Paper - The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Saunders JW Jr. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. (1948) J Exp Zool. Aug;108(3): 363-403. PMID: 18882505
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Proximo-distal Sequence of Origin of the Parts of the Chick Wing and the Role of the Ectoderm
Department of Biology, The Johns Hopkins University, Baltimore, Maryland
Nineteen Figures
I. Introduction
The present study is the outgrowth of experiments designed to analyze the factors that control, the orderly spatial distribution, orientation, and tract specificity of feather germs in the wing of the chick embryo. It has been found that when rectangular blocks of tissue (prospective skin, muscles, and bone) are removed from the dorsal side of the wing bud (72 to 96 hour chicks), the feather germs develop in normally arranged tracts in a high percentage of the cases. Similar results are obtained when the blocks are reoriented 180° and replaced in their original site. Even the substitution of isolated leg-bud tissues for the excised blocks frequently gives typical wings with the feather germs normally distributed. The wing bud, therefore, shows considerable powers of regulation (Saunders, ’47).
It was noted, however, that in cases where atypical wings resulted from operations on early limb buds (ca. 72 hours), the defects were usually confined to the upper arm. This raised the question whether the early limb outgrowth might not consist almost entirely of tissues destined for the future upper arm. If so, where are the materials for the more distal wing parts located at this stage, and how are they formed?
To answer these questions, the method of carbon reference marks (Spratt, ’46) was used to examine the future value of the wing-bud tissues. It was shown by this method that localized materials for the future parts of the wing originate in the apex of the bud and are laid down in proximo-distal sequence as the wing bud elongates.
In the course of this study it was observed that reference marks placed in tl1e dorsal ectoderm and underlying mesoderm of the initial wing outgrowth are later found. in the upper arm region as discrete masses of carbon particles. whereas those inserted in or immediately subjacent to the ectodermal cap of the bud (a ridge of thickened ectoderm which rims the apical border of the bud and looks like a nipple in cross section) are sometimes dispersed through almost the entire length of the forearm. This suggested that the apical ectoderm might be playing a role in the formation of the wing parts from elbow to hand, or, perhaps, of the upper arm also. To test this possibility, the apical ectoderm was excised from a number of wing buds, with the result that defective wings were formed. In control experiments, the excision of equivalent amounts of ectoderm from the dorsal surface of the bud had no effect on the development of the wing.
It is the purpose of this paper to examine the organization of the chick wing bud during the early phases of its outgrowth, with particular reference to (1), the sequence in which tissues for the future wing parts are laid down; and (2), the role of the apical ectoderm in this orderly process.
I wish to express my gratitude t.o Prof. B. H. Willier for his stimulating interest, constructive criticism, and kindly guidance throughout this investigation. I am indebted to Dr. Mary E. Rawles for her many helpful technical suggestions and for the use of her excellent normal series of chick embryo sections; and to Mr. John Spurbeck for his careful work in preparation of the illustrative material.
II. Materials and Operative Procedures
All the experiments were performed on embryos from eggs, principally VVhite Leghorn, incubated at 37.5°—38.0°C. for 72 to 96 hours. Embryos were prepared for experimental treatment at the desired stage of development by the usual methods of candling, sawing a window in the shell, and removing the shell membranes beneath the opening (Hamburger, ’38; Willier and Rawles, ’40).
Fine glass needles, drawn to an [_-shaped point, were used to remove the ectodermal cap of the wing bud. The point of the needle was hooked into the thickened ectoderm at the desired place and then pulled sharply upward, thus tearing loose the ectodermin immediate proximity to the needle. The hooking and tearing were continued until as much tissue was removed as desired. This process normally did not injure the mesoblast. Excision of the ectoderm from the dorsal surface of the wing bud was accomplished by a similar procedure.
A camera lucida sketch was made of each experimental wing before the window was replaced and sealed with melted paraflin. Development was allowed to proceed until about the 10th day of incubation; the embryos were then sacrificed and fixed in Bouin’s fluid. Those specimens with carbon marks were left unstained and were cleared in oil of Wintergreen. In order to facilitate the analysis of skeletal primordia, many of the others were stained with methylene blue (Hamburger, ’42) so as to demonstrate cartilage.
Carbon particles placed in the wing bud do not adhere well to the cells, in contrast to Spratt’s (’46) experience with explanted blastoderms, for the pulsations of the amniotic fluid tend to displace them. The following technique was therefore adopted for placing the marks in the wing tissues. A small amount of finely divided carbon (purified blood charcoal of M erck) is transferred on the point of a needle to the surface of the shell membrane at the edge of the window and moistened with a drop of saline. The porous membrane absorbs the excess fluid and leaves the wet carbon on its surface. A small clump (0.02—0.05 mm diameter) of rather coherent particles is then selected under the binocular dissecting microscope, picked up on the point of a fine steel needle, and transferred directly to the surface of the wing bud. The moistened clump passes through the surface film of the amniotic fluid more readily than if dry. The particles are then gently pushed into the tissues of the limb bud until they are firmly embedded in the ectodermal and mesodermal layers (fig. 1). This method insures against loss of the carbon from the site of implantation in most cases.
Tiny masses of carbon inserted in this fashion become more or less dispersed as development proceeds, depending on the mutual coherence of the particles and the extent of the tissue movements in the marked region. Marks placed in the dorsal tissues of the wing bud may be found iii the skin, muscle, or bone when the embryo is sacrificed and cleared.
III. Normal Development of the Wing
To t'urnish a background for interpreting the experiments, it is necessary to describe the origin of the wing bud and to trace its growth through the period during which the experiments were performed. Arbitrary stages will be characterized for the period from 72 to 96 hours of incubation as a basis for mapping the localization of materials for the fut.ure wing parts and for analyzing the role of the ectoderm in relation to their sequential origin.
Hamburger (’38, ’39) pointed out that the stages for the chick embryo outlined by Duval (1889) and Keibel and Abraham (’00) did not furnish details of the early development of the wing bud. Accordingly he set up 6 provisional stages of a closely graded series of morphological changes, covering the period from approximately 50 to 72 hours of incubation. In the present study, Hamburger’s series has been extended to a total of 10 stages by utilizing, as an index to the clianging morphology of the wing outgrowth, the ratio of its anteroposterior dimension as measured along the body wall (L) to the distance from the body wall to its apex (W).
The first indication of a wing bud is found in embryos of about 30 somites, at which stage there is evident 21 slight thickening of the body wall which extends from the level of the 14th or 15th somite to the 20th. Cross sections through this region (fig. 2) show that the mesenchymal proliferation is covered with an epithelium consisting of an inner euboidal layer and an outer, or epitrichial, layer of cells. The cells of the inner layer, in the region of the future apex of the wing, are somewhat columnar; as development proceeds they assume the character of a pseudo-stratified layer of columnar epithelium, thus forming the apical cap of the bud.
Growth of the wing primordium, as characterized in Hamhurger’s description of stages 1 to 3, leads to formation of the typical 72~hour wing bud. At this stage the embryos have 35 to 38 soinites, and the leg bud has already surpassed the wing bud in size. The latter is a low swelling that projects directly outward from the trunk, extending from the level of the anterior end of the 15th somite to the posterior end of the 20th. Internally it consists of a mass of mesohlastie cells, closely packed and staining heavily with hematoxylin at the tip and on the dorsal and ventral sides, but more loosely arranged and showing less aflinity for the dye in the central core (fig. 1).
The ectodermal thickening at the apex is prominent at this stage. In the region of its greatest height 4 or 5 layers of nuclei may be counted in the euboidal layer, and, while cell membranes are not distinct, the cells appear to show the beginnings of a radial arrangement about the tip of the mesoderm (fig. 3). Anteriorly and posteriorly this apical cap tapers gradually to a single-layered condition, merging with the surrounding ectodermal tissues at the limits of the bud; dorsally and ventrally it tapers rather abruptly. In this, as in later stages, the ectodermal cells are separated from the underlying mesoblast by a well-defined basement membrane (cf. Patterson, 1888).
This description is characteristic of wing buds in the earliest stage used in the present experiments. Hamburger’s seriation assigns this bud to stage 4. The account given below traces its developmental history to about the 96th hour of incubation. It may be noted that the L/W ratios for stages 4, 5 and 6 do not agree precisely with Hamburger’s, but this may be attributed to differences in the method of measuring the antero-posterior dimension of the bud. In the stages listed below, the L/VV ratios were obtained from measurements of camera lucida drawings of wing buds in 0100.
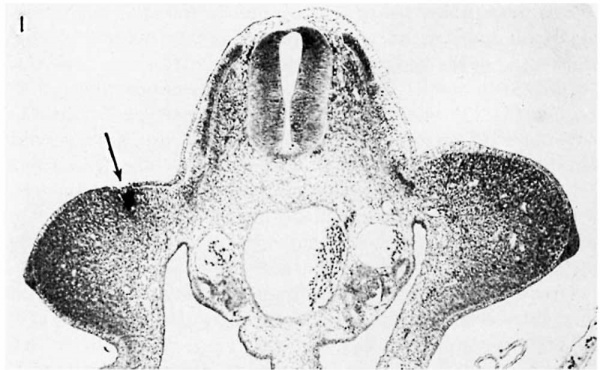
Fig. 1 Cross section through the wing-bud level of a 72-hour embryo (stage 4), showing a small mass of carbon particles (arrow) in the right wing bud. X 75. | |
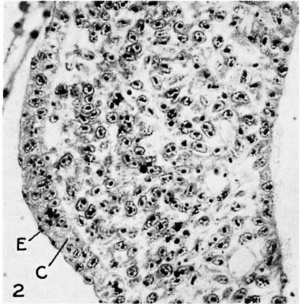
Fig. 2 The mesoblast and covering epithelium of the wing-forming region of a 30 somite embryo, showing epithelial (E) and cuboidal (0) layers of the ectoderm X 450. |
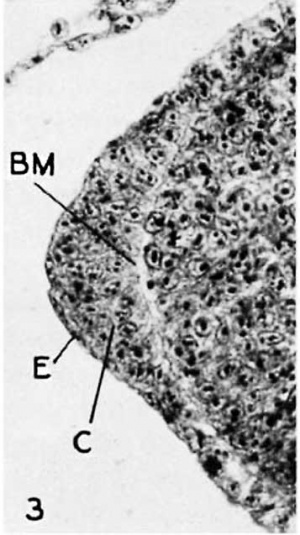
Fig. 3 The apical ectoderm of a wing bud in stage 4. The single-layered epithelium covers the greatly tlniekened euboidal layer. A welI-defined basement membrane (BM) separates the ectodermal cells from the mesoderm. x450 |
A. Stages of development
Stages 1 to 3. Refer to Hamburger’s (’38) account.
Stage 4. L/W = ca. 6.5. Wing bud of typical 3-day chick (ride supra).
Stage 5. L/W = 6.5 to 4.0. 38 to 40 somites. The wing bud occupies the same somite levels as in stage 4. Histologically there is little change (fig. 4a).
Stage 6. L/W =3.9 to 2.8. 40 to 43 somites. The wing outgrowth e.\'tends from the anterior end of the 16th to the posterior end of the ‘.2.Otl1 somite levels. The apical cap has formed a definite ridge or crest, like a nipple in cross section; the cells of the “nipple” are tightly packed, their long axes radiating from a common point at the tip of the mesoblast (figs. 4b and 19).
Stage 7. L/W: 2.7 to 2.3. 43 to 44 somites. The bud occupies the level between the middle of the 16th somite and the posterior end of the 20th. Its less basophilie core has become more compact and is penetrated by more numerous blood channels. There is a pronounced ventral bending of the bud, and a rapid elongation of its posterior half. The tissues of the base of the bud (the VVolt'fian ridge; of. Bardeen and Lewis, ’01) now begin to enlarge, forming a slight swelling lateral to the Ventral ends of the somites and extending somewhat forward of the anterior angle between wing bud and body wall (fig. ale).
Stage 8. L/W = 2..‘.Z to 1.8. 45 or more somites. There is little or no change at this stage in the somite levels associated with the wing bud. Histological cross sections show that the internal mesoblast is arranged as a definite central core of compact cells, the boundary of which is delimited by blood spaces. This is the beginning of the skeletomuscle co»ndc'ns(zHoe: described by Streeter (’48) for the human embryo. The swelling of the basal part of the wing bud has continued (fig. 4d).
fig. AL Cross sections of the right wing bud in stages 5 stage 8; 0, stage 9.
Stage 9. L/W = 1.7 to 1.4. The wing bud now extends posteriorly from the level of the middle or posterior end of the 16th somite to the middle of the 20th. The buds are fiat, padd1e—shaped appendages, beginning to be directed posteriorly as well as ventrally. Sections show the beginnings of the cellular condensation which precedes cartilage formation (fig. 4e).
Stage 10. L/W 1.3 to 1.0. The principal changes consist in a further elongation of the limb bud, a continuation of the prechondral condensation, and the further enlargement of the basal region of the bud. This basal region now is a prominent dorsal swelling of the \Voli’fian ridge, extending beyond the anterior angle of bud and body wall, and caudal to its posterior angle. The appendages are still flat and paddle—shaped; they are directed postero-ventrally and have a sharply defined apical ectodermal ridge.
The description of stage 10 completes the developmental history of the wing bud through the 4th day of incubation. Since the embryo at this time becomes covered by the rapidly growing allantois, later stages of the bud are usually inaccessible to experimentation in am.
The somite counts for the stages described above may vary considerably from those reported. The L/W ratios appear to be a more accurate index of the changing conditions of the wing bud than are somite counts. It should be noted also that the somite levels associated with the wing outgrowth may vary in each stage as much as 1 somite from those reported above. They elongate in the antero-posterior dimension during this period of development, and thus their number at the wing level gradually diminishes. The length of the bud, as measured at its origin on the body wall, remains quite constant, however, and so does the distance from the base of the wing outgrowth to the ventro-lateral ends of the somites. Camera lucida sketches have shown that the average value for the antero-posterior dimension of buds in each stage is close to 1.33 mm. The constancy of these measurements makes it possible to superimpose outline sketches of typical wing buds in the difierent developmental stages, using the ventrolateral borders of the somites and the anterior and posterior angles of t]1e bud as reference points. This is done in the next section in order to analyze the origin of the wing parts. figure 5 shows the outlines of wing buds in stages 4 to 10 as they appear from dorsal aspect, thus enabling one to picture the changing shape of the bud during the period of development just described.
fig. 5 Limb-bud outlines for stages 4 through 10, drawn to the same scale.
IV. Order of Origin of the Wing Parts
A. Maps of prospective wing parts
The tissues which form the future wing parts have been located for each stage of development by inserting carbon reference marks in the ectoderm and mesoderm of the early wing bud and following their subsequent disposition. At first, only 1 mark was used in each embryo. A camera lucida drawing recorded the position of this mark, and then the embryo was allowed to develop until about the 10th day of incubation. From the drawing the location of the marked tissues was plotted in the outline of a wing bud typical of the stage operated on. From data as to the ultimate disposition of the carbon, it was then possible to assign a future. value to the designated area of the typical bud. The data collected from numerous experiments of this sort were assembled as maps showing the prospective significance of the wing-bud tissues for different developmental stages.
In later experiments, wing buds were marked at an early stage with from 1 to 6 reference marks, in order to confirm and supplement the maps. The results of employing this technique verified the inferences made from the preliminary maps.
figure 6 summarizes the results of 144 marking experiments for stages 4, 5, 6, and 7. Those cases are excluded in which the marks were inserted in intimate association with the apical ectoderm. These maps show the approximate outlines of the girdle, upper arm, forearm, and hand areas during the operated stages, as well as their future proximo-distal axes. It will be noted that no maps are shown for stages 8, 9, and 10. The movements of the embryo during these stages, and the ventrally directed orientation of the wing bud, make it almost impossible to record the limb outlines and the positions of the carbon marks accurately in camera lucida drawings.
fig. 6 Maps shows illdicnte the for tissues of the future wing axes of the wing p:u-ts dosignnt. .
'3 in stages 4 through 7. The
B. The scquclnt-ial 01'igiu of tissues for the flutme 'wi12g parts
The maps show that almost the entire wing bud in stage 4 consists of materials for the future upper arm and girdle. As the bud elongates there are progressively formed at its apex the tissues for first the forearm and then the hand. The future parts of the wing arise, therefore, in proximodistal sequence-—-in the same order as that in which sub» sequent processes of differentiation occur; i.e., chondrification, ossification, sepa.ration of the muscle masses, etc. Noteworthy, too, is the fact that the future skeletal tissues of the upper arm and forearm arise in the flexed position, as indicated by the arrows showing the directions of their long axes
fig. 7 Outlines of wing buds in stages 4 and 5 superimposed on a map of the wing parts for stage 6. Note that the future wing areas included within the distal borders of stages 4 and 5 are the same as those shown in maps for these stages. Designation of wing parts as in figure 6.
These considerations raise the problem as to how the tissues for the several parts of the wing are proliferated in this sequence. The maps show that the elongation of the bud and the origin of successively more distal parts of the wing occur in the apical portion of the bud. This is shown more specifically by the following results: ( 1) when maps of prospective wing parts in stages 4, 5, and 6 are superimposed, the areas of the girdle and upper arm coincide, while the forearm region progressively increases apically (fig. 7); (2) carbon marks placed in the upper arm or forearm area remain at a fixed distance from reference marks on the somites during these stages, while the bud as a whole becomes progressively longer. During stage 7, and subsequently, all regions of the bud elon o.4oI.IM 0.33MM. 0_4OMM 0.44am 0.64 ml 0.35 mm
fig. 8 Camera Iucida drawings of the same wing bud in diiferent stages, sltowing the movements of a carbon mark placed in tissues of the future upper arm, with reference to another mark in a somite of the wing level.
gate. Carbon marks placed in these areas then move distally with respect to the somites (fig. 8).
It is apparent from the evidence above, that cells which progressively form the prospective areas of the distal wing parts are localized in the apex of the bud. Since histological preparations give no evidence that the apical eetoderm contributes to the wing mesoblast, it is to be presumed that these cells are in the apical mesoderm. It should be possible, therefore, to mark them with carbon particles in an early stage and thus to obtain wings in which the carbon is dispersed through the tissues from elbow to hand. As previously noted, these cells have been marked in a few cases, with subsequent dispersal of the particles of carbon through the tissues of the forearm (fig. 9), although it has not been possible to define for any one stage the limits of the tissues which contribute the distal parts of the wing. Technically it is very difficult to make reference marks adhere to the apical mesoderm;' but, since marks placed only slightly proximal to the apex of the bud do not move distally, it is assumed that only a very narrow region at the tip is concerned in increasing the length of the bud.
fig. 9 The dispersal of carbon particles inserted immediately subjacent to the apical ectoderm of the 72-hour wing bud. The position of the carbon is shown by stippling in A (stage 4) and in B (7 days). Redrawn from camera lucida sketches.
The question then arises how apical growth provides the tissues for the successive proximo-distal levels of the wing. In plants, growth of a shoot results largely from the elongation of cells locally proliferated iii the actively dividing apical meristem. Counts of division figures in the wing bud show, however, that the percentage of cells in mitosis is not appreciably greater in the apical mesoblast than in other regions. Thus there is apparently little similarity between the mechanism of apical growth in plants and in the chick wing bud.
The changing histological picture of the wing mesoblast suggests an interpretation of apical growth in the chick. During stage 4, the mesoderm of the apex and of the dorsal and ventral sides of the bud is quite compact, while centrally and proximally it consists of loosely arranged cells (fig. 1). In the succeeding stages the central mesoderm becomes denser, and by stage 7 the cell populations of all proximo-distal levels are nearly equal (fig. 4c). Apparently, during stages 4 to 7, proliferation from the compactly arranged cells of the dorsal and ventral sides, and from the proximal portion of the apex, co11tributes cells to the looser tissue of the central region, causing it to become more compact, while the distal portion of the apical mesoclerm contributes to the elongation of the bud. Since the cells are of approximately equal density througllout the bud in stage 7, cell division in all regions then begins to contribute to its increasing length.
0. The accm‘acy of H26 C-(II“bO’H;-’)'}'lallT't’I'1g maps
Before the conclusions drawn fro1n a study of the maps are to be accepted, the reliability of the method of tagging tissues with carbon particles to determine their future value must be demonstrated experimentally. A method of testing the accuracy of the maps may be based on the limited extent of regulative and regenerative powers in chick tissues. Wing bud isolates developing in explant, or in l1eterotopic positions form essentially only those parts of the wing which they would normally form if left irn. Sim (l\*[urray and Huxley, ’25; Murra_\f, ’26; Selby and Murray, '28; Hunt, ’3'.2). Moreover, the stump of a. limb bud from which more or less of the tip, or the anterior or posterior side has been removed does not regenerate the missing parts, nor does it regulate to form a normal, but smaller, limb (Lillie, ’0=-1; Peebles, ’11; Spurling, ’23; \Varren, ’34). If the maps are accurate, therefore, one should be able, by referring to them, to predict the wing omem or mars or structures which would develop either from an isolated portion of the bud growing in an atypical position, or from a defective bud developing in situ.
In 1 group of experiments the isolated tip of the wing bud was grafted to the coelomic cavity of a 60-l1our host embryo (see Hamburger, ’38; Rawles, ’40 and ’45; Budnick, ’45h,
fig. 10 Diagram showing the wing parts formed in a coelomic graft of the severed tip of a wing bud, and those formed by the wing tissues remaining in the stump. Future wing areas designated as in figure 6.
for the technique of making intraeoeloniic grafts and for an analysis of the development of limb-bud isolates in the coelom). In 3 cases the donors survived, and grafts were re» covered from the host chicks.
In 1 case the tip of the bud was severed in stage 7, reference to the map for that stage showing that the cut passed through the region of the future upper arm. A normal girdle, and a small nodule of cartilage, representing the head of the humerus, formed in the donor. The grafted tip developed a rather typical forearm and hand, the former articulating proximally with an abnormal humerus (fig. 10). An extra bone, slender and curved, formed in association with the upper arm region of the graft; it is believed to have originated from presumptive material of the humerus.
The other cases are of wing buds in stage 9, cut through the region of the future forearm (according to provisional maps for the late stages not reproduced in this paper) and these cases gave equally good results. Only 1 case will be (lescribed. In this instance the wing stump of the donor embryo formed a normal girdle and humerus, the latter terminating at the elbow in typical condyles. In the coelom of the host, the tip of the bud formed a rather normal ulna, somewhat twisted at its proximal end, and a short rod, lacking articulating surfaces, representing the radius. The wrist and hand formed almost normally, except that metacarpal II and its phalanx were present in duplicate.
In another series of experiments, the severed tips of the right wing and leg buds were interchanged in the same embryo, with their antero—posterior axes reversed. The operations were originally undertaken to repeat the work of Peebles ( ’11) who reported that in somewhat similar grafts the leg tissues developed as wing parts, and vice versa. Of 5 cases of successful transplantation, 2 embryos survived until after the 9th day of incubation when wing and leg structures are normally easily distinguished; the description of one of these follows.
The operation was carried out on a wing bud in stage 6, the tip being severed in the middle of the future forearm region and replaced by the tip of the leg bud. The tip of the wing bud was then grafted to the stump of the leg bud. The resulting anterior appendage showed an essentially normal humerus articulating distally with a rather normal shank and foot. From the leg region there developed a normal femur and an atypical hand. Neither graft showed regulation of the original axial relations. The forearm area was apparently destroyed or disorganized when the tip of the wing bud was removed, for neither bones nor feather germs characteristic of this region could be found on either of the chimaeric appendages (fig. 11). Essentially similar results were found in the other surviving case.
fig. 11 Diagrammatic representation of the interchange of severed tips of wing and leg buds in the same embryo. Chimaeric appendages redrawn from camera lucida sketches. Wing parts labelled as in figure 6. Note inverted orientation of interchanged tips.
The essential feature in both these groups of experiments is that the wing parts forming in situ and in grafts are approximately those to be expected on the basis of the carbon marking maps. The duplication of skeletal parts which was observed in the coelomic grafts is not unusual (Rudnick, ’45a), and is apparently an expression of the limited powers of regulation possessed by the chick limb bud in the stages studied (Rudnick, ’46; Rawles, ’47; “Wolff and Kahn, "47a and ’47b; Saunders, ’47).
It may be concluded from these results that the wing parts formed by apical and basal portions of the wing bud may be predicted from the carbon-marking maps. The studies of W'arren (’34) show, in addition, that similar predictions may be made for tl1e wing parts formed by either anterior or
fig. 12 Wing parts formed from ant-erior and posterior halves of the wing bud developing in situ in the absence of the other half; scheinatized from data of Warren (’3~l). Skeletal parts of the wing which form after the operation indicated on the left are designated in solid black in the outlines on the right.
.-1, parts formed b}-' anterior halves of the wing bud; B, parts formed by posterior halves.
posterior halves of the bud growing in situ in the absence of the other half. Warren’s experiments were performed on p chicks of 72 to 96 hours of incubation. He excised either the anterior or the posterior half of the bud from apex to body wall, leaving the other half in place. Anterior halves formed the head and parts of the shaft and of the distal expanded region of the humerus, the radius, intermedio-radiale, metacarpgll a11d phalanx of digit II. From posterior halves there developed the postero-distal part of the humerus, the ulna, centralo-ulnare, metacarpals and digits III and IV.
The close agreement between W'arren’s findings and the present results on the localization of prospective skeletal parts is shown in figure 12, where \Varren’s separation of the halves of the Wing bud is superimposed on a map of the wing bud for stage 6. The wing parts which form from anterior or posterior halves, under the conditions of Wa1'1'e11’s experiments are almost exactly those which would be expected on the basis of the distribution of prospective Wing materials shown in the map.
The accuracy of the maps is thus attested by the developmental performance of both apico-basal and antero-posterior fragments of the wing bud under experimental conditions. The reliability of the method of marking Wing tissues with carbon reference marks to determine their future value having been confirmed, the conclusion, that apical elongation of the bud gives rise to tissues for the future wing parts in proximodistal sequence, is further validated.
V. The Role of the Ectoderm
As was noted above, reference marks inserted in the early wing bud subjacent to the apical ectoderm may be dispersed later through the mesodermal tissues of the forearm, whereas those introduced proximal to the tip are later found in the pper-arm region as discrete clumps of carbon particles. Study of the maps showed that in each stage of development the ectodermal thickening is confined to those regions of the periphery of the bud which are actually elongating by apical growth. It was further noted that the greatest thickness of the apical ectoderm is to be found in the posterior half of the bud where its elongation is most rapid (fig. 6). These observations suggested that the apical ectoderm might have an influence on the origin of the wing parts.
A. The development of the wing bud in the absence of the apical ectoderm
As a test of its role in development, the apical cap was excised from wing buds in different stages of development. Embryos operated upon in stage 4 showed an almost complete absence of the right wing; those operated on in later stages showed a suppression of the distal wing parts to a greater or less extent. Carbon reference marks placed in the mesoderm near the denuded region showed that apical growth ceases when the ectodermal cap is removed (fig. 13).
fig. 13 The behavior of a reference mark (c) inserted near the apex of the wing bud after excision of the ectodermal cap. A, wing bud in stage 7; the broken line indicates the excised apical cap. The same wing bud after 28 hours of further development is shown in B, and after 10 days of incubation in 0. Drawings retraced from camera lucida sketches.
Experiments were then carried out to see if extirpation of the ectoderm of the dorsal surface of the wing bud might have a similar effect on formation of the wing parts. The ectoderm was partially or entirely removed from the dorsal surface of 22 wing buds in diflerent stages of development. The resulting embryos had wings with all proximo-distal segments present, although in those cases in which more than approximately three—fourths of the dorsal surface was denuded, defective feather tracts and muscles were found. Thus, whereas an extensive loss of dorsal ectoderm may prevent the completely the approximate boundaries of the future wing areas in the stages operated (cf. fig. 6).
fig. 14 A tabulation of the 1_'esu1t.s of excising the entire apical cap from wing buds in successively later stages of development. Stippled outlines show normal development of the wing, it does 11ot suppress the orderly formation of its parts, as does the absence of the apical ectoderm.
Since apical growth and the orderly formation of the wing parts apparently requires the presence of an intact ectodermal cap, then, by extirpating this structure from wing buds in progressively late stages of development, it should be possible to produce a series of specimens that would show various degrees of suppression of the wing parts, according to the stage at which the operation was performed. It should further be possible to find a cor1‘elation between those wing parts which do develop under these conditions and the prospective value of tissues known (from the maps) to be present at the time the operation was made.
figure 14 shows diagrammatically the results of excising the apical ectoderm from wing buds in stages 4 to 7. It may be noted here that the level to which the wing parts form shifts distally as the apical cap is excised in successively later stages of development. The proximo-distal levels at which the formation of wing parts ceases after operations at a given stage vary over a narrow range, as indicated in the diagrams.
These results are more strikingly illustrated by photographs of representative specimens for operations at each stage (fig. 15). \Vhen deprived of its apical cap in stage 4 the wing may not develop at all, although the girdle forms normally. In stage 5, the operation results in the formation of the upper arm only; while in stages 6 and 7 it suppresses development at the proximal and distal ends of the forearm respectively. The experiments were not continued beyond stage 7 because the ventrally directed growth of the wing bud usually renders the apical cap inaccessible to operation in later stages.
There seems to be a lag period after tissues for the wing parts are formed, and before they acquire the ability to self-differentiate in the absence of the apical ectoderm. For example, although the future upper-arm area may be mapped out almost completely in stage 4, the wing does not develop
if tl1e apical cap is removed in this stage. In stage 5, however, most of the upper arm does form in the absence of the apical cctoderm, while tl1e forearm, of which much of the prospective material has been laid out, fails to (lii’ferentiate (cf. fig. 6).
In the majority of cases (14 out of 24), the wings formed in the absence of the ectodermal cap reach an abrupt termination (figs. 14 and 15). In some instances, however, atypical devel fig. 15 Photograph of 10-day embryos recovered after excision of the apical ectodcrm from wing buds in successively later stages of derelopment. Embryos resulting from operations in stages -1 to 7 are shown in order from left to right. X 11,53.
development proceeded distally beyond the limits indicated in figure 14. The most distal structures are quite atypical in these cases, the anomalies beginning within the region of terminal development indicated for wings in each stage operated on. Since it is very difi‘icult to ascertain for each experiment that all of the apical ectoderm has been removed, these exceptional results have been interpreted as being due to the localized influence of remnants of the ectodermal cap. To test this View the following experiments were carried out.
In a series of 16 cases, either the anterior or the posterior half of the cap was removed from buds in different stages, and the wing was allowed to develop with the other half intact. The localized effect of an absence of the apical cctoderm is shown in figure 16 for operations in stage 5. Excision of tlie posterior portion of the cap suppresses those wing parts which tl1e maps show to be formed from the posterior half of the bud. Excision of the anterior half prevents formation of the anterior wing structures, allowing development of the posterior parts to proceed. Operations performed in other stages gave similar results.
fig. 16 The effect of excising anterior (A) or posterior (B) halves of the apical cap from wing buds in stage 5. Wing parts which develop following the operation are indicated by diagonal hatching.
In other tests all of the apical cap was removed, with the exception of small fragments, and the wing bud was then allowed to develop. Several hours later, examination of the wings operated upon, showed that apical elongation of the mesohlast had proceeded only in the regions where the cap was left intact.
These findings strengthen the argument that the exceptional cases noted in connection with figure 14 are to be interpreted as due to incomplete removal of the apical cap. They indicate that apical elongation of the bud occurs only within tissues immediately subjacent to a normal ectodermal crest, and that the influence of this eetoderm is exercised quite locally on the underlying mesoblast.
B. Histological changes in the mesoderm aflcr excision of the apical eetoderm
The next question was whether the failure of apical growth and sequential formation of the wing parts after the removal of the ectodermal cap might be accounted for on the basis of injury, either (1) accidental injury to the mesoblast in the operation of excising the cap, or (2) necrosis of the mesoder1nal cells iii the apex of the bud.
To answer this question embryos were fixed for histological study immediately after excision of the apical cap and at intervals up to 24 hours thereafter. Serial sections showed that the mesoblast is usually not damaged by the operation. In most wing buds, the mesoderm presents a smooth contour, just as if it were still covered with eetoderm (fig. 18). In some specimens, however, small defects have been noted, these apparently resulting from accidental penetration of the glass needle. The experiments cited above show that such localized injuries would not account for complete suppression of the wing parts.
Examination of the specimens fixed at intervals after the operation showed no evidence of necrosis. The denuded surface usually becomes completely covered with eetoderm within 24 hours, during which period the underlying mesoblast undergoes the histological changes characteristic of the differentiation of the proximal levels of the wing bud. No apical cap is reconstituted by the ectodermal covering.
fig. 17 Pliotogmpli showing the contraction of the mesoblast of the young wing bud after excision of the apical cap (stage 4). Contrast with the zlppearance of the control left wing bud. X 75.
fig. 18 Apex of :1 right wing bud in stage 6 from which the ect-odermal cap has been excised. Note conti-action of the mesoblast in the denuded region. X 335.
fig. 19 Apex of the left. wing bud of the embryo shown in figure 18. X 335. omorx or
It was noted that young wing buds, fixed immediately after removal of the apical ectoderm, show a rather pronounced contraction or shrinkage of the wing mesoblast (fig. 17). In later stages only those cells immediately subjacent to the denuded area show this reaction (figs. 18 and 19). The possibility that this contraction of the mesoderm is an artefact of fixation, the result of more rapid penetration of the fixing fluid in the absence of the ectoderm, is ruled out by the following considerations. first, the diminished volume of the wing bud may be observed in the living embryo when the apical cap is removed in stage 4, an observation confirmed by comparing camera lucida drawings made before and after the operation. Second, when the ectoderm is removed in stage 4 and the embryo is allowed to develop for several hours prior to fixation, the sections show that the increased cellular density has persisted, whereas older embryos, fixed immediately after the operation, show this reaction only iii the region of the wound.
These observations led next to experiments in which the effect of removing the dorsal ectoderm of the bud was studied histologically. Serial sections of chicks operated 011 in different stages showed that the mesoderm contracts in the same manner as when the apical ectoderm is excised; in young stages, the entire mesoblast contracts if the excision is extensive, but in older stages this reaction is confined to the denuded region. There is thus no apparent difference in the regional response of the mesoblast to removal of the covering layer of ectoderm; yet, when the ectoderm of the apex is excised, formation of the wing parts is suppressed, whereas when the dorsal ectoderm is removed, normal development of the wing follows.
The significance of these results is thus far rather obscure. It is hoped that future experiments will disclose whether or not the contraction of the apical mesoblastic cells plays any part in the failure of the wing parts to form when tl1e apical cap is excised.
VI. Discussion
A. The sequential origin of the wing parts in proximo-distal order
The carbon-marking experiments clearly show that when the wing bud first appears it consists almost entirely of materials for the proximal parts of the future wing. The capacity for forming the distal parts appears to reside in a limited region of the mesoderm at the apex of the bud, a region of “apical growth.” Elongation of the bud by apical growth progressively forms the materials for the wing parts in proximo-distal order and in the spatial pattern of subsequent differentiation.
Many experiments, particularly those of Hamburger (’38 a11d ’39) and Rudnick ( ’45), show that the wing primordium has the ability to self—differentiate into a complete and essentially normal foreliml), when grafted to the coelom of a host chick at a stage before it has beco1ne a distinct outgrowth. It is thus indicated that the potency for the formation of all parts of the wing is present in the wing—for1ning area, or bud, very early in development. Up to the present, however, there has been very little evidence as to the pattern of growth and difi"crentiation by which the formation of tissues for the future wing parts is realized, prior to the appearance of regional difi°erences in the bud.
The present results show that the segregation of cells normally destined to form localized regions of the future wing occurs principally in the zone of apical growth. This zone gradually lays down the tissues for first the proximal, and then, in succession, the more distal parts of the wing, thus establishing a primordium in which the materials are laid out i11 the morphogenetic pattern as blocks of a mosaic. When development proceeds with these relationsl1ips undisturbed, each block carries out the developmental assignment to which it is relegated by its position in the whole. The cells of the wing bud are not, however, rigidly fixed as to developmental potency by the order in which they are formed, for the future value of cells from any part of the pattern may be altered by a change of position relative to the other constituents of the bud (Saunders, "47).
The carbon-marking data on the sequential origin of the wing parts receive full confirmation in the results of those other experiments in which the activity of the apical growth zone was suppressed by removing the ectodermal cap from wing buds in successive developmental stages (figs. 1-1 and 15). The defective wing develop1ne11t which resulted makes almost inevitable the conclusion that, in the chick, the wing parts arise in proximo-distal sequence; for, the later the stage of the operation, the more wing parts there are subseqliently formed.
In the Amphibia, also, parts of the limb apparently arise in the same order. The experiments of Balinsky ( ’35) showed that in Tritoaz the formation of successive proximo—distal levels of the limb requires the presence of eetoderm. Balinsky grafted limb-forming mesoderm, free of ectoderm, from donor embryos in stages 24 to 29, 36 to 37, and 39 to 40 (interpreted in terms of Hal-rison’s stages for Amblysfo-ma pmzcfafmn) to hollowed orbits of the eyes of older embryos. The mesoderm from the youngest donors formed only small cartilages representing a part of the shoulder girdle and the proximal region of the humerus; that from the middle group gave origin to girdle, the entire humerus, and 1 or 2 small cartilages, probably derived from prospective forearm tissues; whereas implants from the oldest donors formed, in addition to the proximal elements, the entire radius and ulna, and occasionally, 1 or 2 of the wrist eartilages.
The close parallelism between these findings and those shown in figures 14 and 15 of the present paper affords good indication that in the salamander, as in the chick, the parts of the appendage arise in proximo-distal order. Possibly a similar zone of apical growth is present in Triton, too. It is also noteworthy that Swett (’23) observed no movement distally of a marker tissue implanted in the forelimb disc of an AH’I.()i"lj.S‘li-0’J’l2.a embryo, a result comparable to that obtained when the proximal parts of the future chick wing are marked with carbon particles.
A survey of the literature on the development of amniotes shows that within this group the origin of the anterior appendage follows a pattern of morphological changes similar to that of the chick. It seems likely, therefore, that in amphibians, reptiles, birds, and mammals the sequential formation of the parts of the limb proceeds in much the same manner.
This view has not been generally held. The prevailing opinion, as expressed in modern textbooks of anatomy (Broman, ’1].; Boenig, ’38; Walter, ’39; Young, Robinson, and Brash, ’-13) is that the first outgrowth of the limb bud consists of materials for the future hand (foot), the tissues of the forearm and upper arm (shank and thigh) being proliferated later from the base of the bud. It is thus implied that the tissues for the future segments of the appendage arise in apico—basal order.
It is difficult to trace the origins of this concept. Ever since the time of von Baer (1837), descriptions of the developing tertapod limb have emphasized the fact that the morphological features of the hand are recognizable at an earlier stage than are those of the other limb parts. Thus it is quite possible that over a period of many years the tacit assumption has been made that the tissues of the distal part of the appendage arise first in development, the proximal portions being formed later— despite the fact that other features of the differentiation of the limb proceed in the opposite sequence.
This idea has become so widely accepted that few writers have cited evidence to substantiate their statements on the order of origin of parts of the appendages. One of these writers was Peter (’03), who studied the morphogenesis of the limbs of the lizard, chick, and dog. He noted that, at the time the hand becomes distinctly recognizable in these forms, the apical ectoder1nal cap of the bud borders the periphery of only this most distal segment of the limb outgrowth. Considering the cap to be a “marker” for the hand tissues, he concluded that the first limb materials to appear in the outgrowing bud are those for the future distal part, for the thickened ectoderm is present at the time the bud first becomes visible. As demonstrated in the present study, however, the ectodermal cap borders the mesoblastic cells from which all the limb parts successively take origin, and thus it does not designate the hand-forming region until tissues for that part of the limb are being formed terminally.
(‘ertain aspects of severe cases of human phokomelia might be regarded as offering evidence for the concept of an apiecbasal order of origin of the tissues for the parts of the limb. In individuals who develop with this syndrome of hereditary defects, the long bones are foreshortened to a greater or less degree. In extreme cases the hands and feet may be attached almost directly to the girdles, the long bones having been greatly reduced. In the light of the precocious delineation of the hand in normal development, it might be reasonable to interpret these facts as indicating that the hand tissues are formed first and that, as the tissues for the future bones of the forearm and upper arm are proliferated from the trunk, they fail to differentiate properly, thus leaving the hand quite close to the girdle (cf. Broman, ’11, p. 228).
The morphological picture in such cases may be just as logically explained, however, by assuming that the parts of the human arm arise in the same manner as do those of the chick wing. Landauer (’27) has demonstrated that avian phokomelia is similar to the human defect, in that it is characterized by the same pattern of abnormal chondrification and ossification in the bones of the appendages. In the phokomelic chick, development of the limb bud is essentially normal until the processes of chondrification begin. By this time, according to the results of the carbon-marking experiments, all prospective tissues of the limb are already laid out in their normal spatial pattern. If these-results may be applied to the origiiis of the lnnnan limb, then chondrodystrophic development. of the humerus and forearm would cause the terminally forming hand to be located either closer or farther from the girdle, according to the severity with which the process of chondrification was distorted. Since the cases of human phokomelia are capable of interpretation in the light of the present findings for the chick, it would appear that they offer no basis for postulating an apico-basal order of origin of the parts of the vertebrate limb.
Thus there is apparently no evidence which would tend to disprove the conclusions reached in this study as to the proxiino-distal order of origin of the tissues forming the future wing parts. Since other aspects of embryonic development about the antero-posterior axis proceed in a mediolateral direction, it would really be rather surprising to find the process reversed in the special case of formation of the parts of the appendages.
B. On the role of the apical ectoderm in the orrlcwly origwiexz of the parts of the wing
It has been shown that the wing parts are normally laid down in sequence by the mesodermal cells of the apical growth zone, and that excision of the apical eetoderm overlying this zone results in a wing that shows distal defects extending proxinially to a level that depends on the stage of development at which the operation is performed. In other words, removal of the apical cap, even though followed b_v the regeneration of an ectodermal covering over the wound, suddenly arrests formation of the wing parts by this specialized region of the inesoderni. Furthermore, this property is confined to the apical cap, for excision of equivalent amounts of eetoderm from the dorsal surface of the bud during the same stages has no effect on the subsequent development of the wing.
Is the cessation of the formation of wing parts due to absence of the apical cap of cctodermal cells‘? Or is it due to chance injury to the mesoderm of the growth zone in the performance of the operation? The latter interpretation ORIGIN OF mars or THE CHICK wrxe 397
seems unlikely, since no necrosis of the cells of the growth zone is evident after the operation, and since rather severe mechanical disturbance of this region is followed by normal development of the wing. Carbon marks inserted subjacent to the cap have no effect on the formation of the wing parts, nor does excision of the ectoderm from the dorsal surface of the bud next to the apical cap. In these operations the cap may be loosened from the underlying mesodermal cells, the latter contracting just as they do when only the cap is removed, and yet normal growth of the wing ensues. It is to be inferred, therefore, that incidental injury to the apical mesoderm does not lead to the suppression of the wing parts when the apical cap is excised, and that the presence of the latter is necessary to the normal developmental performance of the growth zone.
In contrast to the suppression of the wing parts which results from absence of the ectodermal cap is the formation of typical wing parts by the growing zone when it is grafted with an intact ectodermal covering to a heterotopic position. It was noted above that when the tip of a right wing bud is grafted to the stump of a right leg bud in reversed anteroposterior and dorso—-ventral orientation, all those parts of a typical wingwhich were not already laid down prior to the operation areformed; these parts being oriented according to the original axes of the grafted growing zone. When, however, a block of tissue composed of ectoderm with underlying mesoderm is excised from the dorsal surface of a wing bud and is grafted in reversed antero-post.erior orientation to the site of a similar excision on the leg bud, it regulates completely to its new surroundings in the majority of cases, forming leg tissues in a normal orientation (Saunders, ’-17). Apparently, therefore, the normal relationship of the apical ectoderm and mesoderm of the growing zone provides a unique and highly integrated system capable of forming a specific developmental pattern under a variety of conditions. A similar situation is present in the apical region of the leg bud. The nature of the relationship between the ectoderm and mesoderm which provides for the unusual properties of the apical zone of the outgrowing limb constitutes a problem for further analysis.
It will be of especial interest in this connection to establish whether the apical ectoderm of a leg bud, for example, grafted to a wing bud from which the cap has been removed, can influence the direction of difi’erentiation of the growing zone. If technical difiiculties attending this operation can be overcome, it may be possible to determine whether or not the ectodermal cap contributes to the axiation or specificity of the limb parts which develop in its presence.
Another problem is whether the cells of the growing zone, which in the present experiments appear to be progressively restricted in their potencies as they lay down in succession the parts of the limb, can show more extensive potencies than they normally exhibit. Since grafts of the tip of the wing or leg buds to a heterotopic position have thus far always included, in addition to the growth zone and its ectodermal covering, some of the proximal tissues, the possibility that the tissues already laid down may have restricted the performance of the mesodermal cells of the apical region has not been eliminated.
That the apical ectoderm is an essential part of an integrated system which progressively lays down the parts of the chick limb is in sharp contrast to the general view that the ectoderm has only a passive role in limb development. Harrison (’18 and ’25) and his students (Detwiler, ’33; Swett, ’27) especially, as well as Rotmann (’31 and ’33), have demonstrated in striking fashion that in salamanders the responsibility for the localization, polarity, and specificity of the limb outgrowth resides in the mesoderm; the ectoderm passivcly conforming to the activities of the underlying cells. It is to be noted, however, that their experiments did not test the ability of the mesoderm to express its potencies under conditions wherein no ectodermal covering was provided. They appear, therefore, not to have furnished a basis for as suming an indifference of the ectoderm for development of the mesodermal limb parts, although they do show that the mesoderm forms its structures without regard to the kind of ectoderm which is present.
This was recognized by Balinsky (’35) who, as noted above, tested the development of ectoderm-free limb mesoderm of Tiriton. in grafts made to the hollowed eye orbit. He found that such grafts are capable of only limited proximo—distal differentiation, depending on the stage of development in which the ectoderm was removed. His results receive support from earlier findings of Steiner (’28). The latter, who studied both Urodeles and Anurans, showed that absence of the apical ectoderm of the beginning limb bud suppresses the formation of the distal limb parts, the proximal parts continuing to diifereiitiate at at normal rate. Only after the destroyed ectoderm has been replaced by regeneration does further development of the distal structures proceed. The studies of both Balinsky and Steiner thus suggest that in the Amphibia, as in the chick, the apical ectoderm is essential to the formation of the limb.
An apical ectodermal thickening has been reported for the developing limb buds in all major vertebrate groups, including the elasmobranchs and fishes (for a review, see Braus, ’O6). Its characteristic structure is similar to that of the chick in all forms except the Amphibia, where a simple thickening of the skin ectoderm over the conical apex of the limb bud is found, instead of a longitudinal crest. On the basis of the present findings for the chick, as supported by the results of Balinsky and of Steiner for the Amphibia, it seems not unlikely that the apical ectoderm may be of greater significance in the development of the limbs in all the vertebrates than has been heretofore realized.
VII. Summary and Conclusions
1. This paper treats of the sequence in which the tissues for the future wing parts originate in the wing bud of the chick embryo, and relates the role of the ectoderm in this orderly process.
2. Carbon particles have been inserted in the tissues of the wing bud during a series of selected stages in its early development. By following these particles as the limbs developed, it has been possible to map the prospective value of the tissues for each stage. The maps show that the materials for the future wing parts are laid down in proximo-distal order and in their definitive spatial pattern, apparently by a specialized growth zone in the apical region of the bud.
In all stages examined, extirpation of the thickened ectoderm at the apex of the bud suppresses the formation of wing parts by the zone of apical growth. In contrast, the removal of equivalent amounts of ectoderm from the dorsal surface of the bud, even when involving physical disturbance to the growth zone, does not affect the future wing parts. Excision of the apical cap of ectoderm is followed, in stage 4, by complete absence of the wing; in stage 5, by formation of only the upper arm; in stage 6, by formation of the upper arm and a part of the forearm; and, in stage 7, by development of a wing lacking only the hand.
at. When the apical growth zone with its ectodermal cap is grafted to the stump of a leg bud, typical wing parts are formed appended to the proximal leg structures. In contrast, blocks of tissue composed of ectoderm and underlying mesoderm from the dorsal surface of the wing bud often regulate when grafted to the site of a similar excision in the leg bud. 5. It is concluded on the basis of these results that: (a) the mcsoderm of the apical region of the wing bud progressively lays down mesodermal materials for the future wing parts in proximo-distal sequence and in their definitive spatial pattern; and (b), this process is suppressed by excision of the apical ectoderm, which is probably all essential part of the highly integrated developmental system made up of ectodermal and mesodermal components in the apical region of the bud.
Literature Cited
BAER, K. 1*}. VON 1837 Ueber Entwickelungsgeschichte der Thicre. Zweitcr Theil. Gehr. Dorntriiger, Konigsherg. onrem or PARTS or THE CHICK WING 401
BALINSKY, B. I. 1935 Selbstdiiferenzierung (les Extremitatenmesodernis im I11terplant. Zool. J., Allgemeine Zoologie 1:. Phys. der Tiere, 54: 327-348.
BARDEEN, C. R., AND W. H. LEWIS 1901 Development of the limbs, body wall, and back in man. Am. J. Anat., 1: 1-36.
BOENIG, H. 1938 Leitfaden der Entwicklungsgeschichte der Menschen. G. Thieme, Leipzig.
BRAUS, H. 1906 Die Entwickelung der Form der Extremitiiten und (les Ex" treniitiitenskeletts. 5th chapter in Hertwig’s Hbh. der Entwickelungslehre der Wirbeltiere, 3: 167-338. '-mo.-\.I.-ax, I. 1911 Normale und Abnorme Entwicklung des Mensc-hen. J. F. Bergmann, Wiesbaden. l.)n'rwIL1:R, S. R. 1933 On the time of determination of the antero-pot-erior axis of the forelimb in Amblystoma. J. Exp. Zool., 6'4: 405-414. I)U\'_u., M. M. 1889 Atlas d’Embryologie. G. Masson, Paris.
HAMBURGER, V. 1938 Morphogenetic and axia.l self-differentiation of transplanted limb primordia of 2-day chick embryos. J. Exp. Zool., 7?’: 379-400. —---——-— 1939 The development and innervation of transplanted limb primordia of chick embryos. J. Exp. Zool., 80: 347-390. 1942 A Manual of Experimental Embryology. University of Chicago Press, Chicago.
HARRISON, R. G-. 1918 Experiments on the development of the forelimb of Amblystoma, a self-differentiating equipotential sytem. J. Exp. Zool., 25: 413-461. 1925 The effect of reversing the medio-lateral or transverse axis of the fore-limb bud in the salamander embryo (Amblystoma punctatum Linn.). Arch. Entwmech. Org., 106': 469-502.
HUNT, E. A. 1932 The differentiation of chick limb buds in chorioallantoic grafts, with special reference to the muscles. J. Exp. Zool., 61?: 57-91.
KEIBEL. F., AND K. ABRAHAM 1900 Normentafeln zur Entwicklungsgeschichte der Wirbelthie1'e. II. Normentafel zur Entwicklungsgeschichte des Huhnes (Gallus domesticus). G. fischer, Jena. ' L.«ND.+\Ur.a, W. 1927 Untersuchungen iiber Chondrodystrophie. I. Allgemeine Erscheinungen und Skelett chondrodystropischer Hiihnnerembryonen. Arch. Entwmech. Org., 110: 195-278.
LILLIE, F. R. 1904 Experimental studies on the development of organs in the embryo of the fowl. 11. The development of defective embryos and the power of regeneration. Biol. Bull., 7: 33-54. Muss.-\Y, P. D. F. 1926 An experimental study of the development of the limbs of the chick. Proc. Linn. Soc. N. S. W., 51 : 187-263.
MURRAY, P. D. F., AND J. S. HUXLEY 1925 Self diiferentiation in the grafted limb-bud of the chick. J. Anat., Lond., 5.9: 379-384.
PATTERSON, A .M. 1888 On the fate of the muscle plate and the development of the spinal nerves and limb plexuses in birds and mammals. Quart. Jour. Micr. Sc.i., .‘.?.S‘.' 109-129.
PEEBLES, F. 1911 On the interchange of the limbs of the chick by transplantation. Biol. Bull., 20: 14-18. 402 JOHN W. SAUNDERS, JR.
PETER, K. 1903 Mitteilungen zur Entwicklungsgesehichtc der Eidechse. IV. Die Extrcmitiitenseheitelleiste der Arnnioten. Arch. Mikr. Anat., 61: 509-521.
R.n\'I.Es, M. E. 1940 The development of melanophores from embryonic mouse tissues grown iii the coelom of chick embryos. Proc. Nat. Acad. Sci., Wash., :-36'.‘ 673-680.
—1945 Behavior of melanoblasts derived from tl1e coeloinic lining in interbreed grafts of wing skin. Physiol. Zool., 18: 1-16.
— 1947 Some observations of the developmental properties of the presumptive hind-limb area of the chick. Anat. Rec., 99: 648-649.
ROTMANN, E. 1931 Die Rollo des Ektoderms und Mesoderms bei der Formbildung'des Kiemen und Extremitiiten Von Triton. I. Operation im Gastrulastadium. Arch. Entwmeeli. Org., 1.94: 747-794.
—1933 Die Rolle rles Ektoderms und Mcsoderms bei cler Formbilrlung (ler Extren'1it'ziten Von Triton. II. 0perat.ionen im Gastrulauml Schwanzknospenstatliuin. Arch. Entvvmech. Org., 12.9: 85-119.
RUDNICK, D. 1945a Limb-forming poteneies of the chick blastoderm: including notes on associated trunk tructures. Trans. Con11. Acad. Arts, Sci., 36: 353—371.
1945b Differentiation of prospective limb material from Creeper chick embryos in coelomie grafts. J. Exp. Zool., 100: 1-17.
1946 Regulation a.nd localization in t.he hind limb bud of the chick embryo. Anat. Rec., 94: 492-493.
SAUNDERS, J. W. 1947 An experimental study of the distribution, orientation, and tract specificity of feather germs in the wing of the chick embryo. Anat. Rec., 99: 647.
SELBY, D., AND P. 1). F. HURRAY 1928 Grafts of longitudinal halves of limbbuds of the four day chick. Austr. J. Exp. Biol. Med. Sci., 5: 181-186.
SPR.A'1'r_. N. '1‘. 1946 Formation of the primitive streak in the explanted chick blastoderm marked with carbon particles. J. Exp. Zool., 103: 259——304.
SPURLING, 1?. G. 1923 The effect of extirpation of the posterior limb bud in the development of the limb and pelvic. girdle in chick embryos. Anat. Ree., .:?-6‘: 41-56.
STEINER, K. 1928 Eiitwickliingsiilecllaniche Untersuehungen iiber (lie Bedeutung dos ektodermalen Epithels der Extremitiitenknospe von Amphibienlarven. Arch. Entwmech. 0rg., 113: 1-11.
S'rREI«:TI-:r.., G. L. 1948 Developmental horizons in human embryos. Fourth Issue ——A review of the histogenesis of cartilage and bone. (Supplemental Issue).'Carneg. Inst. Wash. Contr. Embryo]. (In press.)
SwE'rT_. F. H. 1923 The prospective significance of the cells contained in the four quadrants of the primitive limb disc of Amblystoma. J. Exp. Zool., 37: 207-217.
— 1927 Differentiation of the Amphibian limb. J. Exp. Zool., 47.385-439.
WALTER, II. E. 1939 Biology of the Vertebrates. Revised Edition. Mac.mi1l:ui, New York.
WARREN, A. E. 1934:. Experimental studies on the development of the wing in the embr_\'o of Gallus domesticus. Am. J. Auat., 54: 449-486. ORIGIN or PARTS or THE CHIC‘-K WING 403
WILLIER, B. H., AND M. E. R.-m-was 1940 The control of feather color pattern by melanophores grafted from one embryo to another of a different breed of fowl. Physiol. Zool., 13: 177-199.
WOLNF, E., AND J. KAHN 1947a Production expérimentale de la polydactylie ehez 1’embryon d’oiseau. C. R. Acad. Sci., Paris, 224: 1583-1584. '
—1947b La regulation de ]’ébauehe des membres chez les oise.-aux. C. R. Soc. Biol., Paris, 141: 915-916.
YOUNG, A. H., A. ROBINSON AND J. C. BRASH(rev.) 1943 Human Embryo}, in Cunningham ’s Textbook of Anatomy, Eigllth edition (Brash, J. C., and E. B. Jumieson, eds.). Oxford University Press, London.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_proximo-distal_sequence_of_origin_of_the_parts_of_the_chick_wing_and_the_role_of_the_ectoderm
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G


