Paper - The Anatomy of the Head End of a 20 mm Human Embryo
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Dickie JK. The anatomy of the head end of a 20 mm human embryo. (1914) J Anat Physiol., 48(4): 445-60. PMID 17233010
| Online Editor Note |
|---|
| This historic 1914 paper by Dickie is a description of the development of the human embryo Carnegie stage 20 in week 8.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Anatomy of the Head End of a 20 mm Human Embryo
By J. K. Milne Dickie, M.D., F.R.C.S. Edin.,
Demonstrator of Anatomy, University of Edinburgh.
Introduction
The embryo here described is in the collection of the University of Edinburgh. It was removed by operation and was fixed in a 5 per cent. aqueous solution of formaldehyde. After fixation the vertex-breech measurement was 20 mm. There are no data as to its age, but, judging from its length and general development, it may be placed at approximately a little over seven weeks. The specimen was stained in bulk with acid haemalum and eosin, and cut in serial transverse sections of 10“. A series of models of the head region, at a magnification of 50 diameters, was reconstructed by the wax-plate method. It is upon these models that the following description is based.
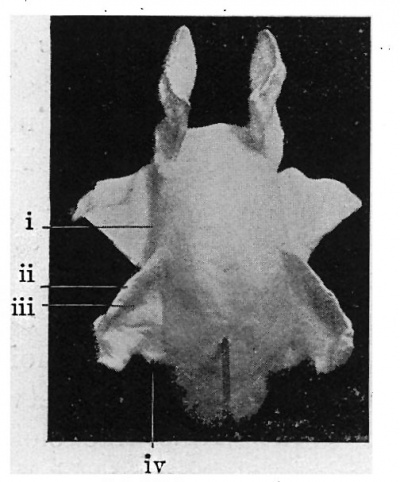
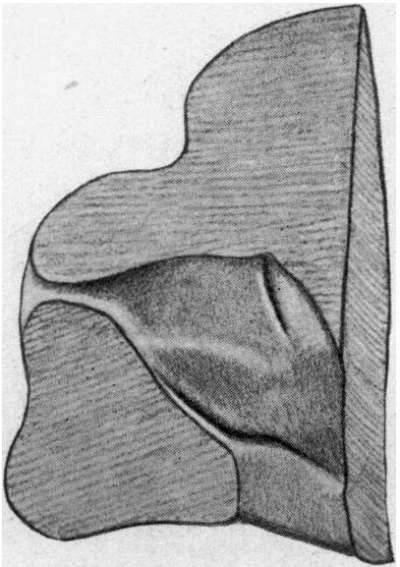
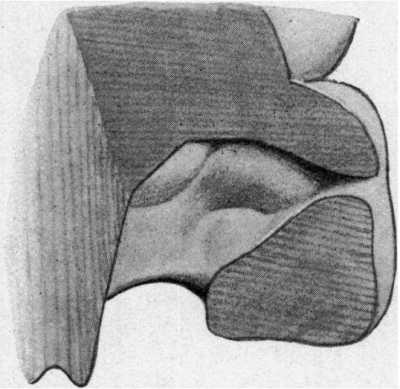
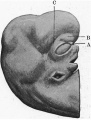
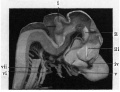
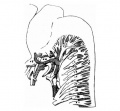
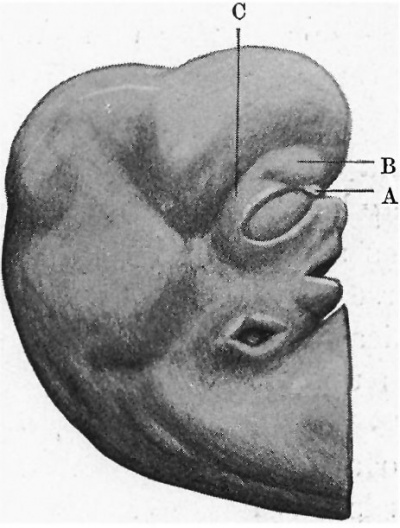
The general characteristics of the embryo are very similar to those of others of about the same age, of which records have been published, and which are figured in Keibel’s Normentafeln (1). The cervical flexure is still so well marked that the chin almost touches the chest wall. The outlines of the brain can be easily distinguished through the superficial tissues (fig. 1). The head in the region of the hind-brain is somewhat wrinkled, but its outline presents a fairly uniform curve, while in Meyer’s 18'5-mm. embryo (1) the hind-brain forms a distinct projection. The external acoustic meatus is represented by a deep oval pit. Its edges are raised and thickened, but there are no longer any distinct tubercles. It is similar to embryos of 18 and 186 mm. in the Normentafeln, while in embryos of 20 and 22 5 mm. the cavity of the meatus is much diminished. The forehead is a large rounded prominence, separated from the nose by a deep transverse fronto-nasal groove, which is prolonged laterally into the upper eyelids. The eyelids are still widely separated. They are better defined than appears to be the case with some other embryos of comparatively similar age, for they are thick raised folds surrounding the eye. The other eyelid is grooved by the lateral continuation of the frontonasal groove, which divides it into three segments, viz. a small triangular part at the medial commissure (fig. 1, A), a larger intermediate part (B), and a lateral part (C), which becomes continuous with the lower lid at the lateral commissure. The lower lid is smooth, and blends with the cheek and the upper lip at the angle of the mouth, and is continuous medially with the root of the nose. The nose is broad and upturned. The nares are widely separated and directed forwards (fig. 2). Their openings are small and partly occluded by plugs of epithelium. They are bounded laterally by thick alas nasi; The mouth is open, exposing the tip of the tongue. The upper lip has a shallow median notch. The lower lip is a smooth rounded shelf, with no trace of its bilateral origin. There is a distinct double chin, which is seen also in an embryo figured by His (2).
|
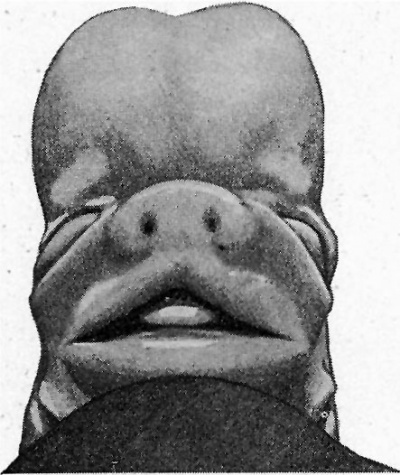
|
| Fig. 1. Profile view of the embryo | Fig. 2. Front view of embryo. showing general form. |
Nervous System
Brain
The three flexures of the brain are still well marked, the cephalic flexure forming the most acute angle of the three.
Primitive Forebrain and its Dimensions
The primitive forebrain is distinctly separated into telencephalic and diencephalic portions. The telencephalon has divided into two cerebral hemispheres, and each hemisphere, which is ovoid in form, has grown ventrally (forwards) beyond the lamina terminalis and dorsally (backwards) over the antero-superior portion of the diencephalon (fig. 3). The olfactory bulb on the ventral surface of the hemisphere is distinctly defined, and is limited medially "and posteriorly by a sulcus. In sections it is seen to be definitely connected with the olfactory epithelium by nerve fibres. The eye stalk on each side is attached to the end of the forebrain, about. half—way between the olfactory bulb and the hypophysis. It is very slender, and shows no trace of its originally hollow character. The eye stalk of Keibel’s 18-mm embryo (1) also is solid, but that of Meyer’s 18'5-mm. embryo is still to a great extent pervious. There is as yet no chiasma. The hypophysis is situated at the ventral corner of the forebrain and is flattened dorsoventrally. The buccal lobe is crescentic in form and partly surrounds the smaller infundibular lobe. It is hollow and still connected with the month by a thin cord of cells. ‘
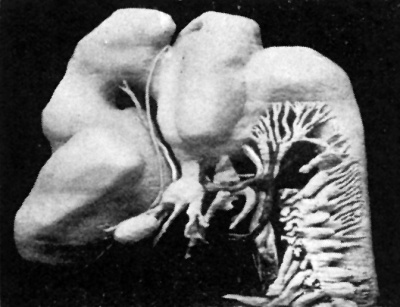
Fig. 3. — Lateral surface model of brain and cranial nerves.
The lateral wall of the third ventricle is distinctly separated by a hypothalamic sulcus into a dorsal thalamic and a ventral hypothalamic region. The hypothalamic sulcus pursues a curved course from the interventricular foramen round the thalamus, and is continuous with a. furrow which‘ traverses the entire length of the mid-brain. The optic recess bounds the hypothalamus anteriorly, and intervenes between it and the corpus striatum. The corpus striatum, again, is separated from the thalamus by the anterior part of the hypothalamic sulcus, and is prolonged laterally through the interventricular foramen of Monro to form part of the floor of the lateral ventricle. The interventricular foramen is a wide opening. through which one can see a large choroid plexus in the lateral ventricle. The hypothalamus is separated by a shallow sulcus into an anterior and a posterior portion. The anterior portion lies just dorsal to the hypophyseal recess, while the posterior part becomes continuous with the floor of the mid-brain. The posterior part of the floor of the diencephalon forms a projection outwards, representing the mammillary protuberance. The thalamus is crossed by a ridge which separates it into dorsal and ventral portions. The posterior or caudal end of this ridge is lost on the lateral wall of the ventricle on a level with a recess in the roof, ‘which probably represents the pineal recess. The anterior end of this ridge is joined by a prominent fold attached to the roof of the diencephalon. Its anterior end blends with the thalamus, and its surface is continuous through the interventricular foramen with. the choroid plexus of the lateral ventricle. This fold is seen in several of His’s specimens (3); and is most probably the commencement of the choroid‘ plexus of the third ventricle, since, according to Elliot Smith (4), that structure is developed from the roof of the forebrain. This choroid ridge extends posteriorly along the roof of the forebrain to a point opposite to where the floor becomes invaginated just posterior to the mammillary eminence. Immediately caudal to its posterior extremity the roof is thickened, and in this thickened portion of its wall are two recesses, which may represent the pineal and prepineal recesses. The anterior commissure is represented’ by a thickening of ‘ the lamina terminalis about its middle.
Fig. 4. — Shows medial aspect of model of brain. 1, Cerebellum ; ll, tegmontum ; Iii, thalamus; iv, Interventriculsr forunen; V, olfactory bulb; vi, vague; vll, uympathetlc.
Mid-brain
The mid-brain or mesencephalon is tubular and curves on itself to join the isthmus. Viewed from the lateral aspect it is somewhat indistinctly separated into a lamina quadrigemina and a peduncular portion. The dorsal part of lamina quadrigemina projects posteriorly over the isthmus so as almost to touch the roof of the fourth ventricle. The cavity of the mid-brain is much smaller than that of the forebrainl Its roof shows several irregularities, probably artificial, as this region of the brain was somewhat damaged. The floor of the mid-brain is thickened on each side of the middle line, this thickening represents the tegmentum of the adult, and is bounded laterally by the continuation of the sulcus hypothalamicus. At the isthmus a small deep pit is seen in the lateral Wall.
Hind-brain
The hind-brain has still distinct pontine and cervical flexures. Its walls vary considerably in thickness in different portions. The thin roof of the fourth ventricle bulges upwards and also laterally, and forms a very prominent feature of the brain in this region. Near the cervical flexure the choroid plexus forms a deep transverse invagination of the roof, and divides it into anterior and posterior portions. The anterior portion of the roof overlaps the alar lamina of the anterior limb of the pontine flexure, so that a deep sulcus appears on the outer surface running parallel with the anterior limb of the pontine part of the brain. The posterior part of the roof of the fourth ventricle is not nearly so prominent as the anterior part, and blends with the medulla oblongata, which tapers off rapidly into the spinal medulla. The diameter of the spinal medulla is fairly uniform, but opposite the origin of the brachial nerves it increases somewhat in thickness. The spinal medulla in the cervical and upper thoracic regions is nearly straight, there being only a slight ventral convexity. The appearance of the hind-brain, as seen from inside, is very striking. The cavity of the fourth ventricle communicates with the mid-brain by means of a narrow isthmus rhombencephali. Immediately posterior to this the cavity Widens out in all its diameters. Its floor is very thick, especially in the posterior limb of the pontine flexure and in the region of the medulla oblongata It has a longitudinal ridge on each side of the middle line which traverses the whole length of the hind-brain. The alar lamina is distinctly separated from the basal lamina in the anterior part of the pons by a furrow. It forms a thick rolled edge and has three bends on it. It begins on the lateral wall of the isthmus near its roof and runs laterally and dorsally a short distance. It then bends at an acute angle and runs ventrally a short distance. Soon it bends slightly laterally, and then again resumes its ventral direction till it reaches the pontine flexure, where it ends. It is much thicker at its pontine end than at the isthmus. Lateral to it the roof forms a very deep recess. The part of the ventricle posterior or caudal to the choroid plexus is comparatively shallow. It is broad at its anterior end, but narrows rapidly into the central canal of the spinal medulla. The cervical flexure occurs in this part of the brain, hence the floor of the cavity is convex in the sagittal plane.
The Spinal Medulla
The spinal medulla has a large central canal, narrow from side to side, but wide in the sagittal plane. The ventral wall is much thicker than the dorsal wall. Its ventral surface shows a shallow median groove. Transverse sections show that the greater part of the spinal medulla is composed of grey matter with a surrounding zone of white matter.
Cranial Nerves
I. The olfactory bulb on each side is represented by a short, thick process from the ventral surface of the cerebral hemisphere, extending between it and the epithelium of the dorsal part of the olfactory cavity.
It is composed of a more or less homogeneous mass of cells with round or oval nuclei.
The optic nerve on each side is fairly slender and springs from the corresponding angle of the diencephalon. It consists largely of cells, but mixed with them are a number of longitudinal fibres, which can be traced as far as the junction of the optic stalk with the diencephalon. There is as yet no indication of any fibres crossing the middle line to form a chiasma. There is now no cavity in the interior of the optic stalk. Keibel’s 18-mm embryo also has a solid optic nerve, while the nerve of Meyer’s 18.5-mm embryo is still hollow.
III. The oculomotor nerve is very long. It arises from the floor of the mid-brain by a bunch of five or six fine rootlets, and passes ventrally between the forebrain and the hind-brain till it comes into relation with the semilunar ganglion, when it curves laterally between the ophthalmic and maxillary divisions of the trigeminal nerve to end in the neighbourhood of the eye.
IV. The trochlear nerve springs from the root of the isthmus by a single root. It arches round the mid-brain and passes forwards a little lateral to the oculomotor nerve. Near the eye it curves forward in intimate relation to branches of the trigeminal nerve, and ends near the olfactory bulb.
V. The trigeminal nerve arises as a very thick trunk a little anterior to the apex of the pontine flexure and some distance from the median plane. Soon after leaving the brain it expands into the large semilunar ganglion, which at this stage is somewhat triangular in shape. At its central or proximal end the nerve consists mainly of fibres, with which a few cells are intermingled; but as the triangular enlargement which represents the semilunar ganglion is approached, the cells increase in number, more especially round the periphery of the bundle of fibres. As the more distant part of the ganglionic mass is approached, the cells increase in number until they almost entirely replace or conceal the fibres. Along the medial side of the ganglion a small bundle of fibres remains distinct, and can be traced into the mandibular division of the nerve. This bundle represents the motor root.
The ophthalmic division springs from the extreme frontal end of the ganglion and runs obliquely downwards and forwards. It soon divides into two branches, one passing medial to the eyeball to end near its medial commissure, the other ending near the optic nerve.
The maxillary division consists of a single trunk, passing downwards and forwards just under the eye to end in the cheek.
The mandibular division (fig. 5), the largest branch of the trigeminal, springs as a short thick trunk from the posterior angle of the semilunar ganglion. It passes downwards (caudally) for a short distance before breaking up into its various branches. Of these, the most medial is the lingual nerve, which passes in a medial direction as a flattened band to reach the side of the tongue, where it enlarges into a ganglion just lateral to the submaxillary gland. The auriculo-temporal is the most posterior branch, and is much smaller than the lingual nerve. It has a short course, curving laterally to reach the skin round the external acoustic meatus. The inferior alveolar nerve lies at first anterior to Meckel’s cartilage, but gradually comes to assume a position lateral to it, and is continued on into the chin region. During its course it gives off a small branch, which crosses Meckel’s cartilage on its lateral side and ends in the mylohyoid muscle. The nerve to the masseter lies lateral to the inferior alveolar nerve and ends in the masseter muscle. The buccinator nerve lies just under cover of the epithelium at the angle of the mouth.
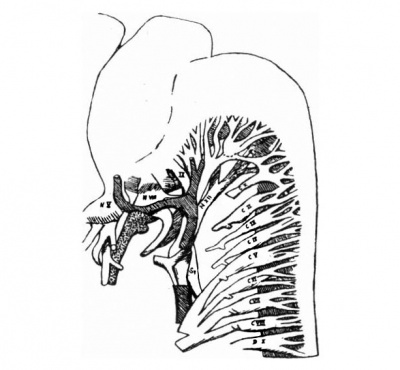
Fig. 5. — Sketch of nerves in occipital region. Meckel’s cartilage is stipled. The lateral hes. vein an part of the internal jugular vein are also shown.
VI. The abducent nerve arises from an area medial and slightly caudal or posterior to the trigeminal and on the same level as the facial nerve by a bunch of small rootlets. It passes downwards and forwards towards the eye, and then curves laterally between the ophthalmic and the maxillary divisions of the trigeminal.
VII. The facial nerve comes from the ventral surface of the brain slightly medial to the acoustic nerve, and curls round its anterior border so as to. lie in a groove in its lateral surface. Leaving the acoustic nerve, it develops a small geniculate ganglion and runs in a caudal direction for some distance, crossing over the long process of the incus, which at this stage lies horizontally. Soon after this it bends downwards and gives off . the chorda tympani, which passes between the malleus and the long process of the incus to reach the lingual nerve. The facial nerve now gives off the nerve to the digastric and passes on to its distribution in the face.
VIII. The acoustic nerve is connected with the pontine part of the hind-brain by two roots. The more medial of the two is the mandibular root, the more lateral is the cochlear root, which, as it enters the brain, becomes associated with a group of cells which represent the dorsal and ventral cochlear nuclei. Outside the brain the two roots are in close apposition. The peripheral end of the trunk becomes associated With a group of cells which is in process of separation into the spiral ganglion and the smaller vestibular ganglion.
IX. The glossopharyngeal nerve lies, in series with the vagus and accessory nerves on the lateral aspect of the hind-brain. It arises as a single thick trunk. which expands slightly to form the superior ganglion. It passes down ventral to the vagus, and, at the level of the auditory ossicles, it shows a much larger petrous ganglion, which gives or!‘ from its ventral side a small twig —the tympanic nerve of Jacobson. Below this point it curves forwards and medially to the tongue.
X. and XI. The vagus and accessory nerves arise by means of a long series of rootlets, which spring from the side of the hind-brain along a line corresponding in direction with the cervical flexure. These run together to form a single large trunk. The spinal root of the accessory can be traced down on the medial side of the cervical nerves as far as the rootlets of the third cervical ganglion. Numerous small ganglionic swellings are seen on the rootlets before they unite to form the vago-accessory trunk. At the upper part of the trunk is a collection of cells which represents the jugular ganglion, and at a lower level the fibres of the trunk ‘become intermingled with cells of the ganglion nodosum. Some of the most caudal rootlets communicate with the first cervical ganglion, and at the point of junction a small ganglion of Froriep is seen. The vago-accessory trunk passes down ventral and lateral to the vertebral column, and opposite the second, third, and fourth cervical vertebrae is the ganglion nodosum, which has been already mentioned. This ganglion is large and spindle-shaped, and gives off from its medial aspect a short superior laryngeal nerve, which passes straight into the larynx. The vagus is continued down into the thorax dorsal to the pericardium.
XII. The hypoglossal nerve arises in a plane medial to the vagus and accessory nerves and in series with the spinal anterior nerve-roots. Its eight or nine slender roots fuse to form a flattened trunk, which comes to lie in contact with the dorsal surface of the ganglion nodosum. It then winds round the ganglion nodosum in a groove on its lateral surface. From this point it turns forwards and medially to the root of the tongue. A descending branch is given off, which accompanies the vagus nerve some distance further.
The first cervical nerve has a small ganglion, which forms communications with the roots of the vago-accessory nerve and with the second cervical nerve. The other cervical ganglia are much larger. The anterior roots are not easily seen. As the nerves emerge from the intervertebral foramina each gives off a small posterior branch. The lower cervical and upper thoracic nerves converge as they leave the spinal medulla and unite to form the brachial plexus.
The sympathetic gangliated trunk in the middle of the neck lies dorsal to the vagus. As it is followed towards the head it comes to lie media] to the vagus, and at the level of the petrous ganglion of the glossopharyngeal nerve it turns abruptly forwards with the carotid artery in the direction of the forebrain. As the trunk is traced towards the thorax it gradually diverges from the vagus, but is still dorsal to it. At the level of the seventh cervical vertebra it forms a loop and is continued into the thorax.
Streeter (5) has reconstructed the nerves in the occipital region of a 17.5-mm. embryo. In most respects the anatomy is very similar to the embryo here described. There are, however, some points of dif'I'erence— thus in Streeter’s specimen the glossopharyngeal nerve arises by several rootlets, and the gangliated trunk of the sympathetic fuses with the ganglion nodosum of the vagus. In this embryo the glossopharyngeal arises by a single root, and the sympathetic is separate from the vagus. The rootlets of the spinal ganglia in Streeter’s embryo are much shorter than in mine.
Vascular System
The carotid artery passes up the neck medial to the vagus, and opposite the first cervical vertebra it curves forwards in the direction of the forebrain. The stapedial artery is present and passes through the hole in the stapes. The two vertebral arteries unite in the concavity of the cervical flexure to form the basilar artery, which at this stage is a long vessel following closely the ventral surface of the pons as far as the cephalic flexure.
The blood from the frontal end of the brain is conveyed by the anterior cerebral veins into the large primitive head vein, which lies medial to the semilunar ganglion and represents the future cavernous sinus. Here it receives a small tributary from the region of the hypophysis. At the posterior edge of the semilunar ganglion it is joined by the middle cerebral vein, which drains the mid-brain and roof of the fourth ventricle. The large vessel (vena capitis lateralis) thus formed is continued posteriorly, lying immediately lateral to the facial nerve and accompanying it out of the cranium. It now lies lateral to the IX., X., XL, and XII. nerves. Opposite to the vagus it receives a large tributary (posterior cerebral vein) from the medulla oblongata, and emerges from the cranium by an opening which later becomes the jugular foramen. The vena capitis lateralis now turns sharply downwards and accompanies the vagus nerve on its lateral aspect, where it becomes the primitive jugular vein.
In the earliest stages of development the _frontal part of the primitive head vein is represented by the vena capitis medialis, which lies medial to the cranial nerves. Later on loops are thrown round the nerves and the medial part disappears, transforming the vessel into the vena capitis lateralis. This series of changes has been fully worked out by Salzer (6) in guinea-pigs, and by Grosser and Brezina (7) in reptiles. Rabl (8) has pointed out that in selachians the persistence of the vena capitis medialis is the normal condition, though in pristiurus there are both medial and lateral vessels. The vena capitis lateralis is identical with the “Basalvene ” described by His (3).
This embryo shows an intermediate stage in the development of the veins of the head. The early vena capitis medialis has been entirely replaced by the vena capitis lateralis, with the exception of that part medial to the semilunar ganglion, which persists as the cavernous sinus. Later the three cerebral veins anastomose and the vena capitis lateralis atrophies and disappears, so that all the blood leaves the cranium through the jugular foramen (Mall, 9).
The Upper Air-Passages
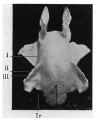
The buccopharyngeal cavity (fig. 6) is widest at the angle of the mouth. From this point backwards it narrows rapidly toithe level of the middle ear recess, where it again widens, and behind which it tapers away gradually into the (esophagus, the lumen of which is uniformly narrow. The cavity as a whole is curved on itself, with a ventral concavity, to the extent of about half a circle. In the roof the primitive choanae are seen as oval openings. The primitive palate, 'i.e. that part of the roof of the mouth between the lip margin and the choanae, presents a shallow groove, the labio-dental sulcus, which runs parallel to the lip margin. The epithelium on the floor of this groove is somewhat thickened. The palatal processes commence from the primitive palate at the anterior end of the choanee, form the lateral boundary of the choanae, and are continued backwards along the roof of the mouth till they reach the medial surface of the mandibular arches, which they cross and fade away on the anterior wall of the first cleft, which at this stage is becoming the lateral wall of the middle ear recess. .
The middle ear recess is a wing—shaped extension from the side of the pharynx (fig. 6). Its upper wall or roof shows a convexity caused by the tip of the cochlea. There is anantero-lateral wall and a postero-lateral Wall separated by a deep cleft, which corresponds with the angle between the first and second arches. The body of Meckel’s cartilage bulges into the antero-lateral wall, while the handle of the malleus similarly causes a bulging on the postero-lateral wall. The most anterior or oral end of the middle ear recess is in the form of a narrow" cleft, which runs from the tympanum forwards and medially along the roof of the buceopharyngeal cavity. This cleft has been named by Hammar (10) and others the sulcus tubo-tympanicus. It is bounded laterally by the continuation backwards of the palatal process on the lower and posterior surface of the mandibular arch. Its medial boundary is the eminentia cochlearis.
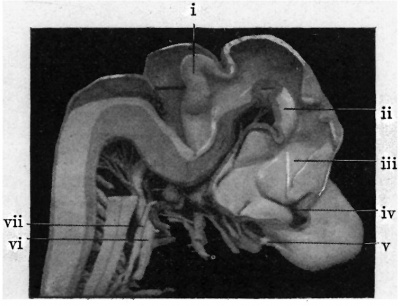
Fig. 6. — Model of the upper air passages. Nasal cavities form togeof molds. slat the side is a groove corresponding with palatal -process. Further down middle ear recess is seen, and beyond that the cavity narrows into the oesophagus. i, Palatal process; ii, sulcus tubo-tympanicns; iii, em nentia cochlearls ; iv, second visceral pouch.
The dorsal extension of the second pouch can be distinguished- as a shallow pocket in the posterior edge of the middle ear recess, -where it opens on to the pharynx (fig. 6). None of the other pouches or clefts can be distinguished in the lateral wall of the pharynx.
The parotid gland is represented by a solid bud of epithelium, growing into the cheek- just behind the angle of the mouth. A The submaxillary gland is also a solid bud of epithelium, growing downwards from the bottom of the alveolo-lingual sulcus. The sublingual gland has not yet appeared.
In the margin of the lower lip there is a labio-dental sulcus similar to that in the upper lip. The tongue occupies most of the floor of the mouth. Its tip reaches nearly to the lip margin, and is bound down by a short frenum. The organ is long and narrow, with a shallow median groove. It is sharply defined from the floor of the mouth by a deep alveolo-lingual sulcus. The root of the tongue can be faintly distinguished from the rest by a shallow transverse groove. There remains no indication of the relative parts played by the tuberculum impar and the lateral tongue rudiments, the anterior two-thirds of the tongue being quite uniform and smooth.
The epiglottis is a thick transverse ridge behind the root of the tongue. The upper aperture of the larynx is bounded behind and laterally by two rounded arytenoid swellings, which are in close apposition to the epiglottis and each other, so that the interval between them is a T-shaped cleft. Below this point the anterior laryngeal wall presents no special features. The laryngeal cavity consists of a transverse and a sagittal part. The transverse cleft is bounded behind by the arytenoid swelling and in front by the epiglottis, and the sagittal cleft lies between the two arytenoid eminences. A large part of the transverse cleft becomes the superior laryngeal aperture of the adult, the arytenoids later becoming separated from the epiglottis. The sagittal cleft is for the most part only a potential cavity, the two arytenoid swellings lying in close apposition, leaving only a narrow channel posteriorly. Lower down the sagittal part of the cavity opens out, the lumen being at first triangular, then oval as it passes into the trachea. There is as yet no trace of the chordal tubercle described by Frazer. The laryngeal cartilages are represented by condensations of precartilaginous tissue.
The Nose
The nasal cavities open in front by the anterior nares and communicate with the buccal cavity by means of primitive choanae, which are oval openings in the roof of the mouth. Their greatest diameters are in the antero-posterior direction. The anterior nares are narrow, and their lumina are almost entirely occluded by epithelial plugs, as is usual at this stage. Behind this point the cavity increases rapidly in its vertical diameter. The floor slopes down from the anterior orifice to the anterior border of the choana, and is formed entirely by primitive palate; while the roof runs first upwards and backwards, and then arches downwards to the posterior end of the choanal orifice. The lateral wall (fig. 7) is as yet fairly simple. Near its lower boundary there is a rounded swelling representing the concha inferior. This is limited below by a furrow, with which the solid naso-lacrimal duct is connected. The infundibulum ethmoidale is represented by a deep oblique cleft in the lateral wall near its roof. Immediately above and behind this the first ethmoturbinal is seen on the roof of the cavity. This illustrates a stage of the process described by Peter (11), in which the ethmoturbinals, which are originally on the medial wall, migrate across the roof on to the lateral wall. On the medial wall of the nose (fig. 8) is an antero-posterior sulcus, situated about midway between the floor and the roof. It deepens as it passes back to become a pocket, and obviously represents part of Jacobson’s organ. In the region of the pocket the epithelium is thick and columnar, and nerve fibres can be traced from it to the olfactory part of the brain. In the upper and posterior part of the septum there is a small bulging area, probably representing the second ethmoturbinal.
The Eye
The retina is a cup-shaped structure formed of two layers, which are as yet distinct. The outer layer already contains some pigment. The hyaloid artery is still present. The lens is a spherical mass lying in the retinal cup. It still shows a crescentic "cavity in its interior.
The extrinsic muscles of the eye are just beginning to appear, and are still in a very rudimentary condition.
I have been unable to discover in this embryo any trace of a lacrimal gland, although there is a well-developed conjunctival sac both above and below. The naso-lacrimal duct is present as a solid column of cells, which arches in from the medial commissure to the inferior meatus of the nose.
The Labyrinth
The labyrinth as a whole is situated slightly dorsal to the external acoustic meatus. Its oral or ventral extremity is formed by the cochlea, the saccus endolymphaticus is its most dorsal extremity, while the vestibule occupies an. intermediate position.
The cochlea (figs. 9 and 10) is beginning to show a constriction at its junction with the vestibule, but the ductus reuniens so formed is as yet very wide. The ductus cochlearis is short and thick, and of its windings only two-thirds‘ of a turn are completed. It lies very close to the middle ear recess and causes a bulging into its roof, as already mentioned.
The vestibule is somewhat elongated, and shows on its medial surface two rounded projections, which represent the beginning of the differentiation into saccule and utricle.
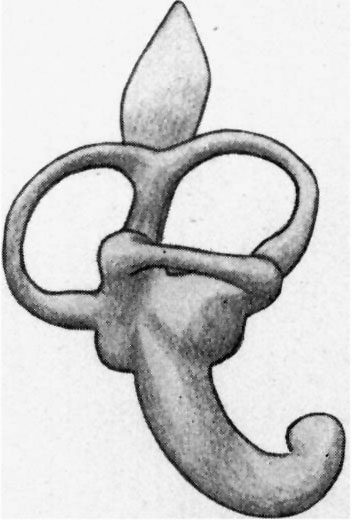
|
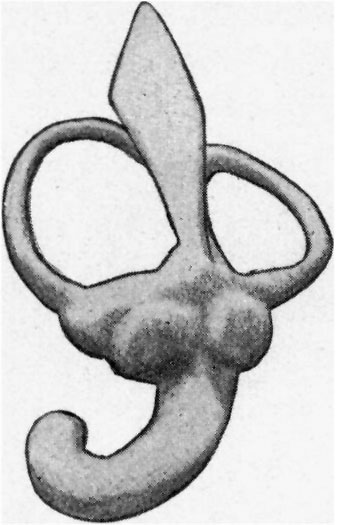
|
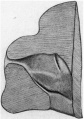
| Fig. 9. — Lateral aspect of the membranous labyrinth. | Fig. 10. — Medial aspect of the membranous labyrinth. The commencing seperation into utricle and saccule is seen. |
The semicircular canals or ducts are well developed and occupy the same relative positions to one another as they do in the adult. The ampullae are already evident. The lateral or horizontal canal is considerably shorter than the superior or posterior semicirculafcanal.
The ductus and saccus endolymphaticus are represented by a spear-shaped diverticulum from the vestibule, medial and parallel to the crus commune.
Streeter (12) has reconstructed and described the labyrinth of a 20-mm. human embryo, which corresponds in every respect with the specimen described in this paper. _
Branchial Derivatives
The thyroid gland is a U-shaped structure. The limbs of the U are flattened at their cranial ends, but as they are followed downwards they become more rounded and meet in the middle line in front of the trachea. The upper or cranial extremities lie lateral to the lower part of the pharynx, though they have no connexion with it. The thyro-glossal duct has also completely disappeared. The thyroid is seen to be composed of loosely-connected columns of deeply—staining round cells. There is no distinct capsule. The thymus is in the form of two rounded cords, which lie close to the vagus carotid. Each cord begins about the level of the third or fourth cervical vertebra, just lateral to the thyroid gland, ventral to the carotid, and medial to the vagus. It passes down the neck, preserving these relationships, and comes gradually forwards towards the middle line, and unites with its fellow of the opposite side in front of the aortic arch. It is a mass of deeply-staining, closely-arranged cells. It has a definite capsule, and, in the greater part of its length,la narrow lumen.
The Skeleton
The base of the skull is composed of precartilaginous tissue, with here and there chondrifying centres. The outlines are as yet somewhat indefinite.
The vertebrae are mostly cartilaginous, while the spaces between the bodies of the vertebrae are filled up by condensed tissue, not unlike cartilage in appearance. The vertebrae and intervertebral substance form a. continuous column in front of the spinal medulla. The neural arches are quite open dorsally, the laminae extending only about half way round the spinal medulla. The transverse processes are flattened and are pierced by the canal containing the vertebral artery.
The notocord runs up the centre of the vertebral column into the base of the skull. It then bends ventrally, and, emerging from the base of the skull, travels forwards between the mucous membrane of the pharynx and the base of the skull to end near the hypophysis.
In conclusion, I wish to express my thanks to Professor Robinson for placing his specimen at my disposal, and for much valuable advice.
References
(1) Keibel F. and Elze C. Normal Plates of the Development of the Human Embryo (Homo sapiens). (1908) Vol. 8 in series by Keibel F. Normal plates of the development of vertebrates (Normentafeln zur Entwicklungsgeschichte der Wirbelthiere) Fisher, Jena., Germany.
(2) HIS, W., “Entwickelung d. menschl. u. tier. Physiognomien,” Arch. f. Anal. 11.. Physiologic Anat. Abt. 1872. -
(3) HIS, W., Die Entttiickelung des menschlichen Gehirns, Leipzig, 1904.
(4) ELLIOT SMIITH, “()1n tge Morphology of the Cerebral Commissures,” Trans. Lin. Soc. Lon. vo . viii., 90 .
(5) Streeter GL. The development of the cranial and spinal nerves in the occipital region of the human embryo. (1905) Amer. J Anat. 4(1):83–116.
(6) SALZER, H., “Ueber die Entwickelung der Kopfvenen des Meerschweinchens,” Morph. Jahrb., Bd. xxiii., 1895.
(7) Gnossnn and BREZINA, “ Ueber die Entwickelung der Venen des Kopfes und Halses bei Reptilien,” Morph. Jahrb., 1895.
(8) RABL, C., Ueber die Entwickelung des Venensystemes der Selachier, Leipzig, 1892.
(9) Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
(10) HAMMAR, J. A., “Studien iiber die Entwickelung des Vorderdarms und einiger angrenzenden Organe,” Arch. f. mikr. Anat., 1902. 5
(11) PETER, K., “Anlage und Homologie der Muscheln des Menschen und der Siiugetiere,” Arch. f. mikr. Anat., 1902.
(12) STREETER, G. L., “On the Development of -the Membranous Labyrinth and Acoustic and Facial Nerves in the Human Embryo,” Amer. Jou/m. of Anat., vol. vi., 1906.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The Anatomy of the Head End of a 20 mm Human Embryo. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Anatomy_of_the_Head_End_of_a_20_mm_Human_Embryo
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G