User:Z3417753
Welcome to the 2014 Embryology Course!
- Links: Timetable | How to work online | One page Wiki Reference Card | Moodle
- Each week the individual assessment questions will be displayed in the practical class pages and also added here.
- Copy the assessment items to your own page and provide your answer.
- Note - Some guest assessments may require completion of a worksheet that will be handed in in class with your student name and ID.
| Individual Lab Assessment |
|---|
|
| Lab 12 - Stem Cell Presentation Assessment | More Info | |
|---|---|---|
| Group | Comment | Mark (10) |
| 1/8 |
|
7 |
| 2 |
|
7.5 |
| 3 |
|
7.5 |
| 4 |
|
8.5 |
| 5 |
|
8.5 |
| 6 |
|
8.5 |
| 7 |
|
7.5 |
Lab Attendance
- Lab 1 --Z3417753 (talk) 12:54, 6 August 2014 (EST)
- Lab 2 --Z3417753 (talk) 11:21, 13 August 2014 (EST)
- Lab 3 --Z3417753 (talk) 11:37, 20 August 2014 (EST)
- Lab 4 --Z3417753 (talk) 11:45, 27 August 2014 (EST)
- Lab 5 --Z3417753 (talk) 11:48, 3 September 2014 (EST)
Online Assessment 1
Article 1
<pubmed>23148203</pubmed>
This study is an analysis of the optimal time from oocyte to preimplantation embryo development for biopsy and preimplantation genetic screening. The discovery of the optimal time can then be used to detect any abnormal chromosomal separation patterns in embryos from older mothers (>40 years old). The study was a longitudinal cohort study involving 9 infertile couples and 21 sets of complete chromosomal screening data, including polar bodies 1 + 2 and their corresponding blastomeres and trophectoderm samples.
METHODS →infertile couples with a good response to controlled ovarian stimulation were enrolled in the study and underwent IVF. Polar bodies, blastomeres and trophectoderm samples were biopsied and analysed by array comparative genomic hybridisation. The chromosomal segregation patterns were analysed from these results and used to deduce the origin of aneuploidy. The results were also used to examine the accuracy of polar body and cleavage-stage preimplantation genetic screening strategies.
RESULTS → Since preimplantation genetic screening tests have been conducted at different times throughout the preimplantation window, it is possible that critical bits of information regarding chromosomal segregation patterns have been missed. Thus, by performing such tests at an optimal time, we are better able to understand these meiotic chromosomal segregation patterns and therefore potentially increase the success rates of in-vitro fertilisation. This study uses a sequential chromosome analysis of polar bodies and their corresponding embryos at both the cleavage and blastocyst stages in order to work out what stage is best to perform these genetic screening tests and biopsies, potentially increasing IVF success rate. The study showed that testing at the polar body stage was least accurate due to the high incidence of post-zygotic events and discovered that performing these tests later on in development (at the blastocyst stage) may produce more reliable results for the screenings, thereby achieving better chromosomal segregation pattern data. These results can now go on to be used for IVF research.
Article 2
<pubmed>23477909</pubmed>
This study examines the accuracy of using array comparative genomic hybridisation (array CGH) techniques for the analysis of first and second polar bodies in predicting aneuploidies of maternal meiotic origin in the cleavage stage embryos of women of advanced maternal age. It is known that aneuploidy is a common cause of pregnancy failure, miscarriage and abnormal pregnancy and most aneuploidy is due to maternal meiotic origin and increases exponentially as the mother approaches menopause.
METHOD → 20 couples requesting preimplantation genetic screening for advanced maternal age (=greater than or equal to 35 years old) and repeated implantation failure (more than 3 cycles), previous aneuploidy pregnancy or recurrent first trimester miscarriage underwent 16 controlled ovarian hyperstimulation cycles and 7 natural fresh cycles. Male partners had sperm parameters within the normal range except for 2 which had oligoasthenoteratozoospermia. Oocytes were retrieved by ultrasound-guided transvaginal aspiration 36 hours after beta-hCG administration. Once the oocytes were retrieved, biopsy of the first polar body was performed and the oocyte was inseminated using intracytoplasmic sperm injection. The following morning, each oocyte was checked for prouclei and extrusion of the second polar body to confirm fertilisation. The second polar body was then biopsied. The polar bodies were then analysed using array CGH analysis and the zona pellucida layer of the oocyte was dissolved. The zona-free embryo then underwent whole genome amplification and array CGH analysis in the cleavage stage.
RESULTS → It has been demonstrated in previous studies that a high correlation exists between the chromosomal status presented from polar body analysis and the actual chromosomes present in the zygotes of older mothers. Due to these results, this study uses polar body analysis and array CGH analysis of mature fertilised oocytes, to identify errors in meiosis within the polar bodies as well as the corresponding cleavage stage embryos. The results of the current study showed that nearly ALL aneuploidies detected in cleavage stage embryos were associated with copy number changes in the polar bodies (93%), indicating the high capability of polar bodies being used to predict aneuploidy and what is actually happening within the embryo.
Online Assessment 2
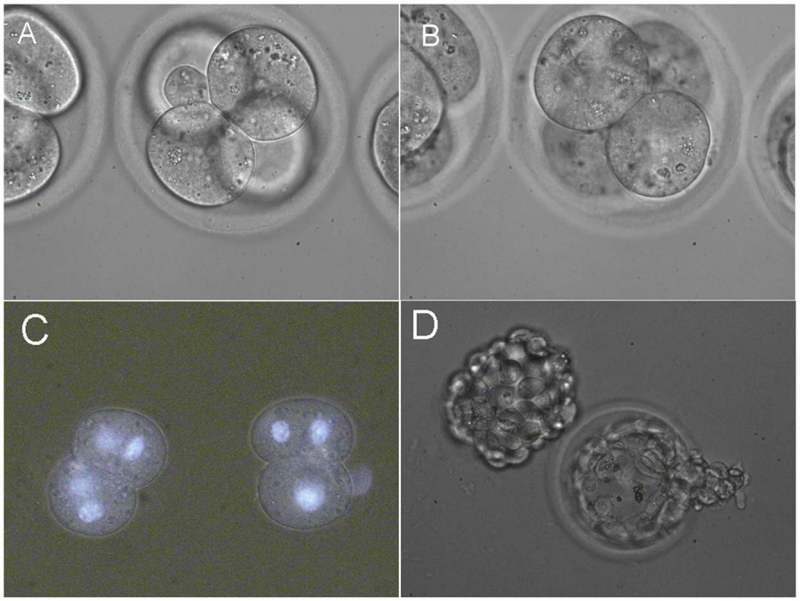
Fusion of two pairs of blastomeres inside 4-cell embryos[1]
Online Assessment 3
Current Research Models and Findings
--Z3417753 (talk) 22:57, 26 August 2014 (EST)
<pubmed>18367374</pubmed> <pubmed>15086026</pubmed> <pubmed>14641326</pubmed> <pubmed>11684660</pubmed> <pubmed>22127979</pubmed>
Online Assessment 4
Cord Stem Cell Article Findings
<pubmed>25101638</pubmed>
This article identifies acute liver failure as a devastating and debilitating illness that occurs within a short period of time, ultimately resulting in death of the patient if proper treatment is unavailable or it is simply too late to treat. It further identifies liver transplantation as the most effective treatment to date however, its application is limited due to an elevated risk of organ rejection and lack of liver donors. It is also known that human umbilical mesenchymal stem cells (hUCMSC) have the potential to differentiate into hepatocyte-like cells, functioning very similarly to hepatocytes as well as secrete certain factors to stimulate the proliferation of nearby hepatocytes, thereby promoting the rejuvenation of the host liver cells. The author hypothesised that by decreasing the amount of manipulation received by the mesenchymal stem cells in vitro, the carcinogenic risk was reduced. As a result, the therapeutic effect (amount of liver repair) of concurrently acting hUCMSC’s and hepatocyte-like cells can be ascertained by studying and comparing the two synchronous actions in acute liver failure mouse models.
The study induced acute liver failure in mouse models using D-galactosamine and lipopolysaccharide, causing the death of approximately 50% of the mice (necrosis of more than 50% of the hepatocytes). The mouse models’ therapeutic effects were then compared before and after the mesenchymal stem cells were differentiated into hepatocyte-like cells, by transplanting and injecting the cells into the tail vein. The results showed that almost ALL mouse were saved by the injection of the hepatocyte-like cells. Similarly, the injection of the hUCMSC’s also demonstrated their capability to repair liver damage, however, the population of these cells tested via the expression/ presence of human hepatocyte growth factor was minimal, suggesting that they allow the reversal of acute liver failure by differentiating into hepatocyte-like cells.
Overall, these results suggest that hUCMSC’s and hepatocyte-like cells are just as effective in therapeutic treatment of acute liver failure in mouse models and that hUCMSC’s play a larger role in stimulating the host hepatocyte repair.
Vascular Shunts
Three major vascular shunts exist within the circulatory system of the foetus:
1. FORAMEN OVALE --> the opening in the interatrial septum (wall between left and right atrium) that allows the flow of blood from the right atrium to the left atrium and has a valve to prevent backflow during the fetal period. It soon closes once right atrial pressure increases. The foramen ovalis then becomes the FOSSA OVALIS postnatally.
2. DUCTUS ARTERIOSUS --> muscular vessel that connects the pulmonary trunk to the aorta, thereby diverting bloodflow to the lungs and going straight into the aorta. After birth, as the amount of oxygen increases, the smooth muscle in the walls constricts closing off the passage. As the ductus arteriosus degenerates, all that is left behind if the LIGAMENTUM ARTERIOSUM.
3. DUCTUS VENOSUS --> a blood vessel that branches from the umbilical vein, allowing oxygenated blood from the placenta to be diverted from the fetal liver to the fetal heart. This shunt closes slowly during infancy and degenerates into the LIGAMENTUM VENOSUM.
Online Assessment 5
Laryngeal-tracheo-oesophageal Cleft is a rare congenital anomaly where there is an abnormal posterior communication between the larynx and pharynx, extending down between the trachea and oesophagus.Cite error: Invalid <ref> tag; refs with no name must have content
Normally, the larynx develops simultaneously from the endoderm (arising from the foregut region) and the mesenchyme (arising from the 4th + 6th pharyngeal arches. The division of the foregut is due to the fusion of the lateral walls of the foregut in the region of the larynx, thereby forming a septum that divides the foregut into a central part = LARYNGEAL-TRACHEAL TUBE as well as a dorsal portion = OESOPHAGUS. The mesenchymal portion (= TRACHEAL-OESOPHAGEAL SEPTUM) is located between the digestive and respiratory tracts and is the result of the separation of the two tracts. Apoptotic epithelial cells are also present at this septum, mainly in the ventral portion, but inactive in the dorsal portion.
There are a few models that explain tracheal-oesophageal anomalies, including Laryngeal-tracheo-oesophageal Cleft:
• INTRAEMBRYONIC PRESSURE → an intense curvature of the cervical region during heart development places pressure upon the oesophagus and as a result displaces it, leading to growth abnormalities.
• EPITHELIAL OCCLUSION → the oesophagus is solid during a stage of development but it is soon recanalised. If the recanalisation does not occur, growth abnormalities may occur.
• VASCULAR OCCLUSION → an abnormally communicating vessel could lead to avascularisation in the foregut, resulting in abnormalities. In the case of Laryngeal-tracheo-oesophageal Cleft, this means the laryngeal region.
• DIFFERENTIAL CELL GROWTH → abnormal cell growth in the ventral or dorsal part of the developing trachea or oesophagus could result in defects of the two tracts.Cite error: Invalid <ref> tag; refs with no name must have content
<pubmed>22151899</pubmed>| [2]</ref>