Paper - Development of the human coelom (1897)
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Mall FP. Development of the human coelom. (1897) J Morphol. 12: 395-453.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Development of the Human Coelom
(1897)
Introduction
Four years ago I wrote a general article on the coelom for Wood's Handbook of Medical Science, in which was emphasized the separation of the body cavity from the extra-embryonic coelom. Since then I have had opportunity to extend my observation to the human embryo, and therefore make this Communication.
Unfortunately, there are no data regarding the beginning of the coelom in the human embryo, and in all probability none will ever be found. The smallest human ovum ever seen is that described by Reichert.[1] It was obtained from a woman who had committed suicide, on account of pregnancy, forty-one days after the beginning of the last menstrual period. It was therefore presumably about thirteen days old. This ovum, which is pictured in every text-book, was 5.5 X 3.3 mm. in diameter, was surrounded by a zone of villi leaving two poles bare, and contained in its interior a mass of cells measuring 1.5 X 1.75 mm. All the space between this inner mass and the chorion is the coelom, and regarding its origin, we can no more than speculate.
During the last few years three other human ova, slightly larger than Reichert’s, have been cut into sections, thus permitting a more careful study of their contents.[2] The dimensions and approximate ages of these embryos are given in the table on the following page.
Table of Young Human Ova
| Observer | Diameter of
Embryonic Vesicle (mm) |
Diameter of
Ova (mm) |
Time between first lapsed
Period and Abortion (days) |
|---|---|---|---|
| Mall (No. XI) 11 | 1.5 X 1 | 10 X 7 | 13 |
| Reichert | 1.75X 1.5 | 5.5 X 3.3 | 14 |
| Von Spee (v. H.) | 1.84 x 1.083 | 6. X 4.5 | 12* |
| Von Spee (Gle.) | 2. x 2 | 10. X 8.5 | 12* |
| Mall (No. XVI) 16 | 2.1 x 2.1 | 18. x 8. | 13 |
| His (Lg.) | 2.15 x 2 | 15. x 12.5 | 12 |
| Von Spee | 2.69 x 3. | 15. x 14. | 14 |
| Janosik | 3. x 4. | 8. | 15 |
- These are all of the authentic young human ova I can collect from the literature giving all of their measurements as well as the menstrual history of the mother.
In both of von Spee’s cases the time between the abortion and the end of the last period is given; in embryo v. H. the time is given as “ exactly five weeks,” while in embryo Gle. “ five weeks ”is given. If we estimate the duration of menstruation as five days and its frequency twenty-eight days, then the time between the first lapsed period and the abortion is twelve days, as I have given it in the table.
It is noticeable that in the three embryos just mentioned, as well as in the remaining four of the table, the size of the whole egg does not correspond with the size of the embryo, nor with its age. I do not think that this great variation in the size of the chorionic vesicle is altogether due to the method of hardening the specimen. Just at this time the growth of the chorion is precocious, as is also the case in the dog[3] rabbit,[4] and monkey.[5]
The papers by Bischoff and by Selenka are worthy of the most careful study by every embryologist, and I take the liberty of rearranging some of Bischoff’s data on the development of the dog. His observations are very extensive, and give us the basis for our present ideas of the passage of the ovum into the uterine tube after fertilization. Unfortunately, they were made before the time of sectioning specimens, yet they are more complete than most researches relating to this subject published since his time.
The portion I tabulate relates to the size of the embryonic mass or vesicle, the size of the ovum, and its approximate age. As far as I have been able to determine, these data taken from the dog are still the most important ones with which we can compare the human ovum. Embryologists are accustomed to state that the age of a human ovum is to be reckoned from the beginning of the first lapsed period, and I think that Bischoff’s observation upon the size and growth of the dog’s ovum corroborates this view. He found that the ova left the ovary during the rutting period, but the exact date could never be determined. Neither did the time of copulation determine the ovulation. As a rule, it took twenty-four hours or less after copulation for the spermatozoa to reach the ovary, and about the same time is required for the ovum to reach the beginning of the uterine tube after ovulation. So if ovulation and copulation took place at the same time, fertilization of the ovum could not take place until twenty-four hours later.
In Bischoff’s tables he often rates the age of an ovum from the first or from the last copulation, or from the beginning or from the end of the rutting period. I have attempted to tabulate his specimens from all four of these dates, but in none of the attempts did the size of the ova correspond with their respective dates. Often eggs of a given date were smaller and developed to a less degree than ova presumably younger. After much difficulty I finally constructed a. table in which the size of the ovum and its age correspond. A number of the ova published by Bischofi were obtained from the same animal by removing half of the uterus at one time and the remaining half the next day. In each portion a number of ova were found, and they were usually of about the same stage of development. By this method of procedure it is possible to determine very accurately the growth of the ovum from one stage to one twenty-four hours later. So, by gradually plodding through the specimens published by Bischoff, it was possible for me to correct his data completely. It is remarkable, as the table shows, how slowly the development takes place in the early stages, and about ten days are required before the ovum is one millimeter in diameter. On the fifteenth or sixteenth day the ovum is about as large as the human ovum described by Reichert (see table).
Table of Age and Size of the Dog’s Ovum
(Compiled From Bischoff.)
| Table of Age and Size of the Dog’s Ovum |
|---|
| To be formatted
H H DIAMTER or AGE. Dmgfgfi? OF |- Eisgfiipivrc STAGE. I day. .15 mm. I cell. 2 days. - .14 “ 2 cells. 3 u J4 It i 4 cc 4 as J6 u -| 13 cc 5 H J6 H ‘ 64 ii 6 “ i .18 “ I Mulberry. 7 N .20 ‘C I {II 8 ti ' _2I at £6 9 u _28 H l as Io “ 4 .30 “ .07 mm. I I “ 1 . “ .1 6 “ I2 [I 2. ‘C '24 H I3 I‘ 3. H .43 I‘ I4 I‘ 4. H .5 H I 5 I‘ 5‘ $6 I . H 16 cs 5. £5 2. it 16% u Ii ii |
| Compiled From Bischoff - It has been somewhat diflicult to compile this table, as Bischoff’s measurements are all given in Paris lines. My measurements are taken in great part from his figures, and I think that these are very accurate. |
Similar results can also be obtained from the various papers published on the rabbit’s embryo. Its development, however, is considerably more rapid than the dog’s, as the period of gestation is but thirty days.
Recently Selenka has given some of his results relating to the development of the monkey. The most valuable specimen relating to the early development of higher animals was unfortunately lost, but its age and dimensions are preserved for us, and are of value in the determination of the age of human ova. The ovum came from a monkey kept in confinement which was killed eight days after copulation. If we estimate one or two days required before fertilization, this ovum cannot be over seven days old. This suggests that the early stage of this variety of monkey is developed more rapidly than that of the dog.
Development of the Monkey
(From Selenka.)
| Development of the Monkey |
|---|
| To be formatted
Dmmnrnn on DIA5lfJ;lf OF Emnnvomc f Vnsrcm. . ‘ f t " r“““l bemnopithecus maurus . . . . . . . . 1.5 mm. .3 mm.1 Semnopithecus pruinosus . . . . . . . 6. “ .5 “ Cercocebus cynomolgus . . . . . . . . 5. t‘ .5 “ Cercocebus cynomolgus . . . . . . . . Io. “ l 2.4 ‘*9 |
| From Selenka.
1 Not an embryonic vesicle, but only a disc. 2 Neurenteric canal present. |
The pictures Selenka gives indicate that the development of a monkey’s ovum is identical with that of the human ovum. At any rate, the few specimens Selenka publishes give results which are equal to the great number of specimens of human ova which have been published. This only indicates that many of the interesting problems relating to early human development will probably be solved by the study of the monkey’s ovum. There is but little doubt now that young monkeys’ ova will soon be obtained for study.
Material Employed
During the last few years I have obtained a number of young human embryos from physicians in different portions, of the United States, and to them I am under all obligation for the
resent stud as well as for some others which are to follow. P Y , Nearly all of the specimens which I givein a table are well preserved, and a number of them ‘are preserved excellently. All of the specimens were stained in alum Carmine, and with the exception of Nos. XVII, XLIII, and LVII were cut transversely. These three were cut in sagittal sections.
All of the specimens were hardened in alcohol, the value of which method I have repeatedly emphasized to my friends, and do continue to emphasize to those who may preserve specimens for my use in the future.[6]
List of Embryos Studied
| No. (Carnegie) | Vertex to Breech Length (mm) | Neck Nape to Breech Length (mm) | From whom obtained | |
|---|---|---|---|---|
| XI | 11 | — | — | Dr. Kittredge, Nashua, N. H. |
| XII | 12 | 2.1 | — | Dr. Ellis, Elkton, Md. III |
| III | 3 | 2.2 | — | Prof. His, Leipzig, Germany. |
| XIX | 19 | 5.5 | 4.5 | Dr. Williams, Baltimore, Md. |
| XVIII | 18 | 7. | 7. | Dr. Douglas, Nashville, Tenn. |
| II | 2 | 3. | 7. | Dr. C. 0. Miller, Baltimore, Md. |
| IV | 4 | — | 7. | Dr. Williams, Baltimore, Md. |
| XLIII | 43 | 15. | 13. | Dr. Booker, Baltimore, Md. |
| VIII | 8 | 17. | 14. | Dr. Ritter, Brooklyn, N. Y. |
| IX | 9 | 17. | 14. | Dr. Eycleshymer, Chicago, Ill. |
| V | 5 | 18.5 | 17. | Dr. Kittredge, Nashua, N. H. |
| XLII | 42 | 18. | 15. | Dr. Wills, Los Angeles, Cal. |
| XVII | 17 | 18. | 16. | Dr. Cottrell, Louisville, Ky. |
| XXVIII | 28 | 19. | 18. | Dr. Sewall, Denver, Col. |
| VII | 7 | 19.5 | 18. | Dr. Booker, Baltimore, Md. |
| XXII | 22 | 20. | 18. | Dr. Snively, Waynesboro, Penn. |
| LVII | 62 | 23. | 20. | Dr. Howard, Cleveland, Ohio. |
| VI | 6 | 24. | — | Dr. C. 0. Miller, Baltimore, Md. |
| X | 10 | 24. | 20. | Dr. W. S. Miller, Madison, Wis. |
| XLV | 45 | 28. | 19. | Dr. Douglas, Nashville, Tenn. |
| XXXIV | 34 | 80. | 60. | Dr. Ellis, Elkton, Md. |
| XLVIII | 43 | 130. | 110. | Dr. Wilson, Worcester, Mass. |
| Ref: Mall FP. Development of the human coelom. (1897) J Morphol. 12: 395-453. | ||||
It is very injurious to these delicate specimens to be wrapped in cotton before they are sent by mail or express. A perfect method is to place the preserved specimen in a bottle filled completely with alcohol, thus imitating the condition of a foetus in utero. If there is no air or cotton in the bottle containing the embryo it is almost impossible to injure the embryo by shaking it.
Since I have emphasized this method of preservation (Johns Hopkins Hospital Bulletin, 1893), I have obtained a number of specimens excellent in every respect. These specimens are not distorted, nor macerated, nor shrunken.
N. B. and N. 13. indicate the length of the embryo measured from the vertex to the breech and from the nape of the neck to the breech, respectively.
cytogenic studies also. In most of the specimens photographs or an additional series of sections were made of the chorion and amnion in order to study the variation of these structures.
Embryos XI,[7] 11 XII 12, and II [8] 2 were completely reconstructed in wax by the method of Born. Nos. IX, VI, and X were reconstructed in part by Born’s method and finished by His’s method of reconstruction. The abdominal viscera of Nos. VI, IX, X, XXXIV, XLV, and XLVIII were modeled by Born’s method.
The mechanical portion of reconstruction has been simplified to a great extent by a special apparatus used in the Anatomical Laboratory,[9] which enables us to employ a modeler. The sections are projected upon a screen, to which the wax plate is attached. By working in a dark room with this apparatus it is easy to direct a modeler to draw the outlines accurately. He can then cut them out, and all that remains to be done is to pile the pieces and then blend them.
The Coelom in Young Ova
All of the young human ova which have been described contain within them a cavity, lined with mesodcrm‘; this is the coelom, bounded by the somatopleure on the outside and by the splanchnopleure on the inside. This arrangement, as shown by a number of diagrams by recent authors, is very unlike the appearance of the blastodermic membranes of many of the lower mammals, and it is necessary therefore that we should revise our conception of the formation of the amnion in the human embryo.
The ova recently published by Graf Spee indicate that the amnion must be formed very early, and, since it is completed before the medullary grooves begin, we must admit now that it is formed much the same as it is in many rodents, i.e., by apparent inversion of the membrane. When Bischoff[10] first described inversion of the membrane in guinea pigs it seemed like a paradox, but, since the comparative methods of study have been introduced, inversion only means that the amnion is completed before the medullary groove begins to form. This alteration of the development of the amnion and the medullary groove makes the body of the embryo develop on a concave surface instead of on a convex one, thus apparently making the embryo inverted, as is the case in the guinea pig.
Closely associated with inversion of the blastodermic membrane is the formation of an additional layer of cells, discovered by Rauber[11] the importance of which has been emphasized by Selenka and others. Rauber’s layer is so marked in the rabbit that it was at first believed to be the true ectoderm. The fate of Rauber’s layer has not been sufficiently studied to interpret it completely, and our ideas regarding it will not improbably require some revision. In many rodents Rauber’s layer becomes markedly thickened on one side of the ovum, forming a support, or Trétger, for the ovum. The relation of Rauber’s layer to the Trziger is shown beautifully by Selenka[12] on Plate XVI of his monograph.
The question which interests us here is whether the inversion of the blastodermic membrane as well as the discovery of Rauber’s layer aids us in advancing a theory of the development of the germ layers of the human embryo, and thus in turn to explain the large coelom as found in all of the earliest human ova. I realize fully that any such effort will not be final, yet I believe that it will aid us to understand better the relation of the membranes as found in the human ovum.
In looking over the illustrations of the development of animals closely related to man, one is struck with the similarity of the arrangement of the membranes to those described for the human ovum by Graf Spee. One must compare only plates XXXV - XXXVIII of Selenka’s[13] paper with the two plates accompanying Graf Spee’s[14] to be convinced that the early development of monkeys is almost identical with that of man. Yet Selenka’s researches on monkeys do not help us a great deal ; they only show us that the monkey’s development is like that of man. In monkeys we have also the precocious chorion and the early amnion and the large coelom between the umbilical vesicle and the chorion. The marked difference is that the amnion is attached to the chorion along its dorsal side, while in the human embryo this is only the case along the posterior end of the amnion. The attachment of the amnion along the chorion suggests that the embryonic plate separated from the exterior of the ovum along this point, as Selenka thinks he observed in a very young ovum only I. 5 mm. in diameter. Unfortunately, the most valuable specimen was injured in its preparation} and Selenka did not trust himself to give any illustrations of it.
With the amnion attached at its dorsal end to the chorion, we understand why the entodermal end of the allantois must grow around an angle to reach the chorion (Selenka, Plate XXXVII, Fig. 5). Somewhat the same arrangement has been described by Graf Spee[15] in his embryo Gle., but the curve is by no means as marked, indicating that the attachment of the embryo to the chorion is along its posterior end, as shown by His[16] in his well-known diagram of the formation of the amnion.
Regarding the very early stages of monkeys and man it is better that we make comparisons with animals most nearly related to them, and now we have careful studies of the very early stages of Chiroptera at our disposal. I believe that Selenka’s[17] study of the development of Pteropus edulis gives us the key for the comparison of the formation of the blastodermic membranes in mammals. Recent investigations by Duval[18] on different families of Chiroptera appear to confirm the work of Selenka on Pteropus.
In order to illustrate these points more clearly I have made diagrams of three of Selenka’s figures of Pteropus. Fig. 1 is from an ovum covered completely with two layers of cells, between which at one pole of the egg there is a mass of scattered cells destined to become the permanent ectoderm. The outer layer of cells has a tendency to grow into the form of villi over the embryonic disc, while on the opposite side of the egg it is composed of but a single layer of cells. Since this outer layer remains well separated from the body of the embryo throughout its development, and since it holds the same position to the egg that Rauber’s layer does in the rodents, I believe it to be identical with Rauber’s layer, and shall speak of it as such. According to Duval this Rauber’s layer disappears over the embryonic disc in the Chiroptera much as in the development of the rabbit and the field mouse. This does not necessarily contradict Selenka’s observations on Pteropus, for the house mouse begins to develop like the field mouse, but continues during the early stages in the same manner as Pteropus does.
4 Selenka: Studien, 1892, p. 209.
Figs. 1-3. —Diagrams of the Development of Pteropus Edulis, after Selenka. Fig. 1 is SeIenka’s Fig. 2; Fig. 2. Selenka’s Fig. 5; Fig. 3, Selenka’s Fig. 9. R, Rauber’s layer; P, placenta; ec, ectoderm; en, entoderm; c/z, chorion; am, amnion; u 2/, umbilical vesicle; mes, mesoderm; {'06, coelom; all, allantois, with the arrow indicating the direction of its future development.
In the next stage the ectoderm has been converted into a hollow mass of cells, Fig. 2, rather by a process of absorption than by an invagination, as I have expressed it in the diagram. The entoderm lines the whole interior of the egg, and surrounds the ectoderm of the amniotic cavity. The ectoderm of the exterior of the egg, Rauber’s layer, is again thickened over the embryonic mass to form the placenta, as Selenka calls it, or the Trézger, if we were discussing rodent embryology.
In the next stage, as expressed in Fig. 3, the mesoderm is beginning to form, and has extended completely over the amnion and partly over the umbilical vesicle. The entoderm has retracted itself and touches the ectoderm ; only the chorda dorsalis is yet to form. Between the amnion and the placenta, or the Trézger portion of Rauber’s layer, there is a marked space, and the mesoderm does not come in contact with it. The allantois grows as a bag into this space and attaches itself to the thickened part of the ectoderm, as shown by Gohre 1 in his figures. In the figure 3 accompanying Gohre’s paper he shows the vesicular allantois attached to the support of the chorion , (black portion of my Fig. 3) leaving on either side of the embryo a coelom. The allantois carries the mesoderm and vessels to the villi of the chorion, and these in turn are imbedded in the decidua of the uterus. In so doing the ectoderm of the chorion receives a second layer of epithelium, and I believe that this must account for the two layers of epithelium we have on the chorionic villi of the human ovum. There has been much written on the subject of the double layer of epithelial cells of the human chorion, and I think that a glance at G‘ohre’s figures 3 and 4, on Pteropus, as well as at Selenka’s figures 11 and 12 (Plate XXXV) and figure 6 (Plate XXXVII) on monkeys, will decide this question more definitely than all the many discussions on the human chorion put together have done.
1 Géihre: Selenka’s Studien, etc., 1892, p. 218.
Having now selected from Selenka diagrams and descriptions of the development of the germ layers of Pteropus, it is easier for me to give a plausible explanation of the beginning of the coelom in the human embryo. If the diagram I have given in Fig. 3 is compared with Selenka’s figures 5 and II (Plate XXXV) and figure 5 (Plate XXXVII) of the monkey, as well as with the sections of young human ova published by Graf Spee 1 and by myself,” one is struck with the great similarity of the two groups of figures.
Fig. 14, given further on, is a diagrammatic outline of a longitudinal section of a young human embryo published recently by Graf Spec. It is the one marked v. H. in the table of young human ova given in the beginning of this paper. When, now, this section is compared with the transverse section of Pteropus, in Fig. 3, the only marked difference is that the umbilical vesicle in Pteropus has retracted, in order to make the arrangement of the membranes as given for the human embryo in Fig. 14.
In order to make the connection complete, I give hypothetical stages in Figs. 4, 5, and 6. Fig. 4 represents the human ovum in the two-layer stage. The outer layer, or Rauber’s layer, is complete as in the rodents and in Pteropus. The inner layer, or cntoderm, is also complete. Between the two is the embryonic shield, or ectoderm of the future embryo. The next figure, 5, shows the beginning of the mesoderm developing towards the tail end of the embryo, as this is the position of the primitive streak, and as the head fold of the amnion in many embryos is often only invested with ectoderm and entoderm. A stage later, Fig. 6, finds the mesoderm enveloping the umbilical vesicle completely, and also partly lining the outer layer, R, of the ovum. The cavity between the two is the coelom. At the tail end of the embryonic disc the mesoderm of the somatopleure and splanchnopleure are still united, and mark the place of the formation of the rudimentary allantois.
1 Graf Spee : His's Archiv, 1889 and I896. 9 Mall: A Human Embryo of the Second Week, Anatom. Anz., Bd. 8, and
Early Human Embryos and the Mode of their Preservation, Johns Hopkins Hospital Bulletin, 1893.
Having carried the development of the human ovum to this stage by means of hypothetical stages, based upon the development of Pteropus, I can now continue the description of the development based upon observation.
Abnormal Ova
Teratologists are accustomed to view a group of abnormal states as arrested development, and in recent years a number of abnormal human ova have been studied by His,[19] by Giacomini,[20] and others. Frequently in the development of an ovum the embryo is destroyed completely, or, according to Giacomini, may wander out of the ovum. In these cases the ova are aborted. Frequently, however, a portion of the embryo is not developed, or it dies and the remaining portion develops for a time, and then the ovum is aborted. I have now in my collection a beautiful example of an ovum of apparently normal structure, the interior of which is lined completely with an amnion, and in place of an embryo there is only an umbilical cord. The ovum was aborted fiftyfour days after the first lapsed period, and was 30 mm. in diameter. The cord was 2 mm. in diameter and 9 mm. long. Its embryonic end seemed to be cut off abruptly, and was covered with a small mass of round cells. I give this example only to show that the embryo may be entirely wanting with a perfect cord and membranes.
FIGS. 4-6. Hypothetical Stages of the Early Development of the Human Ovum. R, Rauber’s layer; ec, ectoderm; en, entoderm; mes, mesoderm; u 12, umb.‘ica.l vesicle; coe, coelom; all, position of allantois.
A large per cent of young ova which come into the embryologist’s hands are abnormal. According to Professor His’s experience over half of the ova less than three weeks old are abnormal, while of those of four and five weeks one quarter are abnormal. In my collection there are ten abnormal ova among twenty-six ova which are less than six weeks old. Of these ten specimens three contained no embryos at all, one (No. XXXVII) contained the cord only, and six were of the nodular form. Of this group of six, three contained a double vesicle with a kind of fibrous capsule, to a great extent similar to the mesoderm of the chorion. One of these is His’s Embryo XLIV, which is frequently described in the books as a normal specimen, but which unfortunately is an abnormal one. My interpretation of these three specimens (Nos. XIII, XIV, and XX) is that the fibrous degeneration overtook the embryonic vesicle after it had reached the stage of Graf Spee’s embryo v. H., my Fig. 14. The remaining three embryos (Nos. XXI, XXXVII, and LVIII) are of the vesicular form, and I believe them to be especially valuable for the proper interpretation of. the early stages of development of the human coelom.
Table of Abnormal Ova
No. Dthgfgfilf OF DI:3fl\lI1:l[;l:::i’E‘;El\{IIgF FROM VVHOM OBTAINED. XIII 3 mm. 1.4 mm. Prof. His’s No. XLIV, Leipzig. xiv * 30 « 1.5 « Dr. Friedenwald, Baltimore, Md. XX I 15 “ 2. “ Dr. Williams, Baltimore, Md. XXI I2 “ 5. “ Dr. Cullen, London, Canada. xxxvn I 25 it I 2. “ Dr. Gould, Philadelphia, Pa. LVIII 20 “ 6. “ Dr. Howard, Cleveland, Ohio. XXXII 3o “ 2x9 “ Dr. Booker, Baltimore, Md. xxlv | 20 « I — Dr. Miller, Baltimore, Md. XXIX 30 “ —— Dr. Booker, Baltimore, Md. LV i 30 “ — Dr. Watson, Baltimore, Md.
- It does not necessarily follow that these embryos are all less than six weeks old, for the menstrual history of the mother indicates that some of them must be considerably older. This is one source of error in obtaining the high per cent of abnormal ova among young embryos. The statistics will not be accurate until the menstrual history accompanies the measurements.
Nos. XXI and LVIII came to me as perfect specimens, both having been hardened unopened, the first in strong formalin and the second in strong alcohol. No. XXI was still enclosed in its decidua, and appeared to be a normal specimen until it had been cut into serial sections. The embryonic vesicle proved to be very large, and was composed throughout of two layers, an inner one giving all the appearance of the entoderm and an apearance of the mesoderm of the umbilical vesicle of young embryos. The mesodermal layer contained within it islands of blood cells, as are also present in normal specimens. The whole vesicle was connected to the chorion with a mass of mesodermal cells somewhat as shown in the diagrammatic Outel. all the ap
FIG. 7. Diagram of a Pathological Ovum which represents an Early Hypothetical Stage.
Fig. 7. The chorion and decidua appeared to be normal.
No. LVIII showed considerable change in the mesoderm of the vesicle and chorion, giving somewhat the appearance of fibroid degeneration rich in cells. The chorion was attached to the vesicle by a strong pedicle, as shown in Fig. 7. The vesicle itself was composed of two layers, an inner and continuous one composed of one layer of cells, and an outer and thickened layer appearing like the mesoderm of the chorion. There were no indications of blood islands. In addition to these two layers there was a third layer fairly well marked near the pedicle and between the vesicle and the chorion. With the exception. of the allantois canal, Fig. 7 is a diagram of this specimen. No. XXXVII is much like No. XXI, but it did not stain well as the specimen was a number of years old when cut.
Giacomini[21] has described a number of similar vesicles, and he expressly states that the vesicles had the structure of the umbilical vesicle, but that there was no trace of the amnion present in any of them. A number of other vesicular forms have been described, and in general they all appear much like the two specimens I have given.
I do not think that it is rash to assert that these vesicles represent an arrested development of an earlier stage, which, due to impaired nutrition, or whatever it might have been, simply allowed the embryonic vesicle to keep
FIG. 8. Diagrammatic Section of Half of the Human Ovum No. XI. Enlarged lo times. The villi are drawn only on the upper side. ac, ectoderm; me, On eXp3.1]Cling_ entoderm ; mes, mesoderm ; we -2;, umbilical vesicle ; coe, coelom; alf, allantois; :2, amnion.
That this expansion can keep on is already shown in the simple enlargement of the chorion after the embryo is distorted or wanting altogether. We have in these specimens a thin chorion with atrophic villi, and why can we not have an expanded and atrophic embryonic vesicle if its development is impaired? In this way I view specimen No. LVIII. It represents a much earlier stage, which has simply expanded and was ultimately aborted. In Nd. LVIII the embryonic vesicle must have ceased its further development a week or so before the abortion, about the time the coelom was beginning to develop. At that time the fibrous degeneration enclosed the embryonic vesicle as Well as extended around the whole chorion into all of its villi. This, then, arrested the further development of the embryo, and the embryonic Vesicle simply continued to expand.
This idea is further strengthened by another ovum whose history I published on several occasions three years ago} The specimen is a good one, having been preserved fairly well, and it has every indication of being normal. Since the specimen has been in my hands I have studied it over and over again, have photographed many of the sections, and have reconstructed it. At first it was very difficult for me to interpret it, but finally it appears to me that something definite can be said regarding the arrangement of the membranes and their relation to older as well as to the pathological and presumably younger specimens.
Embryo No. XI. 11 “The woman, from whom the specimen was obtained, is twenty-five years old, menstruates regularly every four weeks, the periods lasting from four to five days. She gave birth to a child Sept. 19, 1892, and had the first recurrence of menstruation December 19. The second period followed on January 25, and was very profuse; it lasted until February I. The next period should have begun about February 22, but on account of its lapsing the patient concluded that she was pregnant, and called at my office a few days later. I did not examine her, but asked her to remain quiet and await developments, as I thought possible that she might be pregnant. On the evening of March I she fell and sprained herself, and during the same night had a scanty flow. The flow recurred each day, and on the 7th of March she passed the ovum. It was kept in a cool, moist cloth for twenty hours, and when it came into my hands was at once placed in a large quantity of 60% alcohol.” [22]
The ovum is very large for its age, having a long diameter of 10 mm. and a short diameter of 7 mm. It is covered with villi only around its greatest circumference, having two spots with out villi, as was the case with Reichert’s ovum. The villi of the chorion are from 0.5 to 0.7 mm. long and are branched.
1 Mall: Anatom. Anz., 1893, and Johns Hopkins Hospital Bulletin, 1893.
Upon opening the chorion it was found that the germinal vesicle was situated just opposite the edge of the zone of villi. About it was much Coagulated albumen, which I did not remove, and therefore could not obtain good camera drawings. The portion of the chorion to which the vesicle was attached was cut out and stained with alum cochineal and cleared in oil, but even after this treatment it was impossible to obtain any clear picture. The specimen was next imbedded in paraffin and cut into sections 10 p. thick. The series proved to be perfect. From the sections a reconstruction was made in wax, and the accompanying Fig. 8 is a sagittal section of it.
The dimensions of the different portions of the vesicle are as follows :
- Diameter of stem . . . . . . . . . . . 0.4 mm
- Length of stem . . . . . . . . . . . . 0.4 mm
- Length of vesicle . . . . . . . . . . . 1.5 mm
- Width of vesicle. . . . . . . . . . . . 1.0 mm
- Length of invagination . . . . . . . . . 0.8 mm
- Width of invagination . . . . . . . . . . 0. 5 mm
- Diameter of opening of invagination . . . . . 0.03 mm
The sections and reconstruction show that the embryonic vesicle is attached to the chorion by means of a stem (Bazzc/5+ srzlel). The vesicle itself is composed of two layers, between which, at a distance from the stern, there are indications of blood—vessels in the middle embryonic layer. Just beside the attachment of the vesicle to its stem there is a deep, narrow invagination of all layers of the vesicle. The walls of the invagination are somewhat thicker than those of the surrounding vesicle. The accompanying figures give the arrangement of the embryonic layers in different portions of the vesicle. The invagination is in no respect artificial, as suggested by Graf Spee, as the curves are all sharp, and the layers of mesoderm and ectoderm are very definitely outlined. The ectoderm has the sharp contour of the ectoderm of other young embryos published, and gives the pictures which are familiar to all embryologists. The entoderm does not extend all around the sections as I have pictured it, but has fallen off at some points, and this explains why the figures here given do not correspond exactly with those in previous publications. There cannot be any doubt about my interpretation of the arrangement of the embryonic layers in this specimen, nor do I think that it is abnormal. Yet this last point will be decided in the near future, I believe, and therefore it may be dropped until other young embryos are described.
Within the stem of the vesicle there is a sharply defined allantois, which communicates with the cavity of the vesicle just below the invagination of the ectoderm. The cavity of the vesicle extends into the stem at a lower point, and it is this invagination which Graf Spee believes to be the amnion. Gladly would I agree with him were it not that there is no evidence whatever of the presence of ectoderm at this point. Throughout this invagination into the stem there are only two thin layers of cells, one of which runs over into the mesoderm of the chorion and the other into the entoderm of the vesicle.. Yet in this invagination the entoderm is not detected at any point.
FIGS. 9-13. Sections No. 43, 53, 68, 80, and 89 through the Embryonic Vesicle of Embryo No. XI. Enlarged 33 times. The entoderm is a heavy line, the ectoderm is striated, and the mesoderm dotted. zz, amnion; X, cavity of the umbilical vesicle extending into the stem of the vesicle ; R, Rauber’s layer as the ectoderm of the chorion.
The eetodermic plate in the large invagination of the amnion is very broad, but not of equal thickness throughout its extent, and it ends very abruptly beyond the opening upon the surface. As the opening approaches the stem, the cells of the ectoderm are continued somewhat along its surface, as indicated by the black line in Fig. 8.
All of the space between the embryonic vesicle and the chorion is the coelom, and in this specimen it communicates with the amnion. Whether this is transient or unusual cannot, of course, be stated. Should further experience show that the amnion is closed at an earlier stage than indicated in this specimen, it would not materially affect my diagrams or observations. Graf Spee’s recent observation, Fig. I4, makes him think that this is the case, but it is just as easy to interpret the formation of the amnion in Fig. I4 from that in Fig. 8 as by his theory.
The next stages in the development of the embryonic vesicle are taken from Graf Spee, and they are of importance to elucidate the changes which take place preparatory to the formation of the body cavity. In Fig. 14, which represents the younger embryo, the amnion is still surrounded completely with mesoderm, as in embryo No. XI, represented in Fig. 8. The mesoderm crosses the median line, as the sections given by Graf Speel show. The dorsal side of the amnion is covered with a very thick layer of mesoderm, as the closure of the amnion in embryo No. XI would suggest.
From the stage represented in Fig. 14 it is easy to pass to the older embryo represented in Fig. 15. Now the body of the embryo is well marked, the neural folds are just beginning, and the neurenteric canal has just been formed. The chorda dorsalis is not yet separated from the entoderm, and the blood islands encircle completely the umbilical vesicle and have nearly reached the head end of the body of the embryo preparatory to the formation of the heart.
It is not very difficult to imagine the embryonic vesicle of Fig. 8 to be converted into the vesicle of Fig. 14. To be sure, the invagination in Fig. 8 seems to be much larger than necessary, but variations of this kind are frequently encountered in the study of embryology. In the diagrammatic outline of von Spee’s embryo V. H. (Fig. I4), I have emphasized the variation of the thickness of the ectoderm lining the amnion to correspond with von Spee’s Figs. 7, 8, 9 in his recent publication.
1 Von Spee: His’s Archiv, 1896, Plate I, Figs. 4, 5, 9, and I0.
Figs. 14 and 15. Longitudinal Sections of Two Young Human Ova, after Graf Spec. Enlarged to times. Fig. 14, Embryo v. H.; Fig. 15, Embryo Gle. Just half of the chorion is drawn, and the villi are outlined only over a portion of the ovum. R, Ra.uber’s layer; a, amniotic cavity; av, umbilical vesicle; en, entoderm; mes, mesoderm; all, allantois; c, chorda; n c, neurentic canal; H, position of heart.
His longitudinal section, from which my figure is taken, does not emphasize this point, which I consider of importance in this discussion.
In these two ova described by Von Spee, the coelom is much of the same form it was in embryo No. XI, Fig. 8, and therefore needs no special comment. Yet around the head end of embryo Gle. there is a marked accumulation of mesoderm into which the heart is to grow. In the illustrations of the section of this embryo Graf Speel pictures spaces in the mesoderm which he believes to be portions of the body cavity of the embryo, that is, the cavity of the muscle plates, pericardial cavity or peritoneal cavity. It is impossible to determine definitely which portion of the body cavity these spaces represent, but I do not feel inclined to believe that what he marks pericardial cavity in Fig. 23 can possibly represent it, for we are to look for the pericardial cavity between the junction of the pharynx and umbilical vesicle and the head end of the embryo. This portion of the embryo is marked H in my Fig. 15, and falls anterior to Von Spee’s Fig. 16. Von Spee’s Fig. 16 is the 24th section of the embryo, beginning at the head, while his Fig. 23 is the 81st section.
1 Graf Spee : His’s Archiv, I889.
The various small spaces in different portions of the mesoderm cannot be viewed as the real origin of the body cavities without further discussion. In the von Spee embryo v. H. there are indications already of small spaces in the mesoderm at the border of the ectoderm of the embryo. Similar spaces are described by Bonnet[23] for the sheep and by Selenka[24] for the monkey. While Von Spee and Bonnet believe that these spaces belong to the coelom, Selenka simply designates them heart, or vascular.
The blood-vessels are intimately associated with the coelom in their early development, and it is easy to be led into error without an abundance of material. Drasch[25] recently has again emphasized this relation. He has shown in the chick that the blood islands are separated from one another by a number of closed spaces filled only with a fluid. These spaces soon flow together to form the large slit-like coelom of birds. The same condition of things has been shown to be true, but from a very different method, by Budge.[26] He injected the blastoderm of the chick, and showed that the coelom was composed of a network of spaces, which gradually flowed together into the large coelom surrounding the embryo.
Of course in the young human embryos we have at our disposal this stage of the process has long passed, but there is no reason why a remnant of it should not exist at the point of union of the umbilical vesicle with the body. The reason I question von Spee’s interpretation of these small spaces in the mesoderm in embryo Gle. is that I believe that all, or certainly nearly all, of the body cavity is formed by an incorporation of the extra-embryonic coelom within the embryo. What I have observed in human embryos as well as in the injected specimens of Budge shows that this must be true. These small spaces in the mesoderm of the body may belong to the muscle plates and the early blood-vessels, and certainly cannot play any great part in the development of the body cavity. There is no doubt whatever that the whole peritoneal cavity is simply pinched off from the coelom of the outside of the body and it is highly probable that the pericardial cavity and pleural cavities are formed in the same way. The anterior mesentery of the intestine has never existed in the human embryo, and it is therefore needless to explain its mode of disappearance.
My statements are based in great part on embryos Nos. III and XII, and since No. XII is such a perfect specimen it is well for me to describe it in greater detail. The embryo is about the same age as Kollmann’s[27] embryo Bulle, which unfortunately was never fully published. No. III is an embryo given me by Professor His. This embryo had been torn from the umbilical vesicle, and was injured in different portions of the body. Yet the head end of it is fairly well preserved, and it is of value in determining the growth of the body walls covering the heart.
Embryo 2.1 mm long. The history of embryo No. XII is as follows. “ The woman from whom the ovum was obtained is twenty-three years of age and has been married for three years. She is avery intelligent woman, and her statements are reliable. Her menstrual periods recur every thirty days. She had been married some time before she became pregnant, and after passing two periods aborted July 6, 1893. She was unwell the 5th of October and again on the 7th of November, this last period lasting five days. She passed her next period and on December 18th aborted the ovum."[28]
Fig. 3.
The ovum was hardened in strong alcohol without opening it first, and when it came into my hands its dimensions were 18X 18 X 8 mm., that is, it was slightly flattened. It was completely covered with long villi. It was carefully opened, care having been taken not to injure the embryo in any way. The coelom was filled with a clear fluid, and many firm shreds of a fibrine-like body which obscured the embryonic vesicle greatly. With much difficulty the embryo could be outlined, and these drawings proved to be of great service in making the reconstruction. The portion of the chorion to which the embryo was attached and the embryo were stained in Carmine and imbedded in paraffin. The whole was cut into sections, at right angles to thebody, 10 ,u. thick.
FIG. 16. Outline Drawing of a Sagittal Section of the Model of Embryo No. XII. Enlarged 5o times. The heavy line is the aorta. The muscle plates are numbered for occipital, cervical, and dorsal regions, respectively. The mesoderm is striated. am, amnion; a, border between fore—brain and mid-brain; x and x’, extent of closure of spinal canal; S, Seesse1’s pocket; c/z, chcnrda; 5' and 5", first and second branchial pockets; 0 22, otic vesicle; m, mouth; T, thyroid; H, pericardial space ; pk, pharynx; mt, entoderm ; S T, septum transversum; 1, liver; as c, neurenteric canal; all, allantois.
Every other section was enlarged I00 times and drawn on wax plates 2 mm. thick, and from them the model of the embryo was made. The model gives the whole central nervous system, the entoderm throughout its extent, the bloodvessels, and the muscle plates.
The shape of the neural tube is given in the diagrammatic outline. It was closed only along the middle of the body, being open in front down to the beginning of the fourth muscle plate. From the beginning of the fourth plate to the beginning of the fourteenth it was closed, and from there on again it was open. In the figure the portions between X and x’ indicate to what extent the tube is closed. In Figs. 17 and 18 the tube is nearly closed, while in Fig. 20 the tail end of the tube is just beginning to separate from the ectoderm. The cephalic end of the tube already clearly outlines the fore-brain, the mid—brain, and the hind-brain; the constriction, Fig. 16, a, indicates the junction between the first two. On the ventral side of the fore-brain there are two marked pockets, one on either side, just behind the neuropore, which are no doubt the primary optic vesicles. It shows that in the human embryo these are fully outlined before the brain has separated itself from the ectoderm. Farther behind, very near the dorsal median line and about in the middle of the head, there is a short pocket of thickened ectoderm, the otic vesicle. Towards the hinder end of the embryo the spinal cord communicates by means of a solid band of cells with the entoderm, Fig. 20. At no point in this communication is there a canal, so it must be viewed as the last remnant of the neurenteric canal. The location is opposite the twelfth muscle plate, or in the neighborhood of what will later on be the position of the first rib. The chorda dorsalis extends to the neurenteric canal, but not beyond it. There is no chorda in the tail end of the embryo.
FIGS. 17-20. Sections through Embryo No. XII. as indicated by the Lines in Fig. :6. Enlarged 50 times. The black is the coelom within the body. 0‘ and 03, first and third occipital muscle plates; C ‘ and C3, first and eighth cervical muscle plates; D1, first dorsal muscle plate; a, aorta; ‘:1, omphalomesenteric vein ; 1', thyroid; 1, liver: 151:, pharynx ; z’, intestine ; 7: c, neurenteric canal; m r, membrana reuniens.
Throughout the central nervous system, immediately about the central canal, there are many karyokinetic figures, showing that the specimen was excellently preserved. In the greater portion of the neural tube the tissue is already marked by two zones, a central one rich in nuclei, and a peripheral containing none. This corresponds with the description already made familiar to us by His.
The general shape of the whole central nervous system is very unlike that of any other young human embryo ever published. It circumscribes the greater portion of a circle, while in the other human embryos of this size it makes more of a straight line. I think that it is probable that this specimen represents the normal, as it was not injured nor handled in any way before it was cut into sections.
The entoderm, as the figures show, is already divided into fore-gut, mid-gut, and hind-gut. The fore-gut makes the pharynx, from which there are four diverticula on the dorsal side, one on the ventral side, and two near the mouth. The four on the dorsal side mark the first two branchial pockets on either side of the embryo ; the two in front are Seessel’s pocket and the entodermal portion of the mouth; while the one on the ventral side of the pharynx is the beginning of the median portion of the thyroid gland (Fig. 17, t.).
At the junction of the pharynx with the umbilical vesicle there is a large diverticulum into the septum transversum, Fig. 18 1, the beginning of the liver.
Within the tail end of the embryo, behind the neurenteric canal, the hind—gut is enlarged considerably, and from it the entodermal canal of the allantois arises.
The whole umbilical vesicle is covered with blood-vessels which communicate with the veins and arteries of the embryo. Near the origin of the liver there are two veins which collect the blood from the umbilical vesicle and then enter the heart. These are the omphalomesenteric veins. They with a number of their branches are shown in sections in Fig. 18, V. The heart itself is broken, but there is enough of it left to show that it is bent upon itself and contains a large cavity at the point where the veins entered it. From the heart two arteries arise and pass in front of the first branchial pocket, and each follows the course as shown in black in the reconstruction.
The aortae do not unite, but each sends a number of segmental branches to the umbilical vesicle along the tail end of the embryo. These are, of course, temporary ; they may be called collectively the omphalomesenteric arteries. As the permanent omphalomesenteric artery arises more aboral than any of these, it is easy to understand that most of them must degenerate.
The sections show that there are fourteen muscle plates, all of which are hollow and do not in any way communicate with the body cavity in general. Kollman, who described an embryo of this same age, numbers them from before backward, but I think that they can be designated more definitely. Froriep[29] showed that in all amniotic vertebrates there were a number of muscle plates and dorsal ganglia formed in the occipital region, and studied their fate in the chick and in the cow's embryo. Platt[30] has also followed the order of the origin of the muscle plate in the chick, and found that the first division of the mesoderm was between the third and fourth occipital plates. The first three or four of these segments communicate in the chick, according to Dexter,[31] with the coelom, and Bonnet[32] has found also that the same is true in the sheep. Bonnet’s figures (compare his Plate IV) show that a sheep’s embryo of the same stage as embryo XII has muscle plates much more sharply outlined than the human. In order to locate the muscle plates more definitely I have made every effort to count the spinal ganglia in embryo XII, but with no definite result. It is impossible for me to define the spinal ganglia, as often they are represented by a few cells only, then again as a band of cells they extend over several segments. The same is true in the occipital region. Had I been able to number them definitely it would still have been impossible to number the muscle plates from them, for His[33] has shown that there is an occipital ganglion in the human embryo as well as in the lower animals.
The fact that the muscle plates reachto the otic vesicle in embryo XII, as well as in Kol1man’s embryo Bulle, indicate that thetfirst plates must belong to the occipital region, and I have found that there are three occipital muscle plates in embryo No. II.[34] Moreover, there is every indication of a. degeneration of the first two plates in XII, so on this account I am inclined to number them as they are numbered in Fig. 16. I do not think that any of them ever communicate with the pericardial cavity as Bonnet found them in the sheep. The cavities in all of the other plates are small, and they are separated by a large mass of mesoderm from the coelom. This all confirms my view.
The chorda extends from Seessel’s pocket to the neurenteric canal.
There are also a few segmental ducts, some completely and some partly separated from the ectoderm, as was the case in Kollman’s embryo. The ducts are small, and extend over one or two sections only, and occasionally one of them is arising at several different points between a given two segments. They are present on both sides between the first and second cervical segments, second and third segments, third and fourth segments, fourth and fifth segments, and only on the left side in the region of the fifth and sixth cervical segments.
The coelom of this embryo is especially instructive. A sagittal section of the embryo and ovum is given in Fig. 21. This embryo, when drawn connected with the ovum, is very similar to Graf Spee’s embryo Gle. as shown in Fig. 15. It is very easy for us to conceive the von Spee embryo converted into this embryo, for about all the change that is necessary is that the embryo grow somewhat and bend upon itself. In so doing the attachment of the umbilical vesicle becomes smaller as the amnion encircles the body of the embryo more. The position of the neurenteric canal, the shape of the allantois, and the formation of the pericardial cavity, all show that the curving must be a normal one.
Nearly all other young embryos of this stage, or, a little older, which have been published show a straighter body or even a curve in the opposite direction. I have also in my collection two embryos of this stage, Nos. I and XV, which had been taken out of the chorion and torn from the umbilical vesicle, and both of them are straight like Kollman’s embryo Bulle and His’s[35] embryo L. It is difficult to conceive how my embryo XII could possibly be torn out of its membranes without straightening it. We need only recall our experience in hardening embryos of lower animals to be reminded how easily a curved embryo is straightened when it is handled the least bit roughly before it is hardened.
His, in his great monograph on human Cm 5' embryos, emphasizes a curve in the back of the embryo just the reverse of the one given in Fig. 21. I refer to embryos p °" Sch., BB., and Lg., as ' well as to Minot’s embryo 1952 [36]The fact that this inverted bend
FIG. 21. - Sagittal Section of the Ovum with Embryo - ~ No. XII Attached. Enlarged :0 times. Cae, In the back IS not Con coelom ; at 2!, umbilical vesicle; all, allantois; mp, Stant (Hi5’S _, for zn dullary plate; 2: 4:, neurenteric canal. , , e instance), and that it occurs at the time when any tension upon the umbilical vesicle could produce it, makes me believe that it is an artifact. This view was suggested to me a number of years ago, when I was removing young dogs’ embryos from the uterus, and unwittingly distorted a number of them in this very way before they were hardened. The middle of the back is the weakest part of the embryo’s body, and the umbilical vesicle is attached to it. Under these conditions the simple weight of the vesicle is sufficient to bend the back of the embryo as pictured by His.
To return to the coelom. At the hinder end of the embryo the coelom dips into the body overlapping the hind-gut in the neighborhood of the neurenteric canal, as shown in Fig. 20. This cavity communicates with its fellow on the opposite side through an opening between the umbilical vesicle and the allantois, marked 0 in Fig. 16. This communication has already been described by His 1 for an embryo somewhat older. If, now, the point 0 in Fig. 16 is approximated towards NC, with a flexion of the embryo at the same time, this communication is easily explained. In other words, as the hind-gut is being separated from the umbilical vesicle, a groove-like portion of the coelom is also included in the body of the embryo. At the hinder portion of the embryo, on either side, the coelomic grooves extend deeper into the body of the embryo, and communicate with each other around the aboral side of the stern of the umbilical vesicle. This communication is shown well by His in Fig. I, B, Plate VI of his Atlas, as well as in the same figure, page 299 of Minot’s Emérjyoiogy. Excellent profile views showing this point are given in all the embryos figured on Plate IX of His’s Atlas.
I emphasize this point in order to exclude the ventral mesentery for this portion of the embryo. The fact that this mesentery could never have existed in the human embryo is also proved by a careful examination of His’s models of human embryos made by Ziegler.
As we pass towards the head in embryo XII the coelomic groove communicates freely with the extra-embryonic coelom until the region of the membrana reuniens is reached. This is shown in Fig. I9, MR, with the membrana reuniens complete on one side, but not yet united on the other. The membrana reuniens extends up to the heart, and separates the pericardial cavity from the extra-embryonic coelom, then crosses the ventral median line to return on the opposite side of the embryo. Throughout the extent of the membrana reuniens there is a great increase of mesodermal tissue, which encircles completely the beginning of the liver, as Fig. 18 shows. A portion of this mesodermal tissue has been described by His as the septum transversum} According to His only that portion of the mesodermal tissue is septum transversum which lies between the posterior part of the pericardial cavity (Parietei/zc)'/tie), the wall of the intestine, and the point where the veins enter the heart. It extends across the body, and has within it the beginning of the liver. In transverse section this region is shown in Fig. 18. Now the pericardial cavity communicates by means of a long canal on either side, with the peritoneal cavity, and the omphalomesenteric vein hangs into this, attached to a kind of mesentery, as Fig. 18 shows. Lower down, near the communication (Fig. 19), there is an indication of the beginning of the umbilical vein, which unites with the omphalomesenteric vein through the membrana reuniens. The two canals which communicate with the extra-embryonic coelom are the pleural cavities, and the membrana reuniens aids to separate them from the peritoneal.
1 His: Anat. mensch. Embryonen, I, p. 126.
All of the tissues from the diaphragm to the opening of the liver duct into the duodenum arise from the septum transversum and the membrana reuniens; the stomach from the fore—gut, the liver from the liver diverticulum, and the diaphragm from the septum transversum and the membrana reuniens. The Cuvierian duct must also have arisen in the membrana reuniens, in order to pass around the outside of the body cavity to reach the cardinal and jugular veins, as pictured by His[37] for the human embryo.
In the further development of the pleural and pericardial cavities the Cuvierian veins give us our best landmark, as they define the point where the pleural cavity is to be separated from the pericardial. And it really seems, as if the greater portion of the diaphragm is formed from the portion of the septum transversum on the ventral side of the vein and from the membrana reuniens, rather than from the portion immediately in front of the intestine. In other words, there is a horseshoe-shaped ridge of tissue around the neck of the embryo to the ventral side of the pericardial and pleural cavities and parallel to them. The median portion is composed of the septum transversum, and each wing of the shoe is the membrana reuniens, one on either side of the embryo. Its general direction in this stage is parallel with the long axis of the embryo, and within each wing there is an omphalomesenteric vein. I ,
FIGS. 22—24. Three Stages to show the Development of the Blastodermic Layers at the Head End of the Embryo. Fig. 22, Hypothetical Stage. Fig. 23, Embryo No. III. Fig. 24, Embryo No. XII. V, vein; pk, pharynx; am, amnion; m )5, medullary plate; ;& 4:, pericardial cavity; 5', Seessel’s pocket; m, mouth; t, thyroid ; I and 2, first and second branchial pockets; 15,1:-leural cavity; 29: r, membrana reuniens; 2; 0 m, omphalomesenteric vein, which is expressed as a dotted line; 0, communication between right and left body cavities on the ventral side of the umbilical vesicle.
0rz;gz°7z of Pe2z'ca:rdz'al Caviar. With the pericardial cavity opening into the extra-embryonic coelom on either side as 3. basis, it is possible to trace back the pericardial cavity to its origin. Figs. 16 and 24 show that the ventral wall of the pericardial cavity is composed mostly of mesoderm. This is the portion of the membrana reuniens which is composed of mesoderm, as the sections, Figs. 18 and 19, show. An earlier stage is shown in the diagrammatic Fig. 23. It is taken from embryo No. III. In this specimen, since the ectoderm of the amnion has not reached completely around the body, as both the sagittal and transverse sections show (Figs. 23 and 25), it is evident that the pericardial space is first covered on the ventral side with mesoderm and later the ectoderm is added when the amnion begins to close over the head. In embryo III the canals communicating between the pericardial space and the extra-embryonic coelom are not as long as in embryo XII, and the ventral walls of the pericardial space are composed wholly of mesoderm. This indicates that the growth of this wall was first by a union of the mesoderm, which was followed by the ectoderm of the amnion to complete the body wall. The process is shown in Figs. 22-24. Fig. 22 is a hypothetical stage between Graf Spee’s embryo Gle. and my embryo No. III. As the process from Graf Spee’s embryo continues, the blood-vessels reach the body to form the heart, as indicated by the outlines marked v. in Fig. 22. The mesoderm of the amnion then unites with that of the unibilical vesicle, and the first pericardial space is formed. This is not wholly an imaginary stage, for it is based upon Bonnet’s observations upon the sheep as well as Cadiat’s upon the chick? In a sagittal section of a sheep’s embryo of about the same stage (Plate III, Figs. 16-20, 0 CB) Bonnet gives a similar fold, and after the pericardial walls are well F1“’5""5e°“°“*h’°i“3h‘h° Head formed he gives an illustration of a stage of Embryo No. III. Enlarged _ _ . _ _ _ 55 times. AD/1, pharynx; H,heart. in which it still communicates with the extra-embryonic coelom (Plate 1V, Fig. ure growth Of the ==“""i°“*° °°m- 17, KC). With Graf Spee’s embryo plete the ventral body wall. _ _ Gle. and with Bonnet’s observations upon the sheep as a starting-point, it is not difficult to interpret Figs. 22-24.
Exterzszmz qf t/ze Amniorz. ——After the stage of embryo XII is passed the amnion rapidly envelops the whole body and soon passes out over the cord. The next stage after No. XII which I have studied is No. XIX. I have very perfect photographs of this specimen, and the sections are all good, although the nervous system is macerated. The embryo has rotated in the amnion, throwing the cord to the right side with the left side towards the observer. It would have been impossible to obtain a view of the right side of the embryo without cutting the cord. The outlines of this embryo and ovum are given in Fig. 26. Two sections through the body are given in Figs. 27 and 28.
1 Bonnet: His’s Archiv, I889. 9 Cadiat: JO'l1l'.rClB 1’Ana.t. ct de la Physiol., 1883, Plate V, Figs. 1, 2.
The amnion has become separated from the body with the exception of the part about the cord and also that along the right side of the body, over the heart. The arrow in Fig. 25 shows how the amnion on that side is extended over the ventral body wall to make the condition shown in Fig. 28. No doubt the cause of this is the rotation of the body, throwing the cord to its right side and the amnion with it. In nearly all young embryos the cord is on the right side.1 With the exception of the four instances mentioned below, the rotation has always been so as to throw the left side of the body away from the chorion, and in all of these specimens the amnion must have swept over the from left
Fig. 26. Ovum and Embryo No. XIX. Enlarged 5 times. to right, as Shown in the Just half of the ovum is shown. A, arm; 1, leg; H, heart; at '0, umbilical vesicle; B, branchial arch. figures. I find a similar illustration by His in his great monograph?
Aésmce of a Vertral Mesentery.—After the septum transversum has been formed as it is in embryo XII, there is on its ventral side a pretty sharp groove, which indicates that the umbilical vesicle is being constricted at this point.
It is generally believed that the ventral mesentery of the intestine extends to the umbilicus, and that ultimately the round ligament of the liver represents its remnant after most of it has disappeared. This theory is expressed by two diagrams in Minot’s Emérj/ology, page 767. As the liver begins to grow, and while the heart is being pushed down in front of it, the ventral end of the septum transversum is turned down to the umbilicus. While this is taking place the stem of the umbilical vesicle becomes relatively smaller and smaller, but there is no union between the umbilical vesicle and the septum transversum as expressed in Minot’s diagram. The first stage of this process is shown in my Fig. 24, and its successive stages are shown in His’s Atlas, Plate IX. In all six embryos pictured on that plate the successive stages are represented, and in none of them is the umbilical vesicle attached to the septum transversum to form a ventral mesentery. From these embryos of His we can pass to embryo XIX, in which the umbilical vesicle communicates by a round canal with the intestine, and the tube is completely encircled with a space which extends to the liver, thus cutting off any possible ventral mesentery at that point. The same thing is shown, but in a later stage, in Fig. 30, 0, but a new process has already taken place to complicate matters.
1 The exceptions have been published by Waldeyer: Studien des physiol. Inst. zu Breslau, 1865; Janosikz Arch. f. mik. Anat., Bd. 30; His: Anat. mensch. Embryonen, Plate VIII, Figs. A 1-4; Mall: Journ. of Morph., vol. V.
9 His: Anat. mensch. Embryonen, Plate VI, Fig. 3, No. 10.
FIGS. 27 and 28. Section through Embryo XIX to show the Attachment of the Amnion to the Side of the Body. Enlarged 25 times. Am, amnion; S, stomach; H, heart; c, cardinal vein; :4, umbilical vein; a, aorta.
In embryo XII there is just a beginning of an umbilical vein in the membrana reuniens. In Kollman’s embryo the vein is more marked} The vein extends out into the sornato— pleure, far away from either the intestine or the median line. This same position is again shown in His’s embryos BB. and Lr on Plate IX in his Atlas. The left umbilical vein becomes the more prominent, and as the body wall is developed more and more it moves around towards the ventral median line. This movement takes place in common with the movement of the amnion over the body from left to right, as shown in Fig. 28. In embryo No. II, however, the liver has nearly reached the umbilicus, and the vein has almost moved around to the
FIG. 29. — Embryo No. II Attached to the Chorion. Enlarged 5 times. Just half of the Ovum is shown. 0 2:, otic vesicle ; U E, upper extremity; L 15', lower extremity; N, nose ; 1, II, III, branchial arches.
1 Kollman: His’s Archiv, 1891, Plate III, Figs. 2, 3, 4. V. umbil.
ventral median line, as shown both in the reconstruction and the sections (Figs. 30, 37-39). After the vein has moved around the body to its ventral surface, and after the liver moves away from the umbilicus up to the permanent diaphragm, it is easy to explain the formation of the round and broad ligaments of the liver as a secondary formation, but not as a remnant of a ventral mesentery. It might be called a portion of the septum transversum, as it is directly continuous with it. A ventral mesentery does exist between the abdominal walls and the liver, and only extends slightly below the liver. It is always slightly to the left of the median line, and is in direct connection with the septum transversum (Fig. 30, 0 and S T). Caelom qf Embryo No. II. -— After the body cavity is beginning to separate from the extra-embryonic coelom, the next important stage is the one after the separation is complete, as from now on the adult body cavities are formed by a simple division and expansion of the cavities already within the body. This stage is represented in em- A bryos XVIII, II, and Iv. ’ All of these embryos are nearly of the same size, the successive stages being in the order they are given. No. XVIII is somewhat distorted in the middle of the body, while No. IV is slightly macerated. No. II is a perfect specimen, and has been already described by me several years ago.‘ I shall confine my descrip- ,. ,, tion of it to the body cavity. I p .t _A —+ The external form of the embryo within the ovum is given in -29- The relation of the umbilical vesicle and amnion to the chorion, are all given. The umbilical cord is large and lies on the left side of the body, while in most embryos already published it is upon the right side. The cord is short, and midway between the embryo and its attachment to the chorion it shows a decided enlargement. The umbilical vesicle is large, measuring 5 X 7 mm., and is located between the head end of the embryo and the chorion.
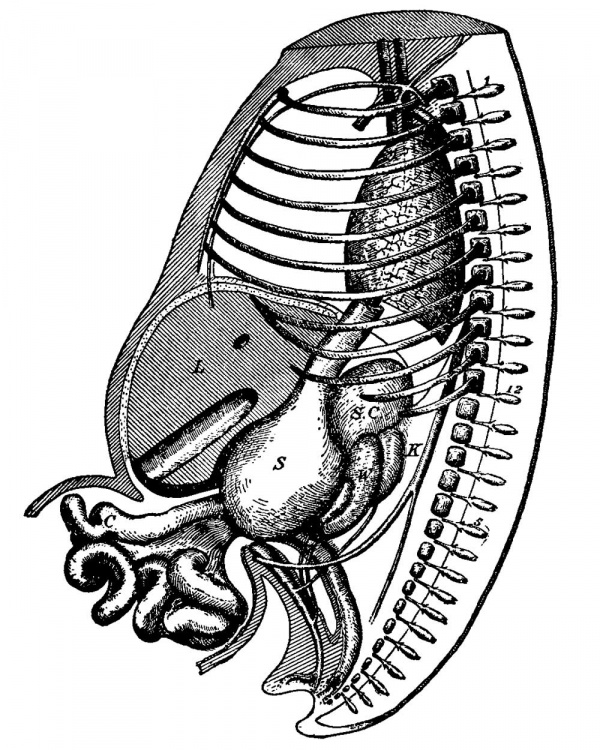
FIG. 30. Reconstruction of Embryo No. II. Enlarged 17 times. V and X, fifth and tenth cranial nerves; 1, 2, 3, and ¢, cast of the branchial pockets; 1 and 8, first and eighth cervical nerves, from the fourth the phrenic arises; 12, twelfth dorsal nerve; A, auricle; V, ventricle; L, lung ; S, stomach ; P, pancreas ; W D, Wolffian body ; K, kidney; III, mesentery; S T, septum transversum ; 0, openings which communicate with the peritoneal cavity of the opposite side. The black line around the heart marks the pericardial cavity.
FIG. 31. Cast of the Body Cavity of Embryo No. . Enlarged 22 times. A , position of the aorta ; posftlon of the umblllcal V, position of the vein ; M’, position of the mesvesicle, as well as the ex- entery; W 8, position of Wolffian body; P. tent of the amnion and the pericardial cavity; L, coelorn over liver.
The amnion has not grown very much, still leaving a great space between it and the chorion, the extra-embryonic coelom.
1 Mall: Journ. of Morph., vol. V. 4
(compare with Fig. 26). Within it hangs this large umbilical vesicle, the lumen of which no longer connects with the alimentary canal. The separation is now complete. Around the stern of the vesicle the extra-embryonic coelom communicates freely with the body cavity, as shown in Fig. 30. This figure is from a reconstruction, and shows the general extent of the body cavity within the embryo. It encircles the heart, and then extends to the lungs and over them and to the stomach, over the intestines, and out into the cord. iA cast of the whole cavity is also given, showing the slit on the dorsal side for the mesentery of the intestine, and the grooves on either side of this for the Wolffian bodies. There are also grooves in the cast for the veins, and the place where the Cuvierian duct enters the heart is marked V The sagittal section of the peritoneal cavity is given in Fig. 32. The striated lin.e indicates where the cavity crosses the median line Fm. 32.—-Outline of Coelom of the body, while the other lines outline the cavity beyond. Lp. outlines the lesser peritoneal cavity. Figs. 3 ;I,—3i3 give the pericardial space; pz, pleural extent of the peritoneal cavity in different cavity; 1 }5, Outline of lesser * ' ° per.mm1mity_ portions of the embryo, as indicated by the lines in Fig. 30.
It is not difficult how to imagine the body cavity of embryo XII converted into the one just described. In that embryo the heart is high in the neck on the oval and dorsal side of the septum transversum. In this embryo it is on the ventral and oral side of the septum transversum, but still above the eighth cervical nerve. The septum transversum has already received its nerve supply from the fourth cervical nerve, as pointed out in the early part of the century by Von Bear. This movement of the septum transversum is accompanied by a movement of all the other organs on their way into the thorax and abdomen of the future individual. In the rotation the Cuvierian duct acts much as the fixed point about which the coelom is bent.
The figures all illustrate this beautifully. But as the heart has rolled over the liver, and the septum transversum has undergone a quarter-revolution, the Cuvierian ducts and all have moved away from the head. This is by no means the end of the excursion of the septum transversum, as its dorsal end must move downtand beyond the twelfth dorsal segment (compare Fig. 30).
The pericardial cavity surrounds the whole heart, as the various figures show. The cavity is traversed only where the large veins enter, and where the aorta leaves the heart. The cavity completely surrounds the bulbus aortae to its origin (Figs. 32-35) in the ventricle. On the dorsal side of the heart the pericardial cavity is separated by a bridge for the transmission of the veins to the heart. Between the bulbus aortae and the entrance of the veins into the heart the pericardial cavity crosses the median line as three distinct openings, as expressed by the black areas in front of the trachea in Fig. 30. On the dorsal side of the heart on either side of the lungs the pericardial cavity communicates with the pleural cavities by means of two openings (Fig. 33), each of which is about .1 x .5 mm. in diameter. Farther on, the pleural cavities extend as two slits which encircle the lobes of the liver and separate them from the alimentary canal on the one hand and from the body wall on the other (Figs. 34-37). The two pleural cavities do not communicate with each other around the lungs, leaving for them both a dorsal and a ventral mesentery.
FIGS. 33 and 34. Sections through Embryo No. II at the Points indicated in Fig. 30. Enlarged 22 times. By, brachial plexus; a, aorta; 5 a, bulbus aortae; plz, pharynx; Ia, heart; I, trachea; a, oesophagus; j, jugular vein ; Z, lung; 22 c, cardinal vein; C, Cuvierian duct.
FIGS. 35 and 36. Sections through Embryo No. II. A, aorta; s, stomach; 2, liver; 14, umbilical vein; x, bulbus aortae; 1:, heart; a, omphalomesenteric vein; g’, lesser peritoneal cavity; f, foramen of Winslow; c, coeliac axis ; w 6, Wolifian body ; -w d, Wolfiian duct.
This appearance of the coelom about the lungs and the liver can be explained by the lungs and liver both growing into the two pleural cavities of embryo XII, and this has often made me think that the membrana reuniens of embryo XII is the main origin of what is called septum transversum in embryo II. If this proves to be the case, then the lower end of the membrana reuniens will form the ventral end of the diaphragm, and not the reverse. A stage between embryos XII and XVIII (Fig. 41) is required to elucidate this point.
In the neighborhood of the stomach the peritoneal cavity on either side of it has become asymmetrical, as Fig. 36 shows. The mesentery has become bent to the left side, leaving a diverticulum from the right side which extends oralwards to the tip of the lung (Figs. 34 and 35) to form the beginning of the lesser peritoneal cavity.1 Further aboralwards the cavities become symmetrical again (Figs. 37, 38), and then unite along the ventral median line, as shown in Fig. 39. The ven~ tral mesentery shown in Fig. 38 does not extend more than a section or two beyond the liver, and is separated by a marked opening from the stem of the umbilical vesicle in this embryo, as is shown in Fig. 30, 0 (see also No. XII, Fig. 16, 0). On the aboral side of the umbilical cord the peritoneal cavities of the two sides unite in both embryos again, marked 0' in both figures.
FIGS. 37-39.— Sections through Embryo No. II. A, aorta; 22 c, cardinal vein; a, omphalomesenteric vein ; p, pancreas ; 2', intestine ; 6, bile duct; 1, liver; I2, heart; :4, umbilical vein; m,mesentery; -w .5, Wolflian body ; all, allantois.
Development of Body Cavity in the Chick. The body cavity of the chick has been carefully studied by Budge,” who followed its course by means of injection. With a fine hypodermic syringe he filled the spaces forming the coelom in the order of their appearance, thus showing their extent in various embryos. The splanchnopleure, according to Budge, may be split into two layers, one dorsal or lymphatic and the other ventral or vascular. Drasch’s3 recent description of the early
1 Mall: Iourn. of Morph., vol. V.
9 Budge: His‘s Archiv, 1880 and 1887. 3 Drasch: Anatom. Anz., Bd. 9.
formation of the coelom confirms this statement. As the first blood-vessels are formed, lymph vessels appear on their dorsal side, which flow together to form a network, and accompany the primitive veins to the axial part of the germinal area. Here the lymphatics form two spaces, one on either side of the body, which soon unite across the body on the ventral side of the heart. In this way the primitive body cavity of birds appears at first as an H, the uprights of which are on either side of the body and the cross-piece on the oral side of the sinus venosus. In its further development the sinus venosus grows to the dorsal side of the cross-piece, thus reversing the relation of the vascular system to the coelom in this portion of the embryo. The uprights of the H fall to the outside of the body, and are swallowed up in the formation of the amniotic folds. According to Budge two diverticula grow from the crosspiece of the H, one on either side of the chorda, towards the tail of the embryo, to form the primitive pleuro—peritoneal cavities. Budge’s paper was
notes after his death, and I am sure that the above state ‘ \. . ~93‘ \\ \‘ ment 1S not correct. Profesm . sor HIS has placed at my
FIG. 40. Section of a Chick to show that the Body Cavity communicates with the extra-embryonic spaces.
Speclmens Coelom. Although the embryo has been injected, Whlch I thlnk Show conclu the injection massesaandc are not continuous. Sively that interpretation of this subject is not correct. Most of the injections were made into the amniotic fold, which is very large in birds. Cross-sections of chicks at this stage show that the large extra-embryonic coelom communicates very freely with the body cavity, and the cross—piece also communicates freely with the cavity at the anterior end of the embryo. This has already been described and pictured by Cadiat,1 and recently again by Duval.2 Around the heart, however, the communication is freest between the extra-embryonic coelom and the body cavity, and it is natural published from fragmentary , that the fluid should find its way along this channel first, and then extend into the body cavity from this point, giving pictures which in transverse section are like Fig. 40. Surface views could not decide that these cavities communicate freely; and these sections which I have studied were no doubt made after Budge had written the rough draft of his manuscript, as they are not referred to in his paper.
1 Cadiat: Jour. de 1’Anat. et de la Physiol., 1883, Plate V, Figs. I and 2. 2 Duval: Atlas d’Embryo1ogie, Plate XXII, Fig. 3 54.
Although the body cavity of the bird is formed after the same manner as it is in the human embryo, there is one marked difference in the formation of the pericardial space. In the bird the mesoderm does not extend throughout the head fold of the blastoderm, leaving a portion of the ectoderm in direct contact with the entoderm. Later the mesoderm grows into this region, and at the same time the pericardial cavity extends to the outside of the body. This condition continues for a long time, allowing the pericardial cavity to communicate with the false amnion after the embryo is well formed. Duval’s excellent Atlas shows how the pericardial cavity first communicates with the exterior of the body, and after the body walls have united the heart still lies in apposition with the liver, as there is no septum transversum. The only trace of a septum transversum that I can find in young chicks is at the point the Cuvierian ducts enter the heart. Here a bridge of tissue passes transversely to the body. The liver does not grow into it, but accompanies the single omphalomesenteric vein before it enters the heart.‘
Development Q)’ the Diapkmgm. — Our knowledge of the development of the diaphragm is based upon the researches of von Baer,” Cadiat,3 His,4 Uskow,5 and Ravn.5 Each contributed his portion : Von Baer that the diaphragm is at first located high in the neck and must descend in its development, and, because of its high position at first, is innervated by a cervical nerve; Cadiat and His recognized the mass of tissue in the embryo which is destined to give rise to the diaphragm ; Uskow and Ravn studied more carefully the separation of the body cavities from one another; and the wandering of the organs was emphasized by Uskow.
1 Since the above has been written this subject has been studied thoroughly by Ravn, whose paper will be found in His’s Archiv, I896.
3 Von Baer: Entwicklungsgeschichte, 1837.
3 Cadiat: Jour. de l’Anat. et de la Physiol., I878.
4 His: Anat. mensch. Embryonen, Th. I, 1880.
5 Uskow: Arch. 1. mik. Anat., 1883.
5 Ravn: His’s Archiv, I889.
It is now no great task for me to give the development of the diaphragm in the human embryo, for I have at my disposal excellent sections, as well as definite knowledge of the anatomy of the surrounding organs contributed by the abovementioned authors.
While the embryo is still straight it is very easy to locate the various organs and their relations to one another, but through their shifting and the flexion and extension of the embryo the relations are constantly changing, and one must not rely too much upon sections, or else erroneous impressions will often be obtained. At first the heart is upon the oral and dorsal side of the septum transversum, then on its ventral side, and finally again on its dorsal side. At first the lungs are on the dorsal side of the heart, then on the lateral side, and finally also on the ventral side of it. At first the liver is on the aboral side of the septum transversum in the head of the embryo, then on the dorsal side of it in the cervical region of the embryo, then as the liver is descending in its excursion it is transferred to the ventral side of the septum and extends into the sacral region. At first the Wolffian body extends high into the thoracic region of the embryo, but while it is degenerating and the diaphragm descends, the upper part of the posterior cardinal vein remains, while the lower part is incorporated with its vena cava inferior, as shown by Hochstetter} As the Cuvierian ducts and cardinal vein descend into the thorax, the segmental veins entering the cardinal veins are gradually shifted, so that veins which originally emptied into the posterior cardinal now empty into the anterior cardinal. While the whole process is taking place the arteries arising from the descending aorta also shift, as I have shown in a previous communication? At that time my collection of human embryos was very limited, and it was necessary to include some observations on lower animals to prove my point, but now I can give a complete table of human embryos in which the point of origin of the coeliac axis is recorded.
1 Hochstetter: Morph. ]ahr., Bd. 20, p. 563. 2 Mall: Journ. of Morph., vol. V, p. 472.
Table Showing Point of Origin of Coeliac Axis
Emsnvo. MI$:f:EH.r;,:s' ORIGIN or COELIAC AXIS. No. XII. 2.1 Opposite 4th cervical nerve} His’s Embryo M 2.6 r “ Ist dorsal “ 9 H as B it znd it u 8 No. II. 7. “ 4th “ “ His’s Embryo A 7. 5 “ 6th “ “ 4 No. XLIII. I3. “ Ioth “ “ No. IX. 14. “ nth “ No. XXII. I8. “ 11th “ No. XVII. 16. « Izth « « No. LVII. 20. Below Izth “ “ Adult. “ I 2th “ “
1 In the first two embryos the omphalomesenteric artery is noted, and not the coeliac axis.
9 Compare Fig. 15, Plate VI, His’s Atlas, with M4, Pl. VII. 3 Compare Fig. 35, Plate II, His’s Atlas, with Fig. 1, Plate 1. 4 Compare Figs. 79 and 86, His’s Atlas, with. Fig. 4, Plate I.
The table shows that the arteries arising from the ventral side of the aorta to supply the stomach and intestines are constantly shifting until their definite origin is finally reached. In these specimens the omphalomesenteric artery is shifted ahead of the coeliac axis. In embryo No. II the omphalomesenteric artery has a double origin from the aorta, which indicates that this movement may be brought about by a new anastomosis forming, which is then followed by an occlusion of the old origin. At any rate it is impossible that the whole aorta shifts with the abdominal viscera, for it is bound to the vertebrae and muscle plates through the segmental arteries.
The various sections and the reconstruction of embryo No. II show the pleural and pericardial cavities still communicating freely. The same is true in embryos XIX, XVIII, and IV.
Immediately after this stage there are no embryos in my collection, so I have no specimen in which the communications between the pleural and pericardial cavities are just closing.
FIG. 41. Diagram to show the Position of the Diaphragm. The numbers on the blocks indicate the embryos from which the diapluagms are taken. K0 is His’s embryo K 0; PP, the out line of the opening between the pleural and peritoneal cavity, which is finally closed when the diaphragm reaches the tenth dorsal segment.
In embryos VIII, V, IX, and XLIII (Figs. 41-43), the pleural and pericardial cavities are separated, while the pleural and peritoneal still communicate. In the embryos with a vertexbreech measurement exceeding 17 mm. the pleural and peritoneal have been separated completely.
The separation of the pleural from the pericardial cavity is dependent upon the complete development of the diaphragm. At first the septum transversum and the membrana reuniens are on the ventral side of the pleural cavity, and both are still located within the head. As the septum transversum descends into the body it is next located on the dorsal side of the heart. In other words, the dorsal end of the septum transversum has not moved as rapidly as the ventral end, and thus the whole mass of tissue has turned a quarter revolution. This is accompanied by the extreme flexion of the head, as represented in embryo No. II. At this time the septum transversum has descended to the lower part of the cervical region. Now the septum begins to turn in the other direction again, for with the development of the neck the ventral end of the septum becomes the fixed point and the dorsal end moves more rapidly. The successive stages in the movement of the septum are best shown in the diagrammatic Fig. 41.
FIG. 42. Reconstruction of Embryo No. IX. Enlarged :7 times. .5‘ T, septum transversum ; L, liver; S, stomach; C‘, caecum; W, Wolffian body; K’, kidney; 1-12, dorsal ganglia; o, ornphalomesenteric artery. The ventral mesentery of the liver is dotted, as it is only a, thin membrane; .5‘ C, suprarenal capsule; X, point of communication between pleural and peritoneal cavities.
FIG. 43. Section through the Point of Communication between the Pleural and Peritoneal Cavities in Embryo No. IX. Enlarged 15 times. 7, seventh rib; L, lung; L2‘, liver; M’, ventral mesen-tery of liver; a, aorta. The diaphragm is complete on one side, X, while it is incomplete on the other.
Fig. 42 shows the septum transversum on the ventral side of the stomach and the pleural cavity communicating with the peritoneal at the point X. The Wolffian body and the suprarenal capsule, which is very large, have receded markedly, and the pleural cavity already forms a pocket on the dorsal side of them. A sagittal section through this region, somewhat distant from the median line, is given in Fig. 44. A transverse section of the embryo pictured in Fig. 42 is given in Fig. 43. This section is just at the point above the opening, and shows the communication between its pleural and peritoneal cavities closed on one side, but open on the other. There is a ridge on the side of the cavity which projects between the lung and the liver and continues down to the suprarenal capsule. This ridge has been well described by Ravn,1 who gives an excellent illustration of the opening with the ridge encircling it.
FIG. 44.Section through Embryo No. XLIII. Enlarged 8 times. H, hand; A, auricle; V, ventricle; L, lung; 5‘ T, septum transversum; P, phrenic nerve; U, umbilical vein; .5‘, stomach; W, Wolflian body; 012, ovary ; A m, amnion; 1-12, ribs.
FIG. 45. - Reconstruction of Embryo No. X. Enlarged 3 times. I-I2, dorsal ganglia; .5‘ C’, suprarenal capsule; W, Wolffian body; K, kidney; 1., liver; S, stomach; C, caecum. The dotted area on the ventral side of the liver indicates the extent of the ventral mesentery of the liver.
In all the embryos in which the pleural and peritoneal cavities still communicate, the vena cava does not yet communicate with the posterior cardinal vein.
Fig. 45 is from an embryo slightly larger than the one from which Fig. 42 was taken. The pleuro.-peritoneal communication has just closed by the walls of the ridge having grown together; the extent and shape of the pleural cavity is much as it is in Fig. 42. The Wolffian body is smaller, and the kidney and suprarenal capsule have come together.
1 Ravn: His’s Archiv, 1889, Plate X, Fig. 16.
The story, then, is brief: as the diaphragm descends, its dorsal end is in apposition with the suprarenal capsule, and finally, when the capsule approaches the twelfth rib, a ridge of tissue which also includes the capsule unites with a ridge from the septum transversum, and the opening is closed. These two ridges, however, are portions of one and the same ridge, as they form a circle and in section appear as two ridges. The circle is closed much after the fashion of tying up a bag.
All of the abdominal organs, with the exception of the kidney, descend; and the descent is not completed until the pelvis is formed to admit some of them. In the stages pictured nearly all the small intestine lies in the umbilical cord, as is the case in many mammalian embryos. In embryo X (Fig. 45) a large portion of the liver also projects into the cord. I have also observed a hernia of the liver in another embryo somewhat larger. I do not consider the form of embryo X altogether normal, but this was not noticed until the reconstruction was complete.
Closely associated with the closing of the pleuro-peritoneal opening is the development of the coeliac ganglion. In these young embryos it is extremely large, and can be outlined already, while the septum transversum is still high in the thorax. As the septum descends, the various communicating branches of the nerves are caught up with the coeliac ganglion and dragged along. This accounts for the high origin of the splanchnic nerve.
Fig. 46 (embryo VI) shows that all the tissues are becoming more definitely outlined, and the whole structure is firmer than in embryo ‘X. The organs of the abdomen are more firmly clustered together, and the intestine has become more convoluted. The lung is much larger, and the pleural cavity extends to the ventral wall of the embryo, obscuring wholly the outline of the heart. In general it confirms everything given in Fig. 45.
Minot[38] has stated that the pleural cavities are to be con sidered a portion of the septum transversum, because they lie on the dorsal side of it. From what has already been said above it will be seen that I consider the septum transversum the mass of tissue between the pericardial cavity, the pleural cavities, and the opening between the two sides of the peritoneal cavity immediately below the liver, marked 0 in Figs. 16 and 30. This tissue includes the membrana reuniens, which is really the wings of the septum transversum as described by His. In my account I have employed the term membrana reuniens whereever possible to avoid confusion, and have usually employed the terms septum and primitive diaphragm as synonyms.
Fig. 46. Reconstruction of Embryo No. VI. Enlarged 8 times. 1-12, dorsal ganglia; S C, suprarenal capsule ; K, kidney ; W, Wolfian body ; S, stomach; C, caecum; L, liver. The dotted area on the ventral side of the liver indicates the extent of the ventral rnesentery.
There are developed within the region of the septum transversum the whole liver, including its ventral mesentery, the lesser peritoneal cavity, the stomach, and the suprarenal capsule. This same region which I have marked out by these three boundaries as the septum transversum is still sharply defined in the adult. The point 0 in Figs. 16 and 30 is still as definably marked as ever by the round ligament, foramen of Winslow, and the duct passing from the liver to the duodenum. The round ligament is developed by the umbilical vein shifting around the side of the abdominal walls into the ventral mesentery of the liver, and then when the liver is retracted from the umbilical cord, the vein and mesentery remain as the round and broad ligaments respectively.
Lesser Peritoneal Cavity
I have already discussed the lesser peritoneal cavity in a separate paper} and find that I can confirm all that I have stated at that time. I can only add that the portion of it extending up under the lung degenerates, while the omental sac is growing rapidly. I have also found that it is extremely easy for the omentum to find its way over the large intestine. At the time this takes place the large intestine is in the median line, while the stomach and the omentum are on the left side of the body. After the intestine is retracted from the cord the caecum falls over to the right side of the body, while the descendingcolon is shifted to the left side, and the omentum then comes to lie on the ventral side of the transverse colon.
E:rpcmsz'o7z of the Body Cczw'z[y and 0blz'2!emz'z'o7z of the extra-embryonic Coelom. —After the pleural and pericardial cavities are separated from each other it is very easy to follow their further development. In embryo II, Fig. 47, the heart is still upright, and a transverse section of it is also transverse to the lung. The pleural cavity lies wholly on the dorsal side of the pericardial, Fig. 32. In the next stage, as the lungs descend more and more, the heart is tilted over so that its base is towards the lung and its apex away from it, as in embryo IX, shown in Figs. 42 and 48. The pericardial space has now become separated completely from the pleural, although both have grown at about the same pace. From now on the pleural space grows more rapidly than the pericardial, as shown in Fig. 49. I have a number of embryos which represent intermediate stages between embryos IX and XXII, and all of them confirm the idea that the pleural space develops first and then is followed by a growth of the lung. Fig. 50, which is a section of embryo No. XLV, shows a marked increase in the size of the lung, but the heart and pericardial space are of about the same size as in embryo XXII. A much later stage is shown in Fig. 51. The scale of enlargement is only half that of Fig. 50, and when this is considered it is again seen that the heart has not grown very much but the lung has developed enormously.
1 Mall: Journ. of Morph., vol. V. 4 5o MALL. [VoL. XII.
FIG. 47-49. Outlines of the Pleural and Pericardial Cavities to show their Relative Position and Size. Enlarged 7 times. Fig. 47, Embryo No. II; Fig. 48, Embryo No. IX; Fig. 49, Embryo No. XXII. H, position of heart; L, position of lung.‘ '
It is therefore seen that at first the pericardial cavity is on the oral side of the pleural, then on the ventral side, and is finally enclosed by the pleural cavity growing over it.
The growth of the pleural cavity over the pericardial accounts for the location of the phrenic nerve in the adult. ‘In Fig. 47 the nerve passes to the septum transversum from the lateral body wall and it is gradually separated from it by the descent of the septum and by the growth of the pleural cavity between the nerve and the body wall, thus locating the nerve in a membrane, as Figs. 48 and 49 will readily explain.
The expansion of the peritoneal cavity is by no means as simple. In it there are many bands and mesenteries, as , suprarenal capsule fill the dorsal side
it must take place very rapidly, for well as a marked shifting of the organs. With the descent of the testis a portion of it is cut off to form the tunica vaginalis.
In embryo II the peritoneal cavity is extremely simple, as the figures show, -—a simple cavity on each side, communicating the one with the other by means of two openings, one above and one below the omphalomesenteric duct. Later, as the diaphragm descends more and more, the liver rotates, and its lobes soon fill the peritoneal cavity, while the intestine develops out into the cord. The Wolflian body, sexual glands, and
of the cavity and the rudimentary pelvis. The whole development of the intestine takes place within the cord, and finally it is drawn into the _ _ embryos when it is about 30 mm. in "":..5.:’.;.“.:?.‘:‘.‘it.‘;°..‘."EfI$::‘.’:‘§:f‘xP:$t length. By what process this takes E“‘a’3'=d7*i“1e5 place I am unable to determine, but
I have never seen a human embryo in which it is only partly retracted. In the pig’s embryo, however, I have found the stages in which the intestine is in process of retraction. The liver now nearly the FIG. 51.——Outline of Pleural and Peri. cardial Cavities in Embryo No.
whole cavity, and extends down to Xxxm E,,1,,,g,d3%,,,,,,,,_ H,
the pelvis’ and in embryo puofiétion of heart; L, Position of
projects over the ovary and is in contact with the rectum. As the intestines are retracted from the cord the liver is relatively higher and higher, for the expansion of the abdominal walls is now greater below the umbilical cord than before, giving more space in this region for the intestine which displaces the liver. In embryos XXXIV and XLVIII the intestines have been studied, and it was found that they were still located in the ventral portion of the peritoneal cavity, as there is no pelvic cavity large enough to hold any portion of them. This question will be discussed in the next paper.
Fig. 52. Embryo No. XXII (22)within the Ovum. Enlarged 3 diameters. The villi of chorion are outlined on one side of the ovum only. The umbilical vesicle, uv, has become shifted around to the dorsal and right side of the embryo. The outline is made from a photograph, and is correct in detail with the exception of the attachment of the chord to the chorion. This in reality attaches itself to the chorion immediately to the right of the embryo.
The extra-embryonic coelom has only a short existence, as it is already completely obliterated in embryo No. XXII. This embryo came to me in an unopened ovum, and on this account is extremely valuable for this purpose. This embryo is about six weeks old, so, reasoning from it, the union of the amnion and chorion takes place earlier than is generally believed. In embryo No. XLIII, which is about five Weeks old, the amnion has expanded over the whole embryo and has nearly reached the chorion. The earlier stages are given in the sagittal sections. They show that the amnion is nearest the chorion at the caudal end of the embryo in the earliest stages, and soon the two unite at this point. As the embryo grows, the union of amnion and chorion extends. At the end of the fourth week the extra-embryonic coelom is still very large ; at the end of the fifth week the space between the embryo and chorion is divided equally between the amnion cavity and the coelom ; at the end of six weeks the extra-embryonic coelom has disappeared.
BALTIMORE, May 5, I896.
Footnotes
- ↑ Reichert: Abhandl. (1. kg]. Akad. d. Wiss., Berlin, 1373.
- ↑ Von Spee: His’s Archiv, 1889; Mall: Anatorn. Anzeiger, 1893; Johns Hopkins Hospital Bulletin, 1893 ; and von Spee : His’s Archiv, 1896.
- ↑ Bischoff: Entwicklungsgeschichte des Hundes Eies, Braunschweig, 1845.
- ↑ Bischoff: Entwicklungsgeschichte des Kaninchen Eies, Braunschweig, 1842.
- ↑ Selenka: Studien iiber Entwicklungsgeschichte des Thiere, Heft 5, Wiesbaden, 1892.
- ↑ Embryologists usually recommend that human embryos should be hardened by placing them in dilute alcohol and then gradually increasing the strength of the alcohol. It has been my experience that by this treatment the specimen is injured by maceration due to the weak alcohol. A few years ago I emphasized the fact that the whole ovum should be placed in a large quantity of strong alcohol as soon as possible. It should be handled as little as possible before hardening it, thus preventing mechanical injury. By leaving the ovum closed the alcohol must first penetrate the chorionic and amniotic fluids before it reaches the embryo, and thus, nearly all of the embryos were drawn or photographed to scale and then carefully cut into sections from ten ,4». to fifty is thick. I find that for purposes of reconstruction it is a mistake to cut the sections very thin. Yet in small specimens, as Nos. XI and XII, the specimens were cut thin to permit of careful without placing the embryo first in weak alcohol, it naturally passes through the successive dilutions of alcohol before it is completely hardened.
- ↑ Mall: Anatom. Anz.
- ↑ Mall: Journ. of Morph., vol. V.
- ↑ Hoen: Johns Hopkins Hospital Bulletin, I896.
- ↑ Bischoff : Entwickl. d. Meerschweinschens, Giessen, 1852.
- ↑ Rauber : Sitzungsber. cl. Naturforcher Gesellsch., Leipzig, 187 5.
- ↑ Selenka: Studien iiber Entwickl. cl. Thiere, Heft 3, 1884.
- ↑ Selenka: Studien, eta, Heft 5, Erste u. Zweite Halfte, 1891, 1892.
- ↑ His’s Archiv, 1889 and 1396.
- ↑ Graf Spee : His’s Archiv, I896, Taf. I, Fig. 1.
- ↑ His: Anat. mensch. Embryonen, Theil I, p. 171.
- ↑ Selenka: Studien, 1891, p. 201.
- ↑ Duval: Jour. de l’Anatomie et de la Physiologic, I895
- ↑ His: Anatomie mensch. Embryonen, Heft 2, 1882, and Internationale Beitriige zur wissenschaftlichen Medecin, Bd._ I, 1891.
- ↑ Giacomini: Ergebnisse der Anatomie und Entwicklungsgeschichte, Bd. 4, 1895. The original papers of Giacomini are in the Archives Italiennes de Biologie, vols. XVIII-XXII.
- ↑ Giacomini: Ergebnisse d. Anat. u. Entwicklungsgesch., Bd. 4, S. 636.
- ↑ Letter from Dr. Kittredge, April 27, 1893.
- ↑ Bonnet: His’s Arehiv, I889.
- ↑ Selenka: Studien, etc., Taf. XXXVIII, Fig. 35.
- ↑ Drasch: Anatom. Anz.. Bd. 9.
- ↑ Budge: His’s Archiv, 1887.
- ↑ Kollman: His’s Archiv, Supplement Bd., 1889, Plate V, Figs. I and 2; von Lenhossék: His’s_Archiv, 1891, Plate I; Kollman: His’s Archiv, 1891, Plate III,
- ↑ Letter from Dr. Ellis, Jan. 7, 1894.
- ↑ Froriep: I-Iis’s Archiv, 1883 and 1886.
- ↑ Platt: Bulletin of the Museum of Comparative Zoology, vol. XVII.
- ↑ Dexter: Anatom. Anz., 1890.
- ↑ Bonnet: His’s Archiv, I889.
- ↑ His: Abhandl. d. siich. Gesellsch. d. Wiss., Bd. XXIV.
- ↑ See also Mall: Journ. of Morph., vol. V.
- ↑ His: Anat. mensch. Embryonen, Plate VI.
- ↑ Minot: Human Embryology, New York, Fig. 169.
- ↑ His: His’s Archiv, 1881, Plate XII, Fig. 9. Also Anat. mensch. Embryonen, Plate IX, Figs. 10-12, 14.
- ↑ Minot : Human Embryology, p. 483.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Development of the human coelom (1897). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Development_of_the_human_coelom_(1897)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G