Paper - Studies on the human oocyte and its follicle 1
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hertig AT. and Adams EC. Studies on the human oocyte and its follicle. I. Ultrastructural and histochemical observations on the primordial follicle stage. (1967) J Cell Biol. 34(2):647-75. PMID 4292010
| Online Editor |
|---|
| This historic 1967 paper by Hertig and Adams uses electron microscopy to study the human oocyte. |
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Studies on the Human Oocyte and its Follicle
I. Ultrastructural and Histochemical Observations on the Primordial Follicle Stage
Arthur T. Hertig and Eleanor C. Adams
From the Department of Pathology, the Harvard Medical School, Boston, Massachusetts 062115
Abstract
Oocytes in primordial (“resting”) follicles in adult human ovaries contain a complex paranuclear structure identified by light microscopists as Balbiani’s vitelline body. By electron microscopy this structure is composed of a mass of mitochondria with associated endoplasmic reticulum, multiple compound aggregates which form a ring around the cytocentrum, and a single stack or coil of annulate lamellae either attached to the nuclear membrane or free in the cytoplasm. The compound aggregates contain vacuoles and finely divided electronopaque material. Evidence is presented for the probable transport of this material between the oocyte and its environment. The cytocentrum contains a central aggregate of amorphous electron—opaque deposits which appear to become periodically aligned on fine fibrils to form the long coarse fibers at the periphery of the cytocentrum. The apparent prevalence of annulate lamellae attached or adjacent to the nuclear membrane of oocytes in ovaries removed during the mid—follicular (estrogenic) phase of the cycle indicates the need for further study of a possible hormonal influence on the resting oocyte. By light microscopy phosphatases were not found within the oocyte, but adenosine—monophosphatase activity is present in the cortical cells surrounding primordial follicles, and also at the periphery of each primitive follicle cell, most prominently at the oocyte side. Glucose—6—phosphate dehydrogenase activity is present within the oocyte cytoplasm.
Introduction
In the adult human ovary, there are only two stages in the growth of the oocyte and its follicle that can be confidently recognized histologically as normal phases of the maturation process. These stages are at opposite ends of the maturation spectrum: one is the resting oocyte in the primordial follicle (40 ,u in diameter); the other is the oocyte with a meiotic spindle forming the first polar body in the fully matured preovulation follicle (1.5 cm). The normal histology of all the intervening stages cannot be recognized since there are as yet no agreed morphological criteria for differentiating follicles that are continuing to develop from those in the earliest stages of atresia. (Certainly mitotic activity in the follicle wall or cumulus is no criterion of normal preovulatory development. In both the human and guinea pig we have seen numerous examples of obviously atretic follicles showing mitoses in their granulosa cells. It is unknown, however, at what stage in the development of any given follicle atresia had begun.) The duration of the developmental processes and its possible correlation with cyclic endocrine events also are unknown in the human female. Moreover the interrelationship of the two ovaries with respect to follicular development is some mammals, however, the morphology of follicular and oocyte developmental processes has been correlated with events in the estrous cycle. For instance, Myers et al. (1936) made a careful histological examination of follicles in ovaries removed at known times of the 16-17 day estrous cycle of the guinea pig. They reported that soon after estrus many multilaminar primary (nonvesicular) follicles had begun to form an antrum thus becoming a secondary or vesicular follicle, and that from these only two or three follicles in each ovary complete their development and ovulate at the next estrus; the remainder becoming atretic at various stages of their growth. In addition to the normal duration of these vesicular follicle stages (one estrous cycle), Myers et al. reported their histological and size criteria in relation to the various succeeding days of the cycle. We have recently described some of the histochemical aspects of oocytes in developing and atretic follicles throughout the estrous cycle of the guinea pig (Adams et al., 1966).
In order to determine the morphological correlation of human follicle and oocyte developmental stages with known events in the menstrual cycle and thus to estimate follicular growth rate, we have begun a careful examination of the total follicular population in human ovaries removed at various times in the menstrual cycle. An electron microscopic study of the first stage examined, oocytes in primordial follicles, is reported here together with the results of a few histochemical studies. In these oocytes from adult human ovaries we have noted ultrastructural differences from oocytes in similar follicles studied in the human prenatal ovary (Lanzavecchia and Mangioni, 1964; Stegner and Wartenberg, 1963) and in ovaries of a variety of other mammalian species. These differences are chiefly characterized by the complexity and organization of the paranuclear structure previously identified by light microscopists as Balbiani’s vitelline body.
Materials and Methods
The follicles were obtained from normal ovaries removed from five fertile women, 28-37 yr of age, who had gynecologic and /or obstetric indications for hysterectomy such as carcinoma in situ of the cervix or leiomyomata of the uterus. One ovary was removed in four instances and both 1n one instance. In addition, we examined primordial follicles from an ovary removed from a patient who had been on the antifertility medication Orthonovum. Also follicles from a wedge biopsy of an ovary, obtained at laparotomy for infertility investigation, were studied. From this material corpora lutea and intact large vesicular follicles are dissected out for other histological, histochemical, or electron microscopic studies. The remaining ovary is then sliced serially at 2-3 mn1 either crosswise or sagitally and fixed for histological, histochemical, and electron microscopic observation of smaller developing or atretic follicles.
For izz'rz‘0clzemz'5try, some slices were fixed 3-12 hr in 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.6, in 2 -,’/'5 glutaraldehyde, or in a combined 2% paraformaldehyde—2%% glutaraldehyde (one-half strength Karnovsky fixative) (1965), both in 0.1 M eacodylate buffer pH 7.4. These were subsequently washed in their respective buffers and rapidly frozen at —70°C. Cryostat sections were reacted for various phosphatases, dehydrogenases, or diaphorases or were stained with Sudan black. Other slices were fresh frozen, sectioned in a cryostat, and reacted for similar enzymes. Although acid phosphatase activity, as visualized by the Gomori method (Becker et al., 1960), was maintained by all three fixatives used, ovarian nucleoside polyphos— phatase, adenosine—5-monophosphatase (VVachstein and Meisel, 1957), and alkaline-phosphatase (Pearse, 1960) activities were maintained only by fixation in 4% paraformaldehyde or in fresh frozen cryostat sections. In view of the doubts recently cast on the reliability of dehydrogenase methods which employ unfixed cryostat sections (Fahimi and Karnovsky, 1966), we have used both 4% paraformaldehydefixed (3 hr) and fresh-frozen cryostat sections for the study of glucose-6—phosphate dehydrogenase and both NADH and NADPH diaphorases by using the methods of Pearse as modified slightly by Karnovsky (1961). In addition, we have added 5 mg/100 ml Nmethyl phenazonium methosulfate (PMS) to some glucose-6-phosphate dehydrogenase incubations in order to bypass diaphorase activity in the production of the formazan.
For electron microscopic studies, the strips of cortex were fixed in 2% glutaraldehyde or in the combined 2% paraformaldehyde——21/2% glutaraldehyde both in 0.1 M eacodylate buffer pH 7.4. After 5 hr fixation, the strips are washed in repeated changes of chilled 0.1 M caeodylate buffer with CaC1g added and are stored in the refrigerator. The slices of ovary thereafter are dissected so that the medulla is separated from a strip of cortex. These are then examined under a dissecting microscope as they are cut into 0.5-0.1 mm transverse slices, each of which is again searched by a dissecting microscope using oblique transillumiprimordial (0.04 mm in diameter), nonvesicular or primary (0.08—0.28 mm in diameter), or small vesicular or secondary (0.30 -1- mm in diameter) follicles. After recording the measurements of individual follicles they are further dissected into orientable blocks of 1.5 mm greatest size, are postosmicated, rapidly dehydrated, and embedded in Epon 812. Before thin sectioning, the preselected cortical blocks are “thick”-sectioned (lp) serially, and successive groups of five serial, thick sections are examined with phase microscopy to search for the presence of primordial follicles. When the edge of a primordial follicle is identified, the block is and serial, thin sections are cut and mounted on 100—mesh grids. After six to eight serial grids of thin-sectioned ribbons are collected, another thick section usually is mounted on a glass slide before collecting the next series of six to eight grids of serial sections. The seriated thick-sections are examined and subsequently photographed for orientation of individual primordial follicles which, in our material, are often present in clusters of two to five oocytes per section. This orientation facilitates the electron microscopic study of serial depths through any one oocyte in a cluster. The thin sections, stained with lead (Venable and Coggeshall, 1965) or with uranyl followed by lead were examined with an RCA EMU-3F or 3H microscope.
For histalogical study, one cortical strip, fixed in 4% paraformaldehyde or in 2% glutaraldehyde followed by Bouin’s fluid, is embedded in paraffin for serial sectioning and is stained with hematoxylin and eosin or periodic—acid Schiff.
trimmed,
Observations
Light Microscopy
In the young adult, primordial follicles about 40 ,u. in diameter can be found in clusters lying in the narrow band of dense cortex just beneath the serosal surface and the tunica albuginea. Primordial follicles, whose oocytes are in the prolonged diplotene phase of meiotic prophase (Baker, 1963), are in a resting stage which can be found continuously from the earliest postnatal ovary (when oogenesis has ceased and earlier meiotic prophase stages have been completed) through the adult reproductive period. Their number is gradually decreased, particularly from adolescence to the menopause, owing to the repeated initiation of development of some of them, first to the primary (nonvesicular) and subsequently to the secondary (vesicular) follicular stages which terminate either with maturation and ovulation or with atresia.
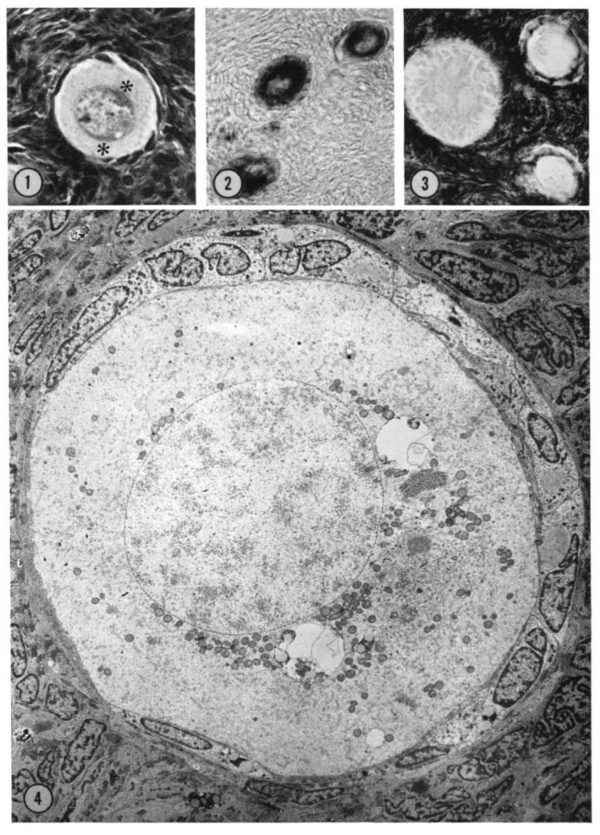
A striking cytoplasmic characteristic of oocytes in primordial follicles is a large paranuclear complex identified previously by light microscopists as the “corps vitelline” surrounded by a “couche mitochondrial” (van der Stricht, 1923), as the “yolk nucleus complex,” (Beams and Sheehan, 1941), or preferably (Raven, 1961) as Ba1biani’s vitelline body (1864). This structure has been studied in our histological and histochemical preparations: with hematoxylin and eosin staining (Fig. 1), it is an eosinophilic complex closely applied to the nuclear membrane; with periodicacid Schiff staining both with and without diastase digestion it is a ring of positive granules surrounding a paler pink central sphere; in frozen sections stained with Sudan black, sudanophilic granules of various sizes are found in this area. In both 4% paraforma1dehyde—fixed frozen sections with and without the addition of PMS in the incubation medium and in unfixed sections without PMS, the entire cytoplasm of the oocyte is strongly reactive for glucose-6-phosphate dehydrogenase activity (Fig. 2). When PMS is added to the incubation medium for unfixed sections, the solubility of this enzyme, G—6—P dehydrogenase, is indicated by the production of the formazan in the incubation medium. The oocyte cytoplasm is moderately reactive for both NADH and NADPH diaphorases in both fixed and unfixed sections. Alkaline and acid phosphatases, as well as neutral phosphatases such as nucleoside poly— or monophosphatases are negative in the oocyte.
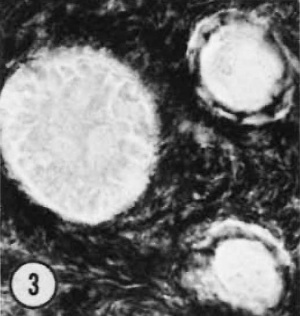
Some of the primitive follicle cells form a crescent—shaped cap of thicker cells over part of the oocyte; the remaining cells form a much thinner wall around the rest of the oocyte. Mitotic activity has not been observed in the follicle wall of this stage. Adenosine—monophosphatase activity, present in the cortical cells which surround the primordial follicles, also appears at the periphery of the primitive follicle cells, most prominently at the surface facing the oocyte (Fig. 3). Acid phosphatase has not been identified in the primordial follicle wall, although it does appear at subsequent stages of development. According to our criteria, when any follicle has thick or cuboidal cells around its entire surface, development to the primary (nonvesicular) follicle has been initiated. Although the oocyte within this early developing follicle is not much larger in diameter than that in the primordial follicle, it begins to show changes at the ultrastructural level.
Electron Microscopy
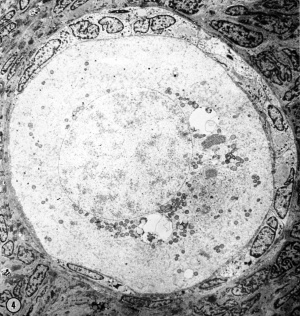
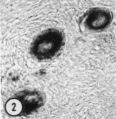
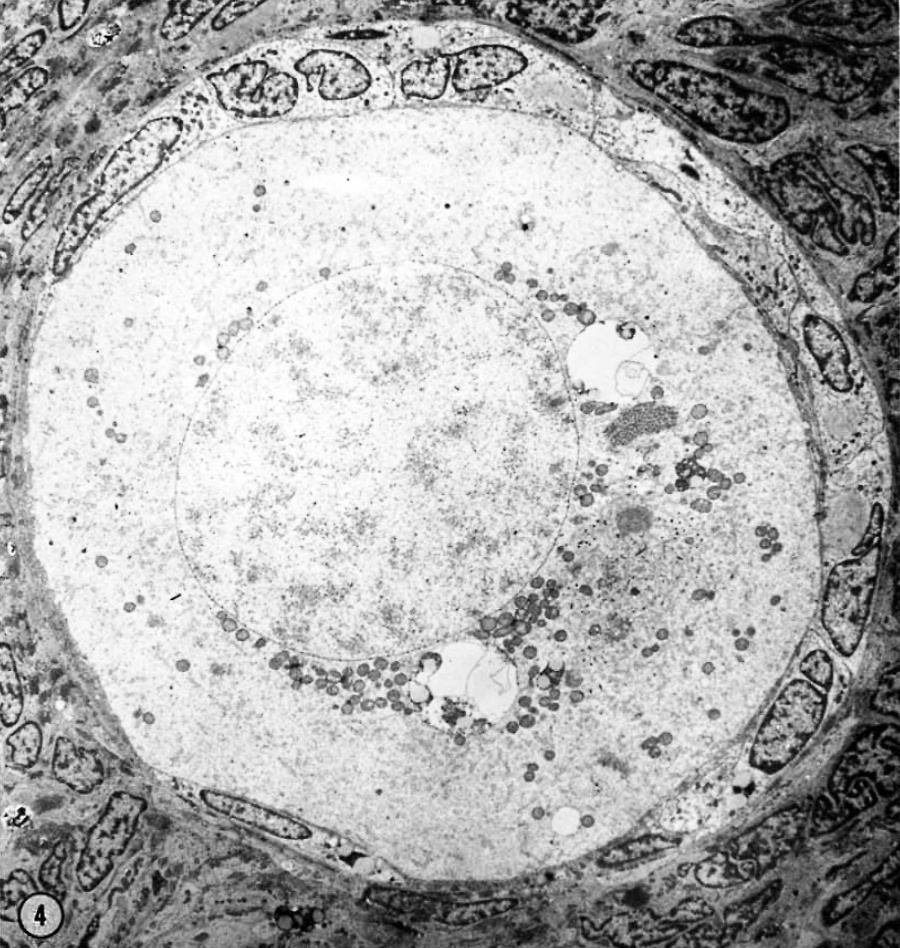
In thin—sectioning blocks of cortex which contain dispersed or clustered primordial follicles, the plane of section with respect to the polarity of any oocyte is necessarily random. Of the 30-40 oocytes serially sectioned in our study, only a very few fortunate micrographs show the interrelationship of all of the cytoplasmic organelles found in the mid—polar region (Fig. 4). (By mid—polar is meant a section which passes through or near the center of the nucleus, through or near the center of the cytocentrum and in addition includes the significant part of the annulate lamellae. This structure is single and eccentric with respect to the true polar axis and may be either attached or detached from the nuclear membrane.) In most oocytes it is necessary to reconstruct mentally the three—dimensional relationships by superimposing micrographs from serial depths through an oocyte.
Study of primordial follicles in ovaries obtained from a variety of phases of the menstrual cycle and from a term pregnancy reveal a constant general relationship of organelles. The nucleus of the oocyte is large and usually spherical and contains a large reticulated nucleolus. Mitochondria, elements of the endoplasmic reticulum and Golgi complexes, are found in variable numbers around the entire circumference of the nucleus. A large paranuclear complex is formed by a concentration of these organelles surrounding large compound aggregates which form a ring around a large cytocentrum. Annulate lamellae are found near or within this paranuclear complex which in its entirety comprises the structure previously described as Balbiani’s vitelline body. The variations between individual oocytes which appear to reflect either metabolic changes or a response to fixation difference will be noted below in the following detailed description of organelle arrangements. The spherical nucleus (Figs. 4 and 25), varying from 22 to 24 p. in diameter, is enclosed by a smooth envelope whose two leaflets exhibit prominent pores (Figs. 5-7). Aggregates of filamentous chromatin with associated perichromatin granules and fine fibrils are distributed throughout the nucleus in wispy strands that apparently represent sections through the diplotene configuration of chromosomes. Clumps of interchromatin granules are also dispersed ‘throughout the nucleus. A large irregularly shaped nucleolus with a nucleolonema is prominent in these oocytes (Fig. 5). Multiple, large clumps of fine, granular material interpreted as heterochromatin are distributed around the periphery of the nucleus or are located near the nucleolus (Figs. 5 and 6). They are surrounded by chromatin and perichromatin granules.
| Abbreviations | ||||
|---|---|---|---|---|
| AL, annulate lamellae
C, Cortex CA, compound aggregates |
CF, coarse fiber
CH, cell chromatin F, follicle cell |
G, Golgi complex
H, heterochromatin I, interchromatin granules |
MT, microtubules
N, nucleus NU, nucleolus |
O, oocyte
P, projection VA, vesicular aggregates |
| Note: In the legends the II number which identifies each patient is followed by another number identifying the particular oocyte represented. | ||||
|
|
|
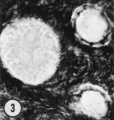
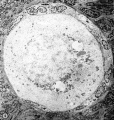
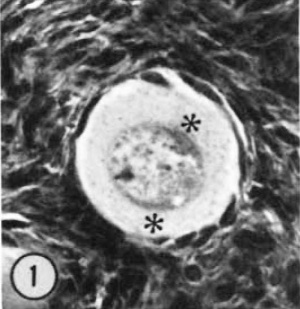
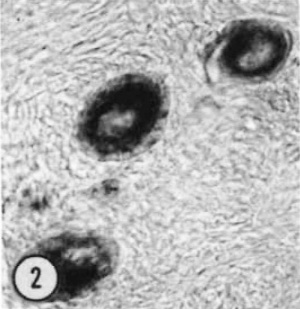
| Figure 1. Mid-polar section through an oocyte in a primordial follicle. Closely applied to the spherical vesicular nucleus is the crescent—shaped Balbiani’s vitelline body (between asterisks at lower right against nucleus) formed by peripheral dense granules (mitochondria) and a central paler area (cytocentrum) surrounding a dense body. H 48, H —l— 14}. X 560. | Figure 2. Note that the cytoplasm of these three oocytes in primordial follicles is strongly reactive for glucose—6—phosphate dehydrogenase. II 54; paraformaldehyde fixative. x 350. | Figure 3. The cortical cells and the periphery of the cells in the Wall of the primitive follicles at right are reactive for adenosine~Inonophosphatase. The pronounced activity at the oocyte—follicle cell junction apparently is located in the numerous projections of the follicle cells present in this intercellular space. The activity of the enzyme is not maintained at the periphery of differentiating follicular cells of the multilaminous primary follicle at left. H 53; paraformaldehyde fixative. x 350. |
| ||
| Figure 4. A low—power survey micrograph through the mid-polar axis of an oocyte in a primordial follicle. Note that most of the organelles are concentrated in one pole of the oocyte; compare with Fig. 9.5. II 44-1; Karnovsky fixative; uranyl and lead stain. X 2700. | ||
FIGURE 5 Nucleus of oocyte showing nucleolonema and associated clump of heterochromatin, aggregates of chromatin with associated perichromatin granules and fine fibrils, and dispersed clumps of interchromatin granules. Pores are prominent on the nuclear membrane. H ‘M-1; Karnovsky fixative; lead stain X 15,000.
The cytocentrum (Figs. 8 and 10) is similar to that in other cell types in that it contains few, if any, mitochondria. At its periphery, a network of sparsely granular endoplasmic reticulum appears to be continuous with many small Golgi complexes and with the more granular endoplasmic reticulum of the peripheral cytoplasm. Elements of this network often are also closely applied to the large compound aggregates. Coarse, long fibers of electron—opaque material are found in the cytoplasmic matrix of this network and extend just beyond the periphery of the cytocentrum where they usually appear to terminate bluntly but occasionally may abut a compound aggregate or a Golgi complex. When serial sections have afforded an opportunity to estimate their three—dimensional relationship, the coarsest fibers appear to form an interwoven basket—like structure at the periphery of the cytocentrum. Smaller caliber fibrils in some oocytes radiate out from near the center of the cytocentrum to join the coarser ones. Although the maximum length of each fiber could not be determined, the greatest diameter is about 0.13 M and each appears to be straight or only slightly curved (Fig. 11). Fixation differences may affect their structure since on cross—section some are homogeneous, some appear as rings with pale centers (Fig. 9), and others as closely packed tetrads. N0 substructures such as tubules have been identified. The microtubules seen within the cytocentrum do not appear to originate from or to be concentrated near these fibers. A feltwork of very fine fibrils often visualized in the matrix of the cytocentrum, appears to merge with these coarse fibers.
FIGURE 6 Multiple clumps of heterochromatin are also found near the periphery of the nucleus. H 48L2; Karnovsky fixative; lead stain. >( 21,000.
FIGURE 7 Bundles of spiral filaments are occasionally attached to the nucleus or lie free in the cytoplasm. H 35-1; 2%. glutaraldehyde. )( 952,000. Inset from same oocyte but adjacent section. X 99,000.
The large, prominent center of the cytocentrum i.s composed of deposits of amorphous, electronopaque material (Figs. 8 and 10). These deposits frequently are seen in periodic alignments which radiate from the center (Fig. 9). When this periodicity is present, the amorphous deposits appear to be aligned on very fine fibrils; this suggests the formation of a transition between the central concentration of amorphous deposits and the more peripheral fibers. Although on occasion the amorphous deposits in the center of the cytocentrum may be reminiscent of the ultra-structural aspect of glycogen, the presence of glycogen in oocytes could not be confirmed by our histochemical studies. Also within the cytocentrum of most oocytes one, or more, large (3.5 p. in diameter), compact aggregate of closely packed vesicles is found (Figs. 8 and 9). Such vesicles are smooth—wal1ed, often appear to contain slightly electron-opaque material, and frequently can be seen to be continuous with the endoplasmic reticulum of this area. In other oocytes these vesicles seem to be dispersed among the amorphous deposits to contribute to a single, central, dense area of the cytocentrum measuring up to 4.5 p. (Figs. 10 and 11). This last arrangement may be the ultrastructural basis for the dense granule visible in the center of the cytocentrum in some H (hematoxylin) and E (eosin) preparations of oocytes (Fig. I).
The resemblance of some of the periodic arrays of deposits and of some coarse fibers to peri»centriolar structures described by others in a variety of somatic cells, led us to hunt systematically for centrioles, particularly in this area of the ooeyte. Since no centriole had been seen in any oocyte routinely examined throughout its entirety, a series of 75 serial grids containing four oocytes was specially prepared without intervening thick sections. In spite of careful search throughout the cytocentrum and elsewhere in the cytoplasm of these four oocytes, no centrioles could be identified.
FIGURE 8 A section through the center of Ba1biani’s vitelline body, showing a cytoplasmic stack of annulate lamellae, ballooned compound aggregates, and a Vesicular aggregate in the cytocentrum near a concentration of clumps of dense amorphous material. For higher magnification see Fig. 9. Straight fibers appear to radiate from this area. Coarser fibers are seen in cross-section at the periphery of the cytocentrum. H 44-1; Karnovsky fixative. X 10,000.
FIGURE 9 A higher power of Fig. 8 to show the clumps of dense amorphous material, some of which appear to be periodically aligned on fine fibrils suggesting a transition between the amorphous deposits and the peripheral coarse fibers at right center. Note feltwork of fine fibrils in the cytoplasmic matrix. A portion of the vesicular aggregate is seen at top left. Note the two instances of helical formation of ribosomes (asterisk) on flat View of endoplasmic reticulum. H sH—1; Karnovsky fixative. )( 32,000.
FIGURE 10 A mid—section through Balbiani’s vitelline body to show the peripheral concentration of mitochondria, the variable ballooning of compound aggregates and the eytocentrum with a dense central area surrounded by endoplasmic reticulum and coarse fibers. A coiled configuration of annulate lamellae, detached from the nuclear inembrane, was seen. beginning in. the adjacent and continuing in subsequent grids. H 48—R1; Karnovsky fixative. X 3800.
FIGURE 1.1 A higher power of Fig. 10, showing in this oocyte, the apparent mingling of the elements of the vesicular aggregates with the clumps of dense amorphous material. Note further the Central cluster of vesicles. At the periphery a.re seen longitudinal and tangential sections through coarse fibers. H 48 R1; Karnovsky fixative. X 91,000, FIGURE 12 Compound aggregates showing variability’ in their structure. Those that are membranebounded are ballooned to a variable degree and in addition to vacuoles and electron-opaque granules also contain Vesicles enclosing electron—opaque material. Others that are not 1'ne1nbrane—bounded frequently have elements of endoplasmic reticulum closely applied to their surface (right). Note mitochondrial rosette at right. H 48-R1; Karnovsky fixative. X £21,000.
Golgi compfexes (Figs. 8, 23, and 24) are closely associated with the periphery of the cytocentrum and in some oocytes appear to form a fenestrated shell around it. They are also interspersed between the mitochondria that form the peripheral region of Balbiani’s vitelline body and in addition are frequently associated with endoplasmic reticulum near the oolemma (Fig. 19). As noted above, their membranes appear to be continuous with the endoplasmic reticulum. Although Golgi complexes frequently are located adjacent to the large compound aggregates, no continuity between these structures has been noted. In some oocytes, Golgi complexes along with endoplasmic reticulum and mitochondria are found closely applied to the entire circumference of the nucleus (Fig. 25). The large electron—opaque compound aggregates (Figs. 8 and 10) at the periphery of the cytocentrum are composed of large vacuoles containing a homogeneous, slightly electron—opaque material surrounded by closely packed, very electronopaque, membranous or granular substance. They present a Variable appearance since some are compact and may or may not be membrane enclosed, while others in the same oocyte are enclosed by a membrane, are ballooned to a variable degree, and appear to have imbibed a clear fluid in which the substructures are dispersed. The latter form contains many small vesicles enclosing dense material (Fig. 12). Smaller com FIGURES 15 and 16 Membranous formations are frequently seen at the intercellular junction between oocyte (top) and follicle cell (bottom). These frequently contain small vacuoles or finely divided electron-opaque material similar to those within the compound aggregates and may represent a mechanism for the bulk transfer of this material between follicle wall and oocyte.
FIGURES 13 and 14 These microgra.phs are from two sections on the same grid through the same area at the periphery of the oocyte. Vacuoles are present tha.t contain either material sin1ilar to myelin figures or elements such as finely divided electron-opaque material (Fig. 13) or small vacuoles (Fig. 14) similar to those within. the compound aggregates. Note coated vesicles at oocyte membrane in Fig. 14. II 44-1; Karnovsky fixative. X 21,000.
H 48-111 and H 44-5; Karnovsky fixative. X 32,000.
FIGURES 17 and 18
Compound aggregates are also seen within the cytoplasm of the follicle cell. They frequently appear to bulge into the oocyte and in such areas the membranes of both cells appear indistinct. H 44-1 and H 44-5; Karnovsky fixative. X 21,000.
FIGURE 19 A compound aggregate in a follicle cell. Note similarity to aggregates in oocyte. The intercellular space between oocyte and follicle conta.ins projections from both cells. Note Golgi complex in close association with endoplasmic reticulum at periphery of oocyte. H 44-3; Karnovsky fixative. X 21,000.
FIGURE 20 Compound a.ggregates are also seen within the cytoplasm of cortical cells outside the primordial follicle wall. Note desmosome at junction between two follicle cells and also concentration of projections i11 intercellular space at the j unction of oocyte membrane and two follicle cells. II 43-1; Karnovsky fixative. X 21,000.
FIGURE 21 Mitochondria to show effect of fixation. With the slightly hypertonic 2% glutaraldehyde fixative, the mitochondria contain arched cristae and a distended channel interpreted to be caused by the infolding of the inner lamina. of the peripheral membranes with or without rupture of the external lamina. The internal vesicle frequently seen within this distended channel could be a cross—section of endoplasmic reticulum which apparently artifactitiously invades the mitochondria (see mitochondrion at lower left). Note distended channels of endopla.smic reticulum with this fixative. H 35-4; 2% glutaraldehyde. X 36,000.
FIGURE 22 With the more hypertonic one-half strength Karnovsky fixative, the arched cristae are prominent a.nd, in addition, multiple secondary tubular cristae are formed by the infolding of the internal lamina. of the-periplieral n_1en'1branes. Note close association of endoplasmic reticulum with mitochondria. Note also electron-opaque material at center of mitochondrial rosette. H 44-3; Karnovsky fixative. X 49,000.
pact aggregates, usually without enclosing mem- 17—20 and 37). Similar compound aggregates are
branes, may be seen in the more peripheral cyto- also present in the serosal epithelium. Vacuoles plasm, within the primitive follicle cells and in containing elements similar to those in the ag many cells of the surrounding cortex (Figs. 4, gregates are present in the peripheral cytoplasm of the oocyte (Figs. 13 and 14-). Frequently a dense, membranous structure is seen lying across and apparently merged with the oocyte membrane (Figs. 15 and 16). This rather amorphous structure, when followed in serial sections, often is associated with vacuoles in the oocyte cytoplasm. When seen in thinner areas of the cytoplasm of the follicle wall, the aggregates often appear to bulge into the cytoplasm of the oocyte (Figs. 17 and 18).
Golgi complexes are seen in the peripheral cytoplasm of the oocyte where they frequently are associated with vacuoles containing multiple vesicles. H 44-1; Karnovsky fixative. x Q1,000.
FIGURE +24 Golgi complexes (right) a.re also prominent at the periphery of the cytocentrum (left) where they appear t.o be in continuity with the network of endoplasmic reticulum surrounding a.nd intermingled with the clumps of amorphous electron-opa.que material. H 44-3; Karnovsky fixative. x 21,000.
A mass of mitochondria (Fig. 10) form the periphery of Balbiani’s vitelline body and a variable number are scattered around the nucleus and in the peripheral cytoplasm. They are spherical—to— ovoid in shape, of variable size, 0.5—l .8 p., and are intimately associated with the endoplasmic reticulum. Both the mitochondria and the endoplasmic reticulum appear to be unusually responsive to differences in tonicity of the fixing fluid. With only slightly hypertonic glutaraldehyde—fixation (Fig. 21), the mitochondria contain a few, prominent, peripheral arched cristae in a pale, homogeneous matrix within which are only a few small granules. In addition, they also contain a small, distended channel interpreted as caused by the swelling of a single crista or by a local infolding of the inner lamina of the peripheral membrane with or without the rupture of the outer lamina. This distortion is frequently associated with the close proximity of endoplasmic reticulum which on occasion even appears (apparently artifactitiously) to invaginate the mitochondria. With the more hypertonic one—half strength Karnovsky paraformaldehyde—gluteraldehyde-fixation (Fig. 22) the very close structural relationship of mitochondria to endoplasmic reticulum is maintained, but the mitochondrial matrix is more electron—opaque. Furthermore, in addition to the usual prominent, arched cristae, the peripheral inner membrane often appears to be infolded to form multiple secondary vesicular or tubular cristae. The membranes forming these structures are not so electronopaque as those of the apparently more stable, arched cristae. Mitochondria in Balbiani’s vitelline body or elsewhere around the nucleus frequently are clustered in rosettes around a dense amorphous, somewhat reticulated substance (Figs. 12 and 22). Although this material may appear to form an adherent cap on one or more of the mitochondria in the rosette formation, examination of serial sections through a rosette indicates that this image is caused by a superimposition of structures on a tangential cut.
Fig. 25. This low-power micrograph shows a section that is not mid-polar through the oocyte since the paranuclear Balbiani vitelline body was present in deeper sections. Note that a stack of annulate lamellae is attached to nuclear mernbrane, that a Variety of organelles are closely applied to the entire circuinference of the oocyte nucleus and that. endoplasmic reticulum is present as distended channels. ll 35-4; 2% glutaraldehyde. X 2800.
FIGURE 26 A higher power of Fig. 25 to show continuity of membranes of a1111ulate lainellae with those of the peripheral endoplasmic reticulum. Note evagination of outer leaflet of nuclear membraile at left of annulate lamcllae. See Figs. 27 a.11d 28 for higher power View of this stack of annulate lamellae as observed in nearby sections. H 35-4; 2% glutaraldeliyde. X 7300.
When closely seriated grids through Balbiani’s vitelline body are available for study, a single mass of stacked or concentric annulate lamellae can be found (Figs. 4, 8, and 29—33). In some oocytes these are in continuity with the nuclear membrane (Figs. 25-28); in others they are found at some distance from the nucleus (Figs. 33 and 34). Whether they are attached to the nucleus or not, the smooth—surfaced paired membranes of the annulate lamellae are continuous at their periphery with the more granular peripheral endoplasmic reticulum (Figs. 27, 28, 31-34) which in turn is closely associated with mitochondria (Fig. 26). In one of a series of sections cut in a fortunate plane through annulate lamellae attached to the nucleus, a continuity of membranes could be clearly visualized (Fig. 27). The outer leaflet of the nuclear membrane is evaginated to form, by infoldings, a large stack of paired membranes whose pore formations are similar to but more closely spaced than those found on the nuclear membrane at a distance from the annulate lamellae (Figs. 29-32). It is noted that in the area of the evagination and infolding of the outer nuclear membrane, the outer loops of the paired annulate membranes are double whereas they are single in areas of the stack where the paired membranes become continuous with the peripheral endoplasmic reticulum. Unfortunately, micrographs through the entire stack of these annulate lamellae were not available for complete three—dimensional study since some of the stack was contained within a thick section. Study of five sections on three serial grids suggests that the evaginating nuclear membrane forms folds that interdigitate either with similar, looped membranes from a second unidentified nuclear evagination or with membranes from the peripheral endoplasmic reticulum. If the latter is the case, the nuclear evagination and infoldings may form a structural template for the induction of “nuclear” pores on the interdigitating membranes of the endoplasmic reticulum. When the pores on adjacent lamellae are aligned, the dense, amorphous substance contained within and between these aligned pores may form a continuous band from the inner nuclear membrane through several layers of the stack (Figs. 27 and 29). The largest mass of annulate lamellae measures 5-7 it in longest diameter. Those that are unattached to the nuclear membranes are often smaller and in some oocytes only one or two fragmented remnants of the larger structure could be found (Fig. 34). Even as they fragment, their continuity with endoplasmic reticulum appears to be maintained since in several oocytes we have seen pores either singly or in clusters on a profile of endoplasmic reticulum (Fig. 35).
In some oocytes, particularly those with annulate lamellae attached or adjacent to the nucleus, the membranes of the endoplasmic reticulum may communicate with the outer leaflet of the nuclear membrane at intervals around its entire circumference (Figs. 25 and 28) and with Golgi complexes scattered throughout the cytoplasm. Other oocytes have most of their endoplasmic reticulum as well as Golgi complexes and mitochondria confined to the area of Balbiani’s vitelline body and to the peripheral cytoplasm in this pole of the oocyte (Fig. 4). Vesicles of endoplasmic reticulum in the peripheral cytoplasm frequently contain an electron—opaque amorphous material (Fig. 35). Although similar vesicles have been observed adjacent to a ballooned, compound aggregate (Fig. 39) we have no clear—cut morphological evidence that the electron—opaque material contained within the endoplasmic reticulum is related to that within the aggregates. The granularity of the endoplasmic reticulum can only be seen in patches on cut view, although a helical configuration (Fig. 9) of polyribosomes can often be seen on fiat view. Single or clustered ribosomes are numerous in the cytoplasmic matrix. The proximity of endoplasmic reticulum to mitochondria was noted above. There appears to be some morphological relationship between the endoplasmic reticulum and the membrane-enclosed com 663 pound aggregates which also is revealed as a fixation eflect. Although the membrane around an aggregate does not appear to be continuous with the endoplasmic reticulum, channels of endoplasmic reticulum often appear to encircle portions of aggregates that are not membrane enclosed (Figs. 12 and 37). The dilation and constriction of the endoplasmic reticulum in any one oocyte appears to reflect differences in the osmolality of the fixation used (see figs. 4 and 25). With 2% glutaraldehyde (only slightly hypertonic) the endoplasmic reticulum is seen as dilated, continuous channels, whereas with the more hypertonic paraformaldehyde-glutaraldehyde solution, it appears constricted and is usually seen as chains of large vesicles. In addition, there is frequently a reciprocal inverse response in some compound aggregates that are membrane bounded. Those oocytes with more constricted endoplasmic retic~ ulum contain some large aggregates so ballooned that they appear to distort the cytoplasm, whereas those with dilated channels of endoplasmic reticulum contain more compact aggregates whose contents are dispersed in more moderate amounts of clear fluid.
Vacuoles containing a variety of structures are seen in the peripheral cytoplasm. Some containing multiple small vesicles and often surrounded by a halo of similar free vesicles are frequently associated with Golgi complexes (Figs. 23 and 38). Others contain either dense material similar to myelin figures (Fig. 39) or a variety of dispersed elements similar to those contained in the compound aggregates: electron—opaque granular material or less electron—opaque small vacuoles (Figs. 13 and 14).
Microtubules present throughout the oocyte cytoplasm are most prevalent around the circum ference of the nuclear membrane where they apparently support the mitochondria and endoplasmic reticulum. Bundles of spiral filaments occasionally abut the nuclear membrane (Fig. 7) or are seen in the more peripheral cytoplasm. Although the fibrils usually appear to possess only one coil they occasionally appear to possess two (see inset, Fig. 7). These fibrils are easily distinguishable from the dispersed microtubules which are present in the nearby cytoplasmic matrix. At the extreme periphery of a few oocytes, the cytoplasmic matrix contains a meshwork of extremely fine fibrils which extend into the evaginations projecting from the cell membrane of the oocyte (Fig. 19).
The cellular junction between the oocyte and the follicle cells is complex. Although a narrow stalk frequently appears to attach the endoplasmic reticulum of the oocyte to a desmosome at the oocyte—follicle cell junction, the neck of this stalk has not been seen to open into the intercellular space (Fig. 36). Occasionally pinocytic vesicles apparently of the “coated” variety are seen on the oolemma (Fig. 14). At intervals, particularly near junctions of two or three follicle cells, the oolemma is evaginated into clusters of projections that interdigitate with the tips of longer projections from the follicle cells (Figs. 20 and 38). Since these projections run at a variety of angles to the oocytecut curface, it is often impossible to determine whether they originate from oocyte or follicle cell. The AMPase activity that is pronounced in this area may indicate the predominance of follicular projections (Fig. 3).
The follicle wall contains both lighter and darker cells (Fig. 4-). Within the cells of the crescentic thicker area is a conspicuous Golgi complex and cytocentrum containing centrioles. These paranuclear organelles are oriented toward the oocyte. These cells frequently contain a large aggregate of fine filaments. Granular endoplasmic reticulum and small mitochondria with transverse cristae are characteristic of the thicker, lighter cells (Fig. 41). As noted above, compound aggregates smaller than but similar to those in Balbiani’s vitelline body are present also in the follicular cells (Figs. 17-19). The denser cells of the follicle wall appear to resemble those in the cortex: they contain many ribosomes free in the cytoplasmic matrix, often have many pinocytic vesicles on their peripheral free surface, and have long extensions that overlap their neighbors. A very thin, amorphous, basal lamina intervenes between the follicle wall and the collagen—containing matrix of the cortex (Figs. 17, 18, and 41). The spindle—shaped cells of the cortex have long, thin projections that are closely applied to, and on occasion invade, this basal lamina. In a few instances peripheral cells within the follicle wall may overlap their neighbors but be partially separated from them by a lake of homogeneous substance morphologically similar to the material of the basal lamina (Fig. 41). At the immediate periphery of some follicle walls, a coneshaped arrangement of cells has been observed that contains at its base a cortical cell that appears to be partially incorporated into the follicle wall (Fig. 40).
FIGURE 27 A micrograpll from a section on the same grid as that in Fig. 26. N 0t.e evagination of outer leaflet of nuclear membrane (bottom) that infolds to interdigitate with membranes from the peripheral cytoplasm thus to form a double loop at this area of the periphery of annulate lamellae. Note single loops (top) where annulate lamellae become continuous with endoplasmic reticulum. Note another evagination of outer leaflet of nuclear membrane at top. H 35-4; 2% glutaraldehyde. )( 252,000.
FIGURE 28 A section on a grid adjacent to that shown in Figs. 96 and 97. Note continuity of distended channels of endoplasmic reticulum with a.nnulate lamellae. Note also that these channels are also continuous with outer leaflet of nuclear membrane (bottom left). H 35-4; 2% glutaraldehyde. X 22,000.
Discussion
Light microscopists have carefully studied the large paranuclear structure in human oocytes in the primordial follicle stage (van der Stricht, 1923; Aykroyd, 1938; Beams and Sheehan, 1941). Raven (1961) in a volume summarizing comparative studies of developing oocytes describes the cytoplasm of this stage as follows: “the earliest oocyte often contains a distinct cytocentrum, situated on one side against the nuclear membrane, sometimes in an indentation of the latter. It consists of one or two small granules, the centrioles, surrounded by a sphere of dense cytoplasm, the arc/zoplasm or z'a’z'o5ome. Sometimes there is an indication of astral radiations in the latter. The archoplasm is often encircled by a ring of Golgi bodies, which in turn is surrounded by a dense cloud of mitochondria. The whole structure thus outlined is known as Balbianfs vitelline body, often wrongly called yolk nucleus.” The complexity of the organelles that form Balbiani’s vitelline body is the distinguishing ultrastructural characteristic of the oocytes in primordial follicles obtained from adult human ovaries. Electron microscopic studies of the unilaminar follicle in other mammalian species have shown a rather simple, large paranuclear aggregate of vesicles or saccules interpreted as a large Golgi complex: rat, Sotelo(1959); guinea pig, Anderson and Beams (1960) and Adams and Hertig (1964); rabbit, Blanchette (1961) and Zamboni and Mastroianni (1966); hamster, Odor (1965) and Weakley (1966). In studying fetal human ovaries, Lanzavecchia and Mangioni (1964) and Stegner and Wartenberg (1963) reported a perinuclear concentration of mitochondria and Golgi complexes in the oocytes in the early stages of meiotic prophase.
It is important to recall in examining the ultrastructural characteristics of the oocytes reported here, that they were obtained from women in their middle reproductive years, and therefore are cells that have remained in an arrested meiotic prophase for 25-35 yr. Nevertheless, they would have continued to be capable of responding to stimuli that would cause them to resume development: to enlarge and differentiate into a mature oocyte,
FIGURES Q9w3‘:2 Sections througli the same concentric arrangement of annulate lamellac which appears to be attached to nuclear nlenibrane (Fig. 392). Note association of pores 011 annulate lamellae with those on nuclear menibranes (Figs. 29 and 32). Also note continuity of membranes of annulate lamellae with endoplasmic reticulum (Figs. 31-32). H 43-1;
Karnovsky fixative. X 91,000.
FIGURE 33
These concentrically ar1'a.ng‘ed annulate lamellae are detached from the
nuclear meiribrane (right). Closely seriated sections never showed any closer morphologic proximity to the nucleus. II 44-9.; Karnovsky fi.\'at.ivc. X 10,000.
FIGURE 34 In some oocytes only fraginents of the larger annulate lamellar structures can be found in the cytoplasm. H 43-2; Karnovsky fixative. X 14,000.
FIGURE 35 Vesicles of endoplasmic reticulum in the peripheral cytoplasm of some oocytes contain an elect.ron—opaque material. The pore formations on the endoplasmic reticulum at right are interpreted to be the residuum of a disintegrated annulate lamellar structure. H 44-1; Karnovsky fixative. X 92Q,000.
to complete the maturation divisions, and thus to become fertilizable. In addition to being aged in terms of somatic cell life span, oocytes in primordial follicles are somewhat unusual cells in that they have no apparent function other than to maintain this readiness to respond to stimulation.
The almost complete localization of organelles, except for profiles of endoplasmic reticulum, to a paranuclear position in many oocytes indicates a predominantly localized metabolism. Lehninger (1965) suggests that, in unfertilized, sea—urchin oocytes whose rate of respiration is low and whose mitochondria are aggregated into bundles, the rate of respiration is limited by the rate of diffusion of oxygen or substrates into the interior of the bundle. In human oocytes, however, the intimate association of endoplasmic reticulum with mitochondria suggests a mechanism for the transport of substrates into the aggregation of mitochondria. We have noted an unusually close morphological relationship of endoplasmic reticulum to mitochondria in these human oocytes regardless of the fixative employed. Whether the variable morphology of the inner lamina of the peripheral membrane of mitochondria, noted as an effect of variation in osmolality of the fixative, reflects any physiological adaptation related to mechanochemical phenomena (Lehninger, 1965) is unknown. Vacuoles containing a variety of internal structures also appear to be evidence of transport to the para 668
nuclear metabolic center from the oocyte surface or vice versa. In some oocytes, the dispersal of limited numbers of organelles around the circumference of the nucleus together with annulate lamellae attached or adjacent to the nuclear membrane may be morphological evidence of some fluctuating distribution of activity in the oocyte cytoplasm. Localized projections from both the oocyte and the follicle cells interdigitate in the intercellular space and appear to reflect a constant active interchange between the oocyte and its follicle wall. The absence of phosphatase activities within the oocyte is similar to our findings in guinea pig oocytes at this stage (Adams et al., 1966). The presence of glucose—6—phosphate dehydrogenase in the oocyte cytoplasm indicates that the pentose—phosphate pathway of carbohydrate metabolism may be operative in this resting phase.
The presence of annulate lamellae in human oocytes of this stage was not unexpected since they have been reported in the cytoplasm of later stages of human oocyte development by Tardini et al. (1960) and by Wartenberg and Stegner (1960) as well as in a human ovum in the pronuclear stage (Zamboni et al., 1966). Annulate lamellae were not observed in guinea pig oocytes in primordial or primary follicles (Adams and Hertig, 1964) nor do they appear to be structures characteristic of most mammalian oocytes other than human ones. The derivation of cytoplasmic
THE JOURNAL or CELL BIOLOGY - VOLUME 34, 1967 annulate lamellae by vesiculation or blebbing of finding a few oocytes with a large stack of annulate the nuclear membrane has been described in lamellae attached to the nucleus, but of a greater oocytes of Necturus and of the echinoderm, T/zyone number with stacked or concentric annulate briareus by Kessel (1963, 1964, and 1966). Our lamellae lying free in the cytoplasm, in or near
FIGURE 36 A narrow stalk of endoplasmic reticulum is in apposition to a desmosolne at the intercellular junctions between the follicle wall (top) and the oocyte (bottom). H 35-3; 2% glutaraldehyde. X 21,000.
FIGURE 37 Endoplasmic reticulum is frequently associated with the compound aggregates in the peripheral cytoplasm of the oocyte. H 35-3; 2% gluta.raldehyde. X 22,000.
FIGURE 38 Vacuoles containing multiple small vesicles (center) and often surrounded by a halo of similar vesicles (left) are present in the peripheral cytoplasm of the oocyte. H 44-3; Karnovsky fixative. X 15,000.
FIGURE 39 Structures resembling myelin figures are separately contained within vacuoles (left) and are not found within the compound aggregates (_rigl1t). Note that a vesicle of endoplasmic reticulum containing electr0n—opa.que material is adjacent to the edge of a ballooned aggregate (lower right). H 444; Karnovsky fixative. X 22,000.
FIGURE 40
Balbiani’s vitelline body, shows that they are repeatedly formed at the nuclear membrane and are subsequently released into the cytoplasm where they apparently fragment. The suggested role for their participation in nucleo~cytoplasmic transfer (Swift, 1956) was based on the resemblance of the membranes of annulate lamellae in the cytoplasm to the ultrastructure of the nuclear membrane. When occasional pores of the nuclear membranes of these human oocytes have been observed in register with those in an attached stack of annulate lamellae, the dense, amorphous substance contained within and between the aligned pores appears to form a continuous band from the inner nuclear membrane toward the base of the stack. Whether this represents transfer of material from the nucleus to either the cytoplasmic matrix or membranes is unknown. When annulate lamellae are attached to the nuclear membrane, it is the presence of their pores and their greatly increased membrane area that distinguish them
A cone~shaped configuration of cortical cells is frequently seen at the immediate periphery of the follicle wall. The tip of the cone lies between two arrows. A projection (P) of the cortical cell at the base of the cone illustrated here appears to be becoming incorporated into the follicle wall. H 4.«4~1; Karnovsky fixative. X 5400.
from the membranes of the slightly granular endoplasmic reticulum that in the same oocyte also may be continuous with the outer leaflet of the nuclear membrane at intervals around its entire circumference.
The varying location of annulate lamellae within Balbiani’s vitelline body became of interest to us since, for a considerable period of our study of primordial follicles, the only oocytes with annulate lamellae attached to the nuclear membrane were three (Adams and Hertig, 1965) from two patients both in the follicular or estrogenic phase of the menstrual cycle, the endometria showing histologically a typical mid-proliferative pattern. In this preliminary series other oocytes from the luteal and menstrual stages of the cycle showed both detached annulate lamellae and poorer development of the peripheral endoplasmic reticulum. Histologically these endometria showed 19-20 day secretory criteria and those of early menstruation respectively. There have been many previous suggestions that the cyclic production of estrogen and /or progesterone in the ovary may have a local influence on a variety of ovarian structures and our initial finding suggested to us that a fluctuating participation by the oocyte nucleus in the cytoplasmic arrangement of organelles might be evidence of such a response to cyclic endocrine events. A hormonal effect on the prevalence and position of annulate lamellae has been noted in human endometrium by Ancla and De Brux (1965). In a series of 40 patients subjected to various forms of hormonal therapy for primary infertility, they reported that in the glandular cells “annulate lamellae may appear before ovulation, but are more frequently seen during the secretory phase if there is hyperestrogenism.” After studying intracellular localization of estrogen in so—called target cells, such as rat uterus, as opposed to cells of the body in general, Segal (1967) has recently formulated concepts of estrogen action which may involve an action at the nuclear level. The difference we noted between oocytes could be
easily explained either by our random sampling of the entire population of primordial follicles in any one ovary or by our failure to locate the annulate lamellae in some oocytes examined at only a few levels in their cytoplasm. Serial grids, although arduous to study, are required even to locate the annulate lamellae and a single fortunate section within a series may produce the only evidence of their attachment to the nuclear membrane. The results of a closer inspection of oocytes from the proliferative, secretory, and menstrual phases of the cycle and from pregnancy near term are shown in Table I.
This is admittedly too small a series for any statistical validity, but in these 27 oocytes studied with great care, there appears to be some evidence that annulate lamellae attached or adjacent to the nuclear membrane are more prevalent when estrogen production is dominant in the normal menstrual cycle. Whether this in fact reflects any environmental hormonal influence will require further detailed study of oocytes in primordial
FIGURE 41 In a few follicles, a peripheral cell may overlap its neighbors but be separated from them by a homogeneous, extracellular material similar to that in the basal lamina at the periphery of the follicle wall. H 43-1; Karnovsky fixative, X 10,000.
TABLE I
The Location of Amzulate Lamellae with Respect to the Nuclear in Phase: of the rlzfenstruai Cycle
Position of the annulate
Case ' . gicéejytifis lamellar s‘t)1(')\(1:ctture in each N0_ Phase of cycle by endometrial dating Cycle day C105 eh, Seriated y e_ ______ sections At nuclear Free in membrane cytoplasm H6* Mid—proliferative l 1 attached 0 H35 Mid-proliferative 4 4 attached (J H5411 Mid-proliferative l 1 adjacent 0 H48 Secretory (19~20th day) 23§ Rt ovary 0 3 (CL side) 3 H48 Secretory (l9—20th day) 23§ Left ovary 1 adjacent 2 3 H37 Menstrual 8 2 attached 6 H44 Menstrual 5 1 adjacent 4 H43 Pregnant, caesarian section 35% wk 2 1 attached 1
Infertility patient; wedge resection of normal ovarian tissue. ii This patient had been on a course of antifertility medication for 4 yr, interrupted by a pregnancy. She had been on same drug, Orthonovum, for 5 months previous to surgery including the 6th and 7th days of the cycle in which surgery was performed.
§ The endometrial dating may or may not coincide with day of cycle owing to normal variation in the length of the menstrual cycle in the human female.
follicles from many more ovaries obtained not only from various cyclic hormonal phases in the adult but also from prepubertal age groups.
The compound aggregates that surround the cytocentrum of these human oocytes in primordial follicles are subject to at least two interpretations: (a) that they are reservoirs of nutritive material analagous to yolk material in oocytes of other species, or (b) that they represent waste deposits of degradation Of organelles similar to lipofuscin pigment commonly found in aging cells. Some of the ultrastructural characteristics of the aggregates, finely divided membranous material and vacuoles, resemble those found pigment in neurons, another nondividing cell (Samarajski et al., 1965). The absence of compound aggregates in oocytes in primordial follicles of the human prenatal ovaries reported by Lanzavecchia and Mangioni (1964) and Stegner and Wartenberg (1963) suggests that the examination of similar follicles in various postnatal and preadolescent years might be of value in evaluating the effects of aging on the oocyte. The lack of demonstrable acid-phosphatase activity, however, indicates that the aggregates may not be lysosomal and, therefore, may not represent autophagic processes by which lipofuscin pigment commonly
in lipofuscin
is localized. Furthermore, myelin figures, usually indicative of membrane degradation, have not been observed among their heterogeneous components but are frequently seen to be separately contained within a vacuole in the peripheral cytoplasm. Thus, it is possible that compound aggregates represent nutritive material. Dense aggregates were not observed in our previous study of guinea pig oocytes (Adams and Hertig, 1964) but have been noted in the cytoplasm of some other immature mammalian oocytes (rabbit: Blanchette, 1961; hamster: Weakley, 1966, and Odor, 1965; rhesus monkey: Hope, 1965). Those observed in the rhesus oocytes have some ultrastructural similarities to the compound aggregates we see in the human oocytes, and it was suggested that they represent developing yolk. The presence of membranes around some aggregates in these human oocytes and the absence of a membrane around others within the same oocyte suggests that not all compound aggregates are in the same physiological state. Those that are membrane enclosed often contain dispersed vesicles of varying size which contain electron—opaque material. Whether this material is related to that seen within the endoplasmic reticulum has not been determined. The close morphological association of endoplasmic reticulum to these aggregates, however, may be related to the inverse reciprocal effects on these two organelles of variations in osmolality of the fixatives used. The presence of some ballooned aggregates, when the more peripheral endoplasmic reticulum is constricted, and the absence of gross ballooning, when the endoplasmic reticulum is dilated, suggests a possible pump mechanism between these two organelles. Merriam (1966) has presented evidence that frog oocytes consist of two osmotically active “compartments.” One, the protoplasmic mass, owes its osmotic properties to the plasma membrane, and the other consisting of interior vesicles owes its osmotic properties to their investing membranes. Because of the magnitudes of experimental swelling in the interior vesicles, he suggests that ion-pumping takes place in the vesicles of the cytoplasm of the frog oocyte.
Whatever the nature of the aggregates seen in a paranuclear location within the oocyte, the presence in the periphery of the oocyte of a variety of vacuoles containing elements of the aggregates together with the presence of compound aggregates in most follicle cells and in some cortical cells suggests their transport to the oocyte from its environment or vice versa. The mechanism for their transfer to the follicle cell from the cytoplasm of the oocyte, or vice versa, is not clear from these studies since in micrographs where a follicular aggregate bulges into the oocyte, complete intervening cell membranes have not been clearly visualized. The repeated observation of dense membranous material which appears to bridge the oocyte-follicle cell membranes may indicate that there is some mechanism for bulk transfer of the elements of the compound aggregates. Such membranous forms frequently are closely associated either in the follicle cell with a compound aggregate or in the oocyte cytoplasm with vacuoles containing elements of the aggregates. The presence in the peripheral oocyte cytoplasm of elements of aggregates within a vacuolar structure suggests that portions of follicular aggregates may be taken up by a phagocytic mechanism at the oocyte surface to be subsequently transported toward the paranuclear location.
The presence of similar compound aggregates in some cortical cells may be evidence that these cells are related to those within the follicle wall. A histochemical similarity between the cortical cells and those of the primitive follicle wall was observed with respect to adenosine—5-phosphatase activity. There is an analogous histochemical similarity in guinea pig ovaries, although the differentiated granulosa cells of developing follicles do not maintain this activity in the human as they do in the guinea pig (Adams et al., 1966). The absence of observed mitotic activity in primitive follicle cells could indicate either that they are as old as the contained oocyte or that they are renewed by some process other than mitosis. In a study of guinea pig follicles in a similar stage (Adams and Hertig, 1964) there was suggestive histological evidence that cortical cells contribute to the follicle wall. In the human primordial follicle, it is possible that cortical cells may move into the thickened, crescentic cap of the follicle wall and gradually slide around the oocyte to move out to the cortex again. Although never clearly visualized in the actual act of becoming incorporated into the follicle wall, occasional follicle Cells overlap the periphery of their neighbors and are partially separated from them by a homogeneous substance morphologically similar to the material of the basal lamina. In addition, many dark peripheral cells in the follicle wall closely resemble cells of the cortex. The coneshaped arrangement of cortical cells noted at the periphery of some primordial follicles has suggested that these may be the areas where cortical cells become incorporated into the follicle wall. Further histochemical study of the nature of the material within the compound aggregates is indicated. Such studies might well lead to a clarification of the relationship of such aggregates within the oocyte to those of the various cells of the surrounding ovarian tissue.
Although light microscopists have over the years identified structures in oocytes as centrioles (Raven, 1961), others have believed that the centriolar apparatus for postfertilization cleavage is introduced into the egg by the spermatozoan (see Mazia, 1961 for discussion). The absence of identifiable centrioles in most electron microscope studies of a variety of oocytes is not surprising in view of the necessarily random sampling of such a large cell. We know of only two reports of the clear visualization of centrioles. Sotelo (1959) illustrated a pair of centrioles in an oocyte from a 2—day—old rat, and Greenfield (1966) has demonstrated centrioles in oocytes of the domestic chicken shortly after hatching. It may be of importance that these oocytes in which centrioles have been identified were from ovaries of the immediate postnatal stage when mitotic activity of oogenesis had recently ceased. Even after specific search in all of our human oocytes, centrioles could not be identified. Recently in a report of spindle—associated chromosomes of meiosis in the rat, Zamboni and Mastroianni (1966) reported that centrioles are not observed. Whether centrioles in oocytes disappear with aging or simply are not found is still unknown. In the human oocyte, at least, it is possible that the one or two areas of dense amorphous deposits found in the center of the cytocentrum, as well as the long coarse fibers supporting the periphery of the cytocentrum, may be adaptations of the centriolar apparatus. Electron microscopic investigations of human oocytes from prenatal and various postnatal stages might clarify this concept. Vesicular aggregates either adjacent to or inter mingled with the central aggregate of dense amorphous deposits were a consistent finding.
This may indicate that the material within these vesicles is related either to the formation of the deposits or to their periodic alignment on "line fibrils which apparently becomes a transition to the peripheral coarse fibers.
This work was supported entirely by grant HD-00137, Division of Human Development and Child Health, formerly C-2451 of the National Institutes of Health.
The authors are greatly indebted to Doctors Charles Easterday, Shirley Driscoll, Christopher Duncan, Sumner Gochburg, Charles Horne, Lorna Johnson, and Alan Rosenfield of the Boston Hospital for Women for their cooperation in providing us with these human ovaries. In addition, grateful thanks are due to Miss Harriet Jopson and Mrs. Barbara Barton for their skilled technical assistance, to Mrs. Audrey Hadfield for the preparation of photomicrographs and electron micrographs, and to Mrs. Nancy Cote for typing the manuscript.
Received for publication 6 February 1967.
Bibliography
Adams EC. and Hertig AT. Studies On Guinea Pig Oocytes. I. Electron Microscopic Observations On The Development Of Cytoplasmic Organelles In Oocytes Of Primordial And Primary Follicles. (1964) J Cell Biol. 21:397-427. PMID 14189912
ADAMS, E. C., and A. T. HERTIG. 1965. Annulate laniellae in human oocytes in primordial and primary follicles. J. Cell Biol. 2T:119A.
ADAMS, E. C., A. T. HERTIG, and S. FosTER. 1966. Studies on guinea pig oocytes. II. Histochemical observations on some phosphatases and lipid in developing and in atretic oocytes and follicles. Am. J. Anat. l9:303—340.
ANCLA, M. and DE BRUX. 1965. Occurrence of intranuclear tubular structures in the endometrium during the secretory phase, and of annulate lamellae in hyperestrogenic human states. Obstet. Gynecol. 26:23-33.
ANDERSON, E. and H. V-V. BEAMS. 1960. Cytological observations on the fine structure of the guinea pig ovary with special reference to the oogonium, primary oocyte, and associated follicle cells. J. Ultra-struet. Res. 3;432—446.
AYKROYD, O. E. 1938. The cytoplasmic inclusions in the oogenesis of man. Z. Zellforsefz. 11/Iz'/rroskop. Andi. 27:691-710.
BAKER, T. G. 1963. A quantitative and cytological study of germ cells in human ovaries. Prue. Roy. Soc. (London) Ser. B. 158: l17—133.
BALBIANI, E. G. 1864. Sur la constitution du germe dans 1’oeuf animal avant la fécondation. Compt. Renal. 58 584-588.
BEAMS, H. W. and J. F. SHEEHAN. 1941. The yolk nucleus complex of the human ovum. Amzt. Record. 81:5-415F554.
BECKER, N. H., S. GOLDFISHER, VV. SHIN, and A. B. NOVIKOFF. 1960. The localization of enzyme activities in the rat brain. J. Bz'o}JIzys‘. Bioefzem. Cytol. 8:649—663.
BLANCHETTE, E. J. 1961. A study of the fine structure of the rabbit primary oocyte. J. Ultrastruct. Res. 5:349-363.
FAHIMI, H. D., and M. J. KARNOVSKY. 1966. Cytochemical localization of two glycolytic dehydrogenases in white skeletal muscle.J. Cell Biol. 29:1 13128.
GREENFIELD, M. L. 1966. The oocyte of the domestic chicken shortly after hatching, studied by electron microscopy. J. Embryoi. Exptf. Moryb/20!. l5:297— 316.
Hope, J. 1965. The fine structure of the developing follicle of the Rhesus ovary. J. Ultrastruet. Res. 12:592-610.
KARNOVSKY, M. 1961. The use of ethylene diamine tetraacetic acid disodium in the histochemical demonstration of triphosphopyridine nucleotide linked dehydrogenases. J. Histoehem. Cytoehem. 9:203—20-<1.
KARNOVSKY, M. J. 1965. A formaldehyde-glutaraL dehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 2T:137A—138A.
KESSEL, R. G. 1963. Electron microscopic studies on the origin of annulate lamellae in oocytes of Necturus. J. Cell Biol. 19:391-414.
KESSEL, R. G. 1964. Electron microscopic studies on oocytes of an echinoderm, Tlzyone briareus, with special reference to the origin and structure of the annulate lamellae. J. Ultrastruct. Res. 10:498514.
KESSEL, R. G. 1966. Some observations on the ultrastructure of the oocyte of Thyone briareus with special reference to the relationship of the Golgi complex and endoplasmic reticulum in the formation of yolk. J. Ultrastruct. Res. 16:305-319.
LANZAVECCHIA, G., and C. MANGIONI. 1964. Etude de la structure et des constituants du follicle humain dans l’ovaire foetal I Le follicle primordial. J. M icroscop. 3 :447—464.
LEHNINGER, A. L. 1965. The mitochondrion. Molecular Basis of Structure and Function. W. A. Beniamin, Inc., New York.
MAZIA, D. 1961. Mitosis and the physiology of cell division. In The Cell. J. Brachet and A. E. Mirsky, editors. Academic Press Inc., New York. 3.
MERRIAM, R. W. 1966. The role of cytoplasmic membranes in the regulation of water concentration within frog oocytes. Exptl. Cell Res. 41:34-48.
MYERS, H. I., W. C. YOUNG, and E. W. DEMPSEY. 1936. Graafian follicle development throughout the reproductive cycle in the guinea pig, with especial reference to changes during estrus (sexual receptivity). Anal. Record. 65:381-401.
ODOR, D. L. 1965. The ultrastructure of unilaminar follicles of the hamster ovary. Am. J. Anal. 116:493— 522.
PEARSE, A. G. E. 1960. Histochemistry, Theoretical and Applied. Little, Brown and Company, Boston.
RAVEN, C. P. 1961. Oogenesis: The Storage of Developmental Information Pergamon Press, New York,
SAMARAJSKI, T., J. M. ORDY, and J. R. KFEFE. 1965. The fine structure of lipofuscin age pigment in the nervous system of aged mice. J. Cell Biol. 26:779-795.
SEGAL, S. 1967. Fetal homeostasis. 11. Arm. N. Y. Acad. Sci. In press.
SOTELO, J. R. 1959. An electron microscopic study on the cytoplasmic and nuclear components of rat primary oocytes. Z. Zellforselz. ;'1-filcroskop. Anal. 50: 749-765.
STEGNER, H. E., and H. H. WARTENBERG. 1963. Elektronenmikroskopische Untersuchungen an Eizellen des Menchen in verschiedenen Stadien der Oogenese. Arclz. Gynaekol. 1992151-172.
STRICHT, O. VAN DER. 1923. Etude comparée des ovules des mammiferes aux différentes périodes de Povogenese, d’aprés les travaux du laboratoire d’histologie et d’embryologie de l’Université de Gand. Arch. Biol. 33: 229-300.
SWIFT, H. 1956. The fine structure of annulate larnellae. J. Bioplzys. Bioclzem. Cytol. 2 (Suppl.): 415-418.
TARDINI, A., L. VITAL:-MAZZA, and F. E. MANSANI. 1960. Ultrastruttura de1l’ovocita umano maturo. 1. Rapporti fra cellule della corona radiata, pellucida ed ovoplasma. Arch “ale l/ecclzz” Anal‘. Pallzol. .-Med. Clin. 33:281-305.
VENABLE, J. H., and R. COGGESHALL. 1965. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 25:407-408.
VVACHSTEIN, M., and E. MEISEL. 1957. Histochemistry of hepatic phosphatases at a physiologic pH. Am. J. Clin. Patlzol. 27:13-23.
W'ARTENBERo, H., and H. E. STEGNER. 1960. Uber die elektronenmikroskopische feinstruktur des menschlichen ovarialeies. Z. Zellforrclz. Mz'kroskop. Anal. 52:450-474.
WEAKLEY, B. S. 1966. Electron microscopy of the oocyte and granulosa cells in the developing ovarian follicles of the golden hamster (Mes0crz'cetus aumtus). J. Anal. l00:503-534.
ZAMBONI, L., and L. MASTROIANNI, JR. 1966. Electron microscopic studies on rabbit ova. I. The follicular oocyte. J. Ultmsmcet. Res. 14:95-117.
ZAMBONI, L., D. R. MISHELL, JR., J. H. BELL, and M. BACA. 1966. Fine structure of the human ovum in the pronuclear stage. J. Cell Biol. 30:579-600.
Figures
Figure 1-4
Figure 25
Fig. 25. This low-power micrograph shows a section that is not mid-polar through the oocyte since the paranuclear Balbiani vitelline body was present in deeper sections. Note that a stack of annulate lamellae is attached to nuclear mernbrane, that a Variety of organelles are closely applied to the entire circuinference of the oocyte nucleus and that. endoplasmic reticulum is present as distended channels. ll 35-4; 2% glutaraldehyde. X 2800.
Copyright
Rockefeller University Press - Copyright Policy This article is distributed under the terms of an Attribution–Noncommercial–Share Alike–No Mirror Sites license for the first six months after the publication date (see http://www.jcb.org/misc/terms.shtml). After six months it is available under a Creative Commons License (Attribution–Noncommercial–Share Alike 4.0 Unported license, as described at https://creativecommons.org/licenses/by-nc-sa/4.0/ ). (More? Help:Copyright Tutorial)
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Studies on the human oocyte and its follicle 1. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Studies_on_the_human_oocyte_and_its_follicle_1
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G