Paper - The development of the venous sinuses of the dura mater in the human embryo
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. The development of the venous sinuses of the dura mater in the human embryo. (1915) Amer. J Anat.18: 145-178.
| Online Editor |
|---|
| This historic 1915 paper by Streeter describes the development of the dural venous sinuses that form the major drainage of the brain to the internal jugular veins in the adult. Below are shown links to modern and historic resources on neural vascular development.
See also Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
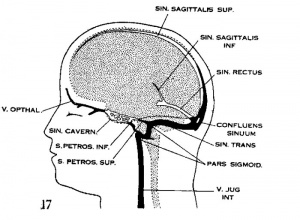
The dural venous sinuses:
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Venous Sinuses of the Dura Mater in the Human Embryo
Department of Embryology, Carnegie Institution of Washington
Seventeen Figures (1915)
Introduction
In the course of a recent study of the structure and topography of the endolymphatic sac in older human embryos it was found necessary to determine in greater detail than is furnished in the present literature, the relations of the blood vessels of this region, and particularly of the large dural veins that eventually form the dural sinuses of the adult. The abundant material in this laboratory that was found available for this purpose has made it possible to fill out the essential stages in the development of these veins with considerable completeness. The work has been much facilitated by the proximity and advice of Professor Mall who, it will be remembered, was the first to give a comprehensive description of these blood vessels in the human embryo and, in a way, this paper forms a supplement to his original publication.
Acknowledgment should furthermore be made to Professor Sabin and Professor Evans. Whose valuable experience in the study of the vascular system and whose unique preparations were generously placed at the Writer’s disposal. Under these conditions the opportunity has been especially favorable for clearing up some of the obscure factors in this interesting field. It is also believed that, in addition to any interest the reader may have in the morphological details of the veins themselves, his attention will be attracted by the bearing they have on the broader principles involved in the establishment of vascular drainage. On following through the successive stages, which are to be outlined in the following paper, it will be noted that they afford striking examples of embryonic change and repeated adjustment of the drainage channels consequent upon the alterations in the form and condition of the particular area drained. Nowhere do we find a clearer picture of these adjustment phenomena than in the region of the brain. Its marked change in form and especially the prolonged relative growth of the cerebral hemispheres make a continuous series of alterations of the veins necessary that extend far into the late embryonic stages.
Material And Methods
The material on which this study is based consists chiefly of serial sections of human embryos taken from the Mall Collection, which new forms the nucleus of the Department of Embryology of the Carnegie Institution. In many of the specimens the blood vessels had been previously injected with India ink or Berlin blue or both. In one instance, No. 458, 54 mm. long, a total preparation of an injected specimen was made and cleared in oil and drawings were made directly from the specimen with the camera lucida. The principal stages, however, were usually based on profile reconstructions prepared from serial sections. These will be specified under their individual descriptions. In some cases portions of the veins were modeled after the Born reconstruction method. The oldest stage examined was in an embryo (No. 234a) 80 mm. crown-rump length. The youngest stage was an embryo (No. 588) 4 mm. greatest length, at which time the otic vesicle has differentiated far enough to make it possible to recognize the situation of the endolymphatic appendage. Before this time certain important phases in the development of the blood vessels of the head have already occurred. These will now be briefly reviewed as an introduction to the subsequent conditions.
Primary Vascularization of Head
The most complete account we have of the vascularization of the brain and its earliest drainage is based on the chick and the pig. For descriptions of these we are largely indebted to Evans (’09, ’ 12). According to this author, the first blood vessels to the brain take the form of a capillary plexus that sprouts out from the aortic arch. These sprouts form a vascular web that closely invests the neural. tube, beginning in the region just caudal to the optic stalk and spreading from there up over the adjacent fore- and mid-brains. By the coalescence of other capillary sprouts from the dorsal aorta a continuous channel is established along the side of the hind-brain that drains these capillaries of the head caudalward toward the venous end of the heart.
A study of the cardinal veins in the chick has recently been made "by Professor Sabin (’14, ’15) in this laboratory, and she has kindly demonstrated her specimens to me. From these specimens the development of the drainage of the primary brain plexus can be plainly made out. In the head region, proper, capillary sprouts from the dorsal aorta fuse into a continuous channel that drains the brain plexus caudalward to the vagus region. At this point it joins a small capillary plexus in which the anterior cardinal vein is to form. This latter plexus is one that develops between the aorta and the vitelline vein. It has been demonstrated by Professor Sabin that it differs from the head plexus in two essential particulars: (1) The sprouts from the aorta from which it is formed bear a definite relation to the cervical myotomes; (2) These sprouts also bear a definite relation to the nephrotomes and thus identify themselves as belonging to the cardinal system. Shortly after the time that this plexus is joined by the head vein its direct connection with the aorta is broken and a circulation is thus established from the primary brain plexus backward- through the cardinal plexus to the venous end of the heart. In the formation of this circulation we are thus dealing with two capillary groups; the cephalic one, belonging intrinsically to the head, and the caudal one, belonging to the cardinal system.
Very soon after the establishment of this circulation in the head it can be seen that the single vessel derived from the fusion of dorsal sprouts of the aorta, which we have described as developing caudalward alongside of the brain, has become the middle segment of a prominent longitudinal channel that extends all the way from the optic stalk to the duct of Cuvier. The cephalic segment of this channel and its tributaries are formed in the more superficial loops of the primary brain plexus; its caudal segment» forms in the cardinal plexus and constitutes the anterior cardinal vein, eventually the internal jugular vein. The whole channel is designated as the ‘prim my head vein’ and with its establishment we may regard the primitive arrangement of the drainage of the brain as completed. Essentially, it cone 5 of a sheet of capillaries that nearly everywhere surrounds the brain tube, and this capillary sheet is drained by many irregularly placed anastomosing loops into the more superficially placed primary head Vein, which runs along the side of the hind-brain and empties finally in the duct of Cuvier at the venous end of the heart.
Stages of Development of the Dural Veins
1. Human embryos 4 mm. long
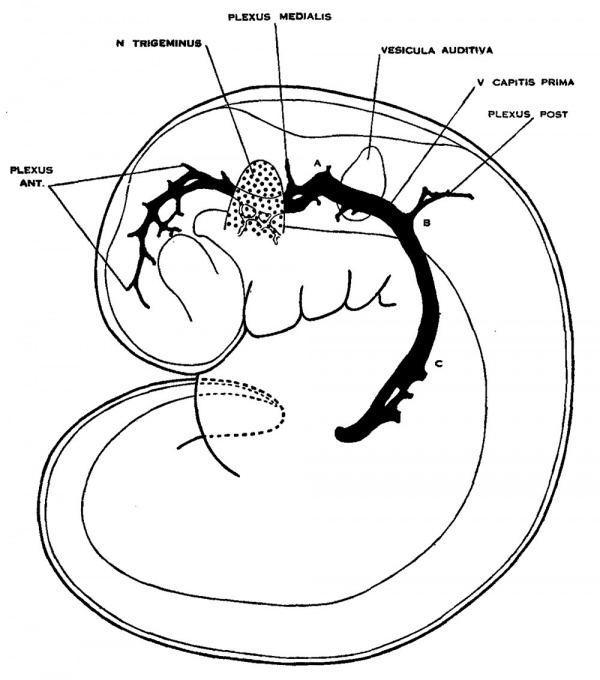
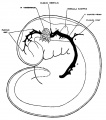
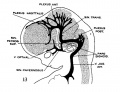
It is this primary arrangement of the drainage of the head, which we have just described, that exists in 4 mm. human embryos, and this is the earliest stage examined in connection with the present study. - In figure 1 is shown a profile reconstruction of such an embryo (No. 588, 4 mm. long, Carnegie Collection). This is slightly younger than the stage shown by Mall ('05) in his figure 3. The conditions in the two, however, are very similar and the relations of the main vein are the same. In our figure the outlines of the neural tube, the semilunar ganglion and the otic vesicle, are indicated and their relation to the venous system of the head is shown. It happens that this embryo is bent transversally in its longitudinal axis, so that its left profile presents a convex surface and its right profile a concave surface. Thus, on account of the oblique position of the head, the large vein of the head in the profile reconstruction shown in figure 1 necessarily assumes a position that is more dorsal than it would be in a true profile. In representing the veins only the principal channels are drawn in; no attempt was made to trace the small venules and their connection with the capillary plexus. Examination of the sections, however, shows that a capillary plexus exists and closely invests the neural tube and the adjacent nerves and sense organs. Minute anastomosing vessels can be seen connecting the plexus with the larger venous channels.
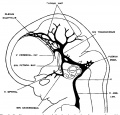
Fig. 1 Profile reconstruction of the primary head vein and its tributaries in an human embryo 4 mm. long (No. 588, Carnegie Collection). At the point marked ‘A’ the vein is bent out of its direct course by the facial and acoustic nerve-mass. The segment included between ‘B’ and ‘C’ represents the cardinal portion of the primary head vein, which eventually becomes the internal jugular vein. Enlarged about 33 diameters.
We thus have a simple system of drainage. The main channel constitutes the primary head vein, a vessel consisting of a single layer of endothelial cells. Its tributaries begin to unite in the region of the diencephalon. In the region of the semilunar ganglion they have coalesced into a main channel that passes median to the ganglion. Further caudal it is bent out of its course to pass lateral and dorsal to the acustico—facial complex. It passes lateral to the otic vesicle and the glossopharyngeal ganglion and then bends inward to become median, and finally dorsal, to the ganglion nodosum of the vagus nerve, whence it passes down to empty into the duct of Cuvier. The primary head vein receives everywhere many tributaries, chiefly Ventral and dorsal. The ventral ones are more numerous near the optic stalk and in the neighborhood of the nerve-ganglion masses. The dorsal ones may be classified in three groups; (1) an anterior group from the region of the diencephalon and mesencephalon; (2) a middle or cerebellar group in the region between the trigeminal nerve and the acustico—facial complex; and (3) a posterior or occipital group from the neighborhood of the vagus rootlets. Especial attention is directed to these three dorsal tributary plexuses, as their arrangement is significant for all the later stages, which will presently be seen.
As we pass to older stages, where the dura mater and the arachnoid spaces are forming, we find that many of the anastomosing channels between the capillaries of the brain and the primary head vein close off, and there is a general separation or cleavage of the more superficial primary head vein and its tributaries from the deeper veins, arising from and draining the capillary sheet that immediately surrounds the brain tube. This deeper system, however, continues to drain into the former at certain restricted places. We can thus distinguish between veins of the dura mater and the cerebral veins. It is the former that are chiefly concerned in the formation of the Venous sinuses and with which we are chiefly interested in the present study.
2. Human embryos 14 mm long
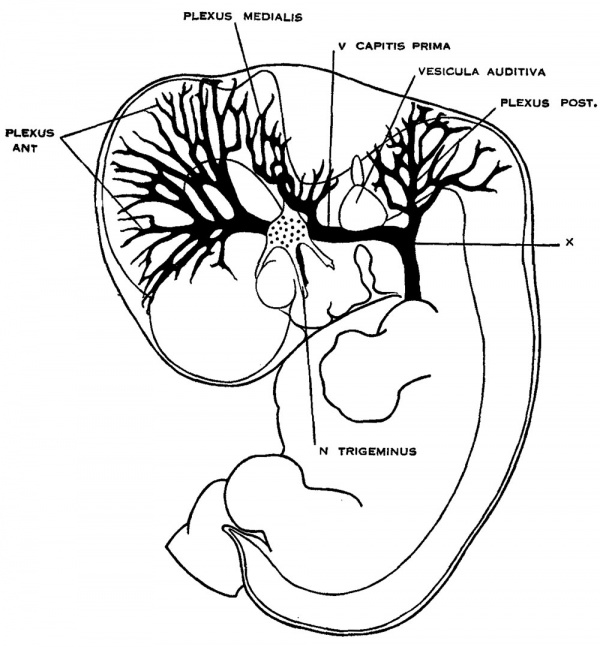
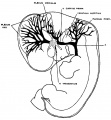
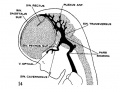
In figure 2 is shown an embryo in which the veins of the dura mater are already separated to a considerable extent from the cerebral veins. The veins of the head of this embryo (No. 940, 13.8 mm long, Carnegie Collection) were distended with a natural blood injection and at the same time the surrounding tissues were quite transparent, so it was possible from a surface examination to determine their arrangement with considerable detail. The specimen was photographed and a print made, which was then elaborated with the details that could be seen in the specimen with the aid of a binocular microscope. Comparisons were also made with serial sections of other embryos of about the same age and in which the blood vessels had been injected with a colored mass. In the collection No. 544 is a particularly good series of that kind, showing about this same arrangement of the drainage of the head. Mall (’05) has pictured about the same stage in his figure 9. This stage is also pictured by Markowski (’11) in his figure 1. In the main points all three figures correspond rather closely. A large venous channel is formed in the region lateral to the diencephalon and passes backward, median to the trigeminal nerve and lateral to the otic capsule, through the region of the future middle ear, where it bends sharply downward in the neck region finally to empty into the duct of Cuvier. All the veins of the cranial region drain into this main channel. This constitutes the primary head vein, with which we are already familiar. It was described by different writers as the ‘anterior cardinal Vein’ until Grosser (’07) showed that only the caudal portion of it——the part that is found in the region of the somites 152 GEORGE L. srnnnrnn and later forms the. internal jugular vein—could be properly spoken of as the anterior cardinal. The portion in the pr segmental region was designated by Salzer (’95) in the guinea-pig as ‘vena capitis medialis’ and ‘vena capitis lateralis,’ depending on whether it was found median or lateral to the cranial nerve trunks. The more cephalic portion, in the trigeminal region, is always found median to the nerve and hence is always vena capitis medialis. Caudal to the trigeminal nerve Salzer describes it as at first coursing medial to the facial, glossopharyngeal and vagus nerves, and subsequently, by a process of ‘island formation,’ migrating lateral to these same nerves, that is, changing from vena capitis medialis to vena capitis lateralis. These terms were advocated on the basis of an homology with similar veins in the lower vertebrates. The importance of vascular homologies practically disappears on the acceptance of the AebyThoma conception of the adaptive capillary formation of blood vessels, which has been so clearly established by the brilliant chick injections of Evans (’09) and therefore in this paper the terms vena capitis medialis and vena capitis lateralis will not be used. It is felt that the term ‘primary head vein,’ covering both of them, will be less confusing and will be entirely adequate from the youngest stages up to embryos about 20 mm. long. The composite origin of this vein, however, should not be forgotten. It has already been pointed out that it belongs in part to the trunk (the anterior cardinal vein) and in part is intrinsic to the head. As we shall presently see, it is the trunk portion, or anterior cardinal, that forms the internal jugular vein, whereas the intrinsic head portion in its more anterior segment becomes the cavernous sinus, the posterior portion (the so-called vena capitis lateralis) disappearing entirely and being replaced by a more dorsally situated channel.
The tributaries draining into the primary head vein are arranged in three plexiform groups (fig. 2), as was pointed out by Mall (’05), the first group emptying into the main channel in front of the semilunar ganglion, the second group between the semilunar and the acustico-facial ganglia, and the third group caudal to the otic capsule. These were designated respectively the ‘anterior,’ ‘middle’ and ‘posterior cerebral veins.’ The latter two each empty into the main channel through a single trunk, but the anterior cerebral Vein maintains the character of the original plexus and has multiple openings into the primary head vein. Furthermore, the veins forming these three groups belong chiefly to the dura mater and the tissues forming the membranous cranium. There is therefore an advantage in adopting a terminology something like that of Markowski (’11). In doing so, a distinction between the lateral and mesial portions will not be made; on the other hand, the three groups as given by Mall will be retained. We will thus speak of the ‘anterior,’ ‘middle’ and ‘posterior dural plexuses,’ or more formally, ‘plexus durae matris anterior,’ ‘ plexus clurae matris medialis’ and ‘plexus durae matris posterior,’ as they are indicated in figure 2. In this figure only the larger channels of the plexus are shown and it is to be understood that an intervening smaller venous mesh connects them more or less completely.
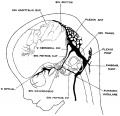
Fig. 2 Drawing of the primary head vein and its tributaries in a human embryo 13.8 mm. long (No. 940, Carnegie Collection). The point marked ‘X’ is the junction of the intrinsic head-portion of the primary head vein with the cardinal portion or internal jugular vein. Enlarged about 7 diameters.
In the ventral portion the dural plexuses are more or less completely separated from the deeper—lying plexus belonging to the wall of the neural tube, from which are developed the cerebral veins. In tracing the plexus dorsalward toward the median line we find an increasing frequency of communication between the two, and near the median line they are so intimately connected that it is impossible to distinguish between them; in other words, in this region the cleavage between these two layers is not yet established. It is interesting to note that there seem to be favorable places for the larger channels to cross dorsally over the median line; one of these is the caudal end of the roof of the fourth ventricle, another at the junction of the mid-brain and hind-brain, and a third over the diencephalon along the caudal margin of the cerebral hemisphere. Where these vessels cross the median line they are usually bilaterally asymmetrical but may anastomose with the plexus of the opposite side. Other than at these three regions, the larger channels as a rule do not reach the median line.
In addition to the three dural plexuses mentioned above, the primary head vein receives a large ventral tributary that lies median to the maxillary division of the trigeminal nerve and also other small veins in that region. These veins drain the tisues that in part are to form the orbit and represent the future ophthalmic veins.
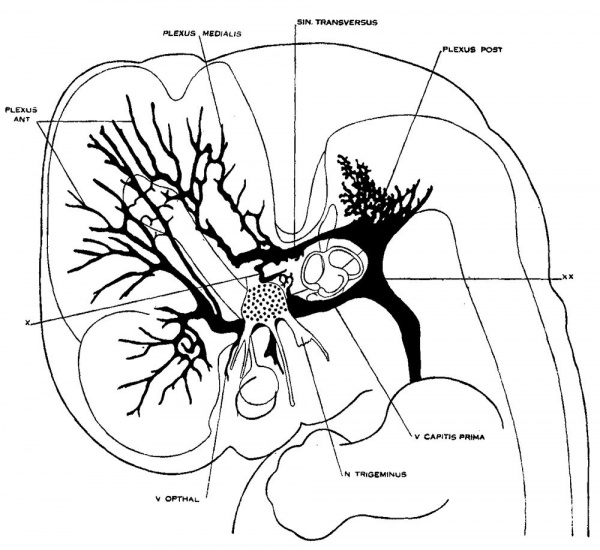
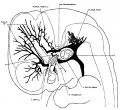
3. Human embryos 18 mm long
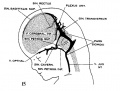
On examining different series of about the 14 mm. period of development and a little older one can see that the primaryhead vein maintains the same course and relations, but the pattern of the dural plexuses is constantly changing. In embryos about 18 mm. long an important change occurs by which the blood from the region of the middle dural plexus drains caudalward into the posterior dural plexus through an anastomosing channel that becomes established between these two plexuses, passing dorsal to the otic capsule and just lateral to the endolymphatic sac. This can be seen in figure 3, which shows a graphic reconstruction of a human embryo, 18 _mm. long (No. 144, Carnegie Collection, crown-rump length 18 mm. formalin; 14 mm. on slide). This is the same embryo shown in Mall’s figure 11 and is about the s-ame age as the embryo pictured in figure 2 of Markowski. In some respects the reconstruction referred to differs from both of these. From Mall it differs in that the greater part of the mid- and forebrain is still drained by the primary head vein. From Markowski it differs in that there is not yet a single large channel passing backward from the anterior and middle dural plexuses, but instead this region still shows an_ extensive anastomosing network not differing much from the pattern we have already seen in figure 2.
An interesting feature in connection with the dural plexuses presents itself in that the trunk that originally drained the middle dural plexus into the primary head vein nearly disappears, owing to the fact that the blood that it heretofore carried (i.e., from the cerebellar region and the posterior part of the mid-brain) adopts the new channel that is established dorsal to the otic capsule and is thus drained into the posterior dural plexus. As a result of this the original trunk that connected the middle plexus with the primary head vein becomes relatively small and partially breaks up into a small plexus. We shall see later, however, that with the next change in the head vein this trunk will open up again as an important channel.
In taking up the question of terminology for figure 3, it is found-that most of the terms used in figure 2 are still applicable.
Fig. 3 Profile reconstruction of the veins of the dura mater in a human embryo 18 mm. long (No. 144, Carnegie Collection). The ‘x’ indicates the original trunk of the middle dural plexus; it corresponds to the superior petrosal sinus. The ‘xx’ marks the upper end of the internal jugular vein. Enlarged about 12 diameters.
There are the three dural plexuses draining into the primary head vein and also the opthalmic veins. The anterior dural plexus, however, can be seen to be reshaping itself so as to come into éloser anastomosis with the middle dural plexus. The middle dural plexus by draining, as it does, over the otic capsule presents the first stage in the formation of the transverse sinus, that is, the sigmoid portion of it. The posterior dural plexus shows less change in its form and connections than any other group of the head veins and this is true also in the later stages. There are some minor alterations in its pattern, but otherwise it simply VENOUS SINUSES OF THE DURA. MATER 157 extends to become the occipital sinus of the adult and at the same time, together with its fellow, develops drainage channels that empty into the plexus of the tentorium. The primary head vein can be subdivided into: the trigeminal portion that is to form the cavernous sinus; the otic portion which passes lateral to the otic capsule accompanying the seventh nerve; and lastly, the cervical portion or internal jugular. vein, the boundary of which is indicated in figure 3. The otic portion already shows a diminution in volume as a result of the establishment of the new drainage channel dorsal to the otic capsule. Dorsal to the otic capsule there is suflicient free space for the development of a vascular channel, whereas the region Ventrolateral to the otic capsule becomes crowded by the development of the cochlea and middle ear. This constitutes a mechanical factor that doubtless has a determining influence upon the change in the course of this blood channel.
4. Human embryos 20 mm long
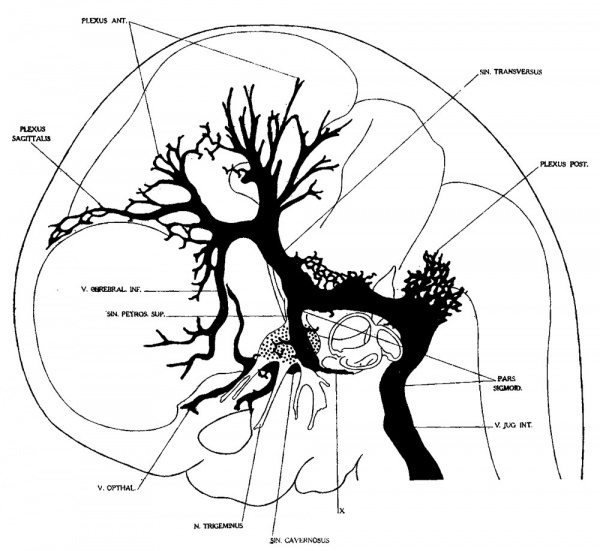
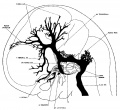
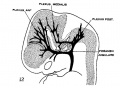
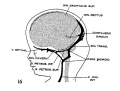
In embryos about 21 mm. long the veins of the head have an arrangement that is intermediate between the embryonic type and the adult type. The veins in the basal portion of the skull closely resemble the adult, while the dorsal veins still have many embryonic features. In figure 4 is shown a graphic reconstruction of the head of such an embryo (No. 460, 21 mm. long, Carnegie Collection). The reconstruction of the veins in this case was greatly facilitated by the work that had already been done on the head of this embryo by Professor Lewis, who kindly put all his tracings and photographs at my disposal. The outlines of the central nervous system are taken directly from a model prepared by him. The study of the veins was facilitated through the fact that the bloodlvessels had been injected through the umbilical vein with India ink by Professor Sabin, while the heart was still beating, so that there is a beautiful injection of the entire vascular system. Before the embryo was cut sketches and photographs of the vessels that could be seen from the surface were made by Professor Evans. There is enough material at hand in relation to this specimen to make a very complete study of its whole vascular system. The writer’s attention, however, was confined to the veins that are under discussion. For the sake of comparison, another embryo slightly older (No. 632, 24 mm. Carnegie Collection) was studied and a profile reconstruction of it is shown in figure 5.
On examination of figure 4 it will be seen that the primary head vein is now separated into its adult parts. In the trigeminal nerve region we can speak of it as the cavernous sinus, receiving as tributaries the ophthalmic veins and a large cerebral vein draining the lateral wall of the diencephalon. This vein belongs to the cerebral vein-system and runs for the most of its course through the pia-arachnoid membranes. It penetrates the dura and runs a short dural course before joining the cavernous sinus. It may be regarded as_ one of the diminishing number of channels that drain the cerebral venous system into the dural system. Besides these there are smaller tributaries from a network in the region of the semilunar ganglion. No tributaries were detected flowing into the cavernous sinus from the cerebral hemisphere, such as were found up to this time; all this blood now flows in the opposite direction, caudalward into the developing transverse sinus.
Tracing the cavernous sinus backward, it can be seen that the interruption between it and the internal jugular vein is complete, though there is still a remnant of that connection, which extends as a blind channel a short Way along the facial nerve. It is interesting to note that we occasionally find in the adult skull a persistent foramen, the ‘foramen jugulare spurium’ of Luschka, which corresponds_to the exit of this decadent channel. The vein itself, however, has never been described as persisting, although it exists normally in lower forms as a drainage for the anterior part of the brain, passing through this extracranial course to empty into the internal jugular vein. In the stage we are studying the drainage of the cavernous sinus is upward over the semilunar ganglion into what may now be recognized as the transverse sinus. This communication is through a short channel that approximately represents the original trunk of the middle dural plexus, and constitutes the superior petrosal sinus. This channel is designated by Markowski (’11) as the ‘vena prootica,’ and he gives a different origin for the superior petrosal sinus. According to him (p. 599), it takes its origin from a small cerebral vein derived from the basal surface of the hind—brain which empties into the V. prootica. In the further development, the opening of the vein migrates by anastomoses along the v. prootica toward the sinus transversus and neither empties into that sinus or into the v. prootica near it. According to him, the superior petrosal sinus has little connection with the cavernous sinus and morphologically represents a metencephalic vein. Regarding the eventual fate of the vena prootica he has apparently made no observations, though he pictures it as a large channel in an embryo 46.5 mm. long. From the specimens I have examined I cannot confirm Markowski’s description of the superior petrosal sinus and I feel convinced that his vena prootica and the superior petrosal sinus are one and the same thing, and that which he regards as the superior petrosal sinus is, instead, one of its tributaries. Mall (’05, p. 17) also described the superior petrosal sinus as the adult form of the ‘vena cerebralis media,’ which, it will be remembered, is the same as the trunk of our middle dural plexus.
Fig. 4 Profile reconstruction of the Veins of the dura mater in a human embryo 21 mm. long (No. 460, Carnegie Collection). The Vein marked ‘X’ is the remnant of the otic portion of the primary head vein which originally connected the cavernous region with the internal jugular vein. The outlines of the central nervous system in this model were taken from a model made by Professor Lewis. Enlarged 10 diameters.
With the alterations in the primary head Vein, the anterior, middle and posterior dural plexuses are drained by means of the new dorsal channel, which empties through the jugular foramen into the internal jugular vein. This channel can at once be recognized as the transverse sinus and the sigmoid portion of it presents relations that are much the same as is found in the adult. The three dural plexuses are still of the embryonic type. The posterior plexus is practically the same as was seen in 18 mm. embryos. Only its coarser meshes are shown in figure 4. A finer plexus extends from this toward the median line and the region of the tentorium.
The whole dural area lying between the cerebral hemispheres and the margin of the cerebellum constitutes the tentorium cerebelli. It is very broad dorsally and is more constricted ventrally, thus in profile it is wedge-shaped. In the loose tissue composing it are found the meshes of the dural plexus. As this region becomes more compressed, consequent upon the growth of the cerebrum and cerebellum, there is a continual adjustment of the contained venous channels and repeated alterations in the pattern of the meshes. In general we find the larger channels radiating upward toward the mid-brain region, and as we approach the median line the plexus becomes finer and there is an intimate anastomosis with the subjacent plexus belonging to the brain wall.
On comparing embryos 21 mm. long with those 18 mm. long there are seen two characteristic changes that occur in the pattern of the anterior dural plexus at this time (compare figs. 4 and 5 with figure 3). In the first place, the anterior dural plexus annexes itself to the middle dural plexus and drains backward through this into the newly established channel dorsal to the otic eapsule. We will therefore, from now on, refer to the combined anterior and middle -dural plexuses as the anterior dural plexus, on the basis that the middle plexus has now lost its identity. In the second place, there is differentiated along the margin of the cerebrum and between the hemispheres, a subdivision of the anterior dural plexus that is eventually to constitute the superior sagittal sinus (marked ‘plexus sagittalis’ in figs. 4-5).
Fig. 5 Profile reconstruction of the dural veins in a. human embryo 24 mm. long (No. 632, Carnegie Collection). The point marked ‘x’ is the junction of the sigmoid portion of the transverse sinus with the internal jugular vein. Enlarged about 4 diameters.
Examination of photographs and sketches of embryos of about this age shows that there is a tendency to the formation of a larger channel along the anterior margin of the anterior dural plexus, that is, along the caudal margin of the cerebrum. This was designated by Markowski (’11) as the ‘anterior marginal vein’ (vordere Grenzvene) and the large tributary, draining the lateral surface of the cerebrum, that empties into it he calls the ‘lateral telencephalic vein,’ of which there may be several. Markowski describes the anterior marginal veins of the two sides as extending forward and toward the median line and uniting in the formation of a plexus out of which is to be derived eventually the superior sagittal sinus. From examination of figures 3, 4 and 5 it can be seen that there is no sharp line between the sagittal plexus as described by us and the more ventral loops of the anterior dural plexus of which it is a part. The ‘anterior marginal vein’ of Markowski is a part of both of them. The discussion regarding the formation of the superior sagittal sinus will be reserved for a subsequent part of this paper. We may point out at this time, however, that the anterior marginal vein of Markowski apparently is not a definite vein but rather a constantly changing channel. What we find is that the more an terior loops of the anterior dural plexus are constantly dropping out and are replaced by the development of the more caudal channels. By comparing figures 4 and 5 we can see this change occurring. Our interpretation of the condition found in figure 5 is that what had been a larger channel along the cerebral margin of the dural plexus is now dwindling into a small mesh, while the main blood stream forms for itself a new course in a more caudal loop of the plexus. In this connection it is well to remember that migration of veins may occur in two ways. There may be a passive change in position or direction of the endothelial tube itself, due to mechanical causes arising from alterations in its environment. This is illustrated by the sigmoid portion of the transverse sinus and its change in form in the later stages (embryos more than 20 mm. long). On the other hand, a vein may change its position by forming or adopting a new endothelial channel and at the same time relinquishing its original endothelial channel. The embryonic plexiform character of the veins in the region of the tentorium is especially favorable for this procedure and we find this type of alteration in the blood channels repeatedly illustrated in this region. In other words, under migration of veins we are to distinguish between passive migration, where there is a change in position due to some flexion or traction on the Vein wall itself, and spontaneous migration, in which there is a change in position of the blood stream only, where by a process of what might be called circumfluent anastomosis or anastomotic progression, the blood stream develops a new channel in the adjacent loops of the plexus with a corresponding dwindling of the previously used loop. The lateral telencephalic veins of Markowski apparently correspond to the inferior cerebral veins of the adult, so we shall label them in that way. Though emptying into the dural system they develop their course through the intra-dural membranes and become typical cerebral veins. It is interesting to note that in the 21 mm. embryo certain definite topographical points in the transverse sinus are already determined, namely, the jugular foramen, the location of the endolymphatic sac, the point of entry of the superior petrosal sinus and of the inferior cerebral veins. Thus we see that more than half the sinus is already established and that it is the terminal or jugular portion that is established first. The remainder of the sinus is relatively late in assuming a permanent form, which is doubtless the result of the prolonged period of growth of the cerebrum, making a continued adjustment of the tentorial plexus necessary. Even in embryos 50 mm. long which we shall now proceed to examine, the proximal end of this sinus is still in the formative stage.
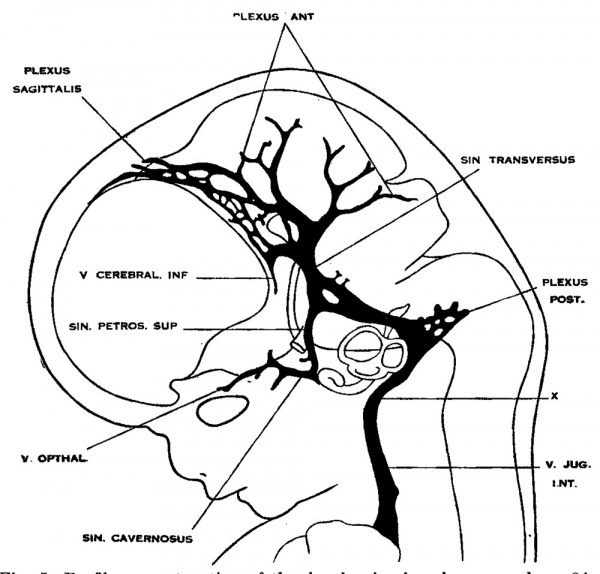
5. Human embryos 50 mm long
To cover the period of embryos about 50 mm. long the writer examined three series belonging to the Carnegie Collection: No. 84, 50 mm. transverse; No. 96, 50 mm. sagittal; and No. 448, 52 mm. sagittal-injected. There was also an embryo of about the same age (Carnegie Embryo No. 458, 54 mm.) that had been injected with India ink. The head was removed and partially dissected, and then cleared after the Spalteholz method. This gave excellent total views of the blood vessels. The profile reconstruction shown in figure 6 is based on series No. 96 and was made by preparing tracings on transparent paper which were then superimposed and a composite tracing made of the whole series. This is about the same stage that is shown by Markowski in his figure 4.
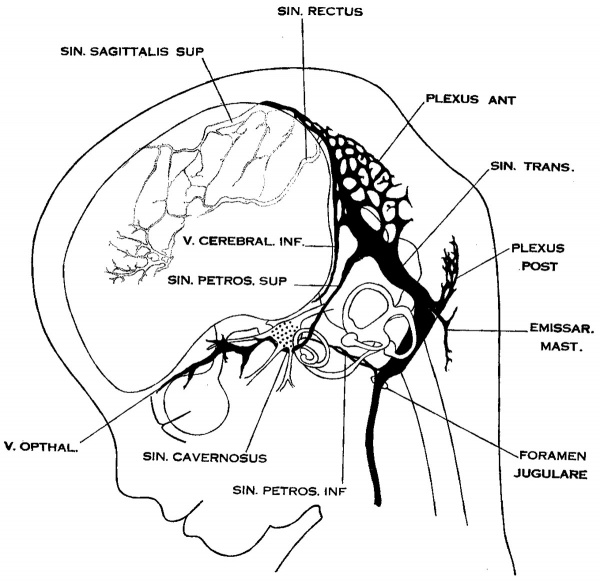
By this time the venous drainage of the cranium is established along channels that correspond fairly well to those found in the adult. It is clearly subdivided into three separate systems: (1) The superficial system draining the integument and soft parts; (2) the dural system lying between the dura and bone; and (3) the cerebral system. All three are originally outgrowths of the same capillary plexus. The separation of the dural veins and the cerebral veins we have traced through, step by step. The superficial veins in embryos 20 mm. long are already separated off from the dural system by the membranous and cartilaginous cranium. They appear first in the lower parts of the head, where they were originally separated off from the deep veins, and form a plexus that gradually spreads upward over the vault. They maintain a few anastomoses with the dural system, which constitute the socalled emissary veins; one of these is shown in figure 6. Aside from the channel maintained through the orbit the chief drainage from the superficial system is through the external jugular vein, which is pictured by Salzer (’95) as already present in guinea-pig embryos 20 mm. long.
On examining the dura in embryos 50 mm. long it will be seen that for the greater part it closely invests the interior of the developing cranium and is relatively poor in blood vessels. This is true especially in those portions where the cartilaginous and bony craniumis more advanced in its differentiation, as in the base ‘of the skull and in the frontal, temporal and lower occipital regions. In other regions "the dura projects within the cranial cavity, being separated from the future bony skull by a layer of areolar tissue, in the meshes of which are found the large blood channels and their tributaries. The largest area of this kind is found situated over the mid-brain and extending from the caudal margin of the cerebral hemispheres to the cerebellum. This area extends laterally down to the base of the skull, narrowing as it does so. It constitutes what is known later as the ‘tentorium cerebelli’ and in it is included the greater part of the dural venous system. A basal extension of the tentorium widens out in the region of the semilunar ganglion and in its meshes is formed the cavernous sinus. A thinner area of the same tissue extends caudalward from the cavernous sinus, medial to the otic capsule, to join the jugular region. The slender plexus of veins extending through this constitutes the inferior petrosal sinus. Along all the sinuses we End this same areolar meshwork. It is not to be confused with the developing arachnoid tissue, from which it is everywhere separated by the dura. Blood vessels supplying and draining the brain are also found in the arachnoid at this time and they are quite numerous in some regions, such as the region of the Sylvian fissure and along the more ventral parts of the mid— and hind—brain,. These cerebral vessels are everywhere separated and distinct from the dural blood channels, with the exception of the few points where they empty into the big dural channels, as occurs in the adult. The connection between the dural system and the cerebral system is no longer by a multiple anastomosis of small vessels, but instead, by isolated larger veins.
Fig. 6 Profile reconstruction of the dural veins in a human embryo 50 mm. long (No. 96, Carnegie Collection). The outlines of the veins of the falx cerebri can be seen through the cerebral hemisphere. Enlarged about 4 diameters.
Examination of figure 6 shows that we have here an arrangement of the dural venous system that in most respects follows the adult arrangement. The cavernous sinus has as yet a simpler character than is found in the adult. It is situated median and ventral to the semilunar ganglion and has the large ophthalmic tributaries in front. Caudally it communicates with the main blood stream by means of the superior and inferior petrosal sinuses. The superior petrosal sinus is a long slender channel that passes over the cochlear part of the otic capsule and empties above into the transverse sinus. The inferior petrosal sinus consists of a plexus of veins that passes median to the otic capsule to empty at the point of origin of the internal jugular Vein.
As regards the transverse sinus, it has been pointed out that the terminal or jugular portion of it is established first. In figure 6 it can be seen that from the point of entry of the superior petrosal sinus to the jugular fossa — in other words, the signoid portion — it consists of a single large channel, and has the same tributaries and the same general relations that are found in the adult. The remainder or proximal portion of the transverse sinus is less well established and the large capillary meshwork found along its dorsal margin shows that the blood channels here are still in the formative stage and must still be spoken of as the remainder of the anterior dural plexus. The main channel is forming along the anterior margin of this plexus, into which the inferior cerebral vein empties. It can be seen how this channel migrates backward in adjustment to the growth of the hemisphere and thus comes to assume a more and more horizontal course. This change in direction, together with growth in length and diameter of the main channel at the expense of the formative meshwork, remains to be completed before the adult condition can be considered as established. The variations found in the adult in the region of the confluens sinuum can be readily understood as variations in channel-selection through this tentorial meshwork.
In the region of the fore-brain a fold of dura is interposed between the two hemispheres and is compressed into a flattened sheet which is to constitute the falx cerebri. This, and the vascular meshwork belonging to it, is directly continuous with the tentorium. Like the tentorium it passes through a prolonged adjustment period. In embryos 50 mm. long two of its permanent channels, which are to belong to the dural sinus system, can be readily recognized. These are the superior sagittal sinus and the straight sinus. In figure 6 the superior sagittal sinus is quite irregular in outline, which is possibly a result of shrinkage of the specimen. Very likely in a normal state, as seen in profile, it would pass evenly along the margin of the cerebrum. The details regarding the vessels belonging to the falx cerebri and the drainage of the chorioidal masses will not be taken up in the present paper, but certain features in the formation of the superior sagittal sinus will. now be referred to.
Sinus Sagittalis Superior
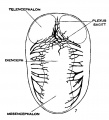
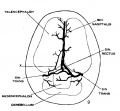
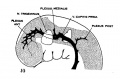
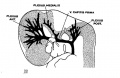
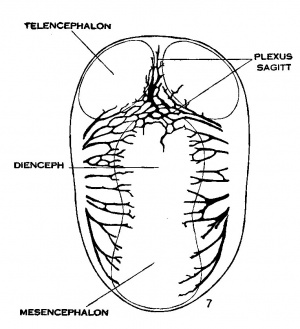
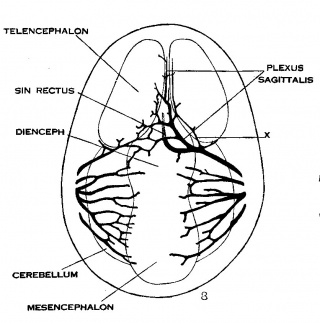
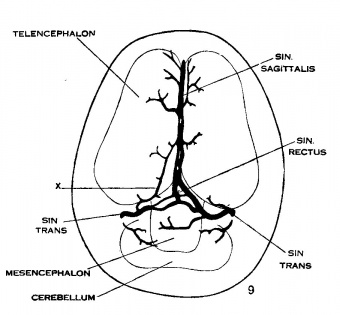
Under the description of embryos 21 mm. long, reference was made to the formation of a ‘plexus sagittalis’ as a subdivision of the anterior dural plexus. At that stage the plexus is clearly differentiated from the remainder of the anterior dural plexus, as can be seen in the dorsal View of an embryo of about that age shown in figure 8. Earlier than this (in embryos about 14 mm. long; figure the plexus can be recognized, though here it is not so clearly separated from the general plexus. In such embryos it can be seen that the larger tributaries of the anterior and middle dural plexuses stop short of the median line——with the exception of anteriorly—where they merge into a longitudinal plexus that dips in between the developing hemispheres. It is in the meshes of this plexus that we find the beginning of the superior sagittal sinus; and the principal steps in its transformation can be seen by comparing figures 7, 8 and 9. Sketches like these necessarily have to be simplified, and on examining them it should be remembered that only the larger channels are shown and in between there is everywhere a fine anastomosing network. Also, the channels do not lie all in the same plane. Furthermore, it is to be noted that there exists in embryos of the same age a considerable variation in the pattern formed by these channels. The three specimens selected, however, may be regarded as illustrating fairly definite stages in this transformation.
In figure 7 is shown a dorsal View of the head of the same embryo previously shown in figure 2 (No. 940, 13.8 mm. long, Carnegie Collection). Here we find the sagittal plexus represented in its simplest form. It will be noted that it possesses two characteristic features: In the first place, there is a tendency to an enlargement of certain portions of the plexus, irrespective of a continuous channel; we thus have a series of small lakelets connected by narrow channels; a definite single superior sagittal sinus cannot yet be said to exist. In the second place, the plexus is distinctly asymmetrical and shows a tendency to drain more freely to one side than the other—in this case, to the right.
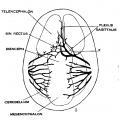
A more definite and simpler channel, system is found in 20 mm. embryos, an example of which is shown in figure 8 (l\‘o. 349, 20 mm. long, Carnegie Collection). Here one might possibly speak of a superior sagittal sinu. The channels, however, are still in the form of a plexus and hence the term ‘plexus sagittalis’ is retained. This view regarding the early identity of the superior sagittal sinus differs from that given by Evans, who pictures the primitive capillary plexus creeping up on each side‘ of the forebrain in 8 mm. pig embryos. A portion of the dorsal margin of this plexus he labels as the ‘primitive superior sagittal sinus’ (Evans ’09, fig. 15 b; Evans ’12, figs. 399-400). According to him, it is thus originally paired and bilaterally) symmetrical. According to the present writer, it is not until later that we can speak of a superior sagittal sinus. It is not until the plexuses, described by Evans, have fused across the middle line and have formed a longitudinal network, in the meshes of which an asymmetrical channel is finally established, that we can speak of a superior sagittal sinus.
|
|
|
Figs. 7, 8 and 9 Three stages in the formation of the sagittal plexus, showing its asymmetrical character and its conversion into the superior sagittal sinus. Draining into it from below is the drainage channel from the chorioidal bodies which becomes the straight sinus. The channels marked ‘x’ are interpreted as undergoing retrogression, being replaced by more caudal channels. figure 7 is a. vertex view of a. human embryo 13.8 mm. long (No. 940, Carnegie Collection). figure 8 is a vertex view of an embryo 20 mm. long (No. 349, Carnegie Collection). figure 9 is a. drawing of an injected and cleared specimen 54 mm. long (No. 458, Carnegie Collection).
Owing to the growth of the cerebral hemispheres in 20 mm. embryos, the dural tissue lying between them begins to take on the form of the falx cerebri. It is in this loose dural tissue that the meshes of the sagittal plexus are found. At this time it can be seen that a larger channel is opening along the dorsal mid~line that will form the superior sagittal sinus, and connected with it by anastomosing loops, is a more ventrally situated large channel that constitutes the ‘sinus rectus.’ This latter extends forWard and drains the lower part of the falx. It has two converging limbs in front that drain the chorioidal masses of the hemispheres. This and the more superficial channels in this embryo drain chiefly to the right side. Smaller anastomosing loops connect also with the plexus in the region of the left transverse sinus.
On coming to embryos 50 mm. long (fig. 9, No. 458, 54 mm. Carnegie Collection) we find that here the superior sagittal sinus is established, at least in part. In its cephalic portion there is a large characteristic channel, lacking only the dural connective tissue investment to make it an adult type. In its more caudal portion it still exhibits a plexiform character that indicates its transitional state. Upon comparison of a number of series the writer is led to interpret the formation of a single channel as the outcome of more than one process; in some segments there seems to be the selection of a favorable loop of the plexus which enlarges and becomes the main channel, and in other segments there is ' apparently an enlargement of two or more collateral loops which subsequently fuse into a more or less common channel. Both processes are apparently represented in figure 9. It is to be expected that we shall find a considerable variation in this in different brains.
No attempt was made to study the histological changes that occur in the completion of the superior sagittal sinus, nor of the cavernous sinus. These involve details with which the present paper is not concerned. The caudalward growth, however, of the superior sagittal sinus in adjustment to the corresponding growth of the hemispheres is of interest in our general problem. By comparing figures 7, 8 and 9 it can at once be seen that this caudal development is accomplished at the expense of the meshes of the anterior dural plexus, in which process the transverse and straight sinuses also take part. These channels gradually obtain a more caudal course by what we have already described as ‘ spontaneous migration.’ The channel repeatedly shifts into a more caudal loop of the plexus, the new loop enlarging and the old loop dwindling. The veins marked X in figures 8 and 9 may thus be interpreted as discarded channels. The eventual ‘confluens sinuum’ (torcular herophili) represents the point at which this caudal development reaches its completion. It usually retains a trace of the plexiform character that is found throughout the embryonic stages.
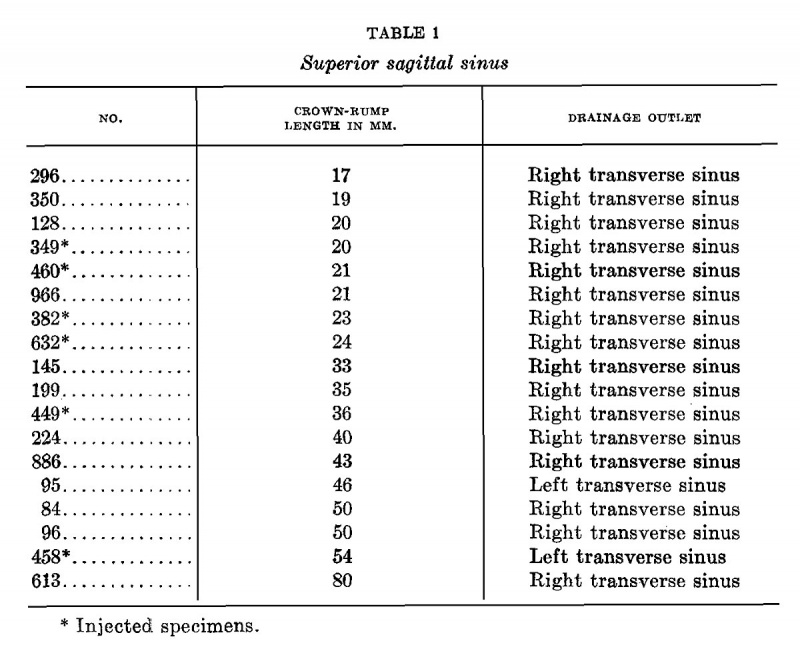
It is interesting to note that the asymmetry of the superior sagittal sinus expresses itself in the embryo, as well as in the adult, by a tendency to drain more to one side of the head than to the other. This becomes established by the time the embryo is 20 mm. long. The drainage is preponderantly toward the right side. It happens that in figure 9 the main drainage was in reality toward the left side. In reproducing the sketch the figure was reversed, right for left, in order to facilitate its comparison with figures 7 and 8. In table 1 is given a list of embryos which were examined as to this point and it will be seen that of eighteen specimens all but two drained predominantly toward the right side, that is, about 89 per cent. In order that account should be taken of the artificial element introduced in those specimens where the vascular system had been injected with coloring matter, such specimens are indicated in the table by an asterisk. No explanation has thus far been reached to explain this interesting asymmetry. The drainage of the straight sinus could not be determined as well in the younger stages and there were not enough of the older stages upon which to base an average. If we may judge from the adult, it would generally drain to the opposite side, that is, the left.
Table 1 Superior Sagittal Sinus
Summary
If now we look back and bring together the essential features in the development of the venous sinuses of the dura mater, we will find their development may be analyzed somewhat as follows: (1) There is first the establishment of the primary arrangement for the drainage of the head; (2) this is followed by a separation of the veins of the head into two and finally three separate layers or strata, of which the middle layer constitutes the dural veins; (3) certain adjustments of the dural channels are made necessary by the environmental changes in the region of the middle and internal ear; (4) similar and still greater adjustments of the dural channels follow the marked growth and change in form of the brain; (5) and finally, there are the late histological changes in the vein walls that convert them into the adult sinuses. This last or histological feature is not taken up in the present paper. The other four features, however, will now be outlined. Simplified sketches of the successive stages have been assembled on one page, as figures 10 to 17, and it is hoped that this will facilitate the identification of the steps in this process. It hardly needs to be pointed out that these steps overlap one another, and also that embryos of apparently the same age exhibit a considerable variation in the pattern of their venous plexuses.
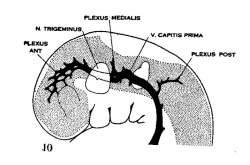
The primary arrangement for the drainage of the capillaries of the head (figs. 10-11) consists of a ‘primary head vein’, which starts in the region of the mid-brain and runs caudalward alongside of the brain tube and terminates at the duct of Cuvier. The primary head vein is composite in origin. That portion of it oral to the vagus nerve is an intrinsic vein of the head; the remaining caudal portion is in reality a neck vein and constitutes the anterior cardinal vein——eventually the internal jugular vein. Together these portions form a continuous channel, the primary head vein, into which the blood from the capillary sheet that immediately invests the brain tube is drained by means of anastomosing venous loops. These loops gradually become arranged more or less in the form of three plexuses, the ‘anterior dural plexus,’ ‘middle dural plexus,’ and ‘posterior dural plexus.’ Other small tributaries empty into the primary head vein which drain the structures ventral and lateral to the brain tube, such as the nerve ganglion masses. The largest of these come from the eye region and these eventually form the ophthalmic vein.
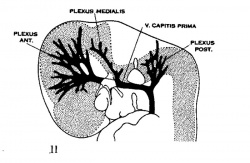
While the three head plexuses are forming (figs 11-12) the outlines of the dura mater and the arachnoid spaces make their appearance, and first of all in the ventral parts. This results in a general separation or cleavage of the more superficial primary head vein and its three tributary-plexuses from the subjacent vessels that arise from and drain the capillary sheet directly investing the brain tube. This deeper system, however, continues to drain into the former at certain places, notably in the more dorsal parts. The primary head vein and its three tributary plexuses thus become established as a true dural system as distinguished from the deeper ‘cerebral veins’ belonging to the arachnoid-pial membrane. The diploic veins are a later subdivision of the dural system. The superficial veins of the head are separated off in the more ventral regions and from there spread upward over the head independently of the dural system. We then have for the head three separate venous systems: (1) the superficial layer belonging to the integument and soft parts; (2) the middle layer belonging to the dura and diploe; and (3) the deep layer of cerebral veins belonging to the brain. It is the middle layer, or dural system, that‘ is exclusively concerned in the formation of the dural sinuses, and whose changes in form and position will now receive our attention.
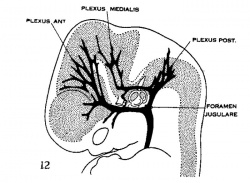
The changes in form of the dural veins that occur after the establishment of the primary arrangement for the drainage of the head (figs. 12—17) are largely due to the mechanical factors involved in the changes of their environment, the two most conspicuous elements being the changes in the region of the cartilaginous capsule of the labyrinth and the changes involved in the growth and alteration in form of the brain. The changes thus produced include the reduction of the plexuses into simple channels, and the total obliteration or change in position of the channels themselves. Under this latter phenomenon we recognize a passive migration, where there is a change in the position of the Vein wall itself, due to some flexion—or traction——force acting upon it. We also recognize a spontaneous migration where there is a changein position of the blood stream only, where by a circumfluent anastomosis the blood stream develops a new channel in the adjacent loops of the plexus with a corresponding dwindling of the previously used channel. An anastomosing plexus is essential in spontaneous migration but is not essential in passive migration. Both of these types of migration are to be distinguished from the formation of replacement channels which is another way, deserving mention, in which the venous channels are changed in position and direction in this process of adjustment.
|
|
|
|
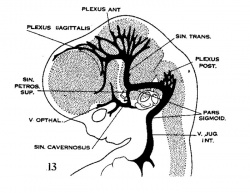
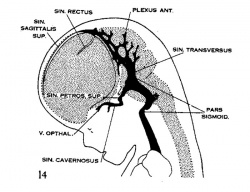
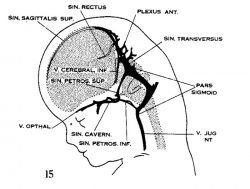
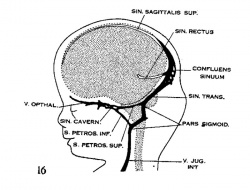
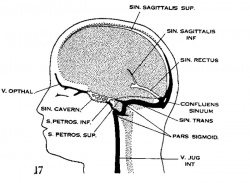
| Figure 10, Embryo No. 588 | Figure 11, Embryo No. 940 | Figure 12, Embryo No. 144 | Figure 13, Embryo No. 460 |
|
|
|
|
| Figure 14, Embryo No. 199 | Figure 15, embryo No. 96 | Figure 16, Embryo No. 234 | Figure 17, adult |
Figs. 10-17 Simplified profile drawings of the dural veins showing the principal stages in their development in human embryos from 4 mm to birth.
It is of particular interest to notice their adaptation to the growth and changes in form of the central nervous system. figure 10, Embryo No. 588, 4 mm.; figure 11, Embryo No. 940, 14 mm.; figure 12, Embryo No. 144, 18 mm.; figure 13, Embryo No. 460, 21 mm.; figure 14, Embryo No. 199, 35 mm.; figure 15, embryo No. 96, 50 mm. crown-rump length; figure 16, Embryo No. 234 a, 80 mm. crown-rump length; figure 17, adult.
In the replacement channel there is the formation of a new channel and the obliteration of an old one, as in migration. It however differs from migration in that it is not a gradual and progressive change in position, but an abrupt and immediately complete one. Furthermore, the new channel lacks the morphological characteristics of the old one. This process is illustrated by the transverse sinus which replaces part of the primary head vein, as will be presently described.
Adjustment of the dural channels occur early in the region of the middle ear. In the same way that the facial nerve is bent out of its original course by the development of the membranous labyrinth and the middle ear, so the dural veins are definitely influenced by the same structures. Owing to the growth of these the course of the primary head vein, ventro-lateral to the otic capsule, becomes an unfavorable one and this segment of it becomes obliterated. To make the necessary adjustment two things happen (figs. 11, 12 and 13). first, an anastomosis is established above the otic capsule through which the middle plexus drains caudally into the posterior plexus. Secondly, the anterior plexus, which originally drained into the primary head vein, fuses with the middle plexus and drains caudally through this and through the newly established channel, dorsal to the otic capsule. This makes a complete trunk for the drainage of the head which is everywhere dorsal to the primary head vein as far as the jugular foramen, Where it is continuous with the internal jugular vein. Of the primary head vein there is left, in addition to the cardinal portion of it or internal jugular vein, only that part in the region of the trigeminal nerve. This may now be called the ‘cavernous sinus.’ Into it drain the veins from the orbit and it, in turn, drains upward through the original trunk of the middle plexus, which is now the ‘superior petrosal sinus,’ into the newly established dorsal channel. By comparing with later stages (figs. 1417) it will be seen at once that this dorsal channel is the ‘transverse sinus’ of which that part between the superior petrosal sinus and the jugular foramen forms its ‘sigmoid portion.’ Thus. in the 21 mm. embryo the dural channels in the region of the temporal bone have acquired essentially all their permanent connections, with the exception of the inferior petrosal sinus which appears a little later (fig. 15). Otherwise, there remains to complete the adult condition only a certain amount of passive migration in accommodation to the changes in the adjacent parts.
The adjustment in the dural channels rendered necessary by the protracted growth of the hemispheres extend much later in fetal life. A large part of this adjustment is accomplished by spontaneous migration of the principal channels and for this a venous plexus is essential. We thus find a continuous persistence in the neighborhood of the advancing occipital pole of the hemispheres of the transitory anterior dural plexus from which are evolved all the veins of the falx cerebri and of the tentorium cerebelli.
An anterior subdivision of the plexus extends forward in the median line as the ‘plexus sagittalis’ which is interposed as a vertical curtain between the hemispheres. Among its dorsal meshes is developed an asymmetrical longitudinal channel which we know as the ‘superior sagittal sinus.’ In its early stages this channel is made up of several collateral anastomosing veins. The eventual single channel is formed in the anterior portions by the selection and enlargement of the most favorable vein with a corresponding disappearance of the others. In the posterior portions there is apparently some coalescence of adjacent veins. The anterior part of the sinus is completed first. As the hemispheres extend backward the sinus correspondingly elongates itself by incorporating the more caudal loops of the plexus. Transverse sections through this part of the sinus in older fetuses thus usually reveal incomplete coalescence of the separate loops. The sagittal plexus very early exhibits a tendency to drain more to one side of the head than to the other and usually toward the right side. As the superior sagittal sinus becomes established we thus find it continuous caudalward usually with the Ventral main channel of the right anterior plexus which eventually forms part of the right transverse sinus. The ‘straight sinus’ is formed in the ventral part of the sagittal plexus and its caudal adjustment is essentially like that of the superior sagittal sinus. It may drain chiefly toward the right or left anterior plexus or equally toward both.
man In embryos between 35 and 50 mm long (figs. 14-15) we can recognize a main channel of the anterior dural plexus, that is, to become the ‘transverse sinus.’ If we disregard the sigmoid portion of it, it forms a fairly straight line with the internal jugular vein. In the interval between the 50 mm. embryo and the adult, the transverse sinus bends backward until it comes to lie at an angle of 90° with the internal jugular. This marked change in position is accomplished in large part by spontaneous migration, by the repeated shifting back of the main blood current into more caudal loops of the plexus with subsequent dwindling of the discarded anterior loops. As the sinus becomes more definitely established the dural plexus becomes relatively smaller (fig. 16) and the final change in position is completed by passive migration, that is, actual traction on the vein wall by its environment. In this change in position of the transverse sinus the superior sagittal sinus and the straight sinus participate and we find in the adult, at the point where they meet, an anastomosis, the ‘confluens sinuum,’ which is usually plexiform in character and represents the last trace of the anterior dural plexus.
Literature Cited
Evans HM. On the development of the aortae, cardinal and umbilical veins, and the other blood vessels of vertebrate embryos from capillaries. (1909) Anat. Rec. 3: 498-518. 1912 The development of the vascular system. Keibel-Mall Manual of Human Embryology, vol. 2.
GROSSER, O. 1907 Die Elemente des Kopfvenensystem der Wirbeltiere. Verh. Anat. Gesell., Wiirzburg. Anat. Anz., Bd. 30, Erganz. Hft.
GRossER, O. UND BREZINA, E. 1895 Ueber die Entwicklung der Venen des Kopfes und Halses bei Reptilicn. Morph. Jahrbuch, Bd. 23.
Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
MARKOWSKI, J . 1911 Ueber die Entwicklung der Sinus durae matris und der Hirnvcnen bei menschlichen Embryonen. Bull. (1. l’Acad. (1. Sciences de Cracovie. Cl. d. Sci. math.-Nat., Serie B, p. 590.
SABIN, F. R. 1915 On the origin of .the duct of Cuvier and the cardinalveins. Proceed. Am. Assoc. Anat., St. Louis, Anat. Rec., vol. 9, p. 11.5. 1915 On the fate of the posterior cardinal veins and their relation to the development of the vena cava and azygos in the embryo pig. Contributions to Embryology, Carnegie Institution of Washington, Pub. No. 223.
SALZER, H. 1895 Ueber die Entwicklung der Kopfvenen des Meerschweiiicliens. Morph. Jahrbuch, Pd. 23.
Figures
fig 1 embryo 4 mm No. 588
fig 2 embryo 13.8 mm No. 940
fig 3 embryo 18 mm No. 144
fig 4 embryo 21 mm No. 460
fig 5 embryo 24 mm No. 632
fig 6 embryo 50 mm No. 96
fig 7 embryo 13.8 mm No. 940
fig 8 embryo 20 mm No. 349
fig 10 embryo 4 mm No. 588
fig 11 embryo 14 mm No. 940
fig 12 embryo 18 mm No. 144
fig 13 embryo 21 mm No. 460
fig 14 embryo 35 mm No. 100
fig 15 embryo 50 mm No. 96
fig 16 embryo 80 mm No. 234a
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Reference
Streeter GL. The development of the venous sinuses of the dura mater in the human embryo. (1915) Amer. J Anat.18: 145-178.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The development of the venous sinuses of the dura mater in the human embryo. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_venous_sinuses_of_the_dura_mater_in_the_human_embryo
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098GCarnegie Embryo 588