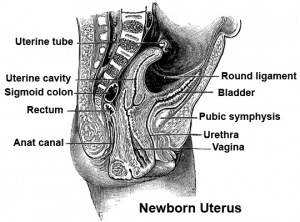
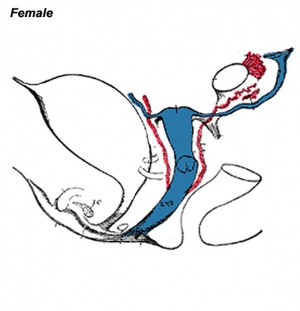
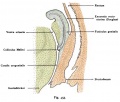
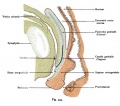
Vagina Development
| Embryology - 3 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The embryonic origin of the vagina has been a historically hotly debated issue with several different contributions and origins described. Current molecular studies show the whole vagina epithelium is derived from the paramesonephric (Müllerian) duct with bone morphogenic protein 4 (BMP4) reshaping the intermediate mesoderm-derived Müllerian duct into the vaginal primordium.[1] Transgenic studies in mice also identified the developmental origin of vaginal epithelium derived solely from Müllerian duct epithelium.[2] Vaginal development is also under negative control of androgens.
See also for external genitalia Integumentary System Development.
History
Acién's hypothesis, related to abnormalities and the embryology of the human vagina as deriving from the Wolffian ducts and the Müllerian tubercle.
Koff (1933)[3] coined the terms "sinovaginal bulb" and "vaginal plate" and proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium,
Bulmer (1957)[4] proposed that vaginal epithelium derives solely from urogenital sinus epithelium.
Robboy etal., (2017)[5] using Carnegie Collection embryos and immunostained for PAX2 (Müllerian epithelium) and FOXA1 (urogenital sinus epithelium). Their results support Bulmer's proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and is different from mouse vaginal development.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Vagina Development | Vagina Embryology |
Paramesonephic Duct
The paired paramesonephic ducts (Müllerian ducts) go through a series of developmental changes recently identified as regulated by a number of molecular factors.
| Female Uterus and Vagina (between week 9 and 20) | ||||
|---|---|---|---|---|
|
The entire vagina is formed from the paramesonephric (Müllerian) duct (red) and does not have a contribution from the urogenital endoderm (yellow). |
Molecular
BMP4
- Links: BMP4
Wnt 4
| Processes regulated by Wnt4 during female reproductive tract development
Wnt4 is required during prenatal and postnatal development of female reproductive tract. The initial MD primordium occurs independently of Wnt4 function (E11.5, the MD primordium in red). After initiation of the process, differentiation of the MD tip cells, prenatal elongation of the MD and postnatal formation of the endometrial gland (eG) all depend on Wnt4 signalling. The daughter cells that are initially Wnt4+ contribute to MD and eG formation. (text from figure legend)
|
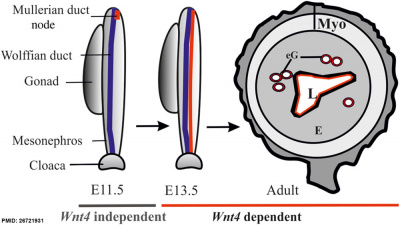
Female reproductive tract Wnt4[9] |
- Links: WNT
Retinoic acid
In mice, the epithelial fate of female reproductive organs is determined by factors secreted from the stroma. Retinoic acid-Retinoic acid Receptor signaling in the Müllerian duct determines the fate of stroma to form the future uterus and vagina.[10]
Initiation
Coelomic epithelium Lim1 expressing cells are specified to a duct fate.
- Lim - proteins named for 'LIN11, ISL1, and MEC3,' are defined by the possession of a highly conserved double zinc finger motif called the LIM domain.
- LIM domain-binding factors - interact with the LIM domains of nuclear proteins are capable of binding to a variety of transcription factors.
Invagination
Duct invagination induced by Wnt4 to reach the mesonephric (Wolffian)
Elongation
Cells at the leading tip proliferate and form the duct elongating to reach the cloaca (urogenital sinus). Mesonephric secretes WNT9b to guide duct elongation. Pax2 also acts in elongation and duct maintenance.
- WNT9b - member of the WNT protein family that encode cysteine-rich secreted glycoproteins that act as extracellular signaling factors.
- Pax2 - member of the paired box protein family.
Tissue Differentiation
The following result come from a recent human developmental study of known epithelial and mesenchymal markers.[11] Note that KRT is keratin protein family with the number representing a specific isoform.
Epithelium
- KRT7, KRT8 and KRT19 - expressed in undifferentiated Müllerian duct and uterovaginal canal, lined by simple columnar epithelia. Later glandular uterine tube, uterine corpus, and endocervix continue expression of these keratins.
- TP63 and RUNX1 - expressed prior to KRT14 in developing Müllerian epithelium.
- KRT6, KRT14 and KRT10 - expressed in exocervix and vagina tissues that undergo stratified squamous differentiation during development in an age-dependent fashion.
- KRT10 - expressed in the vagina after endogenous estrogens transformed the epithelium to a thick glycogenated squamous epithelium. KRT10 is a marker of epithelial terminal differentiation.
- Uroplakin - expressed only in vaginal introitus (entrance), bladder and urethra.
Mesenchyme
- HOXA11 - expressed in uterine mesenchyme.
- ISL1 - expressed in vaginal mesenchyme.
Postnatal Development
A study in mouse has identified Dicer, a riboendonuclease required for microRNA biosynthesis, to be required for postnatal growth if the female reproductive tract.[12]
Innervation
Innervated by the pudendal nerve and the pelvic splanchnic nerves (the uterovaginal nerve plexus):
- sympathetic
- parasympathetic
- nociceptive
Adult Dimensions
A recent study using magnetic resonance imaging (MRI) has accurately measured the dimensions of the adult vagina.[13]
- "Seventy-seven MRI scans were performed on 28 women before gel application to establish baseline vaginal measurements. Average dimensions were calculated for each woman and for the population. The influence of potential covariates (age, height, weight and parity) on these dimensions was assessed. ...Mean vaginal length from cervix to introitus was 62.7 mm. Vaginal width was largest in the proximal vagina (32.5 mm), decreased as it passed through the pelvic diaphragm (27.8 mm) and smallest at the introitus (26.2 mm)."
Other Species
Female cetaceans (whales, dolphins, and porpoises) and hippopotamuses have unusual vaginal folds of tissue that are of unknown function(s).[14]
Female waterfowl have vaginal morphology involving complex convolutions and also a number of dead-end sacs.
Abnormalities
In addition to the genetic abnormalities described below, abnormal sex hormone levels (estrogens, progestins and androgens) have been shown to be teratogenic for female reproductive tract development. See also endocrine disruptors.
Mayer- Rokitansky-Kuster-Hauser syndrome
(MRKH) Abnormality of development of the female genital tract: partial or complete absence (agenesis) of the uterus; absent or hypoplastic vagina; normal fallopian tubes, ovaries, normal external genitalia and normal female chromosome pattern (46, XX). Has an incidence of approximately 1 in 4500 newborn girls and has been associated with a microdeletion at 17q12.[15]
ACOG Committee Opinion No. 728 Summary: Müllerian Agenesis: Diagnosis, Management, And Treatment[6]
- "Müllerian agenesis, also referred to as müllerian aplasia, Mayer-Rokitansky-Küster-Hauser syndrome, or vaginal agenesis, has an incidence of 1 per 4,500-5,000 females. Müllerian agenesis is caused by embryologic underdevelopment of the müllerian duct, with resultant agenesis or atresia of the vagina, uterus, or both. Patients with müllerian agenesis usually are identified when they are evaluated for primary amenorrhea with otherwise typical growth and pubertal development. The most important steps in the effective management of müllerian agenesis are correct diagnosis of the underlying condition, evaluation for associated congenital anomalies, and psychosocial counseling in addition to treatment or intervention to address the functional effects of genital anomalies. The psychologic effect of the diagnosis of müllerian agenesis should not be underestimated. All patients with müllerian agenesis should be offered counseling and encouraged to connect with peer support groups. Future options for having children should be addressed with patients: options include adoption and gestational surrogacy. Assisted reproductive techniques with use of a gestational carrier (surrogate) have been shown to be successful for women with müllerian agenesis. Nonsurgical vaginal elongation by dilation should be the first-line approach. When well-counseled and emotionally prepared, almost all patients (90-96%) will be able to achieve anatomic and functional success by primary vaginal dilation. In cases in which surgical intervention is required, referrals to centers with expertise in this area should be considered because few surgeons have extensive experience in construction of the neovagina and surgery by a trained surgeon offers the best opportunity for a successful result."
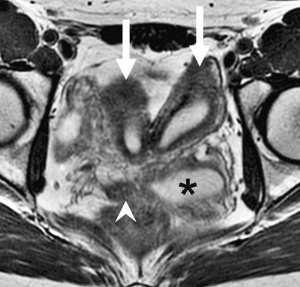
OHVIRA Syndrome
Obstructed HemiVagina and Ipsilateral Renal Anomaly with uterine didelphysis is a syndrome due to lateral non-fusion of the Mullerian ducts with asymmetric obstruction. The presence of vaginal septum also gives rise to other clinical conditions.
OHVIRA Syndrome Magnetic Resonance Images
Endocrine Disruptors
Endocrine disruptors in female reproductive tract development and carcinogenesis.[16]
Additional Images
Historic
References
- ↑ Cai Y. (2009). Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina. Int. J. Dev. Biol. , 53, 925-34. PMID: 19598112 DOI.
- ↑ Kurita T. (2010). Developmental origin of vaginal epithelium. Differentiation , 80, 99-105. PMID: 20638775 DOI.
- ↑ Koff AK. (1933). Development of the vagina in the human fetus. Contrib Embryol , 24, 59-91. PMID: 12332362
- ↑ BULMER D. (1957). The development of the human vagina. J. Anat. , 91, 490-509. PMID: 13475148
- ↑ Robboy SJ, Kurita T, Baskin L & Cunha GR. (2017). New insights into human female reproductive tract development. Differentiation , 97, 9-22. PMID: 28918284 DOI.
- ↑ 6.0 6.1 . (2018). ACOG Committee Opinion No. 728 Summary: Müllerian Agenesis: Diagnosis, Management, And Treatment. Obstet Gynecol , 131, 196-197. PMID: 29266072 DOI.
- ↑ Kurita T. (2011). Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation , 82, 117-26. PMID: 21612855 DOI.
- ↑ Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L & Forney LJ. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. , 108 Suppl 1, 4680-7. PMID: 20534435 DOI.
- ↑ Prunskaite-Hyyryläinen R, Skovorodkin I, Xu Q, Miinalainen I, Shan J & Vainio SJ. (2016). Wnt4 coordinates directional cell migration and extension of the Müllerian duct essential for ontogenesis of the female reproductive tract. Hum. Mol. Genet. , 25, 1059-73. PMID: 26721931 DOI.
- ↑ Nakajima T, Iguchi T & Sato T. (2016). Retinoic acid signaling determines the fate of uterine stroma in the mouse Müllerian duct. Proc. Natl. Acad. Sci. U.S.A. , 113, 14354-14359. PMID: 27911779 DOI.
- ↑ Kehl KJ. (1988). [Annual meeting of the European Nursing Students Group 1988 in Berne, Switzerland]. Krankenpflege (Frankf) , 42, 586-7. PMID: 2905399
- ↑ Gonzalez G & Behringer RR. (2009). Dicer is required for female reproductive tract development and fertility in the mouse. Mol. Reprod. Dev. , 76, 678-88. PMID: 19197916 DOI.
- ↑ Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M & Shaunik A. (2006). Baseline dimensions of the human vagina. Hum. Reprod. , 21, 1618-22. PMID: 16478763 DOI.
- ↑ Orbach DN, Marshall CD, Mesnick SL & Würsig B. (2017). Patterns of cetacean vaginal folds yield insights into functionality. PLoS ONE , 12, e0175037. PMID: 28362830 DOI.
- ↑ Brambati B, Tului L, Simoni G & Travi M. (1991). Genetic diagnosis before the eighth gestational week. Obstet Gynecol , 77, 318-21. PMID: 1988902
- ↑ Ma L. (2009). Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol. Metab. , 20, 357-63. PMID: 19709900 DOI.
Reviews
Costagliola A, Liguori G & Nassauw LV. (2023). Neuronal control of the vagina in vertebrates: A review. Acta Histochem , 125, 151988. PMID: 36566584 DOI.
Zhao F & Yao HH. (2019). A Tale of Two Tracts: history, current advances and future directions of research on sexual differentiation of reproductive tracts. Biol. Reprod. , , . PMID: 31058957 DOI.
Roly ZY, Backhouse B, Cutting A, Tan TY, Sinclair AH, Ayers KL, Major AT & Smith CA. (2018). The cell biology and molecular genetics of Müllerian duct development. Wiley Interdiscip Rev Dev Biol , , . PMID: 29350886 DOI.
Robboy SJ, Kurita T, Baskin L & Cunha GR. (2017). New insights into human female reproductive tract development. Differentiation , 97, 9-22. PMID: 28918284 DOI.
Cai Y. (2009). Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina. Int. J. Dev. Biol. , 53, 925-34. PMID: 19598112 DOI.
Farage M & Maibach H. (2006). Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. , 273, 195-202. PMID: 16208476 DOI.
Cummings AM & Kavlock RJ. (2004). Function of sexual glands and mechanism of sex differentiation. J Toxicol Sci , 29, 167-78. PMID: 15467266
Articles
Lobo Antunes I, Tomás C, Bravo Í, Metello JL, Quintas A & Sá E Melo P. (2019). Double Cervix with Normal Uterus and Vagina - An Unclassified Müllerian Anomaly. Int J Fertil Steril , 13, 83-85. PMID: 30644250 DOI.
Cunha GR, Kurita T, Cao M, Shen J, Robboy S & Baskin L. (2017). Molecular mechanisms of development of the human fetal female reproductive tract. Differentiation , 97, 54-72. PMID: 29053991 DOI.
Reich O & Fritsch H. (2014). The developmental origin of cervical and vaginal epithelium and their clinical consequences: a systematic review. J Low Genit Tract Dis , 18, 358-60. PMID: 24977630 DOI.
Pechriggl EJ, Bitsche M, Blumer MJ, Zwierzina ME & Fritsch H. (2013). Novel immunohistochemical data indicate that the female foetal urethra is more than an epithelial tube. Ann. Anat. , 195, 586-95. PMID: 24172012 DOI.
Kurita T. (2010). Developmental origin of vaginal epithelium. Differentiation , 80, 99-105. PMID: 20638775 DOI.
Deutscher E & Hung-Chang Yao H. (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol. , 307, 227-36. PMID: 17532316 DOI.
Guioli S, Sekido R & Lovell-Badge R. (2007). The origin of the Mullerian duct in chick and mouse. Dev. Biol. , 302, 389-98. PMID: 17070514 DOI.
Kobayashi A, Shawlot W, Kania A & Behringer RR. (2004). Requirement of Lim1 for female reproductive tract development. Development , 131, 539-49. PMID: 14695376 DOI.
Hashimoto R. (2003). Development of the human Müllerian duct in the sexually undifferentiated stage. Anat Rec A Discov Mol Cell Evol Biol , 272, 514-9. PMID: 12740945 DOI.
Mornex F. (2002). [Combined gemcitabine and radiotherapy]. Bull Cancer , 89 Spec No, S127-33. PMID: 12449044
Shapiro E, Huang H & Wu XR. (2004). New concepts on the development of the vagina. Adv. Exp. Med. Biol. , 545, 173-85. PMID: 15086027
Cai Y. (2009). Revisiting old vaginal topics: conversion of the Müllerian vagina and origin of the "sinus" vagina. Int. J. Dev. Biol. , 53, 925-34. PMID: 19598112 DOI.
Sebe P, Fritsch H, Oswald J, Schwentner C, Lunacek A, Bartsch G & Radmayr C. (2005). Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J. Urol. , 173, 1738-42; discussion 1742. PMID: 15821572 DOI.
Drews U. (2007). Helper function of the Wolffian ducts and role of androgens in the development of the vagina. Sex Dev , 1, 100-10. PMID: 18391520 DOI.
Historic
Koff A. Development of the vagina in the human fetus. (1933) Contrib. Embryol., Carnegie Inst. Wash. Publ. 443, 24: 59-60.
Bulmer D. The development of the human vagina. (1957) J. Anat. 91: 490-509.
Search PubMed
Search Pubmed: Vagina Embryology | Vagina Development | vaginal plate development | Mullerian duct
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- The Australian and New Zealand Vulvovaginal Society
- International Society for the Study of Vulvovaginal Disease
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 3) Embryology Vagina Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Vagina_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G