Paper - Cyclic changes in the ovaries and uterus of swine and their relations to the mechanism of implantation (1921)
| Embryology - 1 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Corner GW. Cyclic changes in the ovaries and uterus of swine, and their relations to the mechanism of implantation. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ. 394, :117-146.
| Online Editor | ||||
|---|---|---|---|---|
| This 1921 paper by George Corner describes pig Template:Estrous cycle and implantation. The pig is a polyestrous animal that has estrous cycles throughout the year like: cattle, pigs, mice, and rats.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cyclic Changes in the Ovaries and Uterus of Swine, and their Relations to the Mechanism of Implantation
By George W. Corner (1921)
(4 plates, 2 text-figures) 117-146
From the Anatomical Laboratory of the Johns Hopkins Medical School.
General Introduction
The undertaking set forth in these pages is simple in plan, namely, to follow in one mammal (the sow) the anatomical changes in uterus and ovary which underlie the reproductive cycle. The prospective value of such a study may deserve a word of explanation; it arises from the fact that in different organisms there are varying forms of expression of the cyclic sexual processes. Even within the order of mammals, the reproductive cycle of the female is obscured by as yet unresolved dissimilarities and no satisfactory correlation has been made between phenomena unlike as the urgent rutting of deer, the inconspicuous oestrus of rodents, and the menstrual cycle of the catarrhine apes and man. In our own species we have only conflicting conjectures as to the time of ovulation and the meaning of menstruation, although exact knowledge of this subject would be of value not only for its own sake, but also for the calculations of the embryologist and the gynecologist. The need, therefore, is for a detailed study of individual species, including the determination of the oestrous period and the time relations of ovulation, growth, and retrogression of the corpus luteum, tissue changes in the uterus, and the progress of the ova, whether fertilised or unfertilized.
Some such account has been pieced together for five species, though not with equal completeness: a marsupial, Dasyurus viverrinus (Hlil and O'Donoghue, 1914); the rabbit (Niskoubina, 1909; Ancel and Bouin, 1910; etc.); the guinea-pig (Loeb, 1911, 1914; Stockard and Papanicolaou, 1917; Ishii, 1920); the rat (Long and Evans, 1920-1921)^; and the dog (Marshall and Jolly, 1906; Marshall and Hainan, 1917; Keller, 1909). Something is known of the cycle of ovulation but not of the uterine changes in several other mammals, while in man we know much about the histological cycle of the uterus and almost nothing of the ovarian sequence.
Choice of the domestic pig as subject for a similar study was determined by a combination of circumstances which seemed to outweigh the disadvantages of large size and commercial restrictions upon the collection of material. In this animal oestrus is regularly periodic, frequent, and outspoken; ovulation is spontaneous; study of the ova and embryos is facilitated by the large litters; the ovaries of the ungulates are notably uncomplicated, as contrasted, for instance, with those of the rodents; and the uterine mucosa is of the non-deciduate type with a simple, diffuse placenta. In short, the problem is here reduced to its lowest terms, and the solution promises, therefore, to be the more useful as a contribution to the general theory of the reproductive cycle.
I am indebted to Professors Long and Evans for the opportunity to study in manuscript their forthcoming definitive account of the oestrous cycle of the rat, based on their preliminary studiea referred to in the appended bibliography.
External Manifestations of the Reproductive Cycle in Swine
Investigations in the physiological anatomy of the reproductive system must depend upon material collected at accurately determined periods of the ovarianuterine cycle. For this reason it will be necessary to review briefly the well-known facts of the sexual manifestations of swine. Sexual maturity is attained before the age of one year, sometimes as early as 4 months, often before the uterus has attained its full adult dimensions. Maturity is characterized by the recurrence of periods of sexual activity ("heat," or oestrus) at intervals of 2 to 4 weeks, the usual interval being 21 days. An exact study of the duration of the oestrous cycle has been made by Struve (1911), whose results agree closely with the figures just given; his computations give a mean interval of 20.66 ±0.205 days, with a standard deviation of 2.36. The curve of frequency distribution of observed cases shows the shortest interval to be 15 days, the longest 30 days, but 75 per cent of the animals fall within the limits of 18 to 23 days. The duration of oestrus is commonly 3 days, during which time the rutting sow exhibits excitement in the presence of the male, with ready acceptance of coitus. If no male is present the sow will follow the other females about the pen, sniffing at their genitals and frequently going through an imitation of the sexual act by "riding" upon the others; or she may at times be the recipient rather than the donor of these attentions. In a large herd without boars the females in heat will often be found in a separate group apart from the others, where for hours at a time the exhibition of the oestral urge continues, interrupted only by siesta or feeding time, until at the end of 3 days, or occasionally longer, it subsides, to be followed by an interval during which sexual activities are in abeyance.
At the height of oestrus the vulva is often slightly everted, swollen, and reddened, and there is sometimes a slight serous or mucous vaginal discharge. Occasionally the discharged fluid is flecked or stained with blood, but from internal examination of the reproductive tract it is apparent that in these cases the bleeding is of external origin, caused no doubt by trauma to the vulva; in this detail, as in all others of the description just given, the external manifestations of the sexual cycle are in complete contrast to those displayed by the human and other primate races.
The 21-day cycle appears to continue regularly throughout the year unless interrupted by pregnancy. In pregnant animals received at the packing-houses, fetuses are found in all stages of development without much regard to the time of year. Pregnancy can begin only at an oestral period, since then alone is copulation permitted. The span of gestation is 16 to 17 weeks, usually 116 to 120 days. As with other mammals, the oestral periods do not occur during pregnancy, but according to Struve (1911), oestrus recurs about 4 to 9 days after parturition.
The number of young in a litter varies from 1 to 23, the mean being from about 7 to 9 in different American breeds, according to Surface (1909), who has made a biometric analysis of the data upon this subject.
Collection of the Material
The first step in correlating the organic changes with the outwardly visible functional events which have been recounted is, necessarily, to determine at what point of the cycle ovulation takes place. The results of this part of the investigation, and of a study of the origin of the corpus luteum and its alterations during pregnancy, have already been described in previous publications by the writer (1915, 1917a, 1917b, 1919) and need only be summarized here.
It was found, as had been expected by analogy with previously known species, that the period of oestrus is the time of ovulation. In the stockyards the condition of heat was observed in about 30 animals, which were suitably marked and then were followed through the processes of the abattoir until the genitalia were obtained from the butcher's hands. In such of these animals as were killed during the three days following the onset of oestrus the ovaries contained either mature or recently collapsed Graafian follicles. This much had in fact already been proved by Lewds (1911) in a publication at the time unknown to the writer, but we were able to strengthen the evidence by actual recovery of the ova from the Fallopian tubes. By the use of simple technical methods, which will be described later, a series of segmentation stages and also of unfertilized ova was obtained for study and correlation with the corpora lutea. By good fortune the packinghouse where these first steps were taken was not at the time under great stress of. production ; a few of the swine were retained in the corrals as long as 11 days after the onset of oestrus, and it was therefore possible to follow the development of the corpus luteum up to about the tenth day. It was not until the adoption of a rapid method of locating ova and blastodermic vesicles in the large uterine chambers of the sow that the fate of the ova after their exit from the Fallopian tubes could be continuously followed; but the writer already had in hand the ovaries of sows in all stages of pregnancy from the third week until parturition, to the number of about 140. lipon the sum of this material two previous contributions were based (1915, 1919), covering, therefore, the corpus luteum of pregnancy from the day of ovulation until after parturition and the corpus luteum of unfertilised ovulation until the tenth day of its development.
It was, of course, impossible at the stockyards to follow animals into the latter half of the cycle. Such material, however, was made available by the generous interest of Mr. Walter N. Cooper, manager of the American Feeding Company, of Baltimore, who extended every facility for the undertaking. At the establishment of the company, about 20 miles from Baltimore, large numbers of pigs were kept from an early age until they attained a profitable weight by the consumption of table refuse and other edible garbage collected in the city. The \\Titer made a series of trips to the piggery farm on alternate days throughout a period of 3 weeks, and at each visit selected, with the aid of Mr. Cooper and his assistants, 3 sows which were evidently in active heat. These were marked by ear-tags and isolated from the herds in a special pen. The date of cessation of oestrus was noted by report of the piggery laborers and by personal observation on the days of visit.
At the end of 3 weeks 22 animals had been thus isolated, forming a series of all stages of the cycle. These were then purchased by the University and resold to a packer near the laboratory, by whom all of them were killed on the same day, and the internal genitalia were thus recovered for study. The sows were all in good condition, readily passing government inspection. They were of various breeds and crosses, but were nearly uniform in age (about 10 to 12 months) averaging over 180 pounds in weight, indicative of fairly mature state.
These 22 animals have served as a basis for all statements in the following pages as to the time relations of the reproductive cycle, but, owing to the variability of the interoestral period, it chanced that none of them was killed within the first 3 or 4 days following ovulation. This gap in the series of uteri might have been filled by specimens from the first collection of animals mentioned above, but unfortunately all the uterine preparations of those sows had been put permanently beyond the reach of study by the departure for the war of a pupil to whom they had been intrusted. For this reason a further series of 30 specimens was collected from the slaughter-house. These were not obtained from animals that had been observed during life, but were at least approximately dated by the fact that early corpora lutea were present in all and that the ova or early embryos were recovered from each specimen. By the microscopic structure of the corpora, as worked out from the previous specimens, and by the condition of the ova, it was possible to rank them in a series and to discover from them the earlier uterine changes following ovulation.
It is obvious that there is a limit to the chronological accuracy of data obtained in the various ways described above. Students of the human ovary and uterus may perhaps think enviously of the opportunity afforded in the packing-house and stockyard to collect fresh organs, datable more or less closely, from young, healthy, mature animals to the number of twelve score and to see similar but undated specimens totaling almost 12,000; on the other hand, no such exactness is possible with the large animals of commerce as with animals like the rat, where the method of Stockard, as applied by Long and Evans, permits prediction of the time of ovulation within one hour. In this series of swine it has not been possible, in most cases, to observe oestrus from start to finish, nor do we yet know more than approximately the relation of the moment of ovulation to the usual 3 days of oestrus. Moreover, we are dealing with variable quantities in the duration of the interoestral period and the rate of development of the fertilized ova, both of which factors have been used in establishing correlations. However, the reader will probably not be led astray if we estimate that all references to a given day, assuming a 21-day cycle as a working basis, imply a possible error of =*= 1 day, perhaps even of =1= 2 days as we approach the latter days of the interoestral period.
Methods of Preparation
Since recovery of the ova and early embryos by means of serial section of the tubes and uteri is out of the question in so large an animal as the sow, a procedure was devised which grew out of a suggestion by Professor Evans based upon the practice of Martin Barrj^ (1839), one of the earlier workers on the problems of ovulation, who obtained the tubal ova of rabbits by stroking the tubes with a rod in order to express their contents into a dish. Our improvement, which we have since found had already been used by Sobotta (1897) and others, consists simply in washing out the Fallopian tubes with a stream of isotonic salt solution.
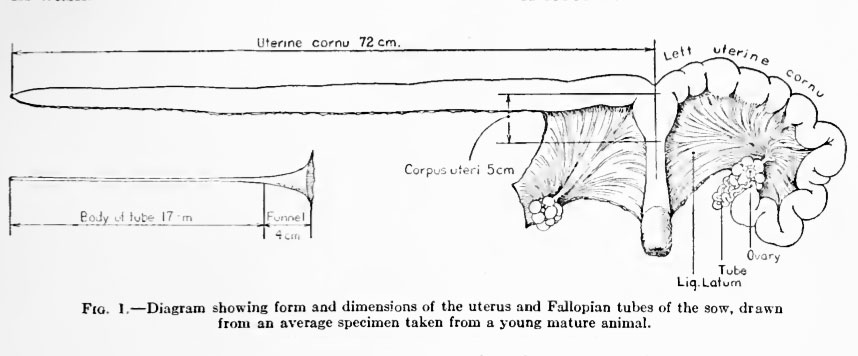
Fig. 1. Diagram showing form and dimensions of the uterus and Fallopian tubes of the sow, drawn from an average specimen taken from a young mature animal.
Reference to figure 1 will make the following description clear. The Fallopian tube is freed from the mesosalpinx by cutting the latter off close to the tubal border and is detached from the uterus at the junction of the tube and cornu. The now straightened tube is suspended by one hand over a Syracuse dish and is filled, by means of a pipette, with sufficient normal saline solution to distend the lumen moderately. The narrow uterine end of the tube prevents the fluid from draining into the dish until the tube is gently "milked" out with the fingers. If ova are present they usually pass out with the first drops of fluid, but in some cases four or five washings are necessary to recover all that are present in the tube. The ova are located in the dish by inspection of the washings under a low-power microscope.
This method is rather awkward when apphed to the whole uterus because of the large quantity of fluid required to distend the canal, amounting often to 300 c.c. For this reason recourse has been had to a plan by which a small amount of fluid (10 to 30 c.c.) is caused to distend successive short portions of the uterus. The uterine cornu having been uncoiled by cutting it from the mesometriimi, a stout clamp is applied to it a few centimeters below the ovarian extremity and the distal portion is dilated with the salt solution; another clamp is now applied a few centimeters below the first, which is removed, and the fluid is forced by gravitj^ and by digital pressure into the second section of the uterus, which is thus in turn distended, and so on. In this manner any ova or unattached embryos which are present are picked up and carried along until the fluid has passed through the whole length of the uterus, and are then deposited with the washings in one or two Syracuse dishes.
The washing-out method is very satisfactory for recovering ova and vesicular embryos from the uterus, but if it is inadvertently applied to a specimen containing embryos of the latter part of the second week or of the third week, which have undergone the extraordinary extension in length of the chorion which is characteristic of the pig and some other ungulate species, the result will be an almost hopeless tangle of chorionic membranes. Therefore, it is a wise precaution, when expecting early embryos of uncertain age, to slit up the cervical end of the uterus for a distance of several centimeters, watching for the delicate, glutinous, threadlike appearance which marks the early chorion. If none is found, then any still earlier embryos which may be present are presumably grouped somewhere in the remaining part of the uterus and may be recovered by washing.
Embryos of the third week and older have been removed by the usual method employed by embryologists, namely by cautious opening of the uterus under salt solution in a well-illuminated glass dish against a dark background. The salt solution used in the work has generally been prepared according to the formula of Locke, except for the omission of glucose.
The large size of the mature Graafian follicle also presents difficulty when it is desired to locate the undischarged ovum. The use of serial sections of the whole follicle is sometimes necessary, in which case much labor can often be saved by embedding in celloidin and employing an assistant to cut the sections, which the observer examines in turn as they are cut, until the cumulus oophorus begins to appear in the sections, after which still greater care is used until the ovum itself is reached. Another method is to lay the hardened ovary into serial slices of about 1 mm. thickness and then to search the follicle walls in the slices under the binocular microscope until the cumuli are found. The ovum-bearing portion of the follicle is cut out of the slice and sectioned in paraffin. As there are usually several follicles in each ovary, the writer's practice has been to try the latter method, reserving enough of the follicles to assure success by the former plan if the latter fails.
Histological preparations have been made by the usual methods. Bouin's picro-acetic-formol has been the chief fixing fluid, supplemented, of course, by a variety of others for special purposes. The stains used will be specified in connection with the illustrations. When it was necessary to wash out the uterus, a block for histological preservation was first cut from the middle of one cornu in order to spare the tissues any harm resulting from the fluid and from the pressure; the portions remaining were then separately washed out for the ova. As it is difficult to cut thin sections of the sow's uterus except from very small blocks, complete transverse sections of each uterus were made by the celloidin technique to serve as reference for the small blocks used for study of cytological and microchemical details.
The writer's thanks are due to the American Feeding Company and to Hohman & Sons of Baltimore, to the Western Meat Company of San Francisco, and to Joseph Stern & Company of New York City for opportunity to collect this material; and to the Department of Embryology of the Carnegie Institution for technical assistance.
Growth of the Graafian Follicle and Maturation of the Ovum
The ovaries of the large animals are not well suited to the study of the various problems connected with the growth of the Graafian follicle, on account of the obvious physical difficulties which prevent systematic examination of large numbers of follicles and ova. Sufficient information has been gained, however, with respect to the pig, to support an outline of follicular growth and to correlate its events roughly with those of the oestrous cycle. It appears that normal follicles of maximum size are found only during the period of a few days previous to ovulation. At all other times there is what might be termed a reserve stock of smaller follicles in the ovary, forming a series of all sizes from the microscopic primordial stage to a diameter of about 5 mm. Judging from the evidence of the 22 mature sows mentioned above, it seems that practically all actively functioning ovaries contain a number of follicles of 3.5 to 5 mm. diameter, which are in readiness for the final enlargement which they are destined to undergo just before rupture. Follicles of larger size are usually found to be atretic, although in a few cases there are normal follicles of 6 and even 7 mm. diameter during the intercestral period.
At a time not as yet accurately determined, but which can not be more than 2 or 3 days before the onset of oestrus, there begins a rapid enlargement of those follicles which are to discharge their ova, bringing them to a diameter of 7, 8, or even 10 mm. ; and at the same time there is a series of histological changes, which, in the smaller animals, have long been known to be connected with the maturation of the ovum. These consist of growth of the theca interna by enlargement of its cellular elements and of a partial dissolution of the cumulus oophorus by separation of its cells, so that the ovum is finally almost freed from its originally firm anchorage to the follicular wall. A full description of these changes will be found in the author's paper of 1919. The ovum itself goes through the first stages of the process of maturation, its nucleus moving toward the periphery of the cell, to form first the t>i)ical germinative vesicle, and then to undergo mitosis and extrusion of the first polar body.
The study of new material has not modified the opinion already expressed in a brief note (1917) that the process of maturation of the ovum follows in the pig the same course as in other mammals. At the moment of rupture of the follicle the first polar body has been discharged and the second polar spindle has been formed, but completion of the second polar body does not take place unless the ovum is fertilized.
Rupture of all the follicles seems to take place simultaneously or at least within a brief space of time, for in a careful examination of perhaps 200 pairs of ovaries containing recently ruptured follicles there has been only one case in which there were also normal, mature, uncoliapsed follicles. A few other specimens which appeared to be of this sort proved upon section to be atretic. This conclusion is contrary to that of Ktipfer (1920), who thinks that an appreciable space of time may be required for the rupture of a group of follicles, but the specimens upon which he bases this statement have apparently not been subjected to microscopic examination. Corner and Amsbaugh (1917) concluded, from a stud}^ of 10 animals, that ovulation probably occurred on the first or second day of oestrus; but the exact time of onset of heat in these animals could not be stated with great exactness, since the pens were visited but twice daily, and in some cases less often. A somewhat different conclusion is drawn by Lewis (1911) from the results of his experiments, unfortunately not known to us at the time of our work. His material consisted of 23 sows, in which the onset of heat and the time of copulation were noted with exactness, the animals being killed at varying times thereafter. In those killed before 30 hours after onset of oestrus the follicles were not ruptured (with one exception), but in most of those killed between 30 and 48 hours the follicles had collapsed. In one case ovulation had not occurred at 70 hours, but as no microscopic examination is mentioned, we can not exclude the possibility that this last case is one in which the follicles had undergone atresia instead of rupture. Le\Ais's interpretation is that "the ovum (in hogs) is not liberated from the ovary until the last part of the period of heat, " but from his table one is forced, rather, to conclude that ovulation usually occurs during the second day of the period of oestrus.
Fate of the Ovum: Passage and Attachment of the Embryos
The time necessary for the passage of the ovum through the Fallopian tube has been set by Assheton (1898) at 3 days, and the present writer's findings are in accord with this statement. This journey of 18 or 20 cm. is therefore traversed in the same time as the far shorter distance in a small mammal like the mouse. If copulation has occurred, the conjugation of ova and spermatozoa takes place in the Fallopian tube and segmentation reaches the stage of 2, 4, or 6 blastomeres by the time the uterine end of the tube is reached. About the fourth day the ova enter the uterus.
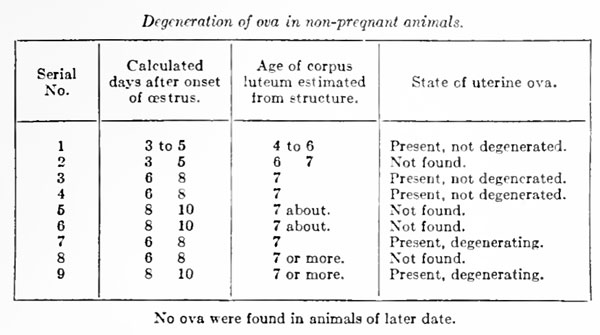
Much interest attaches to the fate of the ova when copulation does not occur. Degenerating ova are but rarely found in the tubes, and one is therefore forced to suppose that all the ova pass into the uterus, whether or not the sow has been impregnated. This conclusion is strengthened by the fact that if from the run of sows at the abattoir one selects those whose ovaries contain fresh-looking solid or nearly solid corpora lutea (i. e., 5 to 7 or more days after ovulation), a considerable proportion of these will be found, upon careful search, to contain either normallooking or degenerating unfertilized ova in the uterus. Scores of such observations, confirmed in numerous cases by microscopic estimation of the age of the corresponding corpora lutea, make it seem clear that the unfertilized ova regularly pass into the uterus and degenerate there, not disappearing completely, however, before at least the seventh day after ovulation.
Some information as to the probable time of their final dissolution can be obtained from the following table of cases in which the ova were sought in the uteri of animals whose last oestrous period had been observed, and which had not been impregnated.
We shall not be far wrong, therefore, in assuming that the unfertilized ovum of the sow disappears by degeneration in utero at about the seventh or eighth day after its discharge from the follicle. Degeneration of the unfertilized ovum is characterized by division of the cytoplasm into rounded masses, which are usually not uniform in size, but sometimes are sufficiently regular to simulate segmentation. These masses ultimately contract so as to present a shrunken appearance within a zona pellucida which is often swollen but at the same time less refractive. The zona pellucida frequently loses its spherical shape and at the last may present only a vague, flattened halo, containing minute punctate granulations, around a few shrunken masses of cytoplasm .
Of still more importance, in affording a basis for correlating the anatomical events of the reproductive cycle, is the question of the rate of groT\i:h and the time of implantation of the fertilized ovum. Fortunately for our purposes, pig embryos of known ages have now been seen at all stages of earl} development. The present writer, with Amsbaugh (1917), had the opportunity of completing the series by the observation of ova immediately after fertilisation, before conjugation of the pronuclei. The careful studies of Assheton (1898) on about 100 embryos carry the history from the stage of two blastomeres until the tenth day, and 30 more specimens of the ninth, tenth, and eleventh days have been described by Wej'sse (1894). From the fourteenth to about the twenty-fifth day we have the detailed data of Keibel's Normentafein (1897). The following account of the early embryology of the pig is based upon the contributions just mentioned and upon personal study of a series of specimens of all stages.
Segmentation begins in the tube and continues after passage of the ova into the uterus; no specimen beyond the stage of 6 blastomeres has yet been observed in the tube. Assheton found that various sows killed on the sixth day contained uterine embryos from 6 blastomeres to fairly well-developed blastodermic vesicles. By the seventh day the zona pellucida has disappeared and two layers, epiblast and hjT^oblast, may be distinguished in the inner cell-mass of the vesicles. 'Qy the eighth or ninth day the vesicles begin to be wTinkled and then to undergo the remarkable elongation which is so characteristic of early pig embryos. By the twelfth day the vesicles are 10 to 12 mm. in length by about 3 mm. in diameter at their widest part; and by the fourteenth day they are 20 to 30 cm. long, with embryonic areas showing the neural groove well developed and possessing 1 to 5 somites. At 17 or 18 days the uterine cavity is completely filled by the embryonic envelopes, which, when their accordion-like folds are eliminated by tension, arc found to have reached their full length of 30 to 40 cm.
It is important to remember that the relations between maternal and fetal tissues are of the simplest character in this species. No decidua is formed from the uterine mucosa, which retains during gestation a structure not widely different from that of the non-pregnant period, and the chorion simply becomes applied, over practically its whole surface, to the epithelium of the uterus (fig. 33, pi. 4). Moreover, the chorion itself remains a simple one-layered membrane, against the inner surface of which, after the third week, the vascular allantois is intimately applied. Nutritive substances passing from mother to embryo must then traverse both the uterine epithelium and the columnar chorionic epithelium in order to enter the fetal (allantoic) vessels. At all periods of pregnancy the chorion is readily detached by gentle traction and at parturition the fetal membranes separate from the uterine wall without lesion or hemorrhage, leaving an intact surface an arrangement which seems excellently adapted to minimize the strain of giving birth to litters sometimes as numerous as 20 or more.
In view of the diffuse non-deciduate placentation, it is not possible to define sharply the time of implantation. We can say only that after about the tenth day growth of the vesicle is so extensive that it must then be considered dependent upon maternal nutrition rather than upon its own resources; by the thirteenth day, if not earlier, its position is fixed; by the fifteenth the chorion has come into relation with a large part of the uterine mucosa; while during the third week the allantois begins to fine the inner surface of the chorion and hence brings fetal and maternal tissues into their definite relationship. It is the period from the tenth to the fifteenth day that is most nearly comparable to the much more precisely limitable act of implantation which occurs in animals like the rabbit and guinea-pig, and presumably in man.
II. Ovary - Origin and Completed Structure of the Corpus Luteum
The author's views as to the origin of the corpus luteum have been fully confirmed by the examination of new material obtained in connection with the study of the uterine cycle, amounting to about 35 animals taken in the first 8 days after ovulation. For a detailed description of the changes undergone by the discharged follicle during its conversion into the corpus luteum, the reader is referred to the author's article of 1919; at the present time a brief summary will suffice as introduction to an, account of the subsequent history and degeneration of the corpus luteum.
When the Graafian follicles collapse at the time of rupture, the extrusion of the follicular fluid and the contraction of smooth-muscle cells in their walls reduce their diameter of 8.5 to 10 mm. to 4 to 6 mm. By the end of one week's growth, however, the corpora lutea are again 8 to 9 mm. in diameter, and if pregnancy ensues there is a further growth until, after 2 or 3 weeks, the maximum size of 10 to 11 mm. is reached. The membrana granulosa is retained intact, except for loss of the cumulus oophorus, after rupture of the follicle. Its cells increase in size without division, their cytoplasm becomes laden -with lipoid substances, and they become the larger elements, commonly known as "lutein cells," in the fully formed corpus luteum. During this process the membrana granulosa is invaded by blood-capillaries from the theca interna, which ramify to form an extensive vascular plexus throughout the new structures. The large lipoid-laden cells of the theca interna of the Graafian follicle are increased in number by mitotic divisions and pass into the corpus luteum to become lodged between the granulosa cells throughout the whole structure. There is no evidence that the cells of the theca interna are ever converted into fibroblasts of the usual spindle-cell type or that they lay down the fibrils of the close-meshed reticulum which is present in the corpus luteum.
The time relations of these changes may be given as follows : ovulation probably occurs on the second day of oestrus ; invasion of the granulosa by blood-vessels and theca interna cells begins on the third or fourth day after onset of oestrus and is completed about the sixth or seventh day; by the seventh day the corpus luteum is usually solid and its cells have become fully differentiated.
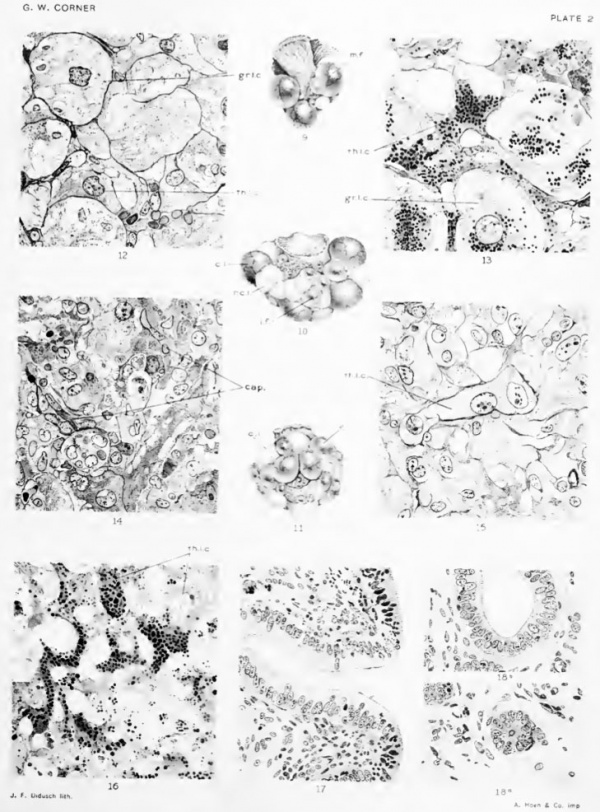
Kupfer (1920) has given a valuable series of colored plates representing the gross appearance of the follicles and of the corpora lutea at various stages of development and retrogression. unfortunately, the figures were not made from specimens of known relation to the oestrous cycle, and for this reason we have provided (plate 2) drawings of ovaries of 3 sows killed at known periods: during oestrus (fig. 9); at 8 days after ovulation, with completely solidified corpora lutea (fig. 10); about 17 days after ovulation, with degenerating corpora lutea and a new group of follicles beginning their preoestrous enlargement (fig. 11).
The corpora lutea of a sow at the tenth day after ovulation are very conspicuous objects by reason of their size, reaching 8 to 9 mm. in diameter and projecting nearly all their bulk from the ovary, so that when numerous corpora are present the remainder of the ovarian substance is dwarfed in comparison. At this stage the corpora are nearly always solid, though at times one or two in an ovary may remain slightly cystic. The cut surface bulges from the capsule and presents a somewhat velvety texture of pink color without trace of the yellow and orange pigments which are so characteristic of the corpus luteum in the bovine and human ovaries. The consistence of the corpus luteum at this time is not unlike that of the pig's liver.
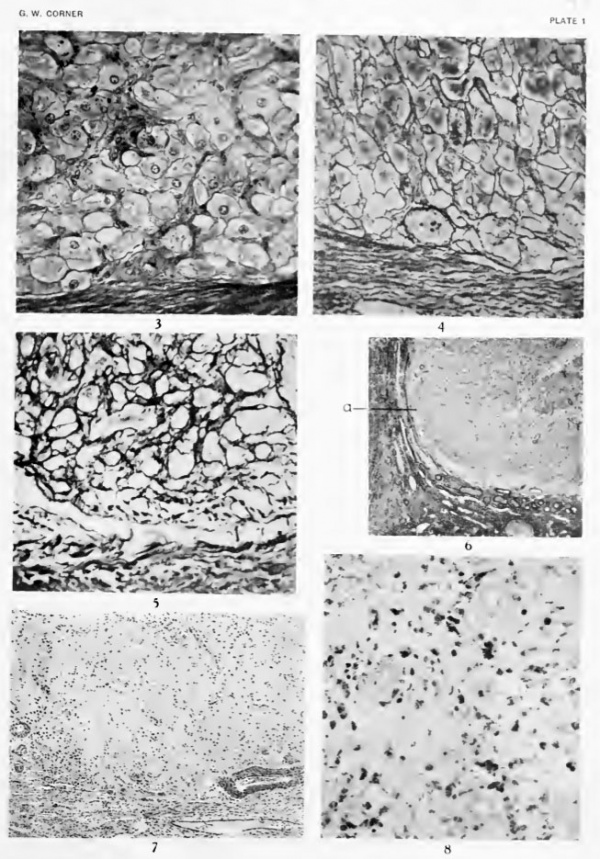
In microscopic sections (fig. 3, plate 1; fig. 12, plate 2) the granulosa lutein cells form the most conspicuous element of the tissue, since they are distinguished both by their large size (reaching the diameter of 30 to 40 micra) and by the elaborate cytoplasmic patterns which they contain after fixation in slow aqueous fixatives hke formol, Bouin's picro-formol-acetic fluid, etc. As previously described (1919), the granulosa lutein cells of certain species contain a large amount of a lipoid or mixture of lipoids, probably associated with proteins, which is sufficiently oily to round up into droplets when the tissue is submitted to the action of water. The droplets thus produced usually surround the pre-existing globules of neutral fat, which are also present in considerable numbers in the granulosa lutein cells of swine, but these latter are readily removed by the alcohol, ether, and xylol of the usual histological procedures, and hence the final appearance is usually such as shown in figure 12 (gr. I. c), in which the hpoid droplets are seen as hollow spheres (appearing as rings in thin sections) more or less retracted from the surrounding cytoplasm. The bodies in question are not seen in fresh tissues, nor in cells fixed very promptly in rapid coagulants like osmium tetroxide, which presumably precipitate the proteids before the oil droplets round up. The appearances described, therefore, are simply the result of methods of fixation which do not preserve certain obscure lipoids in their natural diffused state ; but the artifact has proved useful in several ways, notably in tracing the history of the granulosa derivatives.
Besides the granulosa lutein cells and the blood-vessels, the corpus luteum contains cells of another type, whose origin has been traced by the author to the theca interna of the Graafian follicle. Corpora of the eighth to the tenth day always contain a few distinct clumps of theca interna cells in their original position about the periphery of the corpus luteum and along the vessel-bearing septa which pass radially inward where the follicle-wall was infolded by collapse at ovulation. Many others of the theca cells, however, have wandered among the granulosa lutein cells, from which they can usually be distinguished by smaller size (diameters of 10 to 25 micra), by a more deeply staining cytoplasm, which is often densely packed with minute regular vacuoles, giving a characteristic foamy appearance, and (in osmium preparations) by the presence of plentiful fat globules which vary greatly in number and size (fig. 13, plate 2, th. I. c). The theca lutein cells often have squared or irregular outlines, fitting into the interstices between the swollen rounded surfaces of the larger granulosa lutein cells (fig. 12, plate 2, th. I. c).
The tissue is held together by a framework of reticular connective fibrils which, as illustrated in figure 4, plate 1, from a preparation by Bielschowsky's method, form a dense network about all the lutein cells. In view of the apparent scantiness of fibroblasts of the usual type, the presence of so complete a reticulum gave cause for surprise until it was found (Corner, 1920) that here, as in a number of other organs, the capillary endothelial lining is the source of the reticulum. As the capillary bed is so complex that every cell in the gland is in contact with a blood-vessel, it is not difficult to understand the great density of the reticulum.
Retrogression of the Corpus Luteum
It is a fact of the greatest importance that the changes, just summarized, which lead up to the formation of the corpus luteum, take place with equal completeness, whether or not the sow has been impregnated. It is not possible to distinguish the corpus luteum of pregnancy from that of unfertilized ovulation during the first 2 weeks after discharge of the ova, nor can the observer determine, from the appearance of two specimens of equal age, which of them was destined to further growth in size and a 4 months' span of activity, and which to immediate retrogression.
The time of degeneration of the corpus luteum of unfertilized ovulation can be determined 'with fair accuracy, for the data, though few, are in complete accord with each other. Seven animals of our series were taken 15 or more days after an observed oestrus but before another ovulation had occurred, and all of these contained retrogressing corpora lutea, while all those killed earlier showed no sign of degeneration; 2 of the 7 had begun a second oestral period and thus gave a further check upon the time relations. It is upon the fourteenth or fifteenth day, then, that a sudden change overtakes the structure which has been so elaborately erected. Within 2 or 3 days the diameter of the corpus has decreased from 8.5 or 10 mm. to 6 mm. ; its color has changed from the pink of an active capillary circulation to the whitish tone of scar-tissue, and its texture has much increased in firmness and toughness. Microscopic examination (fig. 6, pi. 1; fig. 14, pi. 2) shows the change to have been so rapid as almost to defy comparison with the previously existent conditions, and exact analysis of what has happened is thus rendered difficult. An example of the suddenness of the break-down is given by one of the animals, killed on the fifteenth day, in which some of the corpora were degenerated while others were intact, but in one corpus there was a patch of advanced degeneration surrounded by unchanged tissue.
The most striking feature of the change is the disappearance of the granulosa lutein cells. In the sows whose ovaries show the least degeneration many of these cells can still be distinguished in various stages, from a fair state of preservation to almost complete degeneration (fig. 14, pi. 2), but in other animals of this period nothing remains but vague vacuolar spaces containing many pycnotic nuclei and nuclear fragments, where but a few days before were closely packed masses of cells, among the most imposing of the animal body. Since these cells in number and bulk formed by far the greater part of the corpus luteum, it is obvious that their breakdown alone will account for the decrease in size and for the relatively greater density of the retrograding corpus. Another result of the degeneration is seen at the periphery of the corpus luteum, where the former sharp demarcation between lutein tissue and the surrounding capsule of connective tissue is now obscured (fig. 6, plate 1). In one or two of the animals there are great numbers of poly-morphonuclear leucocytes in the tissues and also mononuclear wander-cells of the macrophage type, no doubt to serve the purpose of clearing away cellular debris.
The fate of the blood-capillaries is a matter of special interest in view of what has already been said about the capillary endothelium as the source of reticular connective-tissue fibrils. Deprived of much of their circulatory function by abolition of the granulosa lutein cells, the capillaries for the most part collapse, with nuclear degeneration in some cells, but remain in situ with the reticulum. There is at first no increase in the total amount of connective tissue, though the decrease in bulk of the corpus luteum crowds the fibrils into smaller space; but as retrogression proceeds (fig. 5, plate 1) the reticulum gradually becomes denser and thicker until it gains an appearance like that of coliaginous tissue, which is so characteristic of the corpora albican tia (fig. 7, plate 1). Whether in this change the endothelial cells are gradually replaced by inwandering fibroblasts, or themselves retain the fibroblastic function, can not be said with certitude, but there seems to be no a 'priori reason to doubt the latter possibility, in view of what we know of cellular dedifferentiation in general. It may at least be definitely stated that the fibrous tissue of the retrogressing corpus luteum is laid down in situ, for the Bielschowsky preparations make it clear that there is no direct growth of fibers from the surrounding ovarian stroma into the corpus.
It remains to discuss the fate of the cells of the third type, which the writer has described as theca lutein cells. These seem, surprisingly enough, to survive the blow which destroys the granulosa lutein cells; for among the debris and the collapsed capillaries, and also in occasional clumps at the periphery, are found numerous cells which, by reason of their usually angular or elongated shape, foamy cytoplasm, and wealth of osmium-staining fatty material, can hardly be deemed other than the theca lutein cells (figs. 15 and 16, plate 2, th. I. c). In one or two animals the osmium preparations show numerous globules of blackened fatty substance as large as 30 to 40 micra (represented, of course, in ordinary sections by vacuoles) which seem to he in some of these same cells, suggesting that they, too, are temporarily affected by the process of degeneration, to the extent of partial fatty degeneration. When after a few days the nuclear fragments and the vacuolar spaces left by the degenerated granulosa derivatives have disappeared (fig. 15, plate 2), the lipoid-laden cells are more clearly seen, enmeshed in the scar-tissue, where they persist for weeks, acquiring even denser stores of yellow-pigmented fat (fig. 8, plate 1) .
By the time of a new ovulation the corpora lutea have diminished to 6 mm. diameter, at the mid-intercestral period to 4 mm., and by the second ovulation (i. e., when their own age is about 6 weeks) to 3 or 2 mm. Kiipfer (1920) also finds that they can be traced through a second interoestral period. After this they can still be recognized in sections for at least another cycle of ovulation, but finally become so obscure that they can no longer be certainly distinguished from atretic follicles. It is interesting to note that the two specimens of retrogressing corpora lutea of pregnancy (known to be of the seventh and tenth days after parturition) which were briefly mentioned in the previous paper (Corner, 1919) are quite similar in microscopic appearance to those of the non-pregnant sow of the same relative period of retrogression; it is very likely, therefore, that there is no difference in the mode of degeneration of the two tjiJes of corpora lutea.
What is given above is the first attempt, except for that of Leo Loeb (1911a), deahng with the guinea-pig, to describe the Wstological details of retrogression of the corpus luteum from specimens of known age, and in view of the somewhat unorthodox result one is disinchned to enter upon a discussion of its general bearing, except in a tentative way. It may fairly be said, however, that should the writer's views of the structure of the corpus luteum, especially with regard to the fate of the theca interna, be borne out by other work, they will afford a means of reconciling one of the old disagreements of ovarian histology. It has often been pointed out that in cellular arrangement the theca interna of atretic follicles rather closely resembles the corpus luteum. Both tissues consist of large lipoid-laden cells supported by a reticular framework, with a rich capillary blood-supply. In the later stages of retrogression the two tissues are indeed confusingly alike, and, in the writings of Paladino (1879), Clark (1898), and many others, this fact has been one of the mainstays of the theca-origin theory of the corpus luteum. This idea (in connection with the view now held by nearly all investigators, that the so-calied "interstitial cells" found in some mammalian ovaries are derived from the theca interna of atretic folHcles) has been the basis of various attempts to correlate these three elements of the ovary, such, for instance, as that of Ancel and Bouin (1909).
Sobotta (1896), however, with his clear-cut demonstration, now generally accepted, that the granulosa layer of the follicle takes a very important part in corpus-luteum formation, could not agree to any statement of resemblance between the two types of follicle-derived tissue, and so expressed himself, when participating in a general discussion of the fate of the corpus luteum at the 1908 meeting of the Anatomische Gesellschaft. According to his description of events, in the corpus luteum the granulosa persists and the theca interna is used up in the production of connective tissue, while in atresia, of course, the granulosa breaks down and the theca proliferates.
Following, on the other hand, the present Writer's account of formation of the corpus luteum, and assuming that atresia folliculi, which has not yet been adequately studied in the pig, is the same here as in other species, it will be seen that the two processes differ in their course, but not greatly in their end-stages. The granulosa cells, which degenerate in atresia, degenerate also in retrogression of the corpus luteum, after their temporary metamorphosis into granulosa lutein cells; while the theca interna cells, which by our account do not revert to fibroblasts when they enter the developing corpus luteum, persist aHke in the degenerating corpus and in the atretic folhcle. In this way the cells of the membrana granulosa, derived presumably from the "germinal epithelium," are associated with the o\aim rather than with the soma, and those of each follicle run their course and disappear when their own particular ovum leaves the body, either by degeneration or by parturition; whereas the theca interna cells, presumably derived from the ovarian stroma, behave as if morphologically part of the somatic ovarian tissues, only temporarily bound up with the fortunes of the ovum. It is to be hoped that this conception may be tested by work on other species.
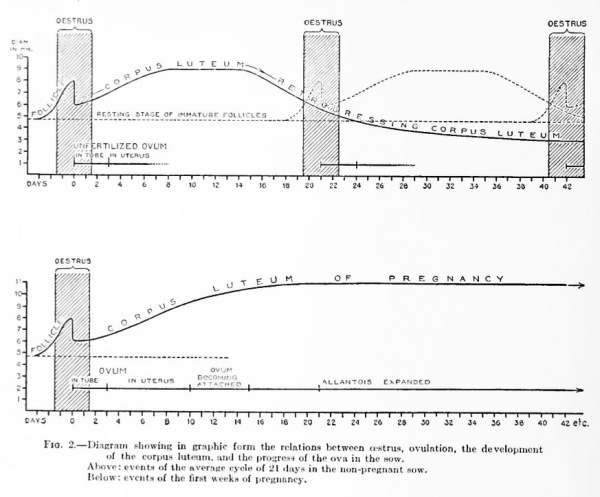
Text-figure 2 gives, in diagrammatic self-explanatory form, a picture of the ovarian cycle as we have described it in the foregoing pages.
Fig. 2. Diagram showing in graphic form the relations between oestrua, ovulation, the development of the corpus luteum, and the progress of the ova in the sow.
- Above: events of the average cyole of 21 days in the non-pregnant sow.
- Below: events of the first weeks of pregnancy.
III. Uterus - General Description of the Sow Uterus
During the period of sexual maturity the sow's uterus undergoes regular alternations of structure, correlated with the ovarian cycle, which are continuously in progress and are varied in their course only by the occurrence of pregnancy. These alternations involve changes in the degree of surface folding of the mucosa, in the morphology and dimensions of the surface epithelium, in the dimensions of the cells of the superficial gland tubules, in the division rate of all the epithelial elements, in the number and kind of wander-cells in the subepithelial portions of the storm, and in the amount of fluids in the interstices of the stroma. In the following pages these changes will be followed in detail.
The general structure of the cornual portions of the sow's uterus is illustrated in plate 3 and plate 4. There is a lining epithelium resting upon a stroma of connective tissue, and this in turn is seated directly upon the muscularis, which, as usual in tubular forms of the uterus, is disposed in two layers, the inner circular and the outer longitudinal. Into the stroma pass a large number of glands having openings in the epithelium.
The epithelium is fundamentally of a simple one-layered type, but (as we shall see) the columnar arrangement is greatly modified at one period of the cycle, so that some histologists (for instance Schmaltz, 1911) have even described it as "many-layered." We may suspect that there is a good deal of incompleteness in the descriptions of the uterine epithelia of other mammals, due, as in this ease, to lack of an understanding of the cychc changes.
The glands open into the uterine lumen by means of tubules which run more or less directly, with but little branching and slight tortuosity, until they are well into the stroma, when they begin to branch rather freely and to exhibit marked tortuosity. In this way the mucosa is divided into two fairly well marked layers, the superficial zone containing but few glands and the basal zone densely packed with glands so twisted and involved that in sections they are cut across in every imaginable way. There is a slight difference between the cells fining the glands of the two zones; those of the superficial tubules are higher, measuring usually 15 to 25 micra in height as against 12 to 18 micra in the glands of the basal zone. There is thus a greater proportion of cytoplasm to nucleus in the cells of the superficial tubules, and they look larger and clearer than those of the smaller and deeper-lying glands. Furthermore, the lumina of the superficial tubules are usually about twice as wide as those of the basal glands, as might be expected from the probability that many of the latter are drained by a few of the superficial tubules. There are no permanent shorter glands or crypts, as in the uterus of the dog and other carnivores. There are no ciliated cells in the surface epithelium of the sow's uterus, but cilia begin to appear immediately within the necks of the glands (fig. 17, plate 2), and may be found throughout the glands. They are better observed m the larger superficial tubules, not only because they are more easily seen in the \\ader lumina, but also because they are larger, stouter, and more numerous here. One may roughly guess that in this region one-fourth of the gland cells are ciliated. In the basal zone the ciliated cells seem fewer and the cilia are slighter and less obvious because packed into a narrow lumen (fig. 18, plate 2, a) ; but when by chance a dilated gland is found they can be seen to good advantage (fig. 18, plate 2, b). If a small piece of the fresh uterine mucosa is snipped off and flattened under a cover-slip, the cilia may be seen beating within the glands. It was by this method, and in fact with tissue from a sow, that the cilia of the uterine glands were first discovered by Nj'lander in Leydig's classroom (Leydig, 1852).
There is no evidence to indicate that there is other than a serous secretion from either glands or surface epithelium. There are no goblet cells in the uterine epithelia of the sow, and careful microchemical tests for mucin in smaller masses give negative results. Equally negative were the results of an effort to demonstrate unsaturated neutral fats by means of osmium tetroxide. The statements of Wegehn (1911), that there is a cyclic variation in the amount of glycogen demonstrable in the human uterine mucosa, suggested a similar study of the sow's uterus, especially since we had already observed glycogen in the fetal membranes in early pregnancy; but no glycogen could be demonstrated, either by Best's carmine method or by iodine after alcohol fixation, in any part of the uterine mucosa of the nonpregnant sow at any stage of the cycle.
The stroma of the uterine mucosa is no more nor less than a rather gelatinous or fluid-infiltrated areolar connective-tissue, through which course the glands, blood-vessels, lymph- vessels, and nerves, and which contains in the meshes of its fibers the various cells of areolar tissue, namely, fibroblasts, macrophages ("clasmatocytes"), plasma cells (often very numerous, but varying without reference to the cycle), and the various leucocytes. There is a slight condensation of the stroma just under the surface epithelium, forming a narrow subepithelial connective-tissue zone, of which the most superficial fibroblasts are flattened against the epithelium to form a basement membrane.
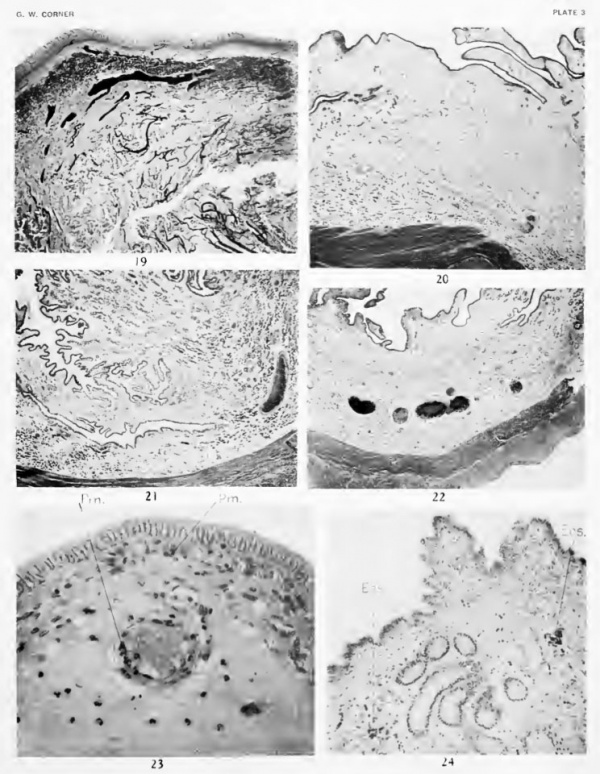
The blood-vessels (fig. 19, plate 3) pass through the muscularis, giving off branches to plexuses in and between the muscular layers, and then form a network of large channels near the base of the stroma. From this network long loops ascend toward the lumen, to end in a delicate capillary plexus in the subepithelial tissue, just below the epithelial cells.
The Uterus During Oestrus
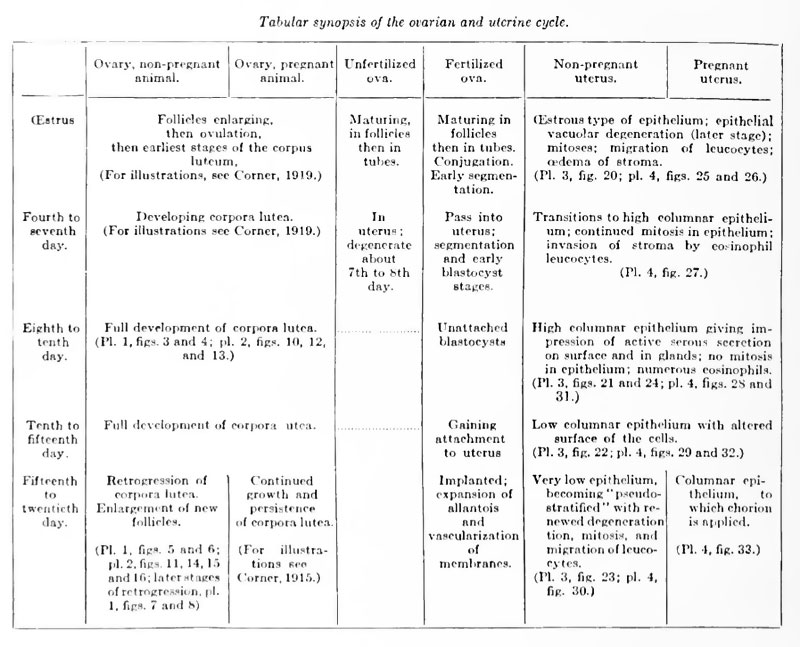
(Figure 20, plate 3; 25, 26, plate 4.)
Ova maturing in the Graafian follicles, or discharged and in passage the through the tubes. Earliest stages of the corpora lutea.
During the days of oestrus the uterine epithelium has a total thickness of 25 to 30 micra. As shown in the figures, it is not obviously columnar, but presents an arrangement which is deceptively suggestive of stratification. The cells are so closely compressed together laterally, and at same time have attained so low a form, that they are rather irregularly packed and the nuclei thus appear to be best arranged m several layers. On careful study many of the cells appear to extend from base to surface of the epithelium, but others seem to be impeded by their neighbors from reaching the free surface. The cells are small, since there is a relatively low proportion of cytoplasm to nucleus; so that, taking all these criteria together, a histologist coming upon such a tissue for the first time might perhaps class it among epithelia like those of the urinary bladder rather than among the secretory types of epithelial tissues.
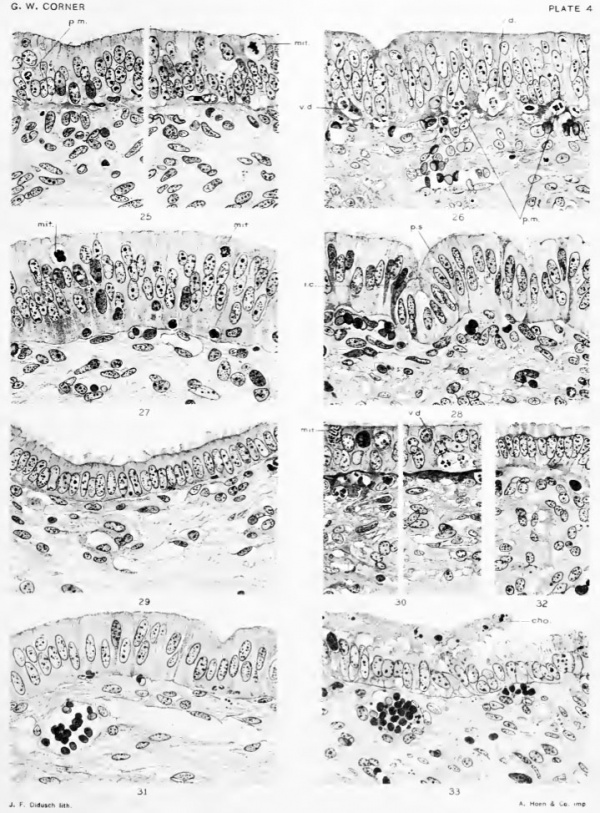
Three details are especially to be noted at this stage: First, mitotic figures are very numerous in the epithelium, in some specimens occurring as often as 1 in every 50 nuclei. The epithelium, therefore, is actively proliferating. Second, a contrary process is also indicated by the presence here and there of phenomena of degeneration; at the base of the epithelium there are points where two or three cells have become vague of outline, with chromatolysis of the nuclei, so that a small vacuole is formed in which he a few nondescript nuclear fragments or granules (fig. 26, plate 4, V. d.). This degeneration is, as we shall see, merely the latter stage of a phenomenon which sets in slightly earlier than the period of oestrus. The same may be said of the third fact of especial interest, namely, the presence, in large numbers, of neutrophilic polymorphonuclear leucocytes in the subepithelial connective-tissue, and even of a few which are embedded in the epithelium, presumably making their way into the lumen (figs. 25, 26, plate 4, p. m.).
During oestrus the stroma of the uterus, m mature animals, is very edematous, so that the interspersed cells are widely spaced (fig. 20, plate 3). There are a few mitoses in the cells of the superficial gland tubules, but none in those of the deep glands. A curious feature is the presence, in some of the gland cells, of highly chromatic extra-nuclear granules, reaching a diameter of 1 to 2 micra. The nuclei of cells possessing these granules are usually of normal appearance; but I have tentatively considered the granulation as a degeneration phenomenon affecting a few cells of the glands. It is seen only during the oestrous period.
Transition During First Week After Ovulation
(Figure 27, plate 4.)
- Ova in transit through the Fallopian tubes; then in the uterus, where they degenerate about the seventh or eighth day. Had they been fertilized the ova would now be passing through the stages of segmentation and blastocyst formation. The corpora lutea are in process of formation.
During the first week after ovulation we see the onset of changes which finally effect a striking alteration in the form of the surface epithelium. The individual cells at first merely grow larger, so that the layer which they form is further piled up to a thickness reaching 35 to 50 micra. The vacuolar spaces indicative of degeneration disappear by the end of oestrus, and by the time the eggs have passed into the uterus there are no more poly-morphonuclear neutrophil leucocytes to be seen in the epithelium or subepithelial stroma. Mitotic divisious of the surface epithelium continue to be very numerous until the end of this period, when they cease altgether, not to be seen again until just before the next oestrus. About the time of their cessation there is a further change in the morphology of the surface epithehal cells, which have been growing m height and which at last are somewhat suddenly ranged into a simple, high columnar epithelium, which will be described in the next section.
There is also a wave of mitotic division in the gland cells, which does not begin, however, until the ova are about to pass into the uterus (3 or 4 days after ovulation). In the superficial gland tubules the mitoses cease simultaneously with those of the surface epithelium, about the sixth or seventh day, but in the basal glands a few mitotic divisions may be seen a day or two longer, even after degeneration of the ova and establishment of the high columnar surface epithelium.
Eosinophil polymorphonuclear leucocytes, which are always present in small numbers in the uterine stroma, especially in the more superficial portions, undergo a great increase in number during the first week after ovulation. linlike the neutrophils of the oestrous stage, the eosinophils do not invade the epithelium, but remain in groups about the vessels and glands of the superficial zone.
There is a marked reduction of the edema of the stroma at the conclusion of oestrus, so that the connective tissue and the structures passing through it are again condensed into somewhat smaller compass.
Stage of Eight to Ten Days After Ovulation
(Figures 21, 24, plate 3; 28, plate 4.)
- The ova have degenerated and disappeared, Had they been fertilized they would now be large blastodermic vesicles, not yet fixed to the mucosa. Corpora lutea fully organized.
The surface epithelium now presents a striking contrast to its former appearance, for its cellular elements are tall and narrow and ranged in simple columnar form. The relative proportion of cytoplasm to nucleus is greatly increased and the nuclei generally occupy a central position. The cells vary in height from 20 to as much as 45 micra, and are arranged in such a way that the surface formed by their free ends is not smooth but wavy, giving rise to little hillocks, between which are depressions or pseudocrypts (fig. 21, plate 3). As we shall See, this arrangement aids in giving the mucosa, in the gross specimen, a soft and velvety texture. The whole appearance is now that of an actively secreting epithelium.
At this stage one notes particularly well a detail which has often been commented upon by observers of other species, including the human, and which is seen at all stages of the cycle, namely, the presence between the normal epithelial cells of others which are compressed laterally, have darker-staining cytoplasm, and usually compressed pycnotic nuclei (fig. 28, plate 4, i. c). They have been called "Stiftchenzellen," "cells with pycnotic nuclei," "intercalar cells," etc., and have been variously interpreted. I can see no reason to doubt that view which holds them to be degenerated cells in the act of extinction. Here and there one also sees small cells of uncertain provenance, with dense, round nuclei, which seem to have intruded themselves into angles at the bases of the tall epithehal cells upon the basement membrane.
At this time the invasion of the more superficial parts of the stroma by eosinophil leucocytes (described in last section) is at its height, as shown in figure 24, plate 3.
The cells of the superficial gland tubules now undergo a slight enlargement. From the seventh to the tenth day of the cycle they measure from 18 to 30 micra, at all other times from 15 to 20 micra. Likewise, the cells of the basal glands gain a httle in height, exceeding their usual limit of 10 to 15 micra and gaining a dimension of 15 to 20 micra. This enlargement of the gland cells is due to an increase in the relative amount of cytoplasm, so that they also gain an appearance as of active serious secretion. It should be mentioned here that at no time are there any marked changes in the morphology of the glands like those described in the human uterus by Hitschmann and Adler (1908) and others.
Stage of the Tenth to Fifteenth Day
(Figure 22, plate 3; 29, plate 4.)
- No ova in the uterus; had they been fertilized the embryos would now be gaining attachment to the uterus. Corpora lutea in a fully developed stage until degeneration sets in on the fifteenth day.
The stage of high columnar epithelium is succeeded about the tenth day after ovulation by another phase, which is characterized by a further modification of the surface epithelium. This consists of a reduction of the cells to a low columnar form, measuring from 15 to 20 micra, and by the extrusion (from the surface of each cell) of cytoplasmic processcs ranging from 3 to 8 micra in height, which will be more readily comprehended by study of the illustrations than from verbal descriptions. In SOme preparations the appearance is as if the free ends of the cells were frayed and eroded; in others the processes are rounded or pointed, and sometimes they might almost be taken for poorly fixed or agglutinated cilia, except for the absence of the basal granules which mark all true cilia in the uterine glands. (Compare fig. 29, plate 4, with fig. 17, plate 2)
We shall have occasion to return, in a later section, to the consideration of this very peculiar change of the epithelium. There appear to be no similar observations on record except those of Hitschmann and Adler (1908), who describe (in the late-interval and premenstrual stage of the human uterus) epithehal cells with frayed-out surfaces, and of Geist (1913), who illustrates, also in the human premenstrual stage, rounded protuberances very much like those of the pig. Geist considers that the protuberances consist of a secreted substance passing out of the cells into the lumen; but as far as our specimens are concerned there seems to be no reason for thinking them other than a modification of the superficial cytoplasm.
Coincident with the lowering of the height of the epithelial cells, there is a disappearance of the complex hillocky arrangement of the surface. The gland epithelium now returns to its usual size and the excess of eosinophil leucocytes disappears from the subepithelial stroma.
Stage of the Fifteenth to the Twentieth Day
(Figure 23, plate 3; 30, plate 4.)
- During the latter part of this stage a new group of follicles is prepared for ovulation. The corpora lutea are degenerating.
During the few days previous to a new ovulation the surface epithehal cells become so low (15 to 20 micra) that in some preparations they are of cuboidal form, hardly higher than their owoi nuclei. The surface protuberances diminish in height and finally disappear as the cells once more begin to be arranged into a pseudostratified epithelium. This stage marks what might be called the completion of the cycle; in the pig it is of brief duration, but in the sheep, where there is a long anoestrous period, the uterine mucosa apparently remains in such a resting stage during the 10 months or more of interval.
During the last days before the onset of oestrus, however, a few mitotic figures are found in the surface epithelium and at the same time there are numerous points of vacuolar degeneration of the epithelial cells. The most remarkable event of this stage is the assembling of large numbers of neutrophil leucocytes in the subepithelial stroma. In some of the specimens (fig. 23, pi. 3), the leucocytes are seen swarming out of every arteriole in the superficial stroma and passing toward the epithelium; a little later they are arranged along the bases of the epithelial cells and a few are to be found passing through toward the lumen. An cedematous state of the stroma also sets in toward the end of this period, and thus, by a combination of all these changes, the uterine mucosa is brought up to the oestrous stage, at which we began the description.
To conclude this cursory description of the uterine cycle, it may be added that the histological changes are not without effect on the general appearance and texture of the uterine mucosa. An opened uterus taken during the oestrous period is paler than at other times, with a firmer and at times slightly gelatinous inner surface (due to the oedema) ; while during the rest of the cycle, and perhaps especially from the ninth to the tenth days, the mucosa is pink or red, soft, and velvety. There is also a periodic change in the external dimension of the uterus, for, by plotting the circumferences (at the mid-points of the cornua) of the uteri of our 22 mature sows of similar age, in the order of their cyclic stages, a significant curve is obtained which is directly correlated with the degree of oedema of the stroma. In other words, the uterus is sHghtly larger just before and during the period of heat, because the stroma is then thickened by oedema. Caution must be used, however, in the interpretation of measurements and gross appearances as seen in specimens obtained at random at the slaughter-house, for there are numerous possibilities of uncontrollable variation, especially in the amount of bleeding of the carcases and in the ages of the animals. Some are taken very young, even before the first oestrus, and therefore long before the uterus has attained full development. A marked state of oestrous oedema does not seem to occur at the earhest heat periods.
For the same reason I have refrained from dogmatic statements on a point of some interest namely, whether there is a postoestrous hypertrophy of the glands, such as has been described, for instance, by Keller (1909) in the dog. Study of our 22 animals of known age and comparable size, though suggesting a positive answer, does not suffice to settle this question, since there are wide variations in the number of glands, and one is further confused by the difference in fluid-content of the stroma. It is difficult to compare specimens in one of which the glands are widely spaced and the stroma thick, while in the other the glands are densely packed in a narrow space.
Another uncertain matter concerns the numerical relations of the epithelial cells. There is a period of very active proliferation of both surface and glandular epithelium, but no time of widespread destruction. One is forced to assume for the present that the postoestrous wave of mitosis is compensated for by the sporadic degeneration of epithelial elements, which must be very frequent if the compressed cells (page 138) are, as suggested, in a moribund or degenerated state, and by the loss of cells due to the vacuolar degeneration of the preoestrous and oestrous periods.
The Uterus Mucosa During the Earliest Weeks of Pregnancy
(Figures 31, 32, 33, plate 4.)
It now becomes a matter of great interest to compare the histological state of the uterus during the earliest weeks of pregnancy with the cyclic alterations just described in the non-pregnant animal. The only available information on this point is given in brief form by Assheton (1906) in his description of the ungulate placenta. It has therefore seemed so important to make renewed observations on this subject that a series of early pregnant uteri has been assembled (by the methods outHned above, pages 123-124), including ova undergoing segmentation in the tubes and uterus, morulae and early blastocysts, and implanting embryos in the shield and earliest somite stages. These have been obtained from the butcher and are therefore wdthout exact data as to age; they have been arranged into a series by comparison of the corpora lutea and the stage of the embryos. The probable ages in days have been estimated from the data of Assheton (1898) on the rate of development of the pig embryo and from our own studies of the development of the corpus luteum (Corner, 1919).
Examination of these very early pregnant uteri gives a result which is as important as it is simple. The same histological changes are found during the first 15 days of pregnancy as during the 15 days following an oestrus without copulation. By the seventh day the CEstrous epithelium has passed into the high columnar stage, with hillocky arrangement of the surface; mitoses have ceased in the epithelium but are numerous in the glands ; eosiniphil leucocytes are present in large numbers in the subepithelial stroma. Thus by the time the ova have developed into large spherical blastocysts (eighth and ninth days) the uterus can in no wise be distinguished from a non-pregnant uterus 8 to 10 days after ovulation. (Compare fig. 28 with fig. 31, plate 4.) Two uteri containing wrinlded vesicles of age estimated at 11 days show low columnar epithelium with smooth surface, diminution of the hillocky arrangement of the epithelium, and few eosinophils. By the fourteenth or fifteenth day, when the chorion is in contact with a large part of the mucosa, the low columnar or cuboidal cells are covered with the curious frayed or rounded protuberances which we have seen to be characteristic of the non-pregnant uterus at the same time after ovulation (fig. 32, plate 4). These changes of course affect the more distant crevices and angles of the mucosa as well as those areas where the chorion is applied to the uterine surface. Where the chorion has been separated from its attachment to the epithelium, as inevitably occurs over large areas during fixation, it may be seen that the trophoblast is pitted and roughened by contact with the irregular surface of the epithelium (fig. 33, plate 4).
After this stage the identity of the histological processes in pregnant and nonpregnant uteri is at an end. At a time when the non-pregnant uterus is again undergoing preoestrous changes (18 to 20 days) the pregnant uterus presents even lower epithelial cells and greater roughening of the cellular surface, so that Assheton speaks even of a degeneration of the epithelium at this time (fig. 33, plate 4). The cells do not die off, however, but on the contrary again become of medium or high columnar type, and so persist, as is well known, throughout pregnancy. The stage of low epithelium with surface protuberances or roughening may quite as plausibly be considered not a degenerative phase, but one of physiological significance, in some way. (See page 143.)
Previous Accounts of the Mucosa of the Sow
Certain previous contributions to the cyclic anatomy of the pig's uterus deserve consideration at this point. Givkovitch and Ferry (1912) attempt a direct comparison between the changes of the human menstrual cycle and those of the pig's uterus. They describe, without giving the time relations, four stages of the corpus luteum: formation, full development, early and advanced involution; and they correlate with these stages four steps in the condition of the uterine mucosa, which they call prehypersemic, hyperaemic, posthyperaemic, and interval. Such a division docs not disagree with our present description, although we have not seen all the histological changes mentioned by Givkovitch and Ferry. It is to be regretted that their preliminary note has not been followed by a definitive account.
Stegu (1912) interested himself chiefly in the question of distribution of the uterine cilia. He had 60 animals at various stages of the cycle and made studies of the fresh mucosa in all of these with fixed and sectioned preparations of 15 of them. As a result of this work he has given the best account of the external features of oestrus that the present writer has seen; he speaks of ovulation during heat; hints at the oestrous oedema of the uterine mucosa, and mentions the degeneration of certain epithelial cells at this time. Being anxious to contrast oestrous with definitely non-oestrous uteri, he had but one animal killed between the end of oestrus and the tenth day thereafter; in this specimen he observed the hillocky arrangement of the epithelium, with crypt-like depressions intervening, which we have found to be characteristic of this stage.
IV. Summary and Discussion
It remains to consider how far our results have justified the hope, with which we began, that the reproductive cycle of the sow might prove illuminating in proportion to its simplicity. We have found, first, that there is a regular periodic chain of events in the ovary; beginning with rupture of the follicle during oestrus, the corpora lutea attain complete organization about the seventh day and hold their full development until the fourteenth or fifteenth day. We have seen that this interval is just long enough to cover the period during which the embryos are becoming attached, should pregnancy result from the ovulation. If no pregnancy occurs, atrophy of the corpora lutea begins about the fifteenth day.
In the uterus we have seen a correlated cyclic alternation. During oestrus there is a characteristic state of the uterine mucosa very similar to that which has been described by Loeb (1914) and by Stockard and Papanicolaou (1917) in the guinea-pig, and very fully and accurately by Long and Evans in their forthcoming monograph on the rat. During the time of formation of the corpora lutea the epithelium, glands, and stroma undergo a series of changes which attain their height about the same time that the corpora become sohd. From the eighth to the tenth day the uterine epithelium presents the appearance of active serous secretion, and then its cells lose height, subside to a low columnar or cuboidal form, and become marked by cytoplasmic protrusions or roughenings on their free ends. After the fifteenth day, when the corpora lutea begin retrogression, there is a slow reversion to the oestrous type of structure. A key to the understanding of these changes is found in the fact that an exactly similar histological progression occurs during the first two weeks of pregnancy, no doubt serving as a mechanism to permit attachment of the embryos. Recalling the elementary type of implantation in this species, one is tempted to form the simple, perhaps crude hypothesis that the uterine epithelium by its serous-secretory stage provides for flotation of the delicate embryonic vesicles and thus facilitates their migration and spacing in the relatively extensive uterine cavities (see Corner, 1921); but during the period of attachment the mucosa is, on the other hand, rendered glutinous by the peculiar surface roughening of the epithelial cells, in order to assist in attachment of the chorions.
Whatever the functional value of these histological details, the reader will agree that the processes described in this paper strongly suggest that the underlying fact of the uterine cycle of the sow is an upbuilding of the mucosa, presumably under control of the corpora lutea, for the purpose of successful implantation. Each act of ovulation is thus accompanied and followed by uterine changes, which either go on to placenta formation or (in the absence of embryos) subside once more, as do the corpora lutea, in preparation for a new ovulation.
Such a general conception has long since been suggested by the work of a number of investigators, including that of Hitschmann and Adler (1908) on the human uterus, L. Loeb (19116, 1914) on the guinea-pig, Kelier (1909) on the dog, Ancel and Bouin (1910) on the rabbit, and Hill and O'Donoghue (1914) on Dasyurus viverrinus. These writers did not see the changes exactly as we have seen them in the pig, nor do they agree in all particulars among themselves, though most of them describe a wave of cell-division in the epithelium and considerable growth of the glands and stroma in the days following ovulation. The discrepancies of detail are no doubt partly due to the fact that each of the animals used for these studies is a law unto itself, both as to anatomy of the reproductive organs and as to outward expression of the cycle. Preparation of the pig's uterus for the diffuse non-deciduate placentation of this species may well involve changes somewhat different from preparation for the elaborate implantation of the guinea-pig and man. Clearly, much remains to be done before satisfactory generalizations can be established.
Plates
Plate 1
|
Fig. 3. Section of the corpus luteum about 10 days after ovulation, showing fully developed granulosa lutein cells. Bouin's fluid, Mallory's connective-tissue stain. Photograph. X210. (Figure 12, plate ?, shows higher magnification of the same preparation.)
|
Plate 2
Plate 3
Plate 4
Bibliography
An-cel, p., and P. BooiN, 1909. Sur les homologies et la signification dea glandea h sfecrfetion interne de I'oiaire. Compt. Rend. Soc. Biol. Paris, vol. 67, pp. 464-466, 497-498.
1910. Reoherches sur les fonctions du corps jaune gestatif. I. Sur le ddterminisme de la preparation de I'utdrus 4 la fixation de I'ceuf. Jour, de Physiol, et de Path, gen., vol. 12. pp. 1-16.
AsSHETON, R., 1898-9. The development of the pig during the first ten days. Quart. Jour. Micros. Sci., n. s. vol. 41, pp. 329-359.
1906. The morphology of the ungulate placenta. Phil. Trans. Roy. Soc. London, Series B, vol. 198, pp. 143-220.
B.vRRT, M., 18.39. Researches in embryology. Phil. Trans. Roy. Soc. London, vol. 129, part 2, pp 307-380.
Clark, J. G., 1898. lirsprung, Wachstum, und Ende des Corpus luteum, nach Bemerkungen am Ovarien des Schweines und des Menschen. Arch f. Anat. u. Physiol., Anat. Abt., pp. 95-134.
Corner, G. W., 1915. The corpus luteum of pregnancy as it is in swine. Carnegie Inst. Wa«h. Pub. No. 222 (Contributions to Embryology , pp. 69-94.
- 1917. Maturation of the ovum in swine. Anat. Rec, vol. 13, pp. 109-112.
- 1919. On the origin of the corpus luteum of the sow from both granulosa and theca interna. Amer. Jour. Anat., vol. 20, pp. 117-183.
- 1920. On the widespread occurrence of reticular fibrils produced by- capillary endothelium. Carnegie Inst. Wash. Pub. No. 272 (Contributions to Embryology), pp. 85-93.
- 1921. Internal migration of the ovum. Johns Hopkins Hospital Bulletin, vol. 32, pp. 78-83.
- and A. E. Amsbaugh, 1917. (Estrus aad ovulation in swine. Anat. Rec, vol. 12, pp. 287-2i/l.
Geist, S. H., 1913. lintersuchimgen liber die Histologic der literusschleimhaut. Arch. f. mikr. Anat., vol. 81, I Abt., pp. 196-219.
GivKoviTCH, J., and G. Ferry, 1912. Sur les rapports de I'ovulation et de la menstruation (note preltminaire) Compt. Rend. Soc. Biol. Paris, vol. 72, pp. 624-626
Hill JP. and O'Donoghue CH. The reproductive cycle in the marsupial (Dasyurus viverrinus). (1914) Quart. Jour. Micros. Sci., 59: 133-174.
HiTSCHM.iNN, F., and L. Adler, 1908. Der Bau der literusschleimhaut des geschlechtsreifen Weibes mit besonderer Beriicksichtigung der Menstruation Monatschr. f. Geburtsh. u. Gynakol., vol. 27, pp. 1-82
IsHii, O., 1920. Observations on the sexual cycle of the guinea pig. Biol. Bull , Woods Hole, voL 38, pp. 237-250.
Keibel F. Normal Plates of the Development of the Pig Embryo (Sus scrofa domesticus). (1897) Vol. 1 in series by Keibel F. Normal plates of the development of vertebrates (Normentafeln zur Entwicklungsgeschichte der Wirbelthiere) Fisher, Jena., Germany.
Keller, K., 1909. Leber den Bau des Endometriums beim Hunde, mit besonderer Beriicksichtigung der cyclischen Veranderungen an den literindrusen. Anat. Hefte, vol. 39, pp. 307-391.
KuPFEK, M., 1920. Beitrage zur Morphologic der weibliohen Geschlechtsorgane bei den Saugetieren. Leber das Auftreten gelber Korper am Ovarium des domestizierten Rindes und Schweines. Vierteljahrsschrift d. naturf. Gesellsch. in Zurich, vol. 65, pp. 377 433.
Lewis, L.L., 1911. Thevitality of reproductive cells. Bull. No. 96, Agric. Exp. Station of Oklahoma.
Letdig, F., 1852. lieber Flimmerbewegung in den literindrusen des Schweines. Arch. f. Anat. u. Physiol., pp. 375-378.
LoEB, L., 1911a. The cyclic changes in the ovary of the guinea-pig. Jour. Morph., vol. 22, pp. 37-70.
- 1911b. lieber die Bedeutung des Corpus luteum fiir die Periodizitat des sexuellen Zyklus beim weiblichen Siiugetierorgaaismus. Deutsch. med. Wochnschr., 37 Jahrg..pp. 17-21.
- 1914. The correlation between the cyclic changes in the uterus and the ovaries in the guinea-pig. Biol. Buli., Woods Hole, vol. 27, pp. 1-44.
Long, J. A., and H. M. Evan'.%, 1920. The oestrus cycle of the rat, etc. Proc. Am. Assoc. Anat., 36th session, Anat. Rec, vol. 18, pp. 241-249. 1921. Further studies on the physiologj' of reproduction. Proc. Am. Assn. Anat., 37th session, Anat. Rec, vol. 21, pp. 56-63.
M.liisHALL, F. H. A., and W. A. Jolly, 1906. Contributions to the physiology of mammalian reproduction. Phil. Trans. Roy. Soc. London, series B, vol. 198, pp. 99-141.
Marsh.\ll, F. H. a., and E. T. Halnan, 1917. On the postoestrous changes occurring in the generative organs and mammary glands of the nonpregnant dog. Proc. Roy. Soc. London, vol. 89, series B, pp. 546-659.
NisKOliBiN.i, N., 1909. Recherches sur la fonction du corps jaune de la grossesse. Thesis, Nancy.
Paladino, G., 1879. Studio sulla fisiologia del I'ovaja. Struttura, genesi, e significazione del corpo luteo. Napoli.
Schmaltz, R., 1911. Die Struktur der Geschlechtsorgane der Haussiiugetiere mit anatomische Bemerkungen, Berlin.
SoBOTTA, J., 1896. lieber die Bildung des Corpus luteum bei der Maus. Arch. f. mikr. Anat., vol. 47, pp. 261-308. 1897. lieber die Bildung des Corpus luteum beim Kaninchen. Anat. Hefte, vol. 8, pp. 469-524.
Stegu, J., 1912. lintersuchungen am Endometrium des Schweines, mit besonderer Berucksichtig\ing des Flimmerepithels und der Brunsterscheinungen. Inaug. Diss., Wien; also in Osterr. Wchuschr. f. Tierhelik., pp. 399-443.
Stock-vrd, C. R., and G. N. P.AP.VNncOLAOu, 1917. The existence of a typical oestrous cycle in the guinea-pig, with a study of its histological and physiological changes. Am. Jour. Anat., vol. 22, pp. 225-265.
Strute, J., 1911. Die Perioden der Brunst bei Riudern, Schweinen, und Pferden. Filhling's Landwirtschaft. Ztg., Jahrgang 60, pp. 832-838.
SuRF.iCE, F. M., 1908-09. Fecundity of swine. Biometrika, vol. 6, pp. 433-436.
Wegelin, C, 1911. Der Glykogengehalt der menschlichen literusschleimhaut. Centralbl. f. aliegem. Path. u. path. Anat. vol. 22, pp. 1-8.
Weysse, a. W., 1894. The blastodermic vesicle of Sits scrofa domeaticua. Proc. Am. Acad. Arts and Sci., vol. 30, 283-321.
Description of Plates
Comparison of the plates will be facilitated by use of the tabular synopsia, page 144.
Plate 1
Fig. 3. Section of the corpus luteum about 10 days after ovulation, showing fully developed granulosa lutein cells. Bouin's fluid, Mallory's connective-tissue stain. Photograph. X210. (Figure 12, plate ?, shows higher magnification of the same preparation.)
Fig. 4. Corpus luteum about 13 days after ovulation, showing reticulum stained by Bielschowsky'a silver-impregnation method. Photograph. X210.
Fig. 5. Corpus luteum (degenerating), about 17 days after ovulation, showing increase in reticulum. Bielschowsky. Photograph. X210. (Same animal as fig. 15, plate 2.)
Fig. 6. Corpus luteum, degenerating, about 20 days after ovulation. Bouin, iron hematoxylin. Photograph. X25. a, blurring of delimitation between lutein tissue and the capsule, as described in text.
Fig. 7. Corpus luteum in well-advanced degeneration, about 4 weeks after ovulation, illustrating complete disappearance of the granulosa lutein cells. Bouin, iron hematoxylin. Drawing. XHO.
Fig. 8. Corpus luteum (degenerate) about 7 weeks after ovulation, showing numerous fat-bearing cells presumably derived from the theca interna. Osmium tetroxide fixation without other stain. Photograph. X 150.
Plate 2
Fig. 9. Ovary of a sow in cestnis. Natural size, m./., mature follicles.
Fig. 10. Ovary of a sow about 8 days after ovulatioh. Natural size. c. I., corpora lutea of the latest ovulation (8 days old). (See fig. 3, plate 1, and figs. 12, 13, plate 2.) r. c. I., retrogressing corpora lutea ol the next previous ovulation (8-|-21 days), i./., immature follicles.
Fig. 11. Ovary of a sow about 17 days after ovulation. Natural size. c. I., corpora lutea of the latest ovulation (17 days old), now retrogressing. (See fig. 14.) /., Graafian follicles enlarging preparatory to the next ovulation, due in about 4 days.
Fig. 12. Corpus luteum about 10 days after ovulation. Bouin, Mallory's connective-tissue stain. X700. gr. c. I., granulosa lutein cell. th. I. c, theca lutein cell.
Fig. 13. Corpus luteum about 10 days after ovulation. Osmium tetroxide fixation without additional staining, to show fat . X700. gr. I. c, granulosa-lutein cell. th. I. c, theca lutein cell.
Fig. 14. Corpus luteum in early degeneration about 17 days after ovulation, showing collapsed capillaries and degenerating lutein cells. Bouin, Mallory's stain. X700. cap., collapsed capillary blood-vessels.
Fig, 15. Corpus luteum in slightly later degeneration about 17 days after ovulation (not the same animal as fig. 14). Most of the cellular debris has disappeared. Bouin, Mallory's stain. X700. th. I. c, persistent cell, presumably a theca lutein cell.
Fig. 16. Corpus luteum in early degeneration about 17 days after ovulation (same animal as fig. 14). Osmium tetroxide for fats. X700. th. I. c, persistent fat-bearing cells, presumably theca lutein cells.
Fig. 17. uterus, showing mouth of gland, to demonstrate that cilia are present in the glands but not on the surface epithelium. Bouin, iron hematoxylin. X330.
Fig. 18. uterus, showing ciliated epithelium in glands lying at the base of the mucosa, near the museularis. Bouin, iron hematoxylin. X330.
Plate 3
Fig. 19. Section of uterus showing blood-veasels. Ink injection, carmine stain. Photograph. XIO.
Fig. 20. uterus during oestrus. Bouin, iron hematoxylin. Photograph. XIO.
Fig. 21. literus about 8 days after ovulation. Bouin, iron hematoxylin. Photograph. XIO.
Fig. 22. uterus about 15 days after ovulation. Bouin, iron hematoxylin. Photograph. XIO.
Fig. 23. Portion of uterine epithelium with subjacent arteriole about 17 days after ovulation, showing migration of polymorphonuclear neutrophil leucocytes from the blood-vessel. Bouin, carmine. Photograph. X500. ptn., polymorphonuclear leucocytes.
Fig. 24. Portion of uterus about 7 daj's after ovulation, showing accumulation of eosinophil leucocytes in the stroma. Photograph. X120. eoa., eosinophil leucocytes.
Plate 4
Sections of sow's uterus at various periods of the cycle. Fixed in Bouin's fluid, cut at 5 micra, and stained with iron hematoxylin. Drawn at uniform magnification of 000 diameters.
Fig. 25. uterus during aatrus.
p. VI., polymorphonuclear leucocyte en route through the epithelium. mit., mitotic figure in dividing cell.
Fig. 26. uterus during astrus. ». d., vacuolar degeneration. p. m., polymorphonuclear leucocytes at base of epithelium.
Fig. 27. uterus (non-pregnant) about 6 days after ovulation, mil., mitotic figure in dividing cell.
Fig. 28. uterus (non-pregnant) about 8 days after ovulation, p. a., pseudocrypt. i. c, intcrcalar cell.
Fig. 29. uterus (non-pregnant) about 1 1 days after ovulation.
Fig. 30. literus (non-pregnant) about 10 days after ovulation. mit., mitotic figure in dividing cell. r. d., vacuolar degeneration.
Fig. 31. literus (pregnant) about 8 days after ovulation.
Fig. 32. literus (pregnant) 14 to 15 days after ovulation.
Fig. 33. literus (pregnant) 18 to 20 days after ovulation, cho., chorion of embryo, partly detached.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 1) Embryology Paper - Cyclic changes in the ovaries and uterus of swine and their relations to the mechanism of implantation (1921). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Cyclic_changes_in_the_ovaries_and_uterus_of_swine_and_their_relations_to_the_mechanism_of_implantation_(1921)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G