X Chromosome
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Humans have 23 pairs of chromosomes, 22 autosomes and a pair of sex chromosomes. Females have a pair of X chromosomes (46, XX) and males have one X and one Y Chromosome (46, XY). This section of notes introduces the X chromosome and its role in development.
There is a separate page discussing X Inactivation that occurs in all female development with one of the X chromosomes to provide the correct gene dosage. There is a separate page discussing Trisomy X, a genetic disorder where there is an additional copy of the X chromosome. There is a separate page discussing Fragile X Syndrome, a genetic disorder where part of the X chromosome is lost.
- Genes such as WNT4 (Wingless-Type Mmtv Integration Site Family, Member 4), NR0B1 (Nuclear Receptor Subfamily 0, Group B, Member 1; Dax-1) necessary for initiation of female pathway ovary development
- An early discovery (1961) was that in order to have correct levels of X chromosome gene/protein expression (gene dosage), females must "inactivate" a single copy of the X chromosome in each and every cell. The initiator of the X inactivation process was discovered (1991) to be regulated by a region on the inactivating X chromosome encoding an X inactive specific transcript (XIST), that acts as RNA and does not encode a protein.
- The genetic content of the X chromosome has been strongly conserved between species because these genes have become adapted to working as a single dose - Ohno's law
- X inactivation occurs randomly throughout the embryo, generating a mosaic of maternal and paternally derived X chromosome activity in all tissues and organs. This can be seen in the fur colour of tortoiseshell cats.
|

|
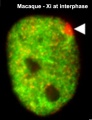
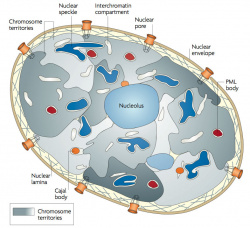
| Chromosome territories (interphase) | Chromosome (Chromatin) structure (mitosis) |
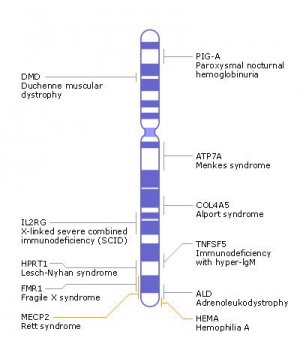
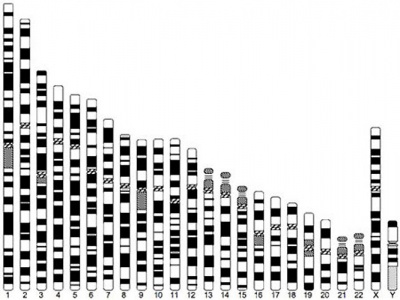
Human idiogram
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: X Chromosome |
X Chromosome Overview
- 1400+ genes
- 150 million base pairs
- Contains about 5% of the haploid genome.
- Genes encode house-keeping and specialized functions.
- Conserved in gene content between species.
- In females, one of the X-chromosomes is inactivated in each and every cell (known since 1961).
- This inactivation occurs during embryogenesis.
- X Inactivation appears to be random in somatic cells. (mosaic pattern)
- The process starts at the "X inactivation centre" and spreads along the chromosome.
Chromosome Statistics
May 2012 (EST)
| Length (bps) | 155,270,560 |
| Known Protein-coding Genes | 812 |
| Novel Protein-coding Genes | 24 |
| Pseudogene Genes | 780 |
| miRNA Genes | 128 |
| rRNA Genes | 22 |
| snRNA Genes | 85 |
| snoRNA Genes | 64 |
| Misc RNA Genes | 52 |
| SNPs | 2,172,609 |
Ohno's law
Ohno's law is a genetic evolutionary theory that suggests that the mammalian X chromosomes are conserved among species. Named after Susumu Ohno (大野 乾 (1928 – 2000) a Japanese-American geneticist and evolutionary biologist, and seminal researcher in the field of molecular evolution.
- Links: Biography | PMID 13730522 | PubMed OHNO S
Monotremes
Contrary to the above theory, the human X chromosome long arm genes are found on the monotreme X chromosome while the short arm genes are found distributed on the autosomes.
Genetic Inheritance
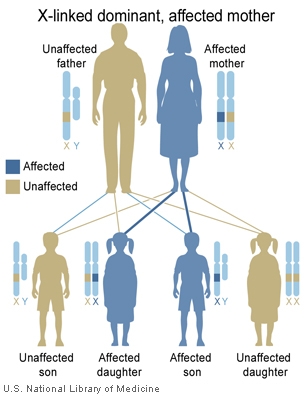
The follow cartoons show how genes located on the X chromosome can have different potential inheritance patterns.

X-Linked dominant (affected father) |
X-Linked dominant (affected mother) |
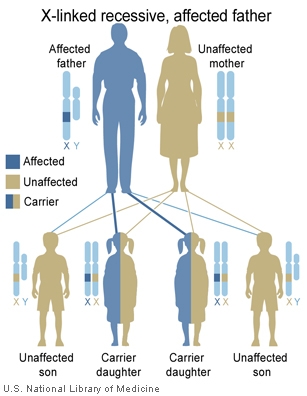
X-Linked recessive (affected father) |
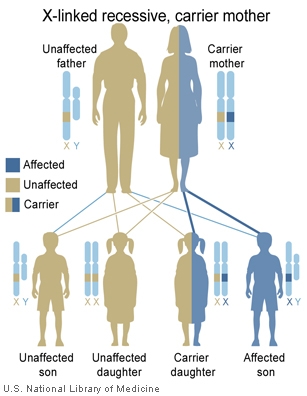
X-Linked recessive (carrier mother) |
- Inheritance Pattern images: Genetic Abnormalities | autosomal dominant | autosomal recessive | X-linked dominant (affected father) | X-Linked dominant (affected mother) | X-Linked recessive (affected father) | X-Linked recessive (carrier mother) | mitochondrial inheritance | Codominant inheritance | Genogram symbols | Genetics
Developmental Genes
BMP
| Table - Human Bmp Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols |
Synonyms | Chromosome |
|---|---|---|---|---|
| BMP15 | bone morphogenetic protein 15 | GDF9B | Xp11.22 | |
| Links: Developmental Signals - Bone Morphogenetic Protein | OMIM BMP2 | HGNC | Bmp Family | Sox Family | Tbx Family | ||||
| Human BMP Family | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SOX
| Table - Human Sox Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols | Synonyms | Chromosome |
|---|---|---|---|---|
| SOX3 | SRY-box 3 | PHP | Xq27.1 | |
| Links: Developmental Signals - Sox | OMIM | HGNC | Tbx Family | ||||
| Human SOX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TBX
| Table - Human Tbx Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols | Synonyms | Chromosome |
|---|---|---|---|---|
| TBX22 | T-box 22 | "CPX, CLPA" | Xq21.1 | |
| Links: Developmental Signals - Tbx | OMIM Tbx3 | HGNC | Bmp Family | Sox Family | Tbx Family | ||||
| Human TBX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abnormalities
Infertility
Primary ovarian insufficiency - depletion or dysfunction of ovarian follicles with cessation of menses before age 40 years.
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| BMP15 | Bone morphogenetic protein 15 | Xp11.22 | 300247 | 1068 | GC0XP050910 | Primary ovarian insufficiency |
| FMR1 | Fragile X mental retardation 1 | Xq27.3 | 309550 | 3775 | GC0XP147912 | Primary ovarian insufficiency |
| Table data source[2] (table 1) Links: fertilization | oocyte | ovary | | Female Infertility Genes | spermatozoa | testis | Male Infertility Genes | Genetic Abnormalities | ART | ||||||
| Female Infertility Genes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| MAGEB4 | MAGE family member B4 | Xp21.2 | 300153 | 6811 | GC0XP030260 | Azoospermia |
| TEX11 | Testis expressed 11 | Xq13.1 | 300311 | 11733 | GC0XM070528 | Azoospermia |
| Table data source[2] (table 1) Links: fertilization | spermatozoa | testis | Male Infertility Genes | Female Infertility Genes | oocyte | ovary | Genetic Abnormalities | ART | ||||||
| Male Infertility Genes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ Sangrithi MN & Turner JMA. (2018). Mammalian X Chromosome Dosage Compensation: Perspectives From the Germ Line. Bioessays , , . PMID: 29756331 DOI.
- ↑ 2.0 2.1 2.2 2.3 Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
Articles
Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, Muzny D, Platzer M, Howell GR, Burrows C, Bird CP, Frankish A, Lovell FL, Howe KL, Ashurst JL, Fulton RS, Sudbrak R, Wen G, Jones MC, Hurles ME, Andrews TD, Scott CE, Searle S, Ramser J, Whittaker A, Deadman R, Carter NP, Hunt SE, Chen R, Cree A, Gunaratne P, Havlak P, Hodgson A, Metzker ML, Richards S, Scott G, Steffen D, Sodergren E, Wheeler DA, Worley KC, Ainscough R, Ambrose KD, Ansari-Lari MA, Aradhya S, Ashwell RI, Babbage AK, Bagguley CL, Ballabio A, Banerjee R, Barker GE, Barlow KF, Barrett IP, Bates KN, Beare DM, Beasley H, Beasley O, Beck A, Bethel G, Blechschmidt K, Brady N, Bray-Allen S, Bridgeman AM, Brown AJ, Brown MJ, Bonnin D, Bruford EA, Buhay C, Burch P, Burford D, Burgess J, Burrill W, Burton J, Bye JM, Carder C, Carrel L, Chako J, Chapman JC, Chavez D, Chen E, Chen G, Chen Y, Chen Z, Chinault C, Ciccodicola A, Clark SY, Clarke G, Clee CM, Clegg S, Clerc-Blankenburg K, Clifford K, Cobley V, Cole CG, Conquer JS, Corby N, Connor RE, David R, Davies J, Davis C, Davis J, Delgado O, Deshazo D, Dhami P, Ding Y, Dinh H, Dodsworth S, Draper H, Dugan-Rocha S, Dunham A, Dunn M, Durbin KJ, Dutta I, Eades T, Ellwood M, Emery-Cohen A, Errington H, Evans KL, Faulkner L, Francis F, Frankland J, Fraser AE, Galgoczy P, Gilbert J, Gill R, Glöckner G, Gregory SG, Gribble S, Griffiths C, Grocock R, Gu Y, Gwilliam R, Hamilton C, Hart EA, Hawes A, Heath PD, Heitmann K, Hennig S, Hernandez J, Hinzmann B, Ho S, Hoffs M, Howden PJ, Huckle EJ, Hume J, Hunt PJ, Hunt AR, Isherwood J, Jacob L, Johnson D, Jones S, de Jong PJ, Joseph SS, Keenan S, Kelly S, Kershaw JK, Khan Z, Kioschis P, Klages S, Knights AJ, Kosiura A, Kovar-Smith C, Laird GK, Langford C, Lawlor S, Leversha M, Lewis L, Liu W, Lloyd C, Lloyd DM, Loulseged H, Loveland JE, Lovell JD, Lozado R, Lu J, Lyne R, Ma J, Maheshwari M, Matthews LH, McDowall J, McLaren S, McMurray A, Meidl P, Meitinger T, Milne S, Miner G, Mistry SL, Morgan M, Morris S, Müller I, Mullikin JC, Nguyen N, Nordsiek G, Nyakatura G, O'Dell CN, Okwuonu G, Palmer S, Pandian R, Parker D, Parrish J, Pasternak S, Patel D, Pearce AV, Pearson DM, Pelan SE, Perez L, Porter KM, Ramsey Y, Reichwald K, Rhodes S, Ridler KA, Schlessinger D, Schueler MG, Sehra HK, Shaw-Smith C, Shen H, Sheridan EM, Shownkeen R, Skuce CD, Smith ML, Sotheran EC, Steingruber HE, Steward CA, Storey R, Swann RM, Swarbreck D, Tabor PE, Taudien S, Taylor T, Teague B, Thomas K, Thorpe A, Timms K, Tracey A, Trevanion S, Tromans AC, d'Urso M, Verduzco D, Villasana D, Waldron L, Wall M, Wang Q, Warren J, Warry GL, Wei X, West A, Whitehead SL, Whiteley MN, Wilkinson JE, Willey DL, Williams G, Williams L, Williamson A, Williamson H, Wilming L, Woodmansey RL, Wray PW, Yen J, Zhang J, Zhou J, Zoghbi H, Zorilla S, Buck D, Reinhardt R, Poustka A, Rosenthal A, Lehrach H, Meindl A, Minx PJ, Hillier LW, Willard HF, Wilson RK, Waterston RH, Rice CM, Vaudin M, Coulson A, Nelson DL, Weinstock G, Sulston JE, Durbin R, Hubbard T, Gibbs RA, Beck S, Rogers J & Bentley DR. (2005). The DNA sequence of the human X chromosome. Nature , 434, 325-37. PMID: 15772651 DOI.
Emerson JJ, Kaessmann H, Betrán E & Long M. (2004). Extensive gene traffic on the mammalian X chromosome. Science , 303, 537-40. PMID: 14739461 DOI.
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Ensembl X Chromosome
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
| |
| Idiogram Chromosome Banding - The term refers to the light and dark pattern, seen after staining with a dye, of individual chromosomes identified in metaphase. It is only in meiosis and mitosis during metaphase that chromosomes can be easily identified, during the normal cell life (interphase) the chromosomes are unravelled and distributed within the nucleus in chromosome territories. A band is that part of a chromosome which is clearly distinguishable from nearby regions by appearing darker or brighter with one or more banding techniques. | |
| Genetic abnormality locations: 1-4 | 5-8 | 9-12 | 13-16 | 17-20 | 21-XY | sSMC | |
| |
| Links: Genetics | Abnormal Development - Genetic |
Cite this page: Hill, M.A. (2024, April 28) Embryology X Chromosome. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/X_Chromosome
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G