hormones, which have a role in the differentiation of internal genital organs, ducts, and gonads. They also transform sex-hormone-secreting cells into interstitial cells or Leydig cells, which are responsible for the release of testosterone. Sertoli cells also affect the differentiation of germ cells; however, this process is arrested until after birth. Testis-determining factor (TDF) is also regulated by the Y chromosome and is responsible for the differentiation of gonadal cords into seminiferous cords or testis cords.
By week 7, the testis starts to develop. As stated, anti-Mullerian hormones have a role in differentiation of gonads. In males, the paramesonephric duct degenerates in the presence of anti-Mullerian hormones. The mesonephric duct, a.k.a Wolffian duct, differentiates in the presence of testosterone. In the embryonic male gonads, there are two main portions, the mesonephric duct and the testis cords. The testis cords contain the Sertoli cells and germ cells. Later in development, it becomes the seminiferous tubules which are responsible for the production of spermatozoa. The mesonephric duct becomes the epididymis. In the mesonephric duct, projections appear that grow towards the testis cords. These projections become the rete testis. The remaining mesonephric duct outside the gonad becomes the ductus deferens. (Week 8)
(Note: add Moore and Persaud reference. Ask how to)
(Note: add reference here as well. Ask how to.)